Abstract
Micro RNAs (miRNAs) constitute a unique class of small, non-coding ribonucleic acids (RNAs) that regulate gene expression at the post-transcriptional level. The presence of two inducible miRNAs, miRNA-125b and miRNA-146a, involved in respectively, astroglial cell proliferation and in the innate immune and inflammatory response, is significantly up-regulated in human neurological disorders including Alzheimer’s disease (AD). In this study we analyzed abundances miRNA-125b and miRNA-146a in magnesium-, iron-, gallium, and aluminum-sulfate-stressed human-astroglial (HAG) cells, a structural and immune-responsive brain cell type. The combination of iron- plus aluminum-sulfate was found to be significantly synergistic in up-regulating reactive oxygen species (ROS) abundance, NF-κB-DNA binding and miRNA-125b and miRNA-146a expression. Treatment of metal-sulfate stressed HAG cells with the antioxidant phenyl butyl nitrone (PBN) or the NF-κB inhibitors curcumin, the metal chelator-anti-oxidant pyrollidine dithiocarbamate (PDTC), or the resveratrol analog CAY10512, abrogated both NF-κB signaling and induction of these miRNAs. Our observations further illustrate the potential of physiologically relevant amounts of aluminum and iron sulfates to synergistically up-regulate specific miRNAs known to contribute to AD-relevant pathogenetic mechanisms, and suggest that antioxidants or NF-κB inhibitors may be useful to quench metal-sulfate triggered genotoxicity.
Keywords: Aluminum sulfate, Alzheimer’s disease, Brain gene regulation, Curcumin, Gallium sulfate, Genotoxicity, Glial cell proliferation, Inflammation, Iron sulfate, Magnesium sulfate, Micro RNA (miRNA), Primary human astroglial (HAG) cells, Pyrollidine dithiocarbamate (PDTC), Reactive oxygen species, Resveratrol analog CAY10512, Synaptic deficits
Micro RNA (miRNA)-mediated RNA interference is a recently discovered genetic mechanism involved in the regulation of gene expression at the post-transcriptional level [1–5]. Human brain cells and tissues utilize only a fraction of the ~900 currently known human miRNAs, and only a highly selective subset of these is altered in neurodegenerative disorders, such as Alzheimer’s disease (AD) [2,3,5–10]. Specifically, increases in the expression of miRNA-146a significantly regulate the abundance of two of miRNA-146a’s major targets, complement factor H (CFH) and interleukin-1 receptor associated kinase (IRAK) mRNAs, key modulators of the brain’s innate immune and inflammatory response [8–11]. The brain-relevant mRNA targets of miRNA-125b, and the brain cell types in which miRNA-125b and miRNA146a are expressed, are not well understood. Specific miRNAs are emerging as important regulators of brain gene expression in both health and disease, and this is typically accomplished by interacting via base pair complementation with the 3′ un-translated region (3′ UTR) of target mRNAs, thereby quenching that specific mRNA’s effectiveness in gene expression [1–12]. Because up-regulated miRNAs have a considerable potential to down-regulate a critical number of target brain mRNAs, they provide an important explanation of why a generalized down-regulation of gene expression is observed in neurodegenerative disorders such as AD [5–12]. Interestingly, NF-κB up-regulation has been implicated in viral-induced, oxidative and pro-inflammatory stress in human brain cells and in AD [13–18]. These experiments are a logical extension of previous work performed in human neuronal and astroglial co-cultures (neural cells; human primary neurons do not grow well without the presence of astroglial support cells) [7]. In the current study we examined the role of magnesium, iron, gallium and aluminum, as sulfates, on reactive oxygen species (ROS) and NF-κB-sensitive miRNA expression in primary human astroglial (HAG) cells, a human brain cell line known to be involved in the innate immune and inflammatory response [7,11,19]. Here we identify two ROS- and NF-κB up-regulated miRNAs, miRNA-125b and miRNA-146a, highly sensitive to iron- and aluminum-sulfate induction in stressed HAG cells. miRNA-125b and miRNA-146a stress-mediated induction was found to be quenched using the electron spin-trapping antioxidant PBN or several independent classes of NF-κB inhibitors. Although other parameters of the inflammatory response need to be investigated, these novel results suggest that antioxidants and NF-κB inhibitors may have specific therapeutic value in reducing the effects of aluminum- and iron-sulfate mediated inflammatory responses and neurotoxicity toward brain-relevant gene expression.
Ultrapure reagents for molecular biology, including MgSO4 (63,133), FeSO4 (44,970), Al2(SO4)3 (11,044; Biochemika MicroSelect©; Fluka Ultraselect©; Fluka Chemical, Milwaukee, WI) and Ga2 (SO4)3 (254207-5G; trace metals grade, Sigma Aldrich, St. Louis MO), freshly prepared as 0.1 M stock solution[20–25], were instilled into either serum-containing or half serum strength human astroglial (HAG) cell maintenance medium (final individual metal concentration 1 μM or 2 μM, pH 7.5) by shaking, followed by filter sterilization using 0.2-μM spin filters (Millipore Corporation, Billerica, MA). Solutions were used at the following concentrations: MgSO4 (2.0 μM), Ga2(SO4)3, (2.0 μM), FeSO4 (2.0 μM), Al2(SO4)3 (2.0 μM), [MgSO4 +Al2(SO4)3] (1.0 μM each) or [FeSO4 +Al2(SO4)3] (1.0 μM each). HAG cells (derived from human embryonic stem cells; CC-2565; Lonza Walkersville, Inc, Walkersville MD) were grown using an astrocyte growth medium (AGM) consisting of astrocyte basal medium (ABM; Lonza CC-3187) supplemented with AGM SingleQuots (Lonza) as previously described by our group [19–21]. HAG cells tested positive for the glial-specific marker GFAP and tested negative for fibroblast contamination using antibodies against fibroblast-specific protein-1 [26–30]. Details of control, magnesium-, iron- and aluminum-sulfate treatment of HAG cells have been previously described [20]. Ultrapure water (18 megohm, Milli-Q, Millipore or Puriss 95305, Fluka) was employed throughout all isolation and biochemical procedures to stringently exclude trace metal contamination; as analyzed by electrothermal atomic absorption spectroscopy, aluminum, magnesium and iron content were ≤5 ppb. Coded isolation reagent and media samples were analyzed for potential trace metal contamination using a Perkin Elmer 5000PC Zeeman-type electrothermal atomic absorbance (EAA) spectrophotometer equipped with an automated sampler and IBM/AT-supported analysis package for trace metal analysis [10,20]. Wherever possible, ultrapure HNO3 washed polysulfone plasticware was used according to the URI-GSO protocols to stringently eliminate trace metal contamination [20,25–27]. As required, HAG cells were treated with (a) curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione diferuloylmethane; purity >98.5%, Axxora, San Diego, CA], dissolved in dimethylsulfoxide as a 100 mM stock solution and used at 5 μM ambient concentration in the ABM cell medium, (b) the metal chelator, anti-oxidant and NF-κB translocation inhibitor pyrollidine dithiocarbamate (PTDC; P8765; Sigma, St Louis, MO) at 25 μM of PTDC, or with (c) the polyphenolic trans-stilbene resveratrol analog CAY10512 (10009536; Cayman Chemical, Ann Arbor, MI) as previously described in detail [11].
Total RNA was isolated using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA), a phenol-guanidine isothiocyanate reagent useful for the simultaneous isolation of DNA, RNA and protein from the same tissue sample [8–12]. Total protein concentrations were determined using dotMETRIC protein microassay (Chemicon; sensitivity 0.3 ng protein/ml) using brain nucleoprotein as a standard [16–20]. All RNA isolation reagents were prepared in 0.2-μM filtered, DEPC-treated water (Ambion 9915G) in acid-washed glass and/or polysulfone plasticware pretreated with RNaseZap (Ambion 9780). Human RNase inhibitors (Ambion 2682 and 2690), ANTI-RNase (Ambion 2692; 1 unit/μl), RNasin (Promega N2115; 1 unit/μl) and/or SUPERase-In (Ambion 2694) were used throughout the isolation procedure to inhibit RNase 1, T1, A, B and C activities. Reactive oxygen species (ROS) levels were assayed in metal-sulfate-treated and untreated 2 week old HAG cells (Fig. 1) using 2′,7′-dichlorodihydro-fluorescein diacetate (H2DCFDA) at 10 μM in cell culture medium in the dark using protocols provided by the manufacturer (Molecular Probes, Invitrogen, Carlsbad, CA). H2DCFDA, cell-permeant indicators that react with the highly reactive singlet oxygen, hydroxyl radicals or superoxide-generating fluorescent signals (collectively termed reactive oxygen species or ROS), were quantified using electronic imaging photography under UV light (Ex 488 nm; Em 530 nm) using a Zeiss Axioskop/Zeiss MC63 photo control unit coupled to a Nikon Optiphot-2 microscope equipped with an additional differential interference contrast/Nikon UFX-DX photo control unit. Probe arrays were scanned with a confocal laser scanner (Agilent Technologies) at 570 nm. Pixel intensities were measured, expression signals were analyzed and features extracted using a commercial software package (Microarray Suite version 5.0, Affymetrix).
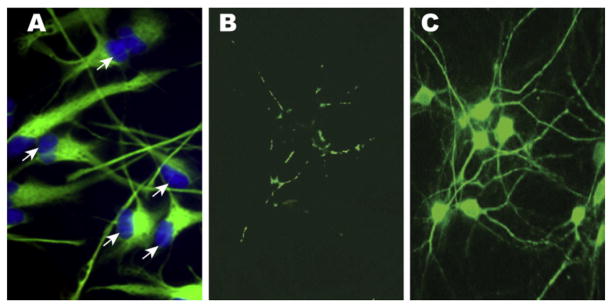
Fig. 1.
(A) 2 week old human astroglial (HAG) cells in primary culture stained with glial-specific glial fibrillary acidic protein (GFAP; green; λmax =540 nm) and Hoechst 33258 nuclear stain (blue; λmax =465 nm); note large diameter nuclei at cellular periphery, an indication of high transcriptional output [20,21]; phase contrast microscopy, (A) magnification 100×; (B) control HAG cells treated with the ROS indicator 2′,7′-dichlorodihydro-fluorescein diacetate (H2DCFDA) indicate basal reactive oxygen species (ROS) abundance; and (C) after treatment with iron- plus aluminum-sulfate (each 50 nM), an effect that was not significant in magnesium-sulfate treated cells (see Fig. 2). HAG cells maintained in primary culture for 2 weeks, display significantly enhanced ROS generation using H2DCFDA assay; note green fluorescent signal (λmax =525 nm) of cell bodies extending into axons and dendrites; (B) and (C) magnification 40×. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Data, statistical analyses and graphic presentations were performed with Data Mining Tool version 3.0, Microarray Suite version 5.0 or GeneSpring version 7.2 (Silicon Genetics) [6,19]. All statistical procedures were carried out using the programs and procedures in the SAS language (Statistical Analysis System, SAS Institute, Cary, NC). All p values were derived from protected t-tests or least square means from a two-way factorial analysis of variance (p, ANOVA); only p-values of less than 0.05 were considered to be statistically significant.
Results are presented in Figs. 1–4, and important points are highlighted in the figure legends and are discussed further below. Magnesium and iron are abundant and useful metals in eukaryotic neurobiology; on the other hand gallium and aluminum are known trivalent retinal- and neural-cell toxins, respectively [10,25,26, unpublished observations]. Five novel results from this study indicate that (a) unlike magnesium and gallium (as sulfates), iron and aluminum together, as sulfates, induce a robust production of ROS in HAG cells (Fig. 1); (b) the trivalent retinal toxin gallium [38] is inactive in inducing ROS in HAG cells when compared to the neural toxin aluminum (Fig. 2); (c) this evolution of ROS is effectively quenched by the antioxidant PBN (Fig. 2); (d) in these same HAG cells under identical treatment conditions, iron and aluminum (as sulfates) synergistically induce signals for the NF-κB p50/p65 complex 8-fold (at 50 nM) to 14-fold (at 100 nM) over controls (Fig. 3); and (e) that this NF-κB induction which appears to drive miRNA-125b and miRNA-146a over-expression, is effectively quenched by 3 independent classes of NF-κB inhibitors that include curcumin, PDTC and CAY10512, with CAY10512 being the most effective (Fig. 4).
Fig. 4.
Up-regulation of an NF-κB-sensitive miRNA-125b and miRNA-146a, in relation to a non-induced, brain abundant miRNA-132, in iron- plus aluminum sulfate-stressed HAG cells, and quenching in the presence of the NF-κB inhibitors curcumin, PDTC and CAY10512; N=5; *p<0.05; **p<0.01, ANOVA.
Fig. 2.
Quantitative comparison of up-regulation of ROS in magnesium-, gallium-, iron-and aluminum-sulfate-treated HAG cells, and quenching using the electron spin trap and anti-oxidant phenyl butyl nitrone (PBN) [21–23]. Combinatorial treatment of trivalent gallium with magnesium, iron or aluminum showed no significant induction of ROS (data not shown). Note synergistic induction of ROS in the presence of iron- plus aluminum-sulfate; N=5; *p<0.05; **p<0.01, ANOVA.
Fig. 3.
Up-regulation of transcription factor the NF-κB p50/p65 complex in iron- and aluminum-sulfate-treated HAG cells; (A) gel-shift assay showing increased DNA-binding of the NF-κB p50 and p65 (activator) complexes from 0 to 100 nM iron- and aluminum-sulfate; lanes marked p65 Ab and 100× are NF-κB oligonucleotide target controls, including a p65 Ab competition assay (that specifically deletes the NF-κB p65 band), and a 100-fold molar excess of NF-κB oligonucleotide (that completely erases the signal for the entire NF-κB p50/p65 dimeric complex) [20,24,29]; (B) signal quantitation of the results in (A); note synergistic induction of NF-κB p50/p65 activator complex in the presence of iron- plus aluminum-sulfate; N=5; *p<0.05; **p<0.01, ANOVA.
In summary, abundant data now indicate that there are significant alterations in gene expression in AD, and that these involve progressive alterations in the expression of genes involved in the innate immune response and pro-inflammatory signaling [30–35]. These current studies further indicate a role for the combination of environmentally common neurotoxic elements aluminum and iron in the miRNA-mediated pathogenetic processes that contribute to inflammatory neurodegeneration [36,37]. Interestingly, no such toxicities on HAG cells were noted with gallium, a known trivalent retinal toxin [38], either alone, or in combination with iron. How neurotoxic metal sulfates specifically access nuclear compartments, target NF-κB-regulated gene expression and alter specific miRNA abundances to trigger these pathogenic changes is currently under intense study.
Acknowledgments
Thanks are extended to Drs. W. Poon, T. Saing and Jian Zhang at brain bank donor institutions. Some of the brain tissues used in these studies were provided by the Memory Impairments and Neurological Disorders (MIND) Institute at the University of California, Irvine Alzheimer’s Disease Research Center (UCI-ADRC); funding for the UCI-ADRC was provided by NIH/NIAgrant P50 AG16573. Thanks are extended to Darlene Guillot for expert technical assistance. Part of this study was presented in abstract form at the 9th International Keele meeting on Aluminum 19–23 February 2011 in Niagara-on-the-Lake, Ontario, Canada. These studies were supported in part by a Translational Research Initiative grant from Louisiana State University (WJL), by an Alzheimer Association Investigator-Initiated Research GrantIIRG-09-131729 (WJL) and by NIH NIAAG18031 (WJL).
Abbreviations
- AD
Alzheimer’s disease
- ANOVA
analysis of variance
- CFH
complement factor H
- HAG
cells human astroglial cells
- mRNA
messenger RNA
- miRNA
micro RNA
- NF-κB
nuclear factor for kappa B RNAs ribonucleic acids
- ROS
reactive oxygen species
- UTR
un-translated region
References
- 1.Mattick JS, Makunin IV. Hum Mol Genet. 2005;14:121–132. doi: 10.1093/hmg/ddi101. [DOI] [PubMed] [Google Scholar]
- 2.Satoh J. J Pharmacol Sci. 2010;114:269–275. doi: 10.1254/jphs.10r11fm. [DOI] [PubMed] [Google Scholar]
- 3.Provost P. Brain Res. 2010;1338:58–66. doi: 10.1016/j.brainres.2010.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltimore D, Boldin MP, O’Connell RM, Rao DS, Taganov KD. Nat Immunol. 2008;9:839–845. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- 5.Lukiw WJ. Neuroreport. 2007;18:297–300. doi: 10.1097/WNR.0b013e3280148e8b. [DOI] [PubMed] [Google Scholar]
- 6.Pogue AI, Cui JG, Li YY, Zhao Y, Culicchia F, Lukiw WJ. Neurosci Lett. 2010;476:18–22. doi: 10.1016/j.neulet.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 7.Pogue AI, Li YY, Cui JG, Zhao Y, Kruck TP, Percy ME, Tarr MA, Lukiw WJ. J Inorg Biochem. 2009;103:1591–1595. doi: 10.1016/j.jinorgbio.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Sethi P, Lukiw WJ. Neurosci Lett. 2009;459:100–104. doi: 10.1016/j.neulet.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 9.Lukiw WJ, Zhao Y, Cui JG. J Biol Chem. 2008;283:31315–31322. doi: 10.1074/jbc.M805371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lukiw WJ, Pogue AI. J Inorg Biochem. 2007;101:1265–1269. doi: 10.1016/j.jinorgbio.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui JG, Li YY, Zhao Y, Bhattacharjee S, Lukiw WJ. J Biol Chem. 2010;285:38951–38960. doi: 10.1074/jbc.M110.178848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Chen XP, Li YJ. Scand J Immunol. 2010;71:227–231. doi: 10.1111/j.1365-3083.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 13.Becaria A, Bondy SC, Campbell A. J Alzheimers Dis. 2003;5:31–38. doi: 10.3233/jad-2003-5105. [DOI] [PubMed] [Google Scholar]
- 14.Campbell A, Becaria A, Lahiri DK, Sharman K, Bondy SC. J Neurosci Res. 2004;75:565–572. doi: 10.1002/jnr.10877. [DOI] [PubMed] [Google Scholar]
- 15.Becaria A, Lahiri DK, Bondy SC, Chen D, Hamadeh A, Li H, Taylor R, Campbell A. J Neuroimmunol. 2006;176:16–23. doi: 10.1016/j.jneuroim.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Lukiw WJ, Cui JG, Li YY, Bhattacharjee PS, Corkern M, Clement C, Kammerman EM, Ball MJ, Zhao Y, Sullivan PM, Hill JM. Neuroreport. 2010;21:922–927. doi: 10.1097/WNR.0b013e32833da51a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higaki S, Gebhardt BM, Lukiw WJ, Thompson HW, Hill JM. Invest Ophthalmol Vis Sci. 2002;43N:1862–1869. [PubMed] [Google Scholar]
- 18.Hill JM, Lukiw WJ, Gebhardt BM, Higaki S, Loutsch JM, Myles ME, Thompson HW, Kwon BS, Bazan NG, Kaufman HE. Virus Genes. 2001;23:273–280. doi: 10.1023/a:1012517221937. [DOI] [PubMed] [Google Scholar]
- 19.Li YY, Cui JG, Hill JM, Bhattacharjee S, Zhao Y, Lukiw WJ. Neurosci Lett. 2011;487:94–98. doi: 10.1016/j.neulet.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexandrov PN, Zhao Y, Pogue AI, Tarr MA, Kruck TPA, Percy ME, Cui JG, Lukiw WJ. J Alzheimers Dis. 2005;8:117–127. doi: 10.3233/jad-2005-8204. [DOI] [PubMed] [Google Scholar]
- 21.Boetkjaer A, Boedker M, Cui JG, Zhao Y, Lukiw WJ. Neurosci Lett. 2007;426:59–63. doi: 10.1016/j.neulet.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruck TP, Percy ME, Lukiw WJ. Neuroreport. 2008;19:245–249. doi: 10.1097/WNR.0b013e3282f4cb7e. [DOI] [PubMed] [Google Scholar]
- 23.Lukiw WJ. In: Aluminum and Alzheimer’s Disease, the Science that Describes the Link. Exley C, editor. Elsevier Publishers; London: 2001. pp. 147–168. [Google Scholar]
- 24.Lukiw WJ, Bazan NG. Neurochem Res. 2000;25:1173–1184. doi: 10.1023/a:1007627725251. [DOI] [PubMed] [Google Scholar]
- 25.Lukiw WJ, Krishnan B, Wong L, Kruck TP, Bergeron C, McLachlan DR. Neurobiol Aging. 1992;13:115–121. doi: 10.1016/0197-4580(92)90018-s. [DOI] [PubMed] [Google Scholar]
- 26.Lukiw WJ, LeBlanc HJ, Carver LA, McLachlan DR, Bazan NG. J Mol Neurosci. 1998;11:67–78. doi: 10.1385/JMN:11:1:67. [DOI] [PubMed] [Google Scholar]
- 27.Lukiw WJ, Percy ME, Kruck TP. J Inorg Biochem. 2005;99:1895–1898. doi: 10.1016/j.jinorgbio.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 28.Bazan NG, Lukiw WJ. J Biol Chem. 2002;277:30359–30367. doi: 10.1074/jbc.M203201200. [DOI] [PubMed] [Google Scholar]
- 29.Moody JR, Lindstrom RM. Anal Chem. 1977;49:2264–2268. [Google Scholar]
- 30.Colangelo V, Schurr J, Ball MJ, Pelaez RP, Lukiw WJ. J Neurosci Res. 2002;70:462–473. doi: 10.1002/jnr.10351. [DOI] [PubMed] [Google Scholar]
- 31.Cui JG, Kuroda H, Chandrasekharan NV, Pelaez RP, Simmons DL, Bazan NG, Lukiw WJ. Neurochem Res. 2004;29:1731–1737. doi: 10.1023/b:nere.0000035809.70905.8a. [DOI] [PubMed] [Google Scholar]
- 32.Lukiw WJ. Neurochem Res. 2004;29:1287–1297. doi: 10.1023/b:nere.0000023615.89699.63. [DOI] [PubMed] [Google Scholar]
- 33.Riazanskaia N, Lukiw WJ, Grigorenko A, Korovaitseva G, Dvoryanchikov G, Moliaka Y, Nicolaou M, Farrer L, Rogaev E. Mol Psychiatry. 2002;7:891–898. doi: 10.1038/sj.mp.4001101. [DOI] [PubMed] [Google Scholar]
- 34.Pogue AI, Lukiw WJ. Neuroreport. 2004;15:1507–1510. doi: 10.1097/01.wnr.0000130539.39937.1d. [DOI] [PubMed] [Google Scholar]
- 35.Loring JF, Wen X, Lee JM, Seilhamer J, Somogyi R. DNA Cell Biol. 2001;20:683–695. doi: 10.1089/10445490152717541. [DOI] [PubMed] [Google Scholar]
- 36.Lynch T, Cherny RA, Bush AI. Exp Gerontol. 2000;35:445–451. doi: 10.1016/s0531-5565(00)00112-1. [DOI] [PubMed] [Google Scholar]
- 37.Lukiw WJ. J Inorg Biochem. 2010;104:1010–1012. [PubMed] [Google Scholar]
- 38.Moon SJ, Han DP. Retina. 2005;25:212–214. doi: 10.1097/00006982-200502000-00016. [DOI] [PubMed] [Google Scholar]