Abstract
Aim
To analyze melanoma incidence and mortality trends in Croatia 1988-2008, compare them with the trends in other populations, and identify possible changes in the trends.
Methods
Incidence data were obtained from the Croatian National Cancer Registry and the mortality data from the Croatian Bureau of Statistics. United Nations population estimates were used for calculating the age-specific rates. Age-standardized rates were calculated by the direct standardization method, using the world standard population as a reference. To estimate incidence and mortality trends, we performed joinpoint regression analysis.
Results
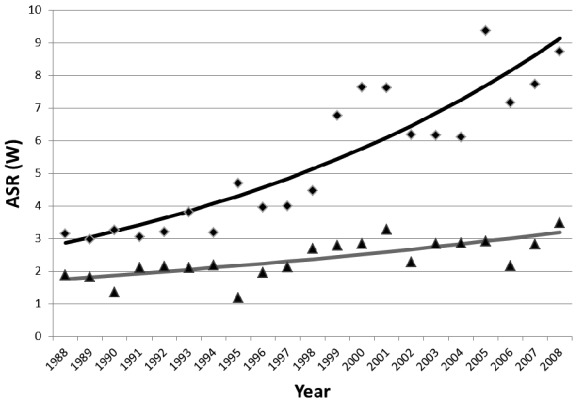
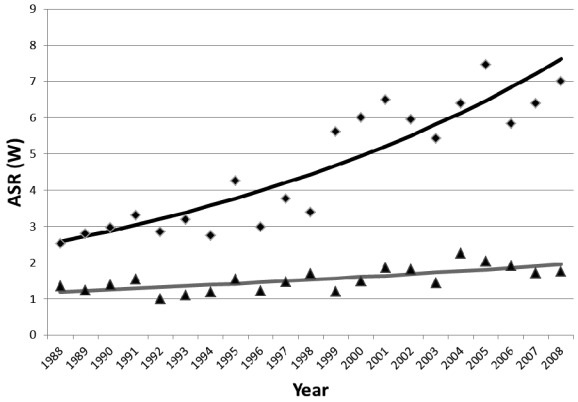
A significantly increasing incidence trend, with estimated annual percent change (EAPC) of 5.9% for men and 5.6% for women, was observed over the whole 21-year period and no additional joinpoints were identified. The overall incidence increase between the first and the last five-year period was 149% for men and 130% for women. Significant increase in the mortality trend was observed, with EAPC of 3.0% for men and 2.4% for women. No joinpoints were identified. The overall increase in mortality between the first and the last five-year period was 45% for men and 50% for women.
Conclusion
Melanoma rates in Croatia are steadily and markedly rising, with similar trends to those in the countries with lower/intermediate incidence. It is important to further investigate the more specific causes of the increasing trends, as well as to implement effective public policies targeting the melanoma burden.
Malignant melanoma of the skin is a cancer originating in melanocytes, the pigment-producing cells of the skin (1). Unlike basal and squamous cell cancers of the skin that are rarely fatal, its five-year relative survival in Europe is 83.1% (2). With 286 new cases and 118 yearly deaths in men and 275 new cases and 79 deaths in women in Croatia in 2008, melanoma represented 2.6% of male cancer incidence and 1.1% of cancer deaths in men, and 2.9% of female cancer incidence and 1.4% of cancer deaths in women (3).
The strongest environmental risk factor for malignant melanoma in white populations is exposure to UV light (4). Intermittent sun exposure, especially before the age of 10 is considered to be a stronger risk factor than a continuous exposure (5,6). Other risk factors for malignant melanoma include invasive melanoma of the skin in one or more first degree-relatives, history of primary invasive melanoma, more than a hundred banal melanocytic nevi, three or more clinically atypical (dysplastic) nevi, pale Caucasian skin (type 1 or 2), red or blond hair, history of one or more severe blistering sunburns, sunbed use (especially before the age of 30), and history of pesticide exposure (6).
Generally, melanoma incidence rates in Caucasian populations increase with proximity to the Equator (7). However, there are variations across Europe, with Switzerland, Denmark, Norway, Sweden, and the Netherlands having the highest rates (15-18/100 000 in men and 16-22/100 000 in women) and Central and Southeastern Europe countries the lowest (4.4/100 000 for men and 4.3/100 000 for women) (8). Until now, recent trends in melanoma incidence and mortality have been less studied in the Mediterranean and Eastern European populations. The aim of this study was to analyze the melanoma incidence and mortality trends in Croatia 1988-2008, compare them with the trends in other populations, and identify possible changes.
Materials and methods
Data sources
Incidence data for the period 1988-2008 were obtained from the Croatian National Cancer Registry. The Registry, founded in 1959, covers the whole Croatian population (approximately 4.4 million persons) and relies on mandatory cancer notifications from primary and secondary health care sources and death certificates from the Croatian Bureau of Statistics. The Registry contributed data to the last three volumes of the Cancer Incidence in Five Continents series (9-11). Melanoma was defined as ICD-9 code 172 and ICD-10 code C43 (12). The number of melanoma deaths was obtained from the WHO Mortality Database (13). For calculating age-specific rates, we used the UN population estimates (14).
Statistical analysis
Age-standardized rates of melanoma incidence in Croatia were calculated by the direct standardization method, using the world standard population as a reference (15). To describe incidence and mortality trends by calendar period, we carried out joinpoint regression analysis using the Joinpoint Regression Software (16). The aim of the approach is to identify possible joinpoints where a significant change in the log-linear trend occurs. To obtain the estimated annual percent change (EAPC), a regression line was fitted to the natural logarithm of the rates using calendar year as the response (17). In describing trends, the terms “significant increase” or “significant decrease” signify that the slope of the trend was statistically significant (P < 0.05). For non-statistically significant trends (P > 0.05), we used the terms “stable” (EAPC between -0.5% and 0.5%), “non-statistically significant increase” (EAPC>0.5%), and “non-statistically significant decrease” (EAPC<-0.5%). All statistical tests were two sided.
Results
From 1988 to 2008, there were 3517 male and 3590 female melanoma cases. The number of new cases ranged from 81 to 303 annually for men, and from 87 to 284 for women (Table 1, 2). The significantly increasing incidence trend, with EAPC of 5.9% (95% confidence interval [CI], 4.5 to 7.5) for men and 5.6% for women (95% CI, 4.3 to 6.9) was observed over the whole 21-year period and no additional joinpoints were identified (Figure 1, 2). The overall incidence increase between the first and the last five-year period was 149% for men and 130% for women (Table 3). There were 1568 men and 1323 women who died of melanoma. The number of annual melanoma deaths ranged between 38 and 118 for men and between 39 and 94 for women (Tables 1, 2). Mortality trend showed a significant increase, with EAPC of 3.0% for men and 2.4% for women (95% CI, 1.6% to 4.4% and 1.3% to 3.6% respectively). No joinpoints were identified (Figures 1, 2). The overall increase in mortality between the first and the last five-year period was 45% for men and 50% for women (Table 3).
Table 1.
Incidence and mortality of melanoma in Croatian men, 1988-2008
| Incidence |
Mortality |
|||||
|---|---|---|---|---|---|---|
| Year | N | crude rate | ASR(W)* | N | crude rate | ASR(W) |
| 1988 |
81 |
3.7 |
3.1 |
51 |
2.4 |
1.9 |
| 1989 |
81 |
3.7 |
3.0 |
50 |
2.3 |
1.8 |
| 1990 |
88 |
4.0 |
3.3 |
38 |
1.7 |
1.4 |
| 1991 |
86 |
3.9 |
3.1 |
55 |
2.5 |
2.1 |
| 1992 |
91 |
4.1 |
3.2 |
60 |
2.7 |
2.1 |
| 1993 |
114 |
5.1 |
3.8 |
66 |
3.0 |
2.1 |
| 1994 |
97 |
4.3 |
3.2 |
67 |
3.0 |
2.2 |
| 1995 |
143 |
6.4 |
4.7 |
38 |
1.7 |
1.2 |
| 1996 |
122 |
5.4 |
4.0 |
61 |
2.7 |
2.0 |
| 1997 |
127 |
5.7 |
4.0 |
70 |
3.1 |
2.1 |
| 1998 |
140 |
6.3 |
4.5 |
84 |
3.8 |
2.7 |
| 1999 |
207 |
9.5 |
6.8 |
86 |
3.9 |
2.8 |
| 2000 |
240 |
11.1 |
7.6 |
91 |
4.2 |
2.9 |
| 2001 |
234 |
10.8 |
7.6 |
106 |
4.9 |
3.3 |
| 2002 |
194 |
9.0 |
6.2 |
71 |
3.3 |
2.3 |
| 2003 |
198 |
9.2 |
6.2 |
96 |
4.5 |
2.9 |
| 2004 |
191 |
8.9 |
6.1 |
92 |
4.3 |
2.9 |
| 2005 |
303 |
14.2 |
9.4 |
98 |
4.6 |
2.9 |
| 2006 |
239 |
11.2 |
7.2 |
73 |
3.4 |
2.2 |
| 2007 |
254 |
11.9 |
7.7 |
97 |
4.6 |
2.8 |
| 2008 | 287 | 13.5 | 8.7 | 118 | 5.6 | 3.5 |
*ASR(W) – age-standardized rates (using world standard population).
Table 2.
Incidence and mortality of melanoma in Croatian women, 1988-2008
| Incidence |
Mortality |
|||||
|---|---|---|---|---|---|---|
| Year | N | crude rate | ASR(W)* | N | crude rate | ASR(W) |
| 1988 |
87 |
3.8 |
2.5 |
53 |
2.3 |
1.4 |
| 1989 |
93 |
4.0 |
2.8 |
44 |
1.9 |
1.2 |
| 1990 |
106 |
4.6 |
3.0 |
50 |
2.1 |
1.4 |
| 1991 |
108 |
4.6 |
3.3 |
58 |
2.5 |
1.5 |
| 1992 |
100 |
4.2 |
2.9 |
39 |
1.6 |
1.0 |
| 1993 |
108 |
4.5 |
3.2 |
43 |
1.8 |
1.1 |
| 1994 |
102 |
4.2 |
2.8 |
45 |
1.9 |
1.2 |
| 1995 |
152 |
6.3 |
4.3 |
62 |
2.6 |
1.5 |
| 1996 |
106 |
4.4 |
3.0 |
44 |
1.8 |
1.2 |
| 1997 |
133 |
5.6 |
3.8 |
59 |
2.5 |
1.5 |
| 1998 |
119 |
5.0 |
3.4 |
65 |
2.7 |
1.7 |
| 1999 |
214 |
9.1 |
5.6 |
54 |
2.3 |
1.2 |
| 2000 |
218 |
9.3 |
6.0 |
65 |
2.8 |
1.5 |
| 2001 |
236 |
10.2 |
6.5 |
78 |
3.4 |
1.9 |
| 2002 |
212 |
9.2 |
6.0 |
71 |
3.1 |
1.8 |
| 2003 |
219 |
9.5 |
5.4 |
65 |
2.8 |
1.4 |
| 2004 |
238 |
10.3 |
6.4 |
94 |
4.1 |
2.3 |
| 2005 |
284 |
12.3 |
7.5 |
94 |
4.1 |
2.0 |
| 2006 |
235 |
10.2 |
5.8 |
81 |
3.5 |
1.9 |
| 2007 |
246 |
10.7 |
6.4 |
80 |
3.5 |
1.7 |
| 2008 | 274 | 12.0 | 7.0 | 79 | 3.5 | 1.8 |
*ASR(W) – age-standardized rates (using world standard population).
Figure 1.
Joinpoint analysis for incidence and mortality of melanoma in Croatian men, 1988-2008. Rhombs – incidence; triangles – mortality; ASR(W) – age-standardized rates per 100 000 (using world standard population).
Figure 2.
Joinpoint analysis for incidence and mortality of melanoma in Croatian women, 1988-2008. Rhombs – incidence; triangles – mortality; ASR(W) – age-standardized rates per 100 000 (using world standard population).
Table 3.
Melanoma incidence and mortality in Croatia (1988-2002 and 2004-2008)
| Period | Person – years* | Incidence |
Mortality |
|||||
|---|---|---|---|---|---|---|---|---|
| N† | ASR(W)‡ | absolute change (%) | N§ | ASR (W)‡ | absolute change (%) | |||
|
Men: |
||||||||
|
1988-1992 |
2.19 |
85 |
3.1 |
- |
51 |
1.9 |
- |
|
|
2004-2008 |
2.13 |
255 |
7.8 |
149 |
91 |
2.7 |
45 |
|
|
Women: |
||||||||
|
1988-1992 |
2.34 |
99 |
2.9 |
- |
49 |
1.3 |
- |
|
| 2004-2008 | 2.30 | 255 | 6.6 | 130 | 86 | 1.9 | 50 | |
*Mean annual male population expressed in million person-years at risk.
†Mean annual number of new cases.
‡Age-standardized rate (using world standard population).
§Mean annual number of deaths.
Discussion
This study was the first to systematically analyze melanoma incidence and mortality trends in the Croatian population. Compared to other European countries from the GLOBOCAN 2008 database, Croatia had an intermediate melanoma incidence, with the ASR of 8.7/100 000 for men and 7.0/100 000 for women. However, with the ASR of 3.5/100 000 for men and 1.8/100 000 for women, it had the third highest male and fourth highest female mortality rate in Europe (8). As opposed to the majority of European countries, which had a higher incidence in women, Croatia had a higher incidence in men, similar to Australia and North America (8). Croatia also had a higher mortality rate in men.
Since the 1970s, melanoma incidence rates in populations of European origin doubled every 10 to 20 years (18). In Northern Europe, they became very high during the 1980s but have been leveling off since the mid-1990s (18,19). In Southern and Eastern Europe, on the other hand, the rates are still increasing (18,20-22). Mortality rates are leveling off in many populations with high incidence rates (such as Australia, USA, Scandinavian countries, the UK) (18,23-27), but are still increasing in most of Eastern and Southern European populations (18,21,22,27). Our results are similar to those in the countries with lower/intermediate incidence, eg, Italy with EAPC of 5.2% for men and 5.3% for women in the period 1985-2003 (22).
Croatian coast and islands have more than 2600 hours of sunshine per year, which contributes to intensive periodical sun exposure of the population (28). Since intermittent sun exposure is a major risk factor, the observed increases in Croatia may be attributed to popularization of tourism and holidaymaking in the 1950s and 1960s and the consumer practices of the 1970s and 1980s (29). In most countries, mortality rates have not increased as rapidly as incidence rates and it is controversial whether the increase in incidence is actually a consequence of increased surveillance and better detection of thin, minimally invasive melanomas (30). Due to the lack of information on stage and thickness of the tumors, it is impossible to determine whether increased awareness and early detection contributed to the increasing incidence trends in Croatia.
There has been only a modest progress in the management of advanced disease, and early diagnosis remains crucial in improving the survival (31,32). There are prevention and early detection strategies available, which have been developed and used in countries with higher incidence rates, including ABCDE guidelines and Glasgow 7-point checklist (33,34). Helping general public in recognizing melanomas and having them removed at an early stage was first done in the Queensland Melanoma Project (35), and then reproduced in the US and some European countries, such as the UK, resulting in earlier diagnosis and increase in the proportion of patients with thinner melanomas and better prognosis (34,36,37). Several population-based case-control studies suggest that whole body skin self-examination, as well as examination by clinicians, reduces the risk of thick melanomas (38,39). However, there are currently no completed randomized control trials that would confirm this. A randomized-controlled trial of a community-based melanoma screening program in Queensland, Australia, is expected to be complete in 2015 (34,40,41). A recent study in northern Germany has reported promising results in terms of feasibility and effectiveness of a systematic, two-step skin cancer screening program for a population of 1.88 million (42).
Croatia participates in Euromelanoma, pan-European prevention campaign against skin cancer that started in Belgium in 1999 (43-45). During Euromelanoma Week, the general public has an opportunity to have a free-of-charge dermatologist screening of the moles (46). It still remains to be seen whether this campaign will have an effect on the incidence and mortality trends in Croatia. Still, more effort should be put into increasing public awareness of melanoma and its prevention. Further research is needed to identify risk groups, their needs, and sun exposure-related behaviors to tailor Croatian prevention campaigns. Integration with the existing public-health activities should be considered, for example with the “Health Promoting Schools” project (45,46), to raise awareness of sun exposure risks and ways of protection among school children, their parents, and teachers.
Current high mortality rates clearly indicate a need to improve the outcomes of Croatian melanoma patients. At present, there is a great variation in the way they are diagnosed, treated, and followed. Policy-makers should ensure that every patient is treated to the same standards. As in other European countries, population aging is expected to occur, resulting in estimated 319 new male and 288 female melanoma cases in 2030, as well as 146 melanoma deaths in men and 88 in women (8). Therefore, an increased demand for melanoma-related medical services is expected, which will also require more detailed incidence and mortality data to enable adequate planning. What remains to be investigated are patterns of service provision and utilization, waiting times, survival patterns, as well as patient pathways, needs, and experiences.
Acknowledgments
Acknowledgment We thank the staff of the Croatian National Cancer Registry for their help with data collection and professor Mirna Šitum, MD, PhD for critical reading of the manuscript and helpful suggestions.
Funding The study was supported by the research grant from the Ministry of Science, Education, and Sports of the Republic of Croatia, No. 005-1080315-0294.
Ethical approval Not required.
Declaration of authorship JB participated in data collection, interpretation of the results, and writing of the manuscript. AZ participated in the study design and coordination, interpretation of the results, manuscript preparation, editing, and review.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Robins P, Perez M. Understanding melanoma. New York (NY): The skin Cancer foundation; 1996. [Google Scholar]
- 2.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R, et al. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009;45:931–91. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Croatian National Cancer Registry, Croatian National Institute of Public Health. Cancer incidence in Croatia 2008. Bulletin No 33. Zagreb (Croatia): Croatian National Institute of Public Health; 2010. [Google Scholar]
- 4.IARC. Solar and ultraviolet radiation. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man: Volume 55. Lyon (France): International Agency for Research on Cancer; 1992. [Google Scholar]
- 5.Armstrong BK, Kricker A. How much melanoma is caused by sun exposure? Melanoma Res. 1993;3:395–401. doi: 10.1097/00008390-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 6.MacKie RM, Hauschild A, Eggermont AM. Epidemiology of invasive cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi1–7. doi: 10.1093/annonc/mdp252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tucker MA, Goldstein AM. Melanoma etiology: where are we? Oncogene. 2003;22:3042–52. doi: 10.1038/sj.onc.1206444. [DOI] [PubMed] [Google Scholar]
- 8.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, cancer incidence and mortality worldwide: IARC CancerBase No. 10. Lyon (France): IARC; 2010. [Google Scholar]
- 9.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, editors. Cancer incidence in five continents, Vol. VIII (IARC Scientific Publications No. 155). Lyon (France): IARC; 2002. [Google Scholar]
- 10.Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J, editors. Cancer Incidence in five continents, Vol. VII (IARC Scientific Publications No. 143). Lyon (France): IARC; 1997. [Google Scholar]
- 11.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al, editors. Cancer incidence in five continents, Vol. IX (IARC Scientific Publications No. 160). Lyon (France): IARC; 2007. [Google Scholar]
- 12.World Health Organization. World Health Organization, mortality database. WHO Statistical Information System. Available from: http://www.who.int/whosis/mort/download/en/index.html Accessed: March 21, 2012.
- 13.International Classification of Diseases (ICD). Available from: http://www.who.int/classifications/icd/en/index.html. Accessed: March 21, 2012.
- 14.United Nations. World Population Prospects, the 2010 Revision. United Nations Population Division Department of Economic and Social Affairs. 2011. Available from: http://esa.un.org/unpd/wpp/index.htm. Accessed: March 21, 2012.
- 15.Doll R, Payne P, Waterhouse JAH, editors. Cancer incidence in five continents, Vol. I. Geneva (Switzerland): Union Internationale Contre le Cancer; 1966. [Google Scholar]
- 16.National Cancer Institute. Joinpoint regression program. 3.4.2 ed. Bethesda (MD): Statistical Research and Applications Branch, National Cancer Institute; 2009. [Google Scholar]
- 17.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 18.de Vries E, Bray F, Coebergh JW, Parkin DM. Changing epidemiology of malignant cutaneous melanoma in Europe 1969-1997: rising trends in incidence and mortality, but recent stabilisations in Western Europe and decreases in Scandinavia. Int J Cancer. 2003;107:119–26. doi: 10.1002/ijc.11360. [DOI] [PubMed] [Google Scholar]
- 19.Stang A, Pukkala E, Sankila R, Soderman B, Hakulinen T. Time trend analysis of the skin melanoma incidence of Finland from 1953 through 2003 including 16,414 cases. Int J Cancer. 2006;119:380–4. doi: 10.1002/ijc.21836. [DOI] [PubMed] [Google Scholar]
- 20.Marcos-Gragera R, Vilar-Coromina N, Galceran J, Borras J, Cleries R, Ribes J, et al. Rising trends in incidence of cutaneous malignant melanoma and their future projections in Catalonia, Spain: increasing impact or future epidemic? J Eur Acad Dermatol Venereol. 2010;24:1083–8. doi: 10.1111/j.1468-3083.2010.03602.x. [DOI] [PubMed] [Google Scholar]
- 21.Pellacani G, Lo Scocco G, Vinceti M, Albertini G, Raccagni AA, Baldassari L, et al. Melanoma epidemic across the millennium: time trends of cutaneous melanoma in Emilia-Romagna (Italy) from 1997 to 2004. J Eur Acad Dermatol Venereol. 2008;22:213–8. doi: 10.1111/j.1468-3083.2007.02388.x. [DOI] [PubMed] [Google Scholar]
- 22.Chellini E, Crocetti E, Carli P, Martini A, Giovannetti L. The melanoma epidemic debate: some evidence for a real phenomenon from Tuscany, Italy. Melanoma Res. 2007;17:129–30. doi: 10.1097/CMR.0b013e3280a60865. [DOI] [PubMed] [Google Scholar]
- 23.Tryggvadottir L, Gislum M, Hakulinen T, Klint A, Engholm G, Storm HH, et al. Trends in the survival of patients diagnosed with malignant melanoma of the skin in the Nordic countries 1964-2003 followed up to the end of 2006. Acta Oncol. 2010;49:665–72. doi: 10.3109/02841861003702528. [DOI] [PubMed] [Google Scholar]
- 24.Montella A, Gavin A, Middleton R, Autier P, Boniol M. Cutaneous melanoma mortality starting to change: a study of trends in Northern Ireland. Eur J Cancer. 2009;45:2360–6. doi: 10.1016/j.ejca.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–74. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baade P, Coory M. Trends in melanoma mortality in Australia: 1950-2002 and their implications for melanoma control. Aust N Z J Public Health. 2005;29:383–6. doi: 10.1111/j.1467-842X.2005.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 27.La Vecchia C, Lucchini F, Negri E, Levi F. Recent declines in worldwide mortality from cutaneous melanoma in youth and middle age. Int J Cancer. 1999;81:62–6. doi: 10.1002/(SICI)1097-0215(19990331)81:1<62::AID-IJC12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 28.About Croatia. Available from: http://www.croatia.hr Accessed: March 21, 2012.
- 29.Grandits H, Taylor K, editors. Yugoslavia’s sunny side: A history of tourism in socialism (1950s-1980s). Budapest: Central European University Press; 2010. [Google Scholar]
- 30.Erickson C, Driscoll MS. Melanoma epidemic: Facts and controversies. Clin Dermatol. 2010;28:281–6. doi: 10.1016/j.clindermatol.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Lasithiotakis KG, Leiter U, Eigentler T, Breuninger H, Metzler G, Meier F, et al. Improvement of overall survival of patients with cutaneous melanoma in Germany, 1976-2001: which factors contributed? Cancer. 2007;109:1174–82. doi: 10.1002/cncr.22511. [DOI] [PubMed] [Google Scholar]
- 32.Lindholm C, Andersson R, Dufmats M, Hansson J, Ingvar C, Moller T, et al. Invasive cutaneous malignant melanoma in Sweden, 1990-1999. A prospective, population-based study of survival and prognostic factors. Cancer. 2004;101:2067–78. doi: 10.1002/cncr.20602. [DOI] [PubMed] [Google Scholar]
- 33.Whited JD, Grichnik JM. Does this patient have a mole or a melanoma? JAMA. 1998;279:696. doi: 10.1001/jama.279.9.696. [DOI] [PubMed] [Google Scholar]
- 34.Weinstock MA. Early detection of melanoma. JAMA. 2000;284:886–9. doi: 10.1001/jama.284.7.886. [DOI] [PubMed] [Google Scholar]
- 35.Beardmore GL. The Queensland melanoma project. Int J Dermatol. 1977;16:831–5. doi: 10.1111/j.1365-4362.1977.tb04302.x. [DOI] [PubMed] [Google Scholar]
- 36.Doherty VR, MacKie RM. Reasons for poor prognosis in British patients with cutaneous malignant melanoma. BMJ. 1986;292:987–9. doi: 10.1136/bmj.292.6526.987. . Clin Res Ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012–5. doi: 10.1136/bmj.304.6833.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aitken JF, Elwood M, Baade PD, Youl P, English D. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126:450–8. doi: 10.1002/ijc.24747. [DOI] [PubMed] [Google Scholar]
- 39.Berwick M, Begg CB, Fine JA, Roush GC, Barnhill RL. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17–23. doi: 10.1093/jnci/88.1.17. [DOI] [PubMed] [Google Scholar]
- 40.Aitken JF, Youl PH, Janda M, Lowe JB, Ring IT, Elwood M. Increase in skin cancer screening during a community-based randomized intervention trial. Int J Cancer. 2006;118:1010–6. doi: 10.1002/ijc.21455. [DOI] [PubMed] [Google Scholar]
- 41.Aitken JF, Elwood JM, Lowe JB, Firman DW, Balanda KP, Ring IT. A randomised trial of population screening for melanoma. J Med Screen. 2002;9:33–7. doi: 10.1136/jms.9.1.33. [DOI] [PubMed] [Google Scholar]
- 42.Breitbart EW, Waldmann A, Nolte S, Capellaro M, Greinert R, Volkmer B, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201–11. doi: 10.1016/j.jaad.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 43.van der Leest RJ, de Vries E, Bulliard JL, Paoli J, Peris K, Stratigos AJ, et al. The Euromelanoma skin cancer prevention campaign in Europe: characteristics and results of 2009 and 2010. J Eur Acad Dermatol Venereol. 2011;25:1455–65. doi: 10.1111/j.1468-3083.2011.04228.x. [DOI] [PubMed] [Google Scholar]
- 44.Lipozecic J, Situm M. Croatian activity in Euromelanoma Day, May 5, 2008. Acta Dermatovenerol Croat. 2008;16:47. [Google Scholar]
- 45.Skin cancer prevention: information, resources and environmental changes. NICE public health guidance 32. 2011. Available from: http://www.nice.org.uk/guidance/PH32 Accessed: March 30, 2012.
- 46.Healthy school. European network of health-promoting schools [in Croatian]. Croatian Institute of Public Health website. Available from: http://www.hzjz.hr/aktivnosti/zdrskol.htm Accessed: March 30, 2012.