Abstract
Aim
To estimate the incidence and mortality trends of gastric and colorectal cancers in Croatia between 1988 and 2008.
Methods
Incidence data for the period 1988-2008 were obtained from the Croatian National Cancer Registry. The number of deaths from gastric and colorectal cancers was obtained from the World Health Organization mortality database. Joinpoint regression analysis was used to describe changes in trends by sex.
Results
Gastric cancer incidence rates declined steadily during the study period, with estimated annual percent change (EAPC) of -3.2% for men and -2.8% for women. Mortality rates in men decreased, with EAPC of -5.0% from 1988-1995 and -2.5% from 1995-2008. Mortality rates in women decreased, with EAPC of -3.2% throughout the study period. For colorectal cancer in men, joinpoint analysis revealed increasing trends of both incidence (EAPC 2.9%) and mortality (EAPC 2.1%).In women, the increase in incidence was not significant, but mortality in the last 15 years showed a significant increase of 1.1%.
Conclusion
The incidence and mortality trends of gastric cancer in Croatia are similar to other European countries, while the still increasing colorectal cancer mortality calls for more efficient prevention and treatment.
The two most important gastrointestinal cancers in Croatia are gastric and colorectal cancer, which together contribute to about one fifth of Croatian cancer incidence and mortality (1).
Gastric cancer
Gastric cancer is the fourth most common cancer and the second leading cause of cancer death worldwide (1,2), with high case fatality rate and five-year survival of less than 20% (3). It displays a substantial geographic variability across the continents, with rates in Asia, some parts of Eastern Europe, and Latin America being several times higher than those in western countries (2).
The etiology of gastric cancer has not been entirely elucidated. The most common risk factors reported in the literature are chronic mucosal infection with Helicobacter pylori (4), consumption of salt and salt-preserved foods (5), low socio-economic status (6), presence of contaminants in drinking water (7,8), smoking (9), and certain occupational exposures (10,11).
During the second half of the 20th century, incidence and mortality rates of gastric cancer have declined steeply throughout Europe (12,13) and around the world (14), mainly as a result of the remarkable improvement in the living conditions (15-18). This improvement may have contributed to a declining prevalence of H. Pylori infection in younger birth cohorts (19) and the use of eradication therapy in older cohorts may have further contributed to the lowering of incidence and mortality rates (20). H. Pylori infection plays an important role in the sequence of events that can lead to gastric cancer and is classified as a type I carcinogen according to the International Agency for Research on Cancer criteria (4).
According to the most recent data from the Croatian National Cancer Registry, the gastric cancer crude incidence rate per 100 000 men in 2008 was 29.0 (617 cases), making it the fifth most common cancer; for women the rate was 17.0 per 100 000 (390 cases), making it the eighth most common cancer (21). Among cancer-related causes of death in 2008, gastric cancer ranked fourth both in men, with a crude mortality rate of 24.2 per 100 000 (514 deaths), and in women, with a crude mortality rate of 15.9 per 100 000 (364 deaths) (22).
Colorectal cancer
Colorectal cancer is a major global public health problem, with approximately 950 000 newly diagnosed cases each year (1). The risk of colorectal cancer increases steeply with age, and in many developed countries colorectal cancer burden is increasing with increasing life expectancy. The incidence is also increasing in many developing countries, as diet and lifestyle become more similar to those in developed countries. Colorectal cancer five-year relative survival in Europe for both sexes combined is 56.2% (23).
Family history of hereditary colorectal cancer is a recognized risk factor, accounting for 15%-20% of cases at the population level (24). Additional 6% excess risk can be attributed to genetic predisposition due to common causal variants in the genome (25-29). Increased colorectal cancer risk is also associated with dietary factors such as high fat and red meat consumption and low vegetable intake, as well as with physical inactivity, excess body weight, and high alcohol consumption (30). On the other hand, it has been inversely associated with the use of exogenous female hormones (31).
Western European countries introduced the secondary cancer prevention in the mid-2000s, most often based on fecal occult blood test followed by colonoscopy (32). Croatia initiated the national colorectal cancer screening program in 2007. Apart from active screening, changes in colorectal cancer trends worldwide could be attributed to standardization and availability of evidence-based surgical, chemo-, and radiotherapy (33,34).
The aim of this study was to analyze gastric and colorectal cancer incidence and mortality trends in Croatia from 1988 to 2008 for both sexes, and to evaluate these findings in comparison to other European countries and in the context of changing environmental and social conditions, with possible implications for the health policies.
Methods
Data sources
Incidence data for the period 1988-2008 were obtained from the Croatian National Cancer Registry. The Registry, founded in 1959, covers the whole Croatian population (approximately 4.4 million persons) and relies on mandatory cancer notifications from primary and secondary health care sources and death certificates from the Croatian Bureau of Statistics. The Registry contributed data to the last three volumes of the Cancer Incidence in Five Continents series (35-37). In addition to incidence data, these publications report indices of data quality (proportion of morphologically verified cases, proportion of cases registered from death certificates only, and mortality to incidence ratio). At the Registry, gastric cancer was defined as ICD-9 (38) code 151 from 1988 to 2000 and ICD-10 (39) code C16 from 2001 to 2008. Colorectal cancer was defined as cancer of the hepatic flexure (153.0), transverse colon (153.1), cecum (153.4), appendix (153.5), ascending colon (153.6), splenic flexure (153.7), descending colon (153.2), sigmoid colon (153.3), recto sigmoid junction (154.0), rectum (154.1), and unspecified (153.8 and 153.9) in ICD-9; and the colon (C18.0-C18.9), recto sigmoid junction (C19.0-C19.9), rectum (C20.0-C20.9), and anus (C21) in ICD-10. The number of deaths with gastric and colorectal cancer as the underlying cause were obtained from the World Health Organization mortality database (40). For calculating age-specific rates, we used the population estimates from the Population Division of the Department of Economic and Social Affairs of the United Nations (41).
Statistical analysis
Age-standardized rates of cancer incidence in Croatia were calculated by the direct standardization method, using the world standard population as a reference (42). To describe incidence and mortality time trends, we carried out joinpoint regression analysis using the software Joinpoint Regression Program, Version 3.5.0. April 2011 (43). The analysis included the logarithmic transformation of the rates, standard error, maximum number of five joinpoints, and minimum of four years between two joinpoints. All other program parameters were set to default values. The aim of the approach is to identify possible joinpoints where a significant change in the trend occurs. The method identifies joinpoints based on regression models with 0-5 joinpoints. The final model selected was the most parsimonious of these, with the estimated annual per cent change (EAPC) based on the trend within each segment (44). To quantify the trend over a fixed period, the average annual per cent change (AAPC) was calculated. The AAPC is computed as a geometric weighted average of the EAPC trend analysis, with the weights equal to the lengths of each segment during the pre-specified fixed interval (45).
In case of non-significant trends (P > 0.05), we used the terms “stable” (EAPC between -0.5% to 0.5%), “non statistically significant increase” (EAPC>0.5%), and “non statistically significant decrease” (EAPC<-0.5%). All statistical tests were two sided. Unless otherwise mentioned, the rates were expressed per 100 000.
Results
Gastric cancer
During the period 1988-2008, there were 25 183 cases of gastric cancer and 21 313 deaths. The age-standardized incidence rate per 100 000 decreased from 29.5 in the first 5-year period (1988-1992) to 16.9 in the last 5-year period (2004-2008) in men (43%), and from 11.7 to 7.0 in women (40%). The age-standardized mortality rate per 100 000 decreased from 25.2 to 14.8 per 100 000 in men (41% decrease), and from 10.0 to 5.8 in women (42% decrease) (Tables 1 and 2).
Table 1.
Male gastric cancer incidence and mortality in Croatia in the period 1988-2008. Number of cases, crude rate and age standardized rate (ASR) per 100 000 (using world standard population)
| Year | Incidence |
Mortality |
||||
|---|---|---|---|---|---|---|
| N | crude rate | ASR | N | crude rate | ASR | |
|
1988 |
847 |
39.0 |
30.9 |
736 |
33.9 |
26.8 |
|
1989 |
844 |
38.8 |
29.8 |
752 |
34.5 |
26.9 |
|
1990 |
872 |
39.9 |
31.2 |
726 |
33.2 |
25.9 |
|
1991 |
821 |
37.3 |
28.6 |
719 |
32.7 |
24.8 |
|
1992 |
786 |
35.5 |
26.7 |
621 |
28.0 |
21.4 |
|
1993 |
704 |
31.5 |
23.2 |
678 |
30.4 |
22.3 |
|
1994 |
754 |
33.6 |
24.1 |
634 |
28.2 |
20.1 |
|
1995 |
756 |
33.6 |
23.0 |
635 |
28.2 |
19.4 |
|
1996 |
720 |
32.1 |
21.7 |
606 |
27.0 |
18.4 |
|
1997 |
782 |
35.1 |
23.1 |
627 |
28.1 |
18.6 |
|
1998 |
683 |
30.9 |
20.0 |
610 |
27.6 |
17.6 |
|
1999 |
831 |
38.0 |
24.3 |
634 |
29.0 |
18.4 |
|
2000 |
719 |
33.1 |
20.8 |
610 |
28.1 |
17.7 |
|
2001 |
710 |
32.9 |
20.4 |
573 |
26.5 |
16.2 |
|
2002 |
711 |
33.1 |
20.1 |
580 |
27.0 |
16.3 |
|
2003 |
718 |
33.5 |
19.9 |
617 |
28.8 |
16.7 |
|
2004 |
662 |
30.9 |
18.1 |
610 |
28.5 |
16.4 |
|
2005 |
667 |
31.2 |
17.8 |
556 |
26.0 |
14.9 |
|
2006 |
604 |
28.3 |
16.1 |
539 |
25.3 |
14.1 |
|
2007 |
628 |
29.5 |
16.4 |
578 |
27.1 |
15.3 |
| 2008 | 617 | 29.0 | 16.0 | 514 | 24.2 | 13.1 |
Table 2.
Female gastric cancer incidence and mortality in Croatia in the period 1988-2008. Number of cases, crude rate, and age standardized rate (ASR) per 100 000 (using world standard population)
| Year | Incidence |
Mortality |
||||
|---|---|---|---|---|---|---|
| N | crude rate | ASR | N | crude rate | ASR | |
|
1988 |
541 |
23.4 |
12.1 |
482 |
20.8 |
10.8 |
|
1989 |
527 |
22.7 |
12.0 |
458 |
19.7 |
10.5 |
|
1990 |
556 |
23.8 |
12.9 |
469 |
20.1 |
10.8 |
|
1991 |
503 |
21.4 |
11.2 |
432 |
18.4 |
9.6 |
|
1992 |
460 |
19.4 |
10.2 |
372 |
15.7 |
8.1 |
|
1993 |
436 |
18.2 |
9.1 |
398 |
16.6 |
8.3 |
|
1994 |
447 |
18.5 |
9.5 |
388 |
16.1 |
8.1 |
|
1995 |
504 |
20.8 |
10.1 |
394 |
16.3 |
8.0 |
|
1996 |
494 |
20.5 |
9.9 |
408 |
16.9 |
8.0 |
|
1997 |
494 |
20.6 |
10.3 |
393 |
16.4 |
7.9 |
|
1998 |
432 |
18.2 |
8.5 |
389 |
16.4 |
7.6 |
|
1999 |
466 |
19.8 |
9.2 |
349 |
14.8 |
6.5 |
|
2000 |
516 |
22.1 |
10.3 |
359 |
15.4 |
6.7 |
|
2001 |
474 |
20.4 |
9.0 |
374 |
16.1 |
6.9 |
|
2002 |
474 |
20.5 |
9.1 |
395 |
17.1 |
7.4 |
|
2003 |
435 |
18.8 |
7.7 |
378 |
16.4 |
6.7 |
|
2004 |
430 |
18.6 |
7.8 |
357 |
15.5 |
6.2 |
|
2005 |
397 |
17.2 |
7.0 |
350 |
15.2 |
6.1 |
|
2006 |
374 |
16.3 |
6.5 |
317 |
13.8 |
5.4 |
|
2007 |
397 |
17.3 |
6.8 |
332 |
14.5 |
5.3 |
| 2008 | 390 | 17.0 | 6.9 | 364 | 15.9 | 6.2 |
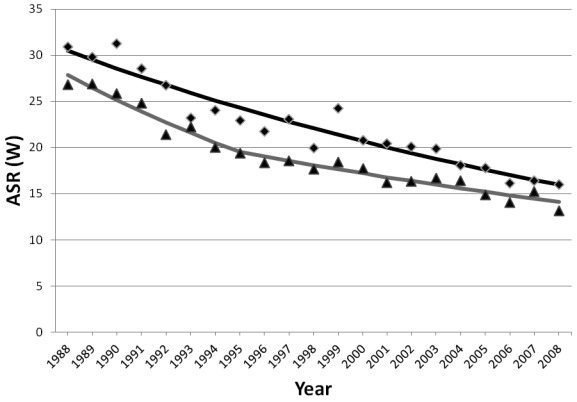
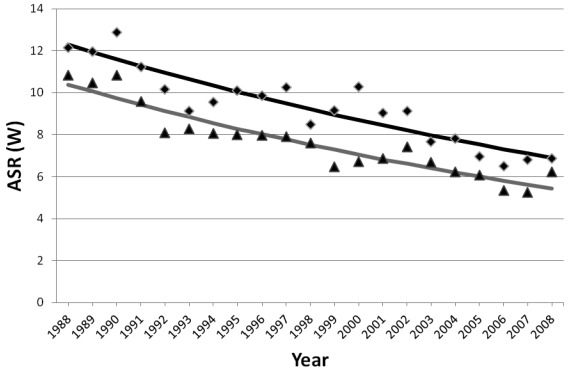
In both sexes, gastric cancer incidence and mortality rates declined steadily (Figure 1 and 2). In men, incidence rates decreased over the entire period (EAPC -3.2%; 95% confidence interval [CI], -3.6 to -2.7), with the best fitting model having no joinpoints, while mortality rates decreased sharply between 1988 and 1995 (EAPC -5.0%; 95% CI, -6.5 to -3.4) and then less sharply between 1995 and 2008 (EAPC -2.5%; 95% CI, -3.1 to -1.8). In women, incidence rates decreased (EAPC -2.8%; 95% CI, -3.5 to -2.2), also with no joinpoints in the final model, and the mortality rates decreased over the entire period (EAPC -3.2%; 95% CI, -3.7 to -2.7) (Table 3).
Figure 1.
Joinpoint analyses of incidence (rhombs) and mortality (triangles) for gastric cancer in Croatia in the period 1988-2008 for men. ASR (W) – age standardized rate per 100 000 (world standard population).
Figure 2.
Joinpoint analyses of incidence (rhombs) and mortality (triangles) for gastric cancer in Croatia in the period 1988-2008 for women. ASR (W) – age standardized rate per 100 000 (world standard population).
Table 3.
Trends in incidence and mortality of gastric cancer in Croatia according to joinpoint analyses, 1988-2008, by sex, with the estimated annual percent change (EAPC) and 95% confidence interval (CI)
| Joinpoint analyses |
||||||||
|---|---|---|---|---|---|---|---|---|
| trend 1 |
trend 2 |
|||||||
| years | EAPC | 95% CI | years | EAPC | 95% CI | |||
| Men: |
||||||||
| incidence |
1988-2008 |
-3.2 |
-3.6 to -2.7 |
|||||
| mortality |
1988-1995 |
-5.0 |
-6.5 to -3.4 |
1995-2008 |
-2.5 |
-3.1 to -1.8 |
||
| Women: |
||||||||
| incidence |
1988-2008 |
-2.8 |
-3.5 to -2.2 |
|||||
| mortality | 1988-2008 | -3.2 | -3.7 to -2.7 | |||||
Colorectal cancer
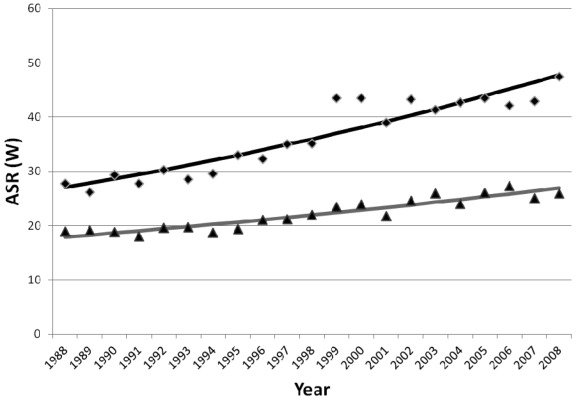
From 1988 to 2008, there were 25 964 cases of colorectal cancer in men (Table 4). The number of male colorectal cancer cases increased more than 2-fold, from 767 in 1988 to 1818 in 2008, while ASR increased from 27.8 to 47.5. In that period, 15 983 male colorectal cancer patients died. The number of colorectal cancer deaths doubled, from 509 in 1988 to 1052 in 2008, while ASR increased from 19.0 to 26.0/100 000. Joinpoint analysis in men did not identify any joinpoints (Table 5 and Figure 3). The incidence trend significantly increased (EAPC 2.9%; 95% CI, 2.3 to 3.9%), as well as mortality trend (EAPC 2.1%; 95% CI, 1.7 to 2.4%).
Table 4.
Male colorectal cancer incidence and mortality in Croatia in the period 1988-2008. Number of cases, crude rate, and age standardized rate (ASR) per 100 000
| Year | Incidence |
Mortality |
||||
|---|---|---|---|---|---|---|
| N | crude rate | ASR | N | crude rate | ASR | |
|
1988 |
767 |
35.3 |
27.8 |
509 |
23.4 |
19.0 |
|
1989 |
729 |
33.5 |
26.2 |
535 |
24.6 |
19.2 |
|
1990 |
837 |
38.3 |
29.3 |
523 |
23.9 |
18.8 |
|
1991 |
780 |
35.5 |
27.7 |
522 |
23.7 |
18.0 |
|
1992 |
886 |
40.0 |
30.2 |
582 |
26.3 |
19.6 |
|
1993 |
876 |
39.2 |
28.5 |
601 |
26.9 |
19.7 |
|
1994 |
947 |
42.2 |
29.6 |
601 |
26.8 |
18.7 |
|
1995 |
1096 |
48.7 |
33.0 |
631 |
28.0 |
19.4 |
|
1996 |
1076 |
48.0 |
32.3 |
702 |
31.3 |
21.1 |
|
1997 |
1172 |
52.6 |
35.0 |
718 |
32.2 |
21.2 |
|
1998 |
1198 |
54.3 |
35.1 |
758 |
34.3 |
22.0 |
|
1999 |
1498 |
68.5 |
43.5 |
801 |
36.6 |
23.5 |
|
2000 |
1503 |
69.3 |
43.5 |
841 |
38.8 |
23.9 |
|
2001 |
1369 |
63.4 |
39.0 |
770 |
35.7 |
21.8 |
|
2002 |
1544 |
71.8 |
43.3 |
885 |
41.2 |
24.6 |
|
2003 |
1482 |
69.1 |
41.3 |
956 |
44.6 |
26.0 |
|
2004 |
1556 |
72.6 |
42.7 |
898 |
41.9 |
24.1 |
|
2005 |
1609 |
75.2 |
43.5 |
998 |
46.7 |
26.0 |
|
2006 |
1595 |
74.7 |
42.1 |
1028 |
48.2 |
27.3 |
|
2007 |
1626 |
76.3 |
42.9 |
982 |
46.1 |
25.1 |
| 2008 | 1818 | 85.5 | 47.5 | 1052 | 49.5 | 26.0 |
Table 5.
Trends in incidence and mortality of colorectal cancer in Croatia according to joinpoint analyses, 1988-2008, by sex, with the estimated annual percent change (EAPC) and 95% confidence interval (CI)
| Joinpoint analyses |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Trend 1 |
Trend 2 |
Trend 3 |
|||||||
| years | EAPC | 95% CI | years | EAPC | 95% CI | years | EAPC | 95% CI | |
| Men: |
|||||||||
| incidence |
1988-2008 |
2.9 |
2.3 to 3.5 |
||||||
| mortality |
1988-2008 |
2.1 |
1.7 to 2.4 |
||||||
| Women: |
|||||||||
| incidence |
1988-1996 |
-0.2 |
-1.9 to 1.6 |
1996-1999 |
10.6 |
-3.7 to 27.1 |
1999-2008 |
-0.5 |
-1.7 to 0.7 |
| mortality | 1988-1994 | -1.9 | -4.1 to 0.3 | 1994-2008 | 1.1 | 0.5 to 1.7 | |||
Figure 3.
Joinpoint analyses of incidence (rhombs) and mortality (triangles) for colorectal cancer in Croatia in the period 1988-2008 for men. ASR (W) – age standardized rate per 100 000 (world standard population).
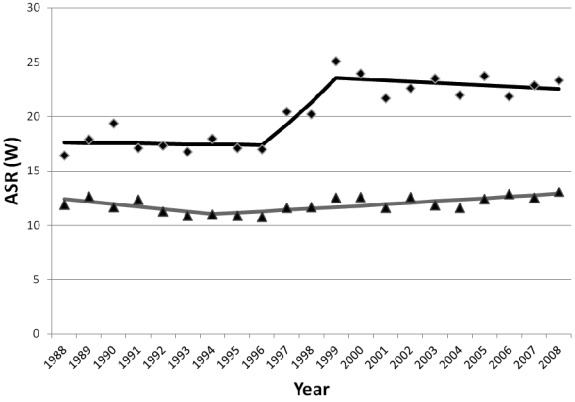
There were 20 752 cases of female colorectal cancer (Table 6). From 1988 to 2008, the number of cases increased almost 2-fold, from 685 to 1254. The ASR increased from 16.4/100 000 to 23.4/100 000. In the same period, 12 999 female colorectal cancer patients died. The number of colorectal cancer deaths increased from 508 in 1988 to 803 in 2008, while ASR increased from 11.9/100 000 to 13.1/100 000. Joinpoint analysis of female colorectal cancer incidence trends (Table 5 and Figure 4) identified two joinpoints, in 1996 and 1999, resulting in three separate trends: from 1988 to 1996 the trend was stable (EAPC -0.2%; 95% CI, -1.9 to 1.6%), from 1996 to 1999 the trend showed a non-significant increase (EAPC 10.6%; 95% CI, -3.7 to 27.1%), and from 1999 to 2008 the trend was again stable (EAPC -0.5; 95% CI, -1.7 to 0.7%). AAPC for the period from 1996 (when the first increase in incidence was noted) to 2008 showed a non-significant increase (AAPC 2.2%; 95% CI, -1.1 to 5.6%). Joinpoint analysis of female colorectal cancer mortality identified one joinpoint in 1994, resulting in two separate trends: the trend from 1988 to 1994 showed non-significant decrease (EAPC -1.9%; 95% CI, -4.1 to 0.3%), while from 1994 to 2008 it showed a significant increase (EAPC 1.1%; 95% CI, 0.5 to 1.7%).
Table 6.
Female colorectal cancer incidence and mortality in Croatia in the period 1988-2008. Number of cases, crude rate, and age standardized rate (ASR) per 100 000 using world standard population
| Year | Incidence |
Mortality |
||||
|---|---|---|---|---|---|---|
| N | crude rate /100 000 | ASR /100 000 | N | crude rate /100 000 | ASR /100 000 | |
|
1988 |
685 |
29.6 |
16.4 |
508 |
21.9 |
11.91 |
|
1989 |
770 |
33.2 |
17.9 |
556 |
24.0 |
12.66 |
|
1990 |
811 |
34.8 |
19.4 |
525 |
22.5 |
11.68 |
|
1991 |
734 |
31.3 |
17.1 |
545 |
23.2 |
12.34 |
|
1992 |
766 |
32.3 |
17.4 |
518 |
21.8 |
11.27 |
|
1993 |
755 |
31.5 |
16.8 |
518 |
21.6 |
10.90 |
|
1994 |
827 |
34.3 |
17.9 |
530 |
22.0 |
10.97 |
|
1995 |
823 |
34.0 |
17.1 |
540 |
22.3 |
10.88 |
|
1996 |
824 |
34.2 |
17.0 |
553 |
22.9 |
10.79 |
|
1997 |
963 |
40.2 |
20.5 |
570 |
23.8 |
11.60 |
|
1998 |
986 |
41.5 |
20.2 |
604 |
25.4 |
11.67 |
|
1999 |
1186 |
50.4 |
25.1 |
639 |
27.2 |
12.50 |
|
2000 |
1197 |
51.3 |
24.0 |
668 |
28.6 |
12.59 |
|
2001 |
1072 |
46.2 |
21.7 |
640 |
27.6 |
11.62 |
|
2002 |
1116 |
48.2 |
22.6 |
673 |
29.1 |
12.59 |
|
2003 |
1213 |
52.5 |
23.5 |
666 |
28.8 |
11.84 |
|
2004 |
1143 |
49.5 |
22.0 |
666 |
28.9 |
11.59 |
|
2005 |
1237 |
53.7 |
23.7 |
749 |
32.5 |
12.38 |
|
2006 |
1175 |
51.1 |
21.9 |
772 |
33.6 |
12.84 |
|
2007 |
1215 |
52.9 |
22.9 |
756 |
32.9 |
12.51 |
| 2008 | 1254 | 54.7 | 23.4 | 803 | 35.0 | 13.07 |
Figure 4.
Joinpoint analyses of incidence (rhombs) and mortality (triangles) for colorectal cancer in Croatia in the period 1988-2008 for women. ASR (W) – age standardized rate per 100 000 (world standard population).
Discussion
This is the first study to systematically analyze the time trends of gastric and colorectal cancer incidence and mortality in Croatia. We chose to use joinpoint regression modeling as the optimal method to detect and depict sharp changes in trends, reflecting changes in cancer prevention and care policies.
Gastric cancer
Our analysis revealed a persistent and steady decrease in gastric cancer incidence and mortality rates in Croatia between 1988 and 2008, with rates at the end of the study period being ~ 40% lower than at the beginning. The decrease was observed in both men and women, and the trends did not level off in the recent years.
Croatia is in the upper half of the list of European countries according to age-standardized gastric cancer incidence and mortality rates for both men and women. According to the GLOBOCAN database, the highest incidence and mortality rates in Europe are observed in Belarus, Albania, and Russian Federation (incidence rates between 25-34/100 000 in men and 12-18/100 000 in women; and mortality rates between 21-30/100 000 in men and 10-15/100 000 in women), with much higher rates in Central and Eastern European countries than in more developed Western or Northern European countries (1).
In the EU from 1980 to 1999, a steady and persistent decrease in gastric cancer mortality rates was observed (from 18.6 to 9.8/100 000 in men and from 8.9 to 4.6/100 000 in women, ~ 50% for each sex) (13,46). Decreases were also reported between 2000 and 2005 (47). The reasons for the decrease are complex, and not completely understood. They include more varied and better nutrition, better food preservation (refrigeration), decreased prevalence of H. pylori infection due to improved living conditions and changes in lifestyle (48-50), and, at least to some extent, decreased smoking prevalence in men (51). The lowering of incidence rates leads to lowering of mortality rates, with a possible contribution of improved medical treatment (52).
During the second half of the twentieth century, Croatian society experienced both industrial and nutritional changes. The availability of various food products, such as fruits and products of animal origin, was facilitated by the introduction of refrigerators (53). Progressive globalization after the war in Croatia (1991-1995) led to a smaller need for homemade food production, which was heavily salted, marinated, and low in antioxidants (54).
The prevalence of H. Pylori infection in Croatia in 1998 in a random sample of individuals aged 20-70 was 60.4%, with higher rates in the southern parts of the country (55). This is considered to be an average value, since the prevalence ranges from 30% in the western countries to 60%-88% in Asian countries (56). Unfortunately, more recent data for Croatia are not available.
The prevalence of smoking in 1995 was 34% among men and 27% among women in 18-65 age group (57), while the prevalence in 2003 was 34% among men and 22% among women in 18+ age group (58). The relatively stable proportion of smokers in Croatia may indicate that the lowering of gastric cancer rates was not influenced by changes in smoking habits.
The use of joinpoint regression model allows a fresh approach to interpretation of trends in cancer mortality over a longer time period. This study, as well as other studies of gastric cancer trends (13,14), shows a decline in mortality, with no signs of leveling off in the recent years. Thus, it is expected that the decrease in incidence and mortality rates will continue (59), especially because such decreases are also observed in the countries that already have relatively low gastric cancer incidence and mortality rates, such as Iceland, Cyprus, and Sweden (incidence rates between 4.7-6.6/100 000 in men and between 2.5 and 3.1/100 000 in women; and mortality rates between 4.1-4.2/100 000 in men and between 1.8-2.4/100 000 in women) (1).
Colorectal cancer
The incidence trend of colorectal cancer showed a significant increase in men (EAPC 2.9%). In women, there was no significant change in trend, though there was a constant non-significant increase. In 2008, with 1818 new cases colorectal cancer was the third most common cancer in Croatian male population and with 1052 deaths, the second cause of cancer mortality (21). In women, with 1254 new cases it was the second most common cancer, and with 803 deaths the second cause of cancer death. Compared to GLOBOCAN 2008 estimates for Europe, Croatia ranked ninth according to incidence of colorectal cancer (ASR 44.4/100 000) in men and fifteenth (ASR 24.3/100 000) in women, but fourth according to mortality in both men (ASR 25.4/100 000) and women (ASR 13.1/100 000) (1).
Incidence trends vary across Europe, with persistent increases or no change in Eastern European countries and persistent slight decreases in Western Europe (17). The increase in the incidence in Eastern Europe (eg, Slovakia or Czech Republic, as well as Croatia) could be attributed to lifestyle changes, presumably introduced by westernization, resulting in obesity and physical inactivity (60). In fact, in 2003 over 1.3 million people in Croatia were either obese or overweight (61). The observed variations of the incidence rates of colorectal cancer reflect the distributions of risk factors across European countries, as well as disparities in the effective cancer prevention and detection (62). However, such results should be interpreted with caution since all registries from which the data have been obtained do not comply with quality requirements, and a substantial number of Eastern European countries eg, Greece, Bosnia and Herzegovina, FYR Macedonia, and Romania, do not have established national cancer registration (63).
In the European Union, colorectal cancer mortality has been declining since the early 1980s by around 1% per year in women, and since the early 1990s by 0.6% per year in men (64). Decreases were found in Austria, Finland, Ireland, Netherlands, Norway, Sweden, Switzerland, United Kingdom, France, and Italy, and even earlier in Belgium, Germany, and Denmark (65). On the other hand, increases were observed in Bulgaria, Poland, Romania, Greece, Portugal, and Spain. In the Czech Republic, Slovakia, and Hungary, the rates were exceedingly high, but remained constant in the recent years (65).
Incidence and the mortality rates may also be affected by screening and early detection. All but three countries (Greece, Russia, and Turkey) (66-68) participate in some form of colorectal cancer screening. However, only five European countries have an organized population-based screening program (Croatia, Finland, France, Italy, the UK) (69-72) and only five have screening guidelines – Finland, France, Germany (73), Italy, and the UK. There is still room for improvement since the participation rate in the voluntary screening is 15% and up to 60% in the organized screening programs (74). In the Croatian national screening, the participation rate was 19.9% (69).
Several randomized trials and one Cochrane review provided strong evidence that fecal occult blood test followed by colonoscopy, if offered every 2 years, reduced mortality rates associated with colorectal cancer by 16% (75,76). In the United States, where screening was introduced in the 1970s, long-term screening programs have played a relevant role in reducing colorectal cancer incidence and mortality over the last two decades. One of the explanations for better survival rates in the United States is an increased detection and removal of colorectal polyps, which often precede colorectal cancer development. Screening programs have been implemented in Europe, including Croatia, only recently, so their impact on the incidence and mortality may become more visible in the future (76). Nevertheless, increased patient awareness of signs and symptoms, recognition in the primary care, and access to diagnostic modalities may have already played a role.
In the past ten years, countries across the EU have initiated national policies for improvement in diagnostics, referral, and assurance of quality of care in specialized centers with attending multidisciplinary cancer teams. The main goals were to improve survival and quality of life and to reduce regional differences in treatment options. Following the publication of the Campbell Report, the UK has implemented specialization of cancer services, which proved to be closely related to a reduction in mortality. Another important step is the shortening of the period between the symptom appearance and the referral to diagnostics (33).
Another important factor that explains the observed patterns of survival and mortality, alongside screening, is the progress in therapy (77). The general improvement in surgical techniques for localized and metastatic tumors substantially prolonged survival (78,79). Only the adoption of surgical training (subspecialization) and pathological evaluation of specimens (quality control) in rectal cancer management resulted in an overall improvement in survival (34,80,81). The wider adoption of new treatment protocols and adjuvant therapies, including progressive increase in sequential adjuvant chemotherapy for advanced non-localized tumors, and preoperative chemo- and radiotherapy for rectal cancers (82), may have favorably influenced colorectal cancer mortality (47,77,83,84). Moreover, in the past fifteen years five new drugs have been introduced (85,86).
We believe that the discrepancy between the incidence (9th in male and 15th in female colorectal cancer) and mortality (4th for both sexes) rank of Croatia in Europe strongly indicates the need for improvement of prevention and treatment.
There are still no standardized protocols for treatment and referral, or requirements for the availability of multidisciplinary teams for colorectal cancer treatment in most Croatian hospitals, with the exception of university hospitals. Due to the high incidence and increasing mortality, Croatian Society of Coloproctology and Croatian Society of Oncology have launched an incentive for quality control and referral improvements for colorectal cancer patients, recognizing the current lack of availability of all treatment modalities at different hospitals (87). Hopefully, establishing accrediting centers for colorectal cancer treatment with trained staff would ensure that every patient receives optimal surgical, neo adjuvant or adjuvant treatment, systemic and local (radiotherapy), and regular follow-up.
Acknowledgments
Acknowledgment We thank Professor Branko Papa for critical reading of the manuscript and helpful suggestions.
Funding This study was partly supported by the Croatian Ministry of Science, Education and Sport grant No. 005-1080315-0294.
Ethical approval Not required.
Declaration of authorship IK performed the study design, data collection, data analysis, and manuscript editing and review. MŠ participated in the data collection, interpretation of the results, manuscript preparation, manuscript editing and review of the manuscript. IŠ participated in data collection, manuscript preparation, and manuscript review. LZ participated in writing the manuscript and made important contribution in the area of the expertise. DVV participated in study design, and manuscript editing and review. DK participated in data analysis, manuscript review, and study design. TK participated in study design, data collection, data analysis, manuscript editing, and manuscript review. AZ participated in study design and coordination, interpretation of the results, manuscript preparation, manuscript editing, and manuscript review.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer incidence and mortality worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr Accessed: March 28, 2012.
- 2.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verdecchia A, Mariotto A, Gatta G, Bustamante-Teixeira MT, Ajiki W. Comparison of stomach cancer incidence and survival in four continents. Eur J Cancer. 2003;39:1603–9. doi: 10.1016/S0959-8049(03)00360-5. [DOI] [PubMed] [Google Scholar]
- 4.Cancer WIAfRo, editor. IARC monographs on the evaluation of carcinogenic risks to humans Shistosomes, Liver Flukes and Helicobacter pylori: Lyon (France): IARC; 1994. [PMC free article] [PubMed] [Google Scholar]
- 5.Joossens JV, Hill MJ, Elliott P, Stamler R, Lesaffre E, Dyer A, et al. Dietary salt, nitrate and stomach cancer mortality in 24 countries. Int J Epidemiol. 1996;25:494–504. doi: 10.1093/ije/25.3.494. [DOI] [PubMed] [Google Scholar]
- 6.Nagel G, Linseisen J, Boshuizen HC, Pera G, Del Giudice G, Westert GP, et al. Socioeconomic position and the risk of gastric and oesophageal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Int J Epidemiol. 2007;36:66–76. doi: 10.1093/ije/dyl275. [DOI] [PubMed] [Google Scholar]
- 7.Barrett JH, Parslow RC, McKinney PA, Law GR, Forman D. Nitrate in drinking water and the incidence of gastric, esophageal, and brain cancer in Yorkshire, England. Cancer Causes Control. 1998;9:153–9. doi: 10.1023/A:1008878126535. [DOI] [PubMed] [Google Scholar]
- 8.Morales-Suarez-Varela MM, Llopis-Gonzalez A, Tejerizo-Perez ML. Impact of nitrates in drinking water on cancer mortality in Valencia, Spain. Eur J Epidemiol. 1995;11:15–21. doi: 10.1007/BF01719941. [DOI] [PubMed] [Google Scholar]
- 9.Tredaniel J, Boffetta P, Buiatti E, Saracci R, Hirsch A. Tobacco smoking and gastric cancer: Review and meta-analysis. Int J Cancer. 1997;72:565–73. doi: 10.1002/(SICI)1097-0215(19970807)72:4<565::AID-IJC3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 10.Aragones N, Pollan M, Gustavsson P. Stomach cancer and occupation in Sweden: 1971-89. Occup Environ Med. 2002;59:329–37. doi: 10.1136/oem.59.5.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocco P, Palli D, Buiatti E, Cipriani F, DeCarli A, Manca P, et al. Occupational exposures as risk factors for gastric cancer in Italy. Cancer Causes Control. 1994;5:241–8. doi: 10.1007/BF01830243. [DOI] [PubMed] [Google Scholar]
- 12.Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1–9. doi: 10.1016/S0895-4356(02)00534-6. [DOI] [PubMed] [Google Scholar]
- 13.Levi F, Lucchini F, Gonzalez JR, Fernandez E, Negri E, La Vecchia C. Monitoring falls in gastric cancer mortality in Europe. Ann Oncol. 2004;15:338–45. doi: 10.1093/annonc/mdh057. [DOI] [PubMed] [Google Scholar]
- 14.Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, et al. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666–73. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 15.Bosetti C, Bertuccio P, Levi F, Lucchini F, Negri E, La Vecchia C. Cancer mortality in the European Union, 1970-2003, with a joinpoint analysis. Ann Oncol. 2008;19:631–40. doi: 10.1093/annonc/mdm597. [DOI] [PubMed] [Google Scholar]
- 16.Lepage C, Remontet L, Launoy G, Tretarre B, Grosclaude P, Colonna M, et al. Trends in incidence of digestive cancers in France. Eur J Cancer Prev. 2008;17:13–7. doi: 10.1097/CEJ.0b013e32809b4cba. [DOI] [PubMed] [Google Scholar]
- 17.Levi F, Lucchini F, Negri E, La Vecchia C. Continuing declines in cancer mortality in the European Union. Ann Oncol. 2007;18:593–5. doi: 10.1093/annonc/mdl437. [DOI] [PubMed] [Google Scholar]
- 18.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 19.Roosendaal R, Kuipers EJ, Buitenwerf J, van Uffelen C, Meuwissen SG, van Kamp GJ, et al. Helicobacter pylori and the birth cohort effect: evidence of a continuous decrease of infection rates in childhood. Am J Gastroenterol. 1997;92:1480–2. [PubMed] [Google Scholar]
- 20.Fuccio L, Zagari RM, Eusebi LH, Laterza L, Cennamo V, Ceroni L, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151:121–8. doi: 10.7326/0003-4819-151-2-200907210-00009. [DOI] [PubMed] [Google Scholar]
- 21.Croatian National Cancer Registry. Cancer incidence in Croatia 2008. Zagreb: Croatian National Institute of Public Health; 2010. [Google Scholar]
- 22.Baklaic Z, Deckovic-Vukres V, Kuzman M, editors. Croatian Health Service Yearbook 2008. Zagreb: Croatian National Institute of Public Health; 2009. [Google Scholar]
- 23.Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, et al. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8:784–96. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 24.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 25.Broderick P, Carvajal-Carmona L, Pittman AM, Webb E, Howarth K, Rowan A, et al. A genome-wide association study shows that common alleles of SMAD7 influence colorectal cancer risk. Nat Genet. 2007;39:1315–7. doi: 10.1038/ng.2007.18. [DOI] [PubMed] [Google Scholar]
- 26.Tomlinson I, Webb E, Carvajal-Carmona L, Broderick P, Kemp Z, Spain S, et al. A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat Genet. 2007;39:984–8. doi: 10.1038/ng2085. [DOI] [PubMed] [Google Scholar]
- 27.Tenesa A, Farrington SM, Prendergast JG, Porteous ME, Walker M, Haq N, et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on 11q23 and replicates risk loci at 8q24 and 18q21. Nat Genet. 2008;40:631–7. doi: 10.1038/ng.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomlinson IP, Webb E, Carvajal-Carmona L, Broderick P, Howarth K, Pittman AM, et al. A genome-wide association study identifies colorectal cancer susceptibility loci on chromosomes 10p14 and 8q23.3. Nat Genet. 2008;40:623–30. doi: 10.1038/ng.111. [DOI] [PubMed] [Google Scholar]
- 29.Houlston RS, Webb E, Broderick P, Pittman AM, Di Bernardo MC, Lubbe S, et al. Meta-analysis of genome-wide association data identifies four new susceptibility loci for colorectal cancer. Nat Genet. 2008;40:1426–35. doi: 10.1038/ng.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giovannucci E, Wu K. Cancers of the colon and rectum. In: Schottenfeld D, Fraumeni JF, Jr, editors. Cancer epidemiology and prevention. New York (NY): Oxford University Press; 2006;809-29. [Google Scholar]
- 31.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington (DC): AICRM; 2007. [Google Scholar]
- 32.Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–38. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenner H, Bouvier AM, Foschi R, Hackl M, Larsen IK, Lemmens V, et al. Progress in colorectal cancer survival in Europe, from the late 1980s to the early 21st century: The EUROCARE study Int J Cancer 2011. May 23Epub ahead of print 10.1002/ijc.26192 [DOI] [PubMed] [Google Scholar]
- 34.Quirke P. Training and quality assurance for rectal cancer: 20 years of data is enough. Lancet Oncol. 2003;4:695–702. doi: 10.1016/S1470-2045(03)01248-8. [DOI] [PubMed] [Google Scholar]
- 35.Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J, editors. Cancer incidence in five continents, Vol. VII (IARC Scientific Publications No. 143). Lyon (France): IARC; 1997. [Google Scholar]
- 36.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, editors. Cancer incidence in five continents, Vol. VIII (IARC Scientific Publications No. 155). Lyon (France): IARC; 2002. [Google Scholar]
- 37.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al, editors. Cancer incidence in five continents, Vol. IX (IARC Scientific Publications No. 160). Lyon (France): IARC; 2007. [Google Scholar]
- 38.World Health Organisation. International classification of diseasses. Geneva (Switzerland): World Health Organization; 1977. [Google Scholar]
- 39.World Health Organisation. International statistical classification of diseases and related health problems. Geneva (Switzerland): World Health Organization; 1992. [Google Scholar]
- 40.World Health Organisation. World Health Organisation, mortality database. WHO Statistical Information System. 2011. Available from: http://www.who.int/whosis/mort/download/index.html Accessed: March 28, 2012.
- 41.United Nations. World population prospects, the 2010 revision. United Nations Population Division Department of Economic and Social Affairs. 2011. Available from: http://esa.un.org/unpd/wpp/index.htm Accessed: March 28, 2012.
- 42.Doll R, Payne P, Waterhouse JAH, editors. Cancer incidence in five continents, Vol. I. Geneva (Switzerland): Union Internationale Contre le Cancer; 1966. [Google Scholar]
- 43.National Cancer Institute. Joinpoint Regression Program. Bethesda (MD): National Cancer Institute; 2009. [Google Scholar]
- 44.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 45.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28:3670–82. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levi F, Lucchini F, Negri E, La Vecchia C. Cancer mortality in the European Union, 1988-1997: the fall may approach 80,000 deaths a year. Int J Cancer. 2002;98:636–7. doi: 10.1002/ijc.10235. [DOI] [PubMed] [Google Scholar]
- 47.Karim-Kos HE, de Vries E, Soerjomataram I, Lemmens V, Siesling S, Coebergh JW. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44:1345–89. doi: 10.1016/j.ejca.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 48.Verdecchia A, Corazziari I, Gatta G, Lisi D, Faivre J, Forman D. Explaining gastric cancer survival differences among European countries. Int J Cancer. 2004;109:737–41. doi: 10.1002/ijc.20047. [DOI] [PubMed] [Google Scholar]
- 49.Corley DA, Kubo A. Influence of site classification on cancer incidence rates: an analysis of gastric cardia carcinomas. J Natl Cancer Inst. 2004;96:1383–7. doi: 10.1093/jnci/djh265. [DOI] [PubMed] [Google Scholar]
- 50.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–92. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 51.Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689–701. doi: 10.1007/s10552-008-9132-y. [DOI] [PubMed] [Google Scholar]
- 52.Hartgrink HH, Bonenkamp HJ, van de Velde CJ. Influence of surgery on outcomes in gastric cancer. Surg Oncol Clin N Am. 2000;9:97–117. [PubMed] [Google Scholar]
- 53.La Vecchia C, Negri E, D'Avanzo B, Franceschi S. Electric refrigerator use and gastric cancer risk. Br J Cancer. 1990;62:136–7. doi: 10.1038/bjc.1990.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kono S, Hirohata T. Nutrition and stomach cancer. Cancer Causes Control. 1996;7:41–55. doi: 10.1007/BF00115637. [DOI] [PubMed] [Google Scholar]
- 55.Babus V, Presecki V, Katicic M, Balija M, Zoric I, Kronja L, et al. Distribution of Helicobacter pylori infection in the adult population of Croatia. Lijec Vjesn. 1997;119:139–42. [in Croatian] [PubMed] [Google Scholar]
- 56.Prinz C, Schwendy S, Voland P. H pylori and gastric cancer: shifting the global burden. World J Gastroenterol. 2006;12:5458–64. doi: 10.3748/wjg.v12.i34.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turek S, Rudan I, Smolej-Narancic N, Szirovitza L, Cubrilo-Turek M, Zerjavic-Hrabak V, et al. A large cross-sectional study of health attitudes, knowledge, behaviour and risks in the post-war Croatian population (the First Croatian Health Project). Coll Antropol. 2001;25:77–96. [PubMed] [Google Scholar]
- 58.Kovacic L, Gazdek D, Samardzic S. Croatian health survey: cigarette smoking. Acta Med Croatica. 2007;61:281–5. [in Croatian] [PubMed] [Google Scholar]
- 59.Amiri M, Janssen F, Kunst AE. The decline in stomach cancer mortality: exploration of future trends in seven European countries. Eur J Epidemiol. 2011;26:23–8. doi: 10.1007/s10654-010-9522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zatonski W, Didkowska J. Closing the gap: cancer in Central and Eastern Europe (CEE). Eur J Cancer. 2008;44:1425–37. doi: 10.1016/j.ejca.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 61.Heim I, Leontic K, Gostovic MJ. Obesity and overweight in Croatia. Acta Med Croatica. 2007;61:267–73. [in Croatian] [PubMed] [Google Scholar]
- 62.Lopez-Abente G, Ardanaz E, Torrella-Ramos A, Mateos A, Delgado-Sanz C, Chirlaque MD. Changes in colorectal cancer incidence and mortality trends in Spain. Ann Oncol. 2010;21(Suppl 3):iii76–82. doi: 10.1093/annonc/mdq091. [DOI] [PubMed] [Google Scholar]
- 63.European Network of Cancer Registries. Available from: http://www.encr.com.fr/ Accessed: April 5, 2012.
- 64.La Vecchia C, Bosetti C, Lucchini F, Bertuccio P, Negri E, Boyle P, et al. Cancer mortality in Europe, 2000-2004, and an overview of trends since 1975. Ann Oncol. 2010;21:1323–60. doi: 10.1093/annonc/mdp530. [DOI] [PubMed] [Google Scholar]
- 65.Fernandez E, La Vecchia C, Gonzalez JR, Lucchini F, Negri E, Levi F. Converging patterns of colorectal cancer mortality in Europe. Eur J Cancer. 2005;41:430–7. doi: 10.1016/j.ejca.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 66.Geitona M, Kanavos P. Colocteral cancer management and prevention policies in Greece. Eur J Health Econ. 2010;10(Suppl 1):S27–33. doi: 10.1007/s10198-009-0193-y. [DOI] [PubMed] [Google Scholar]
- 67.Avksentyeva M. Colorectal cancer in Russia. Eur J Health Econ. 2010;10(Suppl 1):S91–8. doi: 10.1007/s10198-009-0195-9. [DOI] [PubMed] [Google Scholar]
- 68.Tatar M, Tatar F. Colorectal cancer in Turkey: current situation and challenges for the future. Eur J Health Econ. 2010;10(Suppl 1):S99–105. doi: 10.1007/s10198-009-0197-7. [DOI] [PubMed] [Google Scholar]
- 69.Katičić M, Antoljak N, Strnad Pešikan M, Kujundžić M, Štimac D, Šamija M, et al. National Colorectal Cancer Screening Program in Croatia 2007-2010. Bosn J Basic Med Sci. 2011;11(Suppl 1):S67–72. doi: 10.3748/wjg.v18.i32.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chevreul K. Colorectal cancer in France. Eur J Health Econ. 2010;10(Suppl 1):S15–20. doi: 10.1007/s10198-009-0185-y. [DOI] [PubMed] [Google Scholar]
- 71.Masseria C. Colorectal cancer in Italy: a review of current national and regional practice on screening and treatment. Eur J Health Econ. 2010;10(Suppl 1):S41–9. doi: 10.1007/s10198-009-0191-0. [DOI] [PubMed] [Google Scholar]
- 72.Schurer W, Kanavos P. Colorectal cancer management in the United Kingdom: current practice and challenges. Eur J Health Econ. 2010;10(Suppl 1):S85–90. doi: 10.1007/s10198-009-0202-1. [DOI] [PubMed] [Google Scholar]
- 73.von der Schulenburg JM, Prenzler A, Schurer W. Cancer management and reimbursement aspects in Germany: an overview demonstrated by the case of colorectal cancer. Eur J Health Econ. 2010;10(Suppl 1):S21–6. doi: 10.1007/s10198-009-0194-x. [DOI] [PubMed] [Google Scholar]
- 74.European Commission. Health in the European Union. Special Eurobarometer 272e/Wave 66.2. 2007. Available from: http://ec.europa.eu/health/ph_publication/eb_health_en.pdf Accessed: April 4, 2012.
- 75.Towler B, Irwig L, Glasziou P, Kewenter J, Weller D, Silagy C. A systematic review of the effects of screening for colorectal cancer using the faecal occult blood test, hemoccult. BMJ. 1998;317:559–65. doi: 10.1136/bmj.317.7158.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366–78. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 77.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet. 2010;375:1030–47. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 78.Primrose J, Treasure T, Fiorentino F. Lung metastasectomy in colorectal cancer: is this surgery effective in prolonging life? Respirology. 2010;15:742–6. doi: 10.1111/j.1440-1843.2010.01759.x. [DOI] [PubMed] [Google Scholar]
- 79.Primrose JN. Surgery for colorectal liver metastases. Br J Cancer. 2010;102:1313–8. doi: 10.1038/sj.bjc.6605659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–82. doi: 10.1016/S0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 81.Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study that recruited consecutive patients with rectal cancer. Ann Surg. 2011;2011:13. doi: 10.1097/SLA.0b013e31820b8d52. . Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 82.Hospers GA, Punt CJ, Tesselaar ME, Cats A, Havenga K, Leer JW, et al. Preoperative chemoradiotherapy with capecitabine and oxaliplatin in locally advanced rectal cancer. A phase I-II multicenter study of the Dutch Colorectal Cancer Group. Ann Surg Oncol. 2007;14:2773–9. doi: 10.1245/s10434-007-9396-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Javle M, Hsueh CT. Recent advances in gastrointestinal oncology–updates and insights from the 2009 annual meeting of the American society of clinical oncology. J Hematol Oncol. 2010;3:11. doi: 10.1186/1756-8722-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Leon MP, Pezzi A, Benatti P, Manenti A, Rossi G, di Gregorio C, et al. Survival, surgical management and perioperative mortality of colorectal cancer in the 21-year experience of a specialised registry. Int J Colorectal Dis. 2009;24:777–88. doi: 10.1007/s00384-009-0687-1. [DOI] [PubMed] [Google Scholar]
- 85.Saif MW, Kang SP, Chu E. Treatment of metastatic colorectal cancer: from cytotoxic agents to molecular agents and multitargeted strategies. Oncology. 2006;20(Suppl 10):11–9. . Williston Park. [PubMed] [Google Scholar]
- 86.Lucas AS, O'Neil BH, Goldberg RM. A decade of advances in cytotoxic chemotherapy for metastatic colorectal cancer. Clin Colorectal Cancer. 2011;10:238–44. doi: 10.1016/j.clcc.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 87.Vrdoljak E, Plestina S, Dintinjana RD, Tomas I, Sobat H, Separovic R, et al. Clinical recommendations for diagnosis, treatment and monitoring of patients with colorectal cancer. Lijec Vjesn. 2011;133:366–9. [in Croatian] [PubMed] [Google Scholar]