Abstract
Significance: An abundance of experimental evidence suggests that hydrogen sulfide (H2S) plays a prominent role in physiology and pathophysiology. Many targets exist for H2S therapy. The molecular targets of H2S include proteins, enzymes, transcription factors, and membrane ion channels. Recent Advances: Novel H2S precursors are being synthesized and discovered that are capable of releasing H2S in a slow and sustained manner. This presents a novel and advantageous approach to H2S therapy for treatment of chronic conditions associated with a decline in endogenous H2S, such as diabetes and cardiovascular disease. Critical Issues: While H2S is cytoprotective at physiological concentrations, it is not universally cytoprotective, as it appears to have pro-apoptotic actions in cancer cells and is well known to be toxic at supraphysiological concentrations. Many of the pleiotropic effects of H2S on health are associated with the inhibition of inflammation and upregulation of prosurvival pathways. The powerful anti-inflammatory, cytoprotective, immunomodulating, and trophic effects of H2S on the vast majority of normal cells seem to be mediated mainly by its actions as an extremely versatile direct and indirect antioxidant and free radical scavenger. While the overall effects of H2S on transformed (i.e., malignant) cells can be characterized as pro-oxidant and pro-apoptotic, they contrast sharply with the cytoprotective effects on most normal cells. Future Directions: H2S has become a molecule of great interest, and several slow-releasing H2S prodrugs are currently under development. We believe that additional agents regulating H2S bioavailability will be developed during the next 10 years. Antioxid. Redox Signal. 17, 119–140.
Introduction
Organ and cell function are regulated by a myriad of signaling chemical species. Among them, only three are diatomic or triatomic molecules: nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S), the so-called ”gaseous signaling molecules, or gasotransmitters” whose production and metabolism are primarily enzymatically regulated. These small molecules freely diffuse through cell membranes to elicit various responses independently of transporters or membrane receptors or second messenger systems (216), and they modulate many cellular functions through an array of intracellular signaling processes.
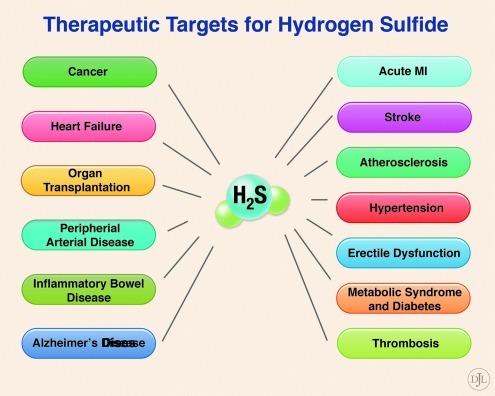
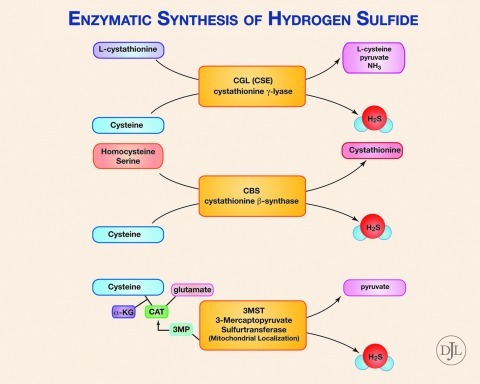
An abundance of recent experimental evidence suggests that H2S plays a prominent role in normal physiology and pathophysiology, and many therapeutic targets exist for H2S therapy (Fig. 1). The molecular targets of H2S include proteins, enzymes, transcription factors, and membrane ion channels. Cysteine is the major source of H2S in mammals, catalyzed by the enzymes: cystathionine beta-synthase (CBS), cystathionine gamma-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST) (Fig. 2). Whereas 3-MST is mainly localized in mitochondria, CBS and CSE exist in the cytosol.
FIG. 1.
Therapeutic targets for hydrogen sulfide (H2S). An abundance of experimental evidence suggests that H2S plays a prominent role in normal physiology and pathophysiology. Therefore, many therapeutic targets exist for H2S therapy, including cancer, heart failure, organ transplant, peripheral artery disease, inflammatory bowel disease, Alzheimer's disease, acute myocardial infarction (MI), stroke, atherosclerosis, hypertension, erectile dysfunction, metabolic syndrome, diabetes, and thrombosis.
FIG. 2.
Enzymatic synthesis of hydrogen sulfide (H2S). Desulfhydration of cysteine is the major source of H2S in mammals and is catalyzed by the trans-sulfuration pathway enzymes cystathionine beta-synthase (CBS), cystathionine gamma-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST). Cystathionine can be converted by CSE to form H2S. CBS can form cystathionine from serine and homocysteine, and additionally can form H2S from cysteine. Cysteine, along with alpha-ketoglutarate (alpha-KG), is converted into 3-mercaptopyruvate (3MP) by cystine aminotransferase (CAT). 3MP can then be broken down by 3MST to form H2S.
CBS and CSE generate H2S by using many different substrates (71). 3-MST catalyzes only sulfur transfer reactions from 3-mercaptopyruvate (3-MP) to various donors, for example:
![]()
The enzymatic sulfur transfer yields a hydropersulfide, not H2S (10). Release of H2S requires a further redox reaction between RSSH and a biological thiol such as glutathione (GSH):
![]()
Recently Kimura et al. demonstrated that 3-MST depends on a biological dithiol-thioredoxin (Trx) or dihydrolipoic acid- for the production of H2S from 3-MP (128).
H2S is enzymatically generated in the vasculature, heart, liver, kidney, brain, nervous system, lung, airway tissues, upper and lower GI tract, reproductive organs, skeletal muscle, pancreas, synovial joints, connective tissues, cochlea, and adipose tissues (105, 112). The key role of H2S in health and disease is clearly borne out by the correlations found to exist between low levels of plasma/tissue endogenous H2S/sulfane sulfur and/or H2S-generating enzymes on the one hand, and on the other the presence and progression of adiposity, marked endothelial dysfunction/insulin resistance, hypertension, hyperhomocysteinemia, diabetes, exacerbated cardiac injury following ischemia-reperfusion injury, Alzheimer disease, cirrhosis, chronic kidney disease, GI tract irritation, asthma, wound healing, and cancer (19, 46, 57, 58, 62, 85, 109, 156, 159, 210, 211, 219, 221, 239).
Physiological Actions of Hydrogen Sulfide
Nutrition, metabolism, and homeostasis
The main dietary sources of sulfur compounds in human nutrition are inorganic sulfates in drinking water and proteins derived from plants and animals. Only two of the twenty amino acids normally present in proteins are sulfur-containing amino acids (SAAs), namely methionine and cysteine. Methionine cannot be synthesized by the human body and must be supplied by the diet, whereas cysteine requirements can, in principle, be met by an excess of dietary methionine. However, cysteine is known as a semi-essential amino acid because humans can synthesize it from methionine to a limited extent (51, 143). Furthermore, the enzymes required for conversion of methionine to cysteine decline with age (17, 21). Dietary excess of cysteine and methionine is stored as GSH (17) (a thiolic antioxidant tripeptide) or, once the GSH pool has been replenished, converted to taurine or oxidized to sulfate (169). In fact, the availability of cysteine appears to be the rate-limiting factor for GSH biosynthesis from glutamate, glycine, and cysteine (9).
The “sulfane sulfur” pool (Fig. 7) performs an essential function in the brain, upon neuron excitation the bound sulfane sulfur releases H2S (80, 194). It is highly likely that H2S formation from sulfane sulfur requires reduced GSH as both hydrogen and electron donor. In the brain, H2S is produced mainly in astrocytes, which contain larger amounts of GSH than neurons.
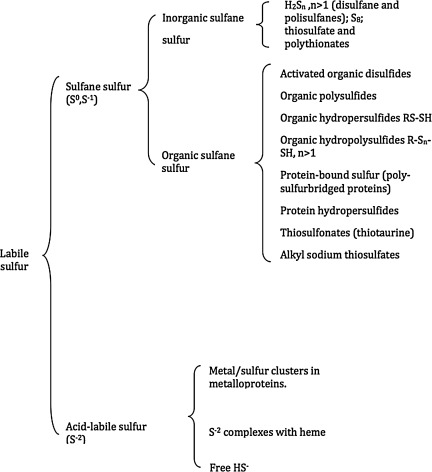
FIG. 7.
Labile sulfur. Labile sulfur can be broken down into acid-labile sulfur, consisting of metal/sulfur clusters in metalloproteins, or heme complexes, as well as free HS-, or sulfane sulfur. Sulfane sulfur can be inorganic or organic in origin.
There is compelling evidence that the reversible formation of mixed disulfides between GSH and low-pKa cysteinyl residues of proteins (e.g., S-glutathionylation) is an important mechanism for dynamic, post-translational regulation of a significant number of regulatory, structural, and metabolic proteins, and signaling pathways (9, 53, 97, 114). Mitochondrial GSH has been shown to act as a “sulfide buffer” when H2S starts to build up in the cell (208). In mice and humans, ethylmalonic encephalopathy (EE) responds well to treatment with high doses of N-acetylcysteine (NAC, a cysteine/GSH prodrug) (208). This disorder is caused by mutations in ETHE1, a mitochondrial matrix sulfur dioxygenase involved in oxidative sulfide catabolism. Mitochondrial GSH can accept the sulfur atom of H2S through the action of sulfide-CoQ reductase, yielding GSH persulfide (GSSH), which is better tolerated by the cell than H2S (70, 71). Importantly, thiosulfate is excreted in massive amounts in the urine of mice and humans presenting with EE, with high thiosulfate and H2S concentrations present in mouse tissues (200). In the five children treated by Viscomi et al, serum thiosulfate concentrations consistently decreased during treatment (208).
In spite of the critical role of sulfur in our diet, and especially of an adequate cysteine intake, dietary consumption of cysteine is generally suboptimal (51, 143). On the other hand, homeostatic regulation of cysteine and GSH pools declines with age, with the onset appearing in men at a younger age than in women (17). Since high dietary intakes of methionine have been shown to raise plasma levels of homocysteine (190), despite adequate intake of B vitamins, and since free cysteine can be a prooxidant (8, 75, 167), cysteine supplementation is nowadays achieved by oral administration of NAC, L-2-oxothiazolidine-4-carboxylate (OTC, another cysteine-GSH prodrug) or IMMUNOCAL (an undenatured protein concentrate rich in SAAs) (8). High-dose oral NAC has been shown to counter the intertwined redox and inflammatory imbalances in cystic fibrosis (201), and in several clinical trials, cysteine supplementation improved skeletal muscle function, decreased the body fat/lean body mass ratio, decreased plasma levels of pro-inflammatory cytokines NF-κB and TNF-α, improved immune function, and increased albumin levels (9, 20, 53, 103, 122, 217). However, Palmer et al. found that oral administration of NAC to mice (10 mg/ml in drinking water) daily for 3 weeks led to development of pulmonary arterial hypertension that mimicked the effects of chronic hypoxia (150). These findings raise the concern that chronic NAC therapy might have similar consequences in patients (124).
The H2S-cysteine-GSH connection has been documented often in the biomedical literature (26, 59, 93, 94, 141, 147, 158). Five factors are currently considered to contribute to the H2S–stimulated increase in intracellular GSH levels: (i) enhancement of cellular glutamate uptake (194), (ii) a H2S–induced increase in the level of gamma-glutamylcysteine synthetase and cystine transporter activity in the cell (94) (iii), reduction of cystine into cysteine by H2S in the extracellular space, and transport of cysteine into cells by the cysteine transporter (93), (iv) H2S stimulation of nuclear transcription factor Nrf2, which in turn upregulates GSH synthesis and transport (9, 26), and (v) a decrease in the activity of GSH-catabolizing enzymes (184). We believe the H2S-cysteine-GSH connection to be strongly dependent on the fact that H2S and L-serine act as co-substrates of cystathionine for CBS to yield L-cysteine (99, 153). This reaction is widely acknowledged to proceed in the opposite direction, producing H2S from cysteine, but its ready reversibility is firmly established (76, 153).
In summary, GSH is the most important intracellular thiolic antioxidant, a major determinant of the thiol/disulfide redox state, and a critical regulator of immune function, cell senescence, apoptosis, and vital redox-sensitive signaling pathways. Adequate levels of GSH are essential for effecting detoxification of xenobiotics and endogenously-generated toxins, for the biosynthesis of many essential biomolecules, and for protecting all cells from oxidative stress. Through the H2S-cysteine-GSH connection, an H2S prodrug may function not only as a source of H2S but also as precursor of L-cysteine and GSH.
Inflammation and immunity
H2S regulates inflammation and cell death, possibly exerting its beneficial effects through action on ATP-sensitive K+ channels (KATP) (196), inhibition of activation of NF-κB and p38 MAPK, scavenging of oxidants, upregulation of intracellular cAMP, and inhibition of caspase-3 cleavage (212). Chronic inflammation is involved in some of the most common human diseases such as rheumatoid arthritis, tuberculosis, asthma, inflammatory bowel disease, vasculitis, and Crohn's disease. Chronic inflammation is an influential factor in type II diabetes, cardiovascular disease, and tumor development (1, 107, 133, 245). Infiltration of macrophages into the cellular mass is a common characteristic of atherosclerotic lesions and tumors. Since Virchow first showed that the inflammatory process influences atherosclerosis and tumor development, a growing body of evidence supports the hypothesis that macrophages play an important role in initiating and promoting both pathologies. In both cases, the combined effects of reactive oxygen species (ROS), cytokines, chemokines, and angiogenic factors, produced by tumor-associated macrophages and other inflammatory cells, explain the abnormal growth of cells: once a cellular mass becomes infiltrated by macrophages, the ability of tumor and atherosclerotic tissue to survive the immune response increases exponentially (163).
Ischemia-reperfusion (I/R) injury is regarded as a form of acute inflammation in which leukocytes play a key role. Experimental studies carried out during the last 20 years contributed to develop the concept that oxidant-induced leukocyte–endothelial tissue interactions are largely responsible for the microvascular dysfunction induced by reperfusion. Recognition of the vital role of the inflammatory process in I/R injury has provided the impetus for an intensive research effort aimed at preventing leukocyte infiltration into post-ischemic tissue (110, 242).
In atherosclerosis, monocyte adhesion to endothelial cells is stimulated by an oxidized cysteine/cystine redox status. The specific mechanism involves intracellular generation of hydrogen peroxide, activation of NF-κB, and transcriptional activation and increased cell surface expression of cell adhesion molecules (CAM's) (83). H2S is an extremely potent inhibitor of leukocyte adherence to the vascular endothelium (243). H2S might interfere with inflammatory processes by diminishing the tissue injury induced by neutrophils via induction of apoptosis and/or scavenging of neutrophil-derived HOCl (220). Importantly, H2S exerts opposite effects on the viability of lymphocytes and granulocytes, which is probably the reason for the potentiation of the acute inflammatory and bactericidal responses and the depotentiation of the chronic inflammatory cellular response (243).
ROS/reactive nitrogen species (RNS) are mediators of NF-κB activation and this process can be blocked by antioxidants, in particular, cysteine and GSH (83). H2S has been shown to downregulate several pro-inflammatory cytokines including NF-κB, TNF-α, IL-1β, IL-6, and IL-8 (55, 98, 144, 151), to modulate leukocyte adhesion and leukocyte-mediated inflammation (55,181), to mediate the cardioprotection induced by ischemic postconditioning (241) and to protect from NF-κB and TNF-α mediated endotoxic shock (113). The powerful reducing/antioxidant/free radical scavenging properties of H2S can explain its wide-ranging anti-inflammatory and cytoprotective effects, including protection against: ischemia-reperfusion injury in heart, brain, retina, liver, and intestine; endothelial dysfunction; hydrogen peroxide-induced damage in rat gastric epithelial cells; hyperhomocysteinemia in rats; methionine- and homocysteine-induced oxidative stress; and hemin-mediated oxidation of low-density lipoprotein (112).
Cytoprotection and pharmacological conditioning
Cardiovascular system
H2S strongly influences the body's redox status through various mechanisms, such as increasing GSH levels in the cytosol, mitochondria, and nucleus of cells, increasing the GSH/GSSG ratio, activating the reperfusion injury salvage kinase (RISK) pathway with upregulation of protective heat-shock proteins, and acting as “master switch” of Nrf2 nuclear translocation, resulting in persistent activation of the antioxidant responsive elements (AREs) of antioxidant genes and concomitant overexpression of antioxidant and phase II enzymes (151, 165). H2S not only exerts anti-apoptotic and anti-inflammatory effects but also anti-nociceptive and blood pressure-lowering effects by activating KATP channels (196). The cardioprotective effect of H2S also involves activation of cardiac extracellular signal-regulated-kinase and/or Akt pathways (196).
Evidence on the cardioprotective effects of H2S has been obtained by many researchers. It has been shown that H2S has profound protective effects on the heart in murine models and that genetic overexpression of CSE in the heart is highly protective from I/R injury (26, 55). Exogenous administration of H2S and its donors in the settings of atherosclerosis, myocardial I/R injury, chronic heart failure, and cardiopulmonary resuscitation shows significantly improved outcomes in small animal models (25, 26, 119, 131, 156). These results are being translated into large animal models (155, 179, 181). The observed protection is associated with improved heart mechanics, reduced myocardial inflammation, preserved mitochondrial function, Nrf2 activation, and reduced cardiomyocyte apoptosis (25, 26, 55, 131, 156).
Yusof et al. reported the first evidence that preconditioning by exposing the small bowel of rats to NaHS induces an anti-inflammatory phenotype, such that postcapillary venules fail to support leukocyte rolling and adhesion when subjected to I/R injury 24 hours later (242). I/R injury is a major source of morbidity and mortality, not only in myocardial infarction, but also in many other clinical settings, including solid organ transplantation and ischemic cerebral and retinal vascular episodes. It is also a cause of irreversible damage to skeletal muscle made ischemic either as the result of pathologic hypoperfusion or of a planned surgical intervention. On the basis of results of both in vitro and in vivo experiments, it was recently concluded that the preischemic or postischemic delivery of NaHS limits I/R-induced cellular damage and confers significant long-term protection, that intravenous or even intra-arterial delivery of an H2S donor would provide more focused treatment of target tissue and, when administered in appropriate doses and within the proper time frame, H2S holds significant promise as a cytoprotective agent (65).
Peripheral arterial disease (PAD) affects over 5% of the older population (>60 years). PAD is considered a marker for systemic atherosclerosis and is frequently complicated by coronary and cerebral events (116). In PAD, oxidative stress is implicated in the correlation of a reduction in flow-mediated dilation (FMD) with a higher risk of developing CV complications. Therefore, treatment with antioxidants, aimed at improving peripheral arterial dilatation, is being investigated (116). In a rat unilateral hind limb ischemic model, treatment with NaHS (50 μmol kg−1day−1) promoted significant angiogenesis and improved regional blood flow. These effects are associated with an increase in vascular endothelial growth factor (VEGF) expression in skeletal muscle and VEGF receptor 2 (VEGFR2) phosphorylation in neighboring vascular endothelial cells. In addition, Akt phosphorylation is increased in ischemic muscles following NaHS treatment. However, treatment with 200 μmol kg−1 day−1 has no angiogenic effect (215).
Angiogenesis is triggered when the effects of pro-angiogenic factors, such as hypoxia inducible factor (HIF) and tumor growth factor (TGF), present in the tissue overcome those of the anti-angiogenic factors. It is possible that, at the higher dose, H2S/HS- inhibits nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (27) and/or binds to multiple cellular targets evoking mechanisms that counteract the pro-angiogenic effects (24, 215). In 2007 Isenberg et al. presented the results of a study on modulation of angiogenesis by certain simple dithiolethiones (DTTs), certain dithiolethione-modified nonsteroidal anti-inflammatory drugs (S-NSAIDs) and valproic acid, and H2S (79). Simple DTTs, S-NSAIDs and S-valproate demonstrated significant anti-angiogenic activities, inhibiting endothelial cell proliferation and vascular cell outgrowth and invasion of extracellular matrix. H2S, on the other hand, dose-dependently inhibited vascular cell outgrowth (at concentrations between 0.1 and 1000 μM) while stimulating endothelial cell proliferation in a dose-dependent manner within the same concentration range. Importantly, vascular outgrowth from muscle tissue was completely abrogated by H2S at a concentration of 0.01 μM, whereas endothelial cell proliferation increased by a factor of less than two between 0.1 and 1000 μM (79). According to Sparatore et al., H2S-donating hybrids-containing a DTT moiety inhibit angiogenesis and cell proliferation, these effects being related to their ability to slowly and gradually release H2S (184).
Nervous system
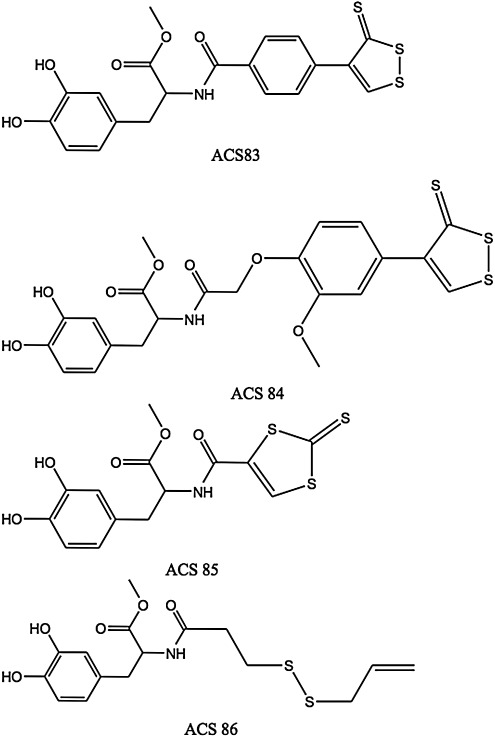
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a neurotoxin that can induce Parkinson's disease (PD)-like symptoms and biochemical changes in animals and humans. Inhaled H2S has been shown by Kida et al. to prevent MPTP-induced movement disorder, neuron degeneration, and neuron apoptosis and gliosis in mice (91). These effects were attributed to upregulation of genes encoding anti-inflammatory and antioxidant proteins, including heme oxygenase-1 (HO-1) and glutamate-cysteine ligase. Levodopa (L-DOPA) is widely used in PD therapy, but it does not prevent loss of substantia nigral dopaminergic neurons. The main factors responsible for this loss are oxidative stress and inflammation, which can be controlled by L-3,4-dihydroxyphenylalanine (L-DOPA) derivatives capable of being converted in vivo into L-DOPA and H2S by chemical and/or enzymatic means such as ACS83, ACS84, ACS85, and ACS86 (Fig. 3).
FIG. 3.
The L-DOPA derivatives capable of being converted in vivo into L-DOPA and H2S by chemical and/or enzymatic means. ACS83, ACS84, ACS85, and ACS86 were synthesized and studied by Sparatore et al. (106). ACS83 and ACS84 are [1,2]-dithiole-3-thione derivatives, ACS85 is a [1,3]-dithiole-2-thione derivative, and ACS86 is a disulfide containing an allylmercapto moiety, which is expected to release H2S upon nonenzymatic reduction by GSH.
The four molecules in Figure 3 were synthesized and studied by Sparatore et al. (106). ACS83 and ACS84 are [1,2]-dithiole-3-thione derivatives, ACS85 is a [1,3]-dithiole-2-thione derivative, and ACS86 is a disulfide containing an allylmercapto moiety, which is expected to release H2S upon nonenzymatic reduction by GSH. ACS84 was converted by isolated mitochondria into H2S. This conversion was also observed in vivo, with a large increase in intracerebral dopamine (30% more than with L-DOPA) and GSH after intravenous administration to rats. The four L-DOPA hybrids reduce release of TNF-α, IL-6, and NO from stimulated microglia and astrocytes. They proved superior to L-DOPA itself as neuroprotectants.
Emerging evidence suggests that H2S may have therapeutic potential in Alzheimer's patients since it reduces mRNA levels and protein levels of beta-site amyloid precursor protein-cleaving enzyme 1 in nerve growth factor differentiated PC12 cells (105, 248). The depletion of H2S in the brains of Alzheimer's patients may be due to high levels of myeloperoxidase. Abnormally low brain levels of endogenous H2S and inflammatory stress are hallmarks of Alzheimer's (219).
Total GSH was shown to be substantially lowered in mitochondria from severely ischemic rat brain tissue (3). Hideo Kimura and colleagues recently showed that exogenous H2S increases GSH production and suppresses oxidative stress in isolated rat mitochondria (93). These in vitro findings were mirrored by in vivo observations that H2S protects ischemic brain by reinstating GSH levels decreased by oxidative stress. H2S has been shown to protect neurons against hypoxic injury by upregulating expression of heat shock protein (HSP) 90 (127, 199). HSP90 is a ubiquitous molecule that contributes to cell survival by regulating the folding of various cellular proteins, including survival factors, and by binding to apoptotic protease activating factor-1 (Apaf-1), thereby preventing apoptosis.
The slow-releasing H2S prodrug ACS67 (a latanoprost-dithiolethione conjugate) was shown to attenuate retinal ischemic damage following experimental elevation of retinal pressure in rats, with ACS67 being more potent than latanoprost (147). The same authors found that ACS67 significantly attenuated hydrogen peroxide-induced damage to transformed neural precursor cells known to exhibit a number of characteristics associated with retinal ganglion cells (RGC). It is pertinent to point out that, according to Osborne et al. (147), the neuroprotective effect of ACS67 probably involves several mechanisms, prominently including stimulation of GSH formation. Biermann et al. (14) recently demonstrated in rats that preconditioning with inhaled H2S (80 ppm in air) significantly attenuates apoptosis of RGCs after retinal ischemia/reperfusion injury. Their results revealed that H2S is able to attenuate caspase-3 cleavage and caspase-3 activity and significantly upregulated induction of cytoprotective chaperone HSP90, and strongly suggest that NF-κB downregulation is one component of this neuroprotective action. Furthermore, Mikami et al. demonstrated that H2S protects the retina from light-induced damage by regulation of intracellular calcium via activation of vacuolar type H+-ATPase (129).
Elevated levels of homocysteine (Hcy) in the blood (hyperhomocysteinemia), may cause mental retardation, seizures, and Alzheimer disease (205) via Hcy-induced oxidative stress and increased cerebrovascular permeability. Tyagi et al. (205) suggest that H2S functions as a type of armor in the brain, and could be a beneficial therapeutic candidate for the treatment of hyperhomocysteinemia-associated pathologies, such as stroke and neurologic disorders.
Digestive system
Exogenous administration of H2S prevents ethanol-induced gastric damage in mice and has a protective role against oxidative stress in rat gastric mucosal epithelium (126, 240), and is effective at preventing damage to the gastric mucosa induced by nonsteroidal anti-inflammatory drugs (NSAIDs) (58) and at promoting resolution of colitis in rats (213). Fiorucci et al. point out that, in addition to the firmly established contribution of exogenous H2S to gastric mucosal protection, its role in accelerating repair of mucosal injury might soon emerge. The therapeutic dose range of NaSH/Na2S was found to be very narrow (240). Takeuchi et al. present evidence supporting the assumption that endogenous H2S is involved in regulation of acid-induced bicarbonate ion secretion and mucosal protection in the duodenum (193).
H2S, as well as precursors containing a dithiolethione moiety, are potent inducers of the antioxidant and cytoprotective enzyme HO-1. This is clinically significant because HO-1 promotes ulcer healing (183). Other mechanisms believed to contribute to the GI-protective effect of H2S are increased epithelial secretion and mucosal blood flow, activation of KATP channels and of capsaicin-sensitive afferent nerves, reduction of leukocyte adhesion/infiltration, downregulation of TNF-α/IL-1β/IFN-gamma expression and scavenging of oxidants (212).
Liver and kidneys
Mounting evidence suggests that H2S regulates intrahepatic blood flow (microcirculation) in the normal and cirrhotic liver (58), with insufficient production of H2S in the cirrhotic liver and downregulation of H2S-producing enzymes in kidney and liver of patients with chronic kidney disease (2). Administration of H2S donors has been found to protect the liver and kidneys from ischemia-reperfusion damage (82, 115). In kidneys, H2S has been found to be beneficial to the prevention or treatment of diabetic kidney disease via alleviating renal glycative injury (115), increasing renal blood flow, glomerular filtration rate, and urinary sodium excretion (230), and ameliorating hyperhomocysteinemia-associated chronic renal failure (173). In the liver, H2S effectively attenuates stress-mediated liver injury and hepatic mitochondrial dysfunction in acutely ethanol-exposed mice (244), and markedly alleviates acetaminophen-induced hepatotoxicity in mice (135). The hepatoprotective and nephroprotective effects of H2S are mostly mediated by the “Nrf2 regulon”, (i.e., by activation of the many cytoprotective and lipogenesis-regulating genes controlled by the Nrf2-ARE pathway) (95,96,174). Additionally, H2S is also of benefit in hyperlipidemic and/or hypercholesterolemic prevention and therapy (75) via both enzymatic and nonenzymatic activities.
Diabetes and metabolism
Diabetes mellitus and its CV complications have been associated with increased production of ROS and perturbations of thiol redox homeostasis. Increased oxidative stress and oxidative damage are considered mediators of vascular injury in CV pathologies, including hypertension and atherosclerosis. In fact, CV disease is the major cause of morbidity and mortality for diabetic individuals. In order to reduce these risks, it is necessary to develop therapies aimed simultaneously at improving energy metabolism, insulin resistance, vascular function, blood pressure, and inflammatory/procoagulant status (125, 161).
The rate of ROS production depends on the metabolic status of the cell, as hyperglycemia increases the steady-state superoxide concentration. The rate of enzymatic reduction of glucose to sorbitol increases as well, with concomitant decreases in NADPH and GSH concentration. This depletion of reducing equivalents results in augmented sensitivity to oxidative stress (123). Thus, oxidative stress from excessive ROS and depleted mitochondrial GSH (mtGSH) can lead to cardiomyocyte apoptosis in the diabetic heart. Similarly, in diabetic retinopathy, superoxide levels in retinal mitochondria of diabetic mice are twice as high as those in nondiabetic controls, and mtGSH levels in the same retinas undergo a 40% decrease due to hyperglycemia (123).
According to Niki and his colleagues, endogenous H2S protects pancreatic β cells of mice from apoptosis induced by oxidative stress and/or glucotoxicity. They also found that NaHS was able to suppress ROS production induced by cytokines or hydrogen peroxide, via activation of Akt signaling (90, 197, 198). These findings are consistent with the effect of DATS (a hydrogen sulfide precursor, see below) on the level of blood sugar and oxidative stress markers in rats with type II diabetes mellitus (54).
It is now apparent that H2S biosynthesis declines as the severity of diabetes increases over time, and that therapies based on administration of different H2S donors to animals or patients in different stages of type I or type II diabetes may be highly successful (19, 46, 109, 221). Interestingly, it has been reported that plasma H2S levels are reduced in overweight individuals, with increasing adiposity being a major determinant of said levels (221). On the other hand, emerging evidence points to diminished Nrf2/ARE activity as a major contributor to increased oxidative stress, disrupted lipogenesis, mitochondrial dysfunction in the vasculature leading to endothelial dysregulation, insulin resistance, and the abnormal angiogenesis observed in diabetes (37, 95, 174, 192, 195, 209). Taken together, the aforementioned findings suggested that, in diabetes, blunted H2S biosynthesis is a major contributor to increased oxidative stress/mitochondrial and endothelial dysfunction and insulin resistance, the causal link being diminished Nrf2/ARE activity.
Last, recent evidence indicates that H2S (or its donors) exerts an anti-atherogenic effect by counteracting the oxidation of low-density lipoprotein (LDL) via HOCl scavenging, H2O2 scavenging, myeloperoxidase inhibition, and inhibiting foam cell formation by downregulating CD36, SR-A (scavenger receptor A) and ACAT1 (acyl-coenzyme A:cholesterol acyltransferase-1) expression via the KATP/ERK1/2 pathway in human monocyte-derived macrophages (100, 250). Lynn and Austin have reviewed experiments demonstrating that H2S supplementation ameliorates atherogenic processes, and therefore that such supplementation may be of therapeutic benefit in the prevention and treatment of atherosclerosis (119). For a full discussion of the relationship between H2S and the metabolic syndrome, please refer to the recent review by Desai et al. (48).
Benavides et al. proposed that endogenous H2S production from garlic-derived organic polysulfides provides the basis for the long-term beneficial effects obtained from the habitual consumption of garlic (13), in particular, the reduction in risk factors associated with the metabolic syndrome such as increased oxidative stress, obesity, hypertension, high blood glucose levels, hypercholesterolemia, hyperlipidemia, platelet aggregation, and blood coagulation, that together greatly increase the risk of developing CV disease and type II diabetes (148). Benavides et al. stressed endogenous H2S production from allyl, di-, and polysulfides derived from garlic (13), but did not mention the presence in garlic extracts of significant amounts of S-substituted L-cysteine derivatives (cysteine S-conjugates) which are also important H2S precursors, such as S-allyl-L-cysteine, S-allylmercapto-L-cysteine, S-propylmercapto-L-cysteine, and S-(penta-1,3-dienyl)mercapto-L-cysteine. These compounds are substrates of CBS (β-cystathionase, which also possesses beta-lyase activity). The mercapto-substituted derivatives are thereby converted into hydropersulfides (RSSH), which readily yield H2S upon reduction by GSH (13, 152). Taken together, these findings suggest that most of the organosulfur compounds in garlic preparations are potential H2S precursors in the body.
H2S as an antioxidant and free radical scavenger
At 37°C and physiological fluid pH (pH 7.4), about 80% of the H2S molecules dissociate to yield HS- (hydrosulfide anion), which is therefore the predominant sulfur-containing species in extracellular fluids and plasma (49), whereas within the cell (pH about 7.2) the amounts of H2S and HS- are nearly equal (145). Hydrosulfide anions are powerful one-electron chemical reductants capable of quenching free radicals by hydrogen atom transfer or by single electron transfer usually at or near diffusion-controlled rates. Their reaction with dioxygen is fast when catalyzed by divalent metal ions. They are also strong nucleophiles as evidenced by their reaction with S-nitrosothiols to release NO (202). The oxidation of hydrosulfide anions by biochemically relevant two-electron oxidants (e.g., hypochlorous acid and hydrogen peroxide) yields initially hydrogen disulfide (H2S2, also known as disulfane) which is also a highly reactive oxidizing agent (139, 160) capable of regenerating H2S by reaction with a thiol (13) or by disproportionation (118, 139). H2S will readily scavenge ROS and RNS, including hypochlorous acid, hydrogen peroxide, lipid hydroperoxides, and peroxynitrite (93, 139). It is also able to scavenge the triplet state of riboflavin (214). However, in the presence of molecular oxygen (dioxygen) autooxidation of H2S generates free radicals (189).
and peroxynitrite (93, 139). It is also able to scavenge the triplet state of riboflavin (214). However, in the presence of molecular oxygen (dioxygen) autooxidation of H2S generates free radicals (189).
Under oxidative stress conditions, H2S may be converted to sulfite by activated neutrophils (132). Mitsuhashi et al. found that when NaHS was added in vitro to the supernatant of activated neutrophils, a significant amount of sulfite could be detected. Furthermore, a NADPH oxidase inhibitor markedly suppressed the production of sulfite. The chemical production of sulfite from H2S by neutrophil oxidative bursts is associated with inflammation, which might be responsible for the high levels of serum sulfite found in patients with pneumonia (132).
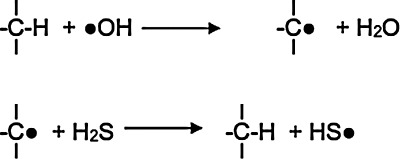
Although seldom acknowledged, simple species containing an SH group such as H2S, HS-, HS-SH, and HSS- excel at undoing the damage inflicted to biomolecules by free radicals through hydrogen atom donation to carbon-centered radicals (Fig. 4) (157, 191). Although hydrogen atom transfer to carbon-centered radicals is a diffusion-controlled reaction, the extremely low concentrations of H2S and H2Sx in blood and tissues limit their efficiency at repairing free radical damage to biomolecules (145).
FIG. 4.
Hydrogen atom donation to carbon-centered radicals. Simple species containing an SH group such as H2S, HS-, HS-SH, and HSS- excel at undoing the damage inflicted to biomolecules by free radicals through hydrogen atom donation to carbon-centered radicals (157, 191). Although hydrogen atom transfer to carbon-centered radicals is a diffusion-controlled reaction, the extremely low concentrations of H2S and H2Sx in blood and tissues limit their efficiency at repairing free radical damage to biomolecules.
Typically, carbon-centered free radicals react with oxygen to yield alkylperoxyl radicals:

which react further by abstracting hydrogen atoms from other biomolecules:
![]()
An inability to repair oxidized DNA, lipids, and proteins contributes to the damage induced by oxidative stress. Examples of macromolecular repair include DNA repair by base or nucleotide exclusion, protein repair by thioredoxin and glutaredoxin (162), and lipid repair by GSH peroxidase.
In order to appreciate the importance and uniqueness of the role of H2S as an antioxidant/free radical shield/cytoprotector, it is essential to recall that single antioxidants as pharmacologically active agents have not been found to exhibit extremely powerful therapeutic effects (177). This rather limited success might seem at first surprising in view of the decreased levels of selected major antioxidants consistently found in a number of disease states, but the limited success of this “single direct antioxidant approach” can be rationalized by recalling that mammals possess highly evolved and well-integrated antioxidant mechanisms that require the concerted and synergistic action of both antioxidant enzymes and low-molecular-weight antioxidants, with different antioxidants operating extracellularly and/or in specific cell compartments and having limited functional overlap: some destroy peroxidic species and/or peroxynitrite, others break free radical chains, and still others quench singlet oxygen (45). In addition, due to their short half-lives, direct antioxidants (vitamins C, E, etc.) must be administered frequently and at relatively high dosages to sustain their physiological efficacy (84). Furthermore, use of high-dose direct antioxidants may elicit pro-oxidant effects (45). However, H2S is not just another antioxidant to be added to the list of “direct antioxidants”, but it is also a powerful cytoprotective agent capable of activating nuclear transcription factor Nrf2 and consequently of inducing the expression of over 200 genes. These Nrf2-dependent genes encode proteins involved in lipid homeostasis, phase 2 detoxifying/antioxidant enzymes, directly acting antioxidant proteins, synthesis of low molecular weight antioxidants, and several P450 enzymes (84, 95, 174).
Cell signaling
The interaction of H2S with nuclear transcription factors has been intensively scrutinized. Many researchers have shown, using both cells in culture and whole animals that, in most cases, H2S inhibits NF-κB (112, 222). Slow-releasing H2S donors such as DATS, GYY4137, and S-diclofenac have also been shown to block NF-κB nuclear translocation in mouse macrophages and rat liver. Administration of GYY4137 to LPS-injected rats resulted in activation of signal transducer and activator of transcription-3 (STAT3), which is known to regulate the expression of many genes that mediate cell survival, proliferation, and angiogenesis (1, 113). H2S administration induces activation of transcription factor Nrf2 (26). In the nematode, Caenorhabditis elegans, H2S upregulates HIF-1 (23).
Many mechanisms of action of H2S may be mediated by protein S-sulfhydration (138,172). Sen et al. recently showed that S-sulfhydration of NF-κB by H2S is responsible for its anti-apoptotic actions (171). Mustafa et al. pioneered the concept of S-sulfhydration (SHY) as a signaling system (138). They define SHY as a physiological process wherein H2S attaches an additional sulfur atom to the thiol (-SH) groups of cysteine (Cys) residues within proteins, yielding a hydropersulfide group (-SSH). SHY usually activates enzymes (138). S-sulfhydration of GAPDH, for instance, results in a 7-fold increase in catalytic activity (138). Among the 49 proteins that were found to be basally S-sulfhydrated by liver-generated H2S are albumin, actin, β tubulin, CSE, CBS, several phosphatases, and catalase, and these authors estimate that from 10 to 25 percent of endogenous GAPDH, β tubulin. and actin are S-sulfhydrated in vivo (138).
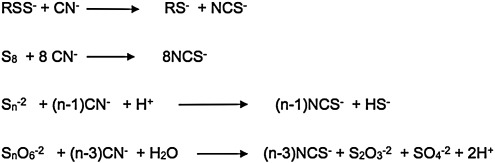
Sulfane sulfur results following sulfhydration, and may also serve as a biological source of H2S. Operationally, sulfane sulfur was defined by Wood in 1987 (224) as sulfur that reacts, at pH 8.5–10, with cyanide to yield thiocyanate (Fig. 5) (88, 89, 225). From a structural viewpoint, a sulfane sulfur atom in an electrically neutral molecule is always attached to another sulfur atom and is either in an oxidation state of zero, or in an oxidation state of −1, and is attached to a hydrogen atom or to an “activating group” such as allyl, benzyl, phenacyl, etc. The “outer” sulfur atom of a hydropersulfide group is highly redox-labile, and is readily converted into H2S by reducing agents such as dithiothreitol, cysteine, or GSH:
FIG. 5.
Sulfane sulfur. Sulfane sulfur was defined by Wood in 1987 (224) as sulfur that reacts, at pH 8.5–10, with cyanide to yield thiocyanate (88, 89, 225). From a structural viewpoint, a sulfane sulfur atom in an electrically neutral molecule is always attached to another sulfur atom and is either in an oxidation state of zero or in an oxidation state of −1 and is attached to a hydrogen atom or to an “activating group” such as allyl, benzyl, phenacyl, etc.
![]()
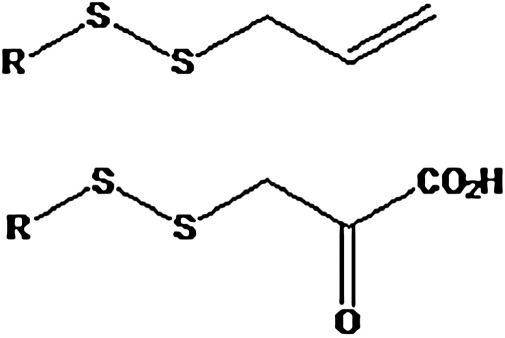
“Activated organic disulfides” such as those shown in Figure 6 are organic sulfane sulfur compounds (12). The molecules of organic hydropersulfides, and hydropolysulfides contain the –S-S-H moiety, and therefore also contain sulfane sulfur. The most important hydropersulfides in biology are probably thiocysteine (Cys-SSH) and GSH hydropersulfide (G-SSH). “Bound sulfur” was defined by Ogasawara et al. as “divalent sulfur that is easily liberated as sulfide by reduction with dithiothreitol” Therefore, the “sulfane sulfur pool” constitutes a major portion of the labile sulfur pool (Fig. 7) present in tissues of plants and animals.
FIG. 6.
“Activated organic disulfides.” These compounds contain “cyanolyzable sulfur” (12) and therefore belong in the category of organic sulfane sulfur compounds.
Acid-labile sulfur comprises various metalloproteins, which contain sulfide ions as part of metal/sulfur clusters (mainly Fe/S and Zn/S clusters) (81). Acidification may liberate the S−2 ions, which are released as SH- and H2S. The brain, heart, and liver contain significant amounts of acid-labile sulfur, whereas lung and muscle contain less (87). The labile sulfur pool (206) comprises both inorganic and organic chemical species, the simplest being disulfane (HS-SH), which is present as  at physiological pH. In this context, it is important to bear in mind that, since sulfane sulfur atoms are in the zero or minus one oxidation state, they must gain electrons (i.e., be reduced) in order to generate S2- or HS-.
at physiological pH. In this context, it is important to bear in mind that, since sulfane sulfur atoms are in the zero or minus one oxidation state, they must gain electrons (i.e., be reduced) in order to generate S2- or HS-.
The conversion of a thiol into a hydropersulfide by H2S requires one equivalent of an oxidant (81, 86, 139):
![]()
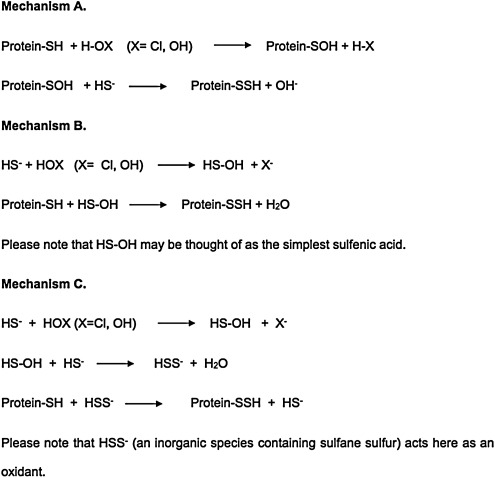
Although Nagy and Winterbourn recently proposed hypochlorous acid as a candidate (139), we believe hydrogen peroxide and the superoxide radical anion to be much more widely available oxidants in living tissues. Three likely mechanisms for S-sulfhydration are shown in Figure 8.
Fig. 8.
Three likely mechanisms for S-sulfhydration. At physiological pH, most cysteine thiol groups in proteins are protonated (-SH) and hence display low reactivity towards H2O2. However, in some proteins where the cysteine residue is flanked by basic amino acids, the cysteine-SH group exists as the highly oxidizable thiolate anion (-S-). This introduces an element of specificity in H2O2-mediated signaling, suggesting that mainly proteins containing low pKa cysteine residues undergo S-sulfhydration.
At physiological pH, most cysteine thiol groups in proteins are protonated (-SH) and hence display low reactivity towards H2O2. However, in some proteins where the cysteine residue is flanked by basic amino acids, the cysteine-SH group exists as the highly oxidizable thiolate anion (-S-). This introduces an element of specificity in H2O2-mediated signaling, suggesting that mainly proteins containing low pKa cysteine residues undergo S-sulfhydration. Hydrogen peroxide is the physiological oxidant of choice because it is constitutively produced inside most cells at various loci such as mitochondria, peroxisomes, and the cytosol mainly via enzymatic processes mediated by SOD, NADPH oxidases, xanthine oxidases, sulfhydryl oxidases, thiol oxidases, and monoamine oxidases (27, 83), and the reactivity of hydrogen peroxide toward thiols and H2S is high (140). Hydrogen peroxide generation in mammals is probably in the vicinity of 50 μmol kg−1min−1 (83).
S-sulfhydration of an enzyme may be accomplished through interaction with the proper substrate and does not require a discrete oxidation step involving a thiol group at the active site (as in mechanisms A, B or C, Fig. 8). Thus, 3-mercaptopyruvate has been reported to react with Trx, yielding pyruvate and Trx hydropersulfide (218). Therefore, H2S might S-sulfhydrate Trx through the following enzymatic pathway:

As efficient mitochondrial pathways for H2S oxidation are available (71), steady state tissue concentration can be held at very low levels and it is possible for H2S to function as an oxygen sensor (146). Thus, under hypoxic conditions, H2S catabolism would be blocked, leading to increased H2S levels with activation of specific responses (146). This hypothesis is consistent with similarities between the effects of hypoxia and H2S, enhancement of hypoxic signaling by H2S precursors, and abolishment of hypoxic signaling by H2S synthesis inhibitors.
Sexual function
Moore and his co-workers (185–187) have described some pioneering studies that provide evidence for the endogenous formation of H2S and its pro-erectile relaxant effect on the corpus cavernosum of mammals, as well as on the effects of H2S in female sexual function. The first set of results were corroborated in a recently published article (40). There is also evidence that oxidative stress is implicated in erectile dysfunction (ED) in diabetic rodents (15,) and that interventions based on administration of tetrahydrobiopterin (182) and upregulation of antioxidant enzymes may be useful (44). For a discussion of the roles of endogenous and exogenous H2S in the endocrine and reproductive systems and the possibility of developing new therapies for ED that target this pathway, please see the recent articles by D´Emanuelle Di Villa Bianca et al. (41) and Zhu et al. (252).
Sparatore et al. have developed an H2S-donating derivative of sildenafil (ACS6) with possible clinical indications in ED, benign prostatic hypertrophy, and low urinary tract symptoms (184). ACS6 is a hybrid obtained by esterification between a phenolic dithiolethione and a carboxylic acid derived from sildenafil by attachment of a carboxyl moiety (CO2H) to the N-methyl group joined to the piperazine ring. The H2S released by S-sildenafil (ACS6) inhibits both PDE5 and NOX expression and activity. Furthermore, H2S applied ex vivo or overexpression of CSE has been shown to increase cGMP levels by phosphodiesterase inhibition in aortic ring preparations (22). Hence, this mechanism may constitute the basis of a new and effective approach to the treatment of patients suffering from ED, benign prostatic hypertrophy, and lower urinary tract symptoms.
In fact, ACS6 and sildenafil citrate relaxed cavernosal smooth muscle equipotently and ACS6 inhibited superoxide formation more than sildenafil citrate (175). Shukla et al. concluded that ACS6 not only promotes erection, but also affords effective protection from oxidative stress through upregulation of GSH synthesis. Additionally, in an investigation of the effect of NaHS on pregnant rat uterine contractility in vitro, Sidhu et al. found that this “hydrogen sulfide donor” produced significant dose-dependent decreases in uterine spontaneous contractility (176).
Life span modulation
Many lines of evidence suggest that oxidative stress plays an important role in aging. In C. elegans and Drosophila melanogaster, mutations resulting in resistance to toxic stresses, oxidative or not, tend to result in increases in longevity. In C. elegans, recent studies have shown that the Nrf2 homologue, SKN-1 (121), is necessary for the life span extension seen with dietary restriction, and overexpression of SKN-1 can increase life span. In D. melanogaster, increased Nrf2 activity correlates with oxidative stress resistance and increased life span of male flies (192). In mice, decreased Nrf2 signaling with age, and increased Nrf2 signaling with caloric restriction have been observed (111). H2S augments the life span of C. elegans through a sirtuin, a process that may involve protein S-sulfhydration (130). Since sirtuins are also found in vertebrates and since H2S signaling pathways are highly conserved, it is possible that this effect/mechanism might be found in mammals as well. According to Powolny et al, treatment of the worm C. elegans with DATS increases its mean lifespan, even if the treatment is initiated during young adulthood (154). Since DATS readily yields H2S in vivo, we consider it likely that this effect of DATS is mediated by H2S.
Leiser and Miller describe a series of studies that lend support to the hypothesis that augmented Nrf2 activity contributes to several forms of stress resistance observed in long-lived Snell dwarf mice that live about 40% longer than littermate controls and show delays in the onset of many aging-related pathologies (111). Importantly, Dwarf-derived fibroblasts exhibit many of the traits associated with enhanced Nrf2/ARE activity, including higher levels of GSH and higher GSH/GSSG ratios. In a related development, Guayerbas et al. concluded that a 4-week treatment of mice with NAC and thioproline protected all animals against early age-associated behavioral impairment, but the improvement was more evident in prematurely aging mice (61). On the other hand, Brown-Borg and collaborators found that in long-lived Ames dwarf mice the flux of methionine through the transsulfuration pathway is enhanced (in part because of upregulation of CBS and CSE), leading to an increased reduced GSH pool, mainly in the liver (136), with heightened resistance to toxic/oxidative challenges, and 50%–64% longer lives than their wild counterparts (males and females, respectively) (207). Importantly, Ames dwarf mice have a delayed occurrence and reduced incidence of presumably fatal neoplastic disease compared with their normal siblings (78).
Protection from NSAID toxicity
Nonsteroidal anti-inflammatory drugs (NSAIDs) also possess analgesic and anti-pyretic effects. The main adverse drug reactions associated with use of NSAIDs are gastrointestinal tract irritation, inhibition of cyclooxygenase (COX)-1 and COX-2 (211), inhibition of enzymatic H2S synthesis (211, 212), development of cardio- and cerebrovascular pathologies, and development of altered renal function. In fact, in the USA, an estimated 5% of all visits to a doctor are related to prescription of NSAIDs, and NSAID-related upper gastrointestinal adverse drug reactions are believed to result in over 100,000 hospitalizations and around 16,500 deaths yearly (204). Recent studies have shown that over 50% of patients taking NSAIDs have suffered mucosal damage to their small intestine (69). In a very recent and comprehensive meta-analysis, Sven Trelle et al. concluded that significantly increased CV risks are associated with taking naproxen, ibuprofen, diclofenac, celecoxib, etoricoxib, lumiracoxib, and rofecoxib (204). Since millions of persons with chronic musculoskeletal symptoms are long-term users of NSAIDs, their doubled risk of heart failure and increased risks of myocardial infarction and stroke are of the utmost concern.
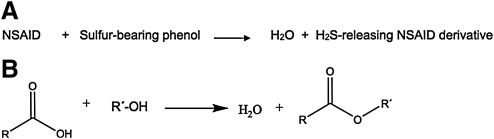
Administration of NSAIDs results in a significant decrease in endogenous H2S enzymatic production. This effect was most profound with indomethacin, but was also observed with aspirin, diclofenac, and ketoprofen (58). Since endogenous H2S contributes significantly to mucosal defense (212), it is reasonable to expect that exogenous administration of this mediator would be effective at preventing NSAID-induced mucosal damage. Indeed, H2S donors such as NaHS (58) and diallyl disulfide (DADS) (212) were shown to confer mucosal protection from NSAIDs, preventing gastric damage in rodents. Furthermore, DADS prevented naproxen-induced decreases in gastric blood flow and increases in leukocyte adherence. Based on these findings, several research groups have developed NSAID derivatives that release H2S in vivo. These are obtained by conjugating a molecule of an NSAID with one of an H2S releasing compound. Typically, these H2S–releasing NSAID derivatives are carboxylic acid esters with general formula RCOOR´, obtainable (at least in principle) by condensing the NSAID molecule, which bears the carboxyl moiety with a sulfur-containing phenolic molecule (Fig. 9). The sulfur-bearing /H2S releasing phenols that have been used are shown in Figure 10.
FIG. 9.
Several research groups have developed NSAID derivatives that release H2S in vivo. (A) These are obtained by conjugating a molecule of NSAID with one of an H2S releasing compound. (B) Typically these H2S–releasing NSAID derivatives are carboxylic acid esters with general formula: RCOOR´. These are obtainable (at least in principle) by condensing the NSAID molecule, which bears the carboxyl moiety with a sulfur-containing phenolic molecule.
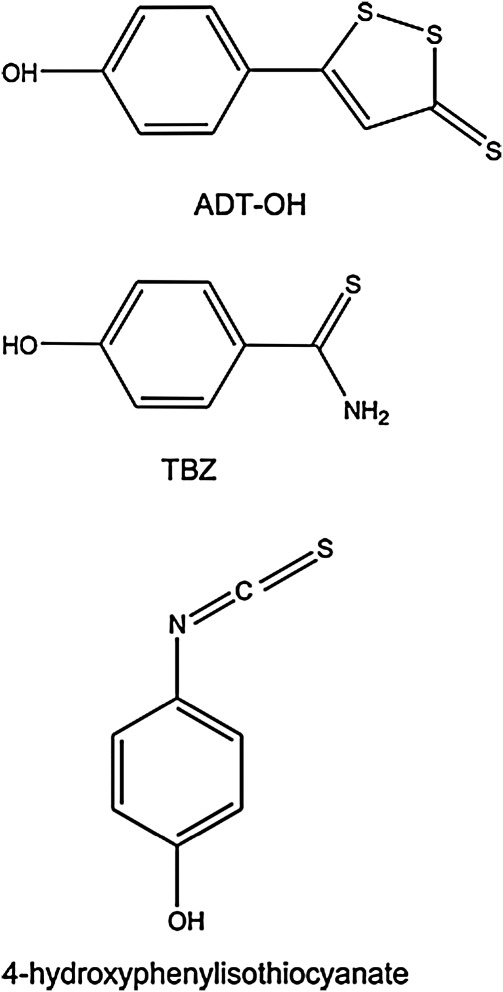
FIG. 10.
Chemical structure of some of the sulfur-bearing/H2S releasing phenols that have been used in the literature. These phenols include: ADT-OH, TBZ, and 4-hydroxyphenylisothiocyanate.
One such H2S-releasing NSAID, S-diclofenac (ACS15, see below), showed greater anti-inflammatory activity than diclofenac at equimolar doses in several experimental models (184). Treatment with S-diclofenac, but not diclofenac, resulted in a marked reduction in severity of pancreatitis-associated lung injury. Moreover, S-diclofenac has much lower gastrointestinal toxicity than diclofenac and provides marked cardioprotection in a well-characterized experimental model of ischemia-reperfusion injury in the rabbit (164). Furthermore, S-diclofenac effects were accompanied by a significant increase in GSH, inhibition of angiogenesis and cell proliferation, and inhibition of NF-κB and TNF-α.
Hibernation and protection against hemorrhage
In 2005, Blackstone et al. revealed that H2S induces a hypometabolic state in naturally nonhibernating mice (16). When exposed to nontoxic H2S concentrations, mice rapidly and reversibly entered a hibernation-like state, which Blackstone et al. designated as “suspended animation-like” (16). An 80 ppm H2S treatment induced, within minutes, a 60% reduction in CO2 production and oxygen consumption, which can be lowered to over 90%. Additionally, core body temperature decreases to near-ambient, and heart rate and breathing frequency are significantly lowered. Oxygen demand is so drastically diminished that H2S-treated mice survive for over 6 hours in an atmosphere containing 5% oxygen, whereas untreated controls die within 15 min. Upon cessation of H2S exposure, the mice awoke without displaying neurological or behavioral abnormalities.
Following up on these highly newsworthy studies, Morrisson et al. showed that inhaled H2S or intravenous-administered Na2S can protect rats from lethal hemorrhage, with surviving rats free from functional or behavioral deficits (134). In the introduction to this article, the authors state that “clinicians and investigators have long hypothesized that reducing metabolic demand could buy time for patients suffering from insufficient blood supply until they can receive definitive treatment”. In effect, this goal is still being actively pursued in many quarters (5, 18, 47, 50), but it is proving extremely difficult to translate the protective effects displayed by H2S treatment of rats to larger mammals.
In fact, attempts to protect piglets and pigs from hemorrhagic shock failed (50), as well as administration of gaseous H2S-via extracorporeal membrane lung ventilation to sheep, in an attempt to avoid the potential pulmonary toxicity of H2S (47). It seems that these failures are related to the fact that the higher doses of H2S required to depress metabolism in larger mammals elicit toxic effects, systemic and/or pulmonary, and the possibility that the ability of H2S to abate metabolism depends on the specific metabolic rate of animals. H2S may reduce metabolism when the baseline metabolic rate is high (i.e., in awake mice), but not when metabolic rate is already depressed, for instance, in anesthetized mice or sheep (47).
Cancer prevention and treatment
Consideration of the many differential anti-cancer effects of H2S (collected in Table 1), shows that H2S and H2S prodrugs seem to be capable of inhibiting all stages in cancer development. These studies will be further expanded upon below. However, we will first discuss briefly the events that lead to the development of cancer to better understand how H2S may be used to intervene.
Table 1.
“Direct” Anticancer Effects of H2S
| |
|
|
Affected stage(s)* |
|
|||
|---|---|---|---|---|---|---|---|
| Effect | Mediator (s) | 1 | 2 | 3 | 4 | References | |
| 1 | Increased immunocompetence | GSH (↑), Taurine (↑) | X | X | X | X | (9, 20, 53, 60, 178, 246, 247) |
| 2 | Inhibition of procarcinogen activation by oxidases (Cyp-450, etc) | Nrf2 (↑) | X | X | X | (11) | |
| 3 | Inhibition of NfkB nuclear translocation | X | X | X | (112, 222) | ||
| 4 | Epigenetic silencing of protooncogenes | SAM (↑) | X | X | X | (39) | |
| 5 | Epigenetic reactivation of tumor suppressor genes | HDAC (↓) | X | X | X | (142) | |
| 6 | DNA protection/repair | GSH (↑), Trx(↑) | X | X | X | (43, 52, 77, 93, 149) | |
| 7 | Abolishment of chronic inflammation | GSH (↑), CAMs in Leukocytes (↓) | X | X | X | (9, 143, 151, 196, 201) | |
| 8 | Prooxidant/proapoptotic “redlining” | Sulfane sulfur (↑) ROS (↑) | X | (56, 104, 166, 223, 235) | |||
| 9 | Antiangiogenesis (at “high levels” of H2S) | X | (79, 184) | ||||
| 10 | Antimetastatic effect | E-Cadherin (↑) | X | (74, 120) | |||
1, Initiation; 2, Promotion; 3, Progression; 4, Metastasis.
Normal cellular homeostasis is maintained by a balance between the processes of cell proliferation and cell death (apoptosis). An imbalance may lead to uncontrolled cell proliferation and cancer. The causal role played by ROS/RNS in carcinogenesis is now firmly established (203) and two mechanisms are thought to operate: (i) modulation of gene expression, with numerous oncogenes and tumor-suppressor genes operating through redox mechanisms that may be amenable to pharmacological intervention (223), and (ii) induction of genetic modifications. Redox dysregulation contributes to mutations and malignant transformation/progression through mitogenic signaling and modulation of apoptotic and survival pathways. Usually, pro-oxidant deviations from redox homeostasis relate to many aspects of the cancerous phenotype including alterations in metabolism, modulation of the cell cycle, upregulation of anti-apoptotic survival signaling, and upregulation of pro-angiogenic signaling.
According to the “differential redox set points” hypothesis, pro-oxidant-induced upregulation of intracellular ROS/disulfide stress specifically targets cancer cells, the therapeutic index being determined by the redox differential between the set points of normal and malignant cells. Wondrak (223) uses a highly descriptive analogy of this process with the operation of a car engine, where the red bar displayed on car tachometers denotes the maximum speed at which the car's engine is designed to operate without being damaged. In cancer cells, with a high set point of oxidative stress, pro-oxidant manipulation induces a redox shift that “redlines” and ruins the cancer cell's proliferative machinery. In contrast, normal cells tolerate the same pro-oxidant shift. It is important to note that many redox-targeted cancer drugs (including H2S donors) have been shown to potentiate the effect of other anticancer agents and radiation, which is consistent with preferential sensitization of cancer cells to the cytotoxicity of the nonredox-directed agent.
H2S is a Janus-faced molecule that can also behave as a pro-oxidant (6, 7, 189) via its interaction with dioxygen and/or the superoxide ion to generate sulfur-centered and oxygen-centered free radicals as well as higher sulfides H2Sn (1<n<8). A likely mechanism for H2S2 formation from NaHS and O2 in aqueous solution at pH close to 7 is:
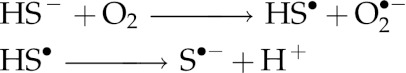
Please note that Ka for HS• is greater than Ka1 for H2S by a factor of about 1000 (118).
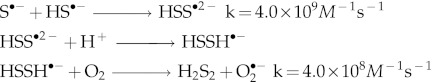
The first step would be the slowest in the sequence, but it is efficiently catalyzed by transition metal ions (117).
In turn, inorganic polysulfides (H2Sn) and organic hydropolysulfides (RSnH, n>1) are known to possess a high tendency to undergo homolysis and generate perthiyl radicals, 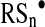 and
and 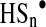 (137).
(137). 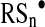 and
and 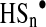 are highly reactive and easily generate ROS, and react rapidly with oxidants, such as dioxygen and oxyhemoglobin, to form ROS through a pseudocatalytic redox cycle (31, 137). In short, the ability of H2S to act as pro-oxidant and the high reactivity as both oxidants and reductants (81) of inorganic polysulfides lead us to consider the possibility that, in many kinds of cancer cells, H2S treatment has pro-oxidant effects that may lead to malignant cell death through redlining.
are highly reactive and easily generate ROS, and react rapidly with oxidants, such as dioxygen and oxyhemoglobin, to form ROS through a pseudocatalytic redox cycle (31, 137). In short, the ability of H2S to act as pro-oxidant and the high reactivity as both oxidants and reductants (81) of inorganic polysulfides lead us to consider the possibility that, in many kinds of cancer cells, H2S treatment has pro-oxidant effects that may lead to malignant cell death through redlining.
There is evidence in favor of pro-oxidant redlining of cancer cells by treatment with H2S or its prodrugs. Treatment of human neuroblastoma SH-SY5Y cells with NAC and ribose-cysteine resulted in elevation of sulfane sulfur level and inhibition of their proliferation (85). Diallyl disulfide (which releases H2S in vivo), was found by Filomeni et al. to induce neuroblastoma cell death (56). These researchers presented evidence that supports mediation of cytotoxicity by a ROS-dependent c-Jun NH2-terminal kinase/c-Jun signaling cascade (56). An extensive series of publications by Singh et al. (4, 66–68, 92, 154, 231–237), Seki et al. (72, 73, 170), Das et al. (42), and Lee et al. (104) demonstrate that DATS selectively targets DU145 and PC-3 cells in prostate cancer models, amazingly without damaging a normal prostate cancer cell line (237), kills cells of human gastric cancer cell lines, arrests the cell cycle in human cancer cell lines, and is cytotoxic towards a human breast cancer cell line (104), lung adenocarcinoma (228), prostate (231), colon (72), and human glioblastoma cells. This differential effect of DATS has been attributed to induction of intracellular oxidative stress through ROS generation. In a recent report, Lee et al. postulate that mitochondria are the main source of ROS generation and that DATS-induced oxidative stress is detected through glutaredoxin (GRX) (104).
DATS contains sulfane sulfur and is an excellent source of H2S in vivo (see section Diabetes and Metabolism), as evidenced by its ability to increase the intracellular GSH level and enhance the antioxidant and detoxification capabilities of rat primary hepatocytes (226). While we consider it likely that the effect of DATS on cancer cells is mediated by H2S, it has yet to be definitively demonstrated. Although the anticancer effects have been attributed in some cases to reversible covalent modification of specific proteins (73), it is estimated that this may be just an epiphenomenon (81, 137). In fact, Hosono's demonstration that cysteine residues Cys-12 and Cys-354 of beta tubulin are oxidized by DATS to S-allylmercaptocysteine residues constitutes indirect proof of H2S formation in this system (73):
![]()
DATS has been intensively studied in China during the last 25 years. However, most research results were published in obscure Chinese periodicals. Validation of the anticancer and cancer chemopreventative activities of DATS is found in that body of literature, of which the following studies are worth mentioning:
DATS inhibits mouse colon tumors in mouse CT-26 cells allograft model in vivo (227). The authors conclude that DATS may represent a colon cancer-preventing agent.
A double blind intervention study was performed on 2526 experimental subjects and 2507 persons in the control group, with those in the first group taking doses of 200 mg synthetic DATS daily plus 100 μg of selenium every other day for one month of each year from November 1989 to December 1991. After a 5-year follow-up, it was concluded that the DATS+Se treatment had the effect of decreasing the incidence of digestive cancer by over 50% (64, 251).
DATS enhances the antitumor function of macrophages either by priming macrophages alone or by synergic action, meanwhile increasing the susceptibility of some tumor cells to macrophage cytotoxicity (247).
DATS augments the activation of T lymphocytes. This effect is related to inhibition of NO production by macrophages. In addition, DATS can antagonize the inhibition of tumor-derived immunosuppressive factors produced by S180 cells and Ehrlich ascitic cancer cells on the activation of T lymphocytes and reduce the inhibitory rate significantly. The authors state that DATS is potentially useful in tumor therapy (246).
Apoptosis of human cholangiocarcinoma FRH-0201 cells can be induced by DATS in vitro in a dose-dependent manner (38).
DATS can induce mitotic arrest in human gastric cell line SGC-7901 (35).
DATS can cause gastric cancer cell (MGC803 and SGC7901 cell lines) arrest in the M-phase, and this may be one of the mechanisms for inhibiting cell proliferation (63).
DATS induces apoptosis in human gastric cancer cell line BGC-823 through downregulation of Bcl-2 and increased caspase-3 expression and activity (101, 102).
There are many studies from several laboratories on the cytotoxic effects of organic isothiocyanates (e.g., sulforaphane and benzyl isothiocyanate) derived from cruciferous vegetables (36, 168, 180, 188, 203, 229, 249). These authors report that organic isothiocyanates selectively kill cancer cells (human prostate, human pancreatic, etc.) in culture through ROS-mediated mechanisms. Since hydrolysis of organic isothiocyanates under physiologic conditions may generate H2S, it is likely that these selective cytotoxic effects are H2S-mediated (238).
Allyl sodium thiosulfate, also known as 2-propenyl thiosulphate (2-PTS) and also found in garlic, has been shown by two research groups to behave similarly to DATS in many respects (28–30, 166). These authors found that 2-PTS reacts with GSH, under physiologically relevant conditions, generating H2S. In vitro, 2-PTS inhibits proliferation of human tumor cell lines WiDR, 293, HL-60, and HuT78 (human T-lymphoblastoid cell line) in a dose-dependent manner, and caused oxidative damage and apoptosis to HL-60 and HuT 78 cells. Cytotoxicity of 2-PTS is related to a blockage in the G2/M phase of the cell cycle, which was linked to an early increment in ROS flux, and to inactivation of rhodanese, with concomitant thiolation to yield a protein disulfide highly sensitive to proteolytic degradation.
Moore, Deng and co-workers provided further evidence on the anticancer effects of H2S (108). They studied the interaction of two H2S donors (NaHS and GYY4137) with cancer cells in vitro and the effect on mice tumors of intraperitoneal injection of 100–300 mg/kg/day of GYY4137, a slow-releasing H2S donor that persists in the culture medium for up to 7 days, versus only 2 hours for NaHS. GYY4137 (but not NaHS) is cytotoxic to human cancer cells in a concentration-dependent manner. The two H2S donors studied did not affect the survival of normal human lung fibroblasts (IMR-90 and WI-38), and GYY4137 promoted cancer cell (MCF-7), but not normal cell (IMR-90) apoptosis. It also induced cell cycle arrest of cancer cells in the G2/M phase. Daily administration of GYY4137 to immunodeficient mice for 14 days caused a dose-dependent reduction in the growth of tumors induced by prior injection of a human leukemia cell line (HL-60 or MB4-11).
Chattopadhyay et al. show, in a recent series of articles, that H2S-releasing-NSAIDS are effective at inhibiting the growth of a variety of cancer cells (32–34). H2S-releasing-NSAIDS inhibited cell proliferation, promoted apoptosis, and caused G0/G1 cell cycle block of eleven different cancer cell lines (33). The H2S-releasing-NSAIDS had potencies of 28- over 3,000-fold compared to their NSAID counterparts in these effects, and H2S-releasing-aspirin (HS-ASA) was consistently more potent than the other H2S-releasing-NSAIDS tested (33). HS-ASA not only inhibits the growth of HT-29 human colon and Hepa 1c1c7 mouse liver adenocarcinoma cells in culture, it also induces Nrf2 expression and Phase-II detoxifying enzymes in vivo (34). HS-ASA also shows promise as a therapeutic agent in estrogen receptor negative breast cancer (32).
Conclusion
H2S has come to the forefront of some very exciting and promising research to treat a variety of diseases, and several H2S-releasing prodrugs are currently under development by the pharmaceutical industry. H2S possesses a very diverse biological profile that includes: potent antioxidant, anti-apoptotic, anti-inflammatory, metabolic, vasoactive, and cytoprotective actions on normal cells that could potentially be harnessed to treat a number of pathological states. The robust antioxidant actions of H2S involving direct scavenging of toxic reactive oxygen species combined with the effects on antioxidant enzyme expression and function are highly attractive features of this gaseous signaling molecule. It is possible that H2S prodrugs and novel agents that modulate H2S bioavailability might be efficacious for acute myocardial infarction, stroke, diabetes, arthritis, peripheral artery disease (PAD), metabolic syndrome, organ transplantation, erectile dysfunction, diabetes, inflammatory bowel disease and pulmonary hypertension. Contrastingly, H2S appears to exert powerful prooxidant and proapoptotic actions on cancer cells of different origins, which suggests that H2S prodrugs might be developed into effective anticancer agents capable of achieving high specificity and broad efficacy across different cancer types. However, it is important to fully consider the highly toxic actions of supraphysiological levels of hydrogen sulfide, and great care must be taken during the development of H2S-based therapeutic agents. This is true of any agent that exerts potent actions on the redox status in both normal cells and cells undergoing oxidative stress during pathological states.
Abbreviations Used
- 2-PTS
2-propenyl thiosulfate
- 3-MST
3-mercaptopyruvate sulfurtransferase
- ACAT1
acyl-coenzyme A:cholesterol acyltransferase-1
- Akt
protein kinase B
- Apaf-1
apoptotic protease activating factor-1
- AREs
antioxidant responsive elements
- ATP
adenosine triphosphate
- cAMP
cyclic adenosine monophosphate
- CAMs
cell adhesion molecules
- CBS
cystathionine beta-synthase
- cGMP
cyclic guanosine monophosphate
- CO
carbon monoxide
- COX
cyclooxygenase
- CSE
cystathionine gamma-lyase
- CV
cardiovascular
- DATS
diallyl trisulfide
- DTT
dithiolethione
- ED
erectile dysfunction
- EE
ethylmalonic encephalopathy
- ERK1/2
p44/42 MAPK
- FMD
flow-mediated dilation
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GDOPs
garlic-derived organic polysulfides
- GRX
glutaredoxin
- GSH
glutathione
- GSSH
glutathione persulfide
- Hcy
homocysteine
- HIF
hypoxia inducible factor
- HO-1
heme oxygenase-1
- H2S
hydrogen sulfide
- HS-ASA
H2S-releasing-aspirin
- HSP
heat shock protein
- IBD
inflammatory bowel disease
- IL
interleukin
- INF-gamma
interferon gamma
- I/R
ischemia-reperfusion
- I-R-I
ischemia-reperfusion injury
- KATP
ATP-sensitive K+ channels
- LDL
low density lipoprotein
- L-DOPA
L-3,4-dihydroxyphenylalanine
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- mtGSH
mitochondrial glutathione
- NAC
N-acetylcysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NMDA
N-methyl-D-aspartic acid
- NO
nitric oxide
- Nrf2
nuclear factor erythroid 2-related factor 2
- NSAIDs
nonsteroidal anti-inflammatory drugs
- OTC
L-2-oxothiazolidine-4-carboxylate
- PAD
peripheral arterial disease
- PAPs
adenosine-3′-phosphate-5′-phosphosulfate
- PD
Parkinson's disease
- RBC
red blood cells
- RGC
retinal ganglion cells
- RISK
reperfusion injury salvage kinase
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RSSH
hydropersulfide
- SAAs
sulfur containing amino acids
- SHY
S-sulfhydration
- S-NSAIDs
dithiolethione-modified nonsteroidal anti-inflammatory drugs
- SR-A
scavenger receptor A
- -SSH
hydropersulfide group
- STAT-3
signal transducer and activator of transcription-3
- TGF
tumor growth factor
- TNF-α
tumor necrosis factor alpha
- Trx
thioredoxin
- VEGF
vascular endothelial growth factor
- VEGFR2
VEGF receptor 2
Acknowledgments
DJL and BLP are supported by grants from National Institutes of Health (NIH) National Heart Lung and Blood Institute (NHLBI) 5R01HL-092141-03, and 1R01HL-093579-03 to DJL, and by the Carlyle Fraser Heart Center at Emory University Hospital Midtown. GG would like to express his gratitude to Marcela del Rocio Rosas del Real for her invaluable assistance in preparing the manuscript.
Disclosure Statement
DJL is a co-founder and consultant for Sulfagenix. GG is a founder and consultant for Sulfagenix. GG is an inventor of several United States patents for the use of hydrogen sulfide-based therapeutics for a number of disease states.
References
- 1.Aggarwal BB. Gehlot P. Inflammation and cancer: How friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aminzadeh MA. Vaziri ND. Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol Dial Transplant. 2012;27:498–504. doi: 10.1093/ndt/gfr560. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MF. Sims NR. The effects of focal ischemia and reperfusion on the glutathione content of mitochondria from rat brain subregions. J Neurochem. 2002;81:541–549. doi: 10.1046/j.1471-4159.2002.00836.x. [DOI] [PubMed] [Google Scholar]
- 4.Antosiewicz J. Herman-Antosiewicz A. Marynowski SW. Singh SV. c-Jun NH(2)-terminal kinase signaling axis regulates diallyl trisulfide-induced generation of reactive oxygen species and cell cycle arrest in human prostate cancer cells. Cancer Res. 2006;66:5379–5386. doi: 10.1158/0008-5472.CAN-06-0356. [DOI] [PubMed] [Google Scholar]
- 5.Aslami H. Juffermans NP. Induction of a hypometabolic state during critical illness. A new concept in the ICU? Neth J Med. 2010;68:190–198. [PubMed] [Google Scholar]
- 6.Attene-Ramos MS. Wagner ED. Gaskins HR. Plewa MJ. Hydrogen sulfide induces direct radical-associated DNA damage. Mol Cancer Res. 2007;5:455–459. doi: 10.1158/1541-7786.MCR-06-0439. [DOI] [PubMed] [Google Scholar]
- 7.Attene-Ramos MS. Wagner ED. Plewa MJ. Gaskins HR. Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res. 2006;4:9–14. doi: 10.1158/1541-7786.MCR-05-0126. [DOI] [PubMed] [Google Scholar]
- 8.Baker DH. Bryant KI. Dilger RN. Parsons CM. Dietary L-homoserine spares threonine in chicks. J Nutr. 2009;139:1298–1302. doi: 10.3945/jn.109.104372. [DOI] [PubMed] [Google Scholar]
- 9.Ballatori N. Krance SM. Notenboom S. Shi S. Tieu K. Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390:191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerjee R. Kabil O. Redox biochemistry of hydrogen dulfide. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bass SE. Sienkiewicz P. Macdonald CJ. Cheng RY. Sparatore A. Del Soldato P. Roberts DD. Moody TW. Wink DA. Yeh GC. Novel dithiolethione-modified nonsteroidal anti-inflammatory drugs in human hepatoma HepG2 and colon LS180 cells. Clin Cancer Res. 2009;15:1964–1972. doi: 10.1158/1078-0432.CCR-08-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beinert H. A tribute to sulfur. Eur J Biochem. 2000;267:5657–5664. doi: 10.1046/j.1432-1327.2000.01637.x. [DOI] [PubMed] [Google Scholar]
- 13.Benavides GA. Squadrito GL. Mills RW. Patel HD. Isbell TS. Patel RP. Darley-Usmar VM. Doeller JE. Kraus DW. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci USA. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biermann J. Lagreze WA. Schallner N. Schwer CI. Goebel U. Inhalative preconditioning with hydrogen sulfide attenuated apoptosis after retinal ischemia/reperfusion injury. Mol Vis. 2011;17:1275–1286. [PMC free article] [PubMed] [Google Scholar]
- 15.Bivalacqua TJ. Usta MF. Kendirci M. Pradhan L. Alvarez X. Champion HC. Kadowitz PJ. Hellstrom WJ. Superoxide anion production in the rat penis impairs erectile function in diabetes: Influence of in vivo extracellular superoxide dismutase gene therapy. J Sex Med. 2005;2:187–197. doi: 10.1111/j.1743-6109.2005.20228_1.x. discussion 197–198. [DOI] [PubMed] [Google Scholar]
- 16.Blackstone E. Morrison M. Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518–518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 17.Blanco RA. Ziegler TR. Carlson BA. Cheng PY. Park Y. Cotsonis GA. Accardi CJ. Jones DP. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr. 2007;86:1016–1023. doi: 10.1093/ajcn/86.4.1016. [DOI] [PubMed] [Google Scholar]
- 18.Bouma HR. Verhaag EM. Otis JP. Heldmaier G. Swoap SJ. Strijkstra AM. Henning RH. Carey HV. Induction of torpor: Mimicking natural metabolic suppression for biomedical applications. J Cell Physiol. 2012;227:1285–1290. doi: 10.1002/jcp.22850. [DOI] [PubMed] [Google Scholar]
- 19.Brancaleone V. Roviezzo F. Vellecco V. De Gruttola L. Bucci M. Cirino G. Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br J Pharmacol. 2008;155:673–680. doi: 10.1038/bjp.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breitkreutz R. Pittack N. Nebe CT. Schuster D. Brust J. Beichert M. Hack V. Daniel V. Edler L. Droge W. Improvement of immune functions in HIV infection by sulfur supplementation: Two randomized trials. J Mol Med. 2000;78:55–62. doi: 10.1007/s001099900073. [DOI] [PubMed] [Google Scholar]
- 21.Brosnan JT. Brosnan ME. The sulfur-containing amino acids: An overview. J Nutr. 2006;136:1636S–1640S. doi: 10.1093/jn/136.6.1636S. [DOI] [PubMed] [Google Scholar]
- 22.Bucci M. Papapetropoulos A. Vellecco V. Zhou Z. Pyriochou A. Roussos C. Roviezzo F. Brancaleone V. Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 23.Budde MW. Roth MB. Hydrogen sulfide increases hypoxia-inducible factor-1 activity independently of von Hippel-Lindau tumor suppressor-1 in C. elegans. Mol Biol Cell. 2010;21:212–217. doi: 10.1091/mbc.E09-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai WJ. Wang MJ. Moore PK. Jin HM. Yao T. Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 25.Calvert JW. Elston M. Nicholson CK. Gundewar S. Jha S. Elrod JW. Ramachandran A. Lefer DJ. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation. 2010;122:11–19. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calvert JW. Jha S. Gundewar S. Elrod JW. Ramachandran A. Pattillo CB. Kevil CG. Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan EC. Peshavariya H. Datla SR. Dusting GJ. Jiang F. Redox regulation of angiogenesis: NADPH oxidase as a drug target. Anti-angiogen Drug Disc Devel. 2011;1:86–115. [Google Scholar]
- 28.Chang HS. Ko M. Ishizuka M. Fujita S. Yabuki A. Hossain MA. Yamato O. Sodium 2-propenyl thiosulfate derived from garlic induces phase II detoxification enzymes in rat hepatoma H4IIE cells. Nutr Res. 2010;30:435–440. doi: 10.1016/j.nutres.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Chang HS. Yamato O. Yamasaki M. Ko M. Maede Y. Growth inhibitory effect of alk(en)yl thiosulfates derived from onion and garlic in human immortalized and tumor cell lines. Cancer Lett. 2005;223:47–55. doi: 10.1016/j.canlet.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Chang HS. Yamato O. Yamasaki M. Maede Y. Modulatory influence of sodium 2-propenyl thiosulfate from garlic on cyclooxygenase activity in canine platelets: Possible mechanism for the anti-aggregatory effect. Prostaglandins Leukot Essent Fatty Acids. 2005;72:351–355. doi: 10.1016/j.plefa.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Chatterji T. Keerthi K. Gates KS. Generation of reactive oxygen species by a persulfide (BnSSH) Bioorg Med Chem Lett. 2005;15:3921–3924. doi: 10.1016/j.bmcl.2005.05.110. [DOI] [PubMed] [Google Scholar]
- 32.Chattopadhyay M. Kodela R. Nath N. Barsegian A. Boring D. Kashfi K. Hydrogen sulfide-releasing aspirin suppresses NF-kappaB signaling in estrogen receptor negative breast cancer cells in vitro and in vivo. Biochem Pharmacol. 2012;83:723–732. doi: 10.1016/j.bcp.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Chattopadhyay M. Kodela R. Nath N. Dastagirzada YM. Velazquez-Martinez CA. Boring D. Kashfi K. Hydrogen sulfide-releasing NSAIDs inhibit the growth of human cancer cells: A general property and evidence of a tissue type-independent effect. Biochem Pharmacol. 2012;83:715–722. doi: 10.1016/j.bcp.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Chattopadhyay M. Kodela R. Nath N. Street CR. Velazquez-Martinez CA. Boring D. Kashfi K. Hydrogen sulfide-releasing aspirin modulates xenobiotic metabolizing enzymes in vitro and in vivo. Biochem Pharmacol. 2012;83:733–740. doi: 10.1016/j.bcp.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Chen C-M. Yin M-C. S-allylcysteine, S-ethyl cysteine and S-propyl cysteine alleviate oxidative stress-induced damage within PC-12 cells. J Sci Food Agric. 2008;88:2493–2498. [Google Scholar]
- 36.Chen YR. Wang WF. Kong ANT. Tan TH. Molecular mechanisms of c-Jun N-terminal kinase-mediated apoptosis induced by anticarcinogenic isothiocyanates. J Biol Chem. 1998;273:1769–1775. doi: 10.1074/jbc.273.3.1769. [DOI] [PubMed] [Google Scholar]
- 37.Cheng X. Siow RC. Mann GE. Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: A role for mitochondria and the nuclear factor-E2-related factor 2-Kelch-like ECH-associated protein 1 defense pathway. Antioxid Redox Signal. 2011;14:469–487. doi: 10.1089/ars.2010.3283. [DOI] [PubMed] [Google Scholar]
- 38.Cong C. Chen H. Sun J. Apoptosis of FRH-0201 cells in human cholangiocarcinoma induced by allitridi. Hebei Medicine. 2008 [Google Scholar]
- 39.Cyr AR. Domann FE. The redox basis of epigenetic modifications: From mechanisms to functional consequences. Antioxid Redox Signal. 2011;15:551–589. doi: 10.1089/ars.2010.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.d'Emmanuele di Villa Bianca R. Sorrentino R. Maffia P. Mirone V. Imbimbo C. Fusco F. De Palma R. Ignarro LJ. Cirino G. Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation. Proc Natl Acad Sci USA. 2009;106:4513–4518. doi: 10.1073/pnas.0807974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.d'Emmanuele di Villa Bianca R. Sorrentino R. Mirone V. Cirino G. Hydrogen sulfide and erectile function: A novel therapeutic target. Nat Rev Urol. 2011;8:286–289. doi: 10.1038/nrurol.2011.45. [DOI] [PubMed] [Google Scholar]
- 42.Das A. Banik NL. Ray SK. Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer. 2007;110:1083–1095. doi: 10.1002/cncr.22888. [DOI] [PubMed] [Google Scholar]
- 43.de la Asuncion JG. Millan A. Pla R. Bruseghini L. Esteras A. Pallardo FV. Sastre J. Vina J. Mitochondrial glutathione oxidation correlates with age-associated oxidative damage to mitochondrial DNA. FASEB J. 1996;10:333–338. doi: 10.1096/fasebj.10.2.8641567. [DOI] [PubMed] [Google Scholar]
- 44.Deng W. Bivalacqua TJ. Champion HC. Hellstrom WJ. Murthy SN. Kadowitz PJ. Superoxide dismutase. A target for gene therapeutic approach to reduce oxidative stress in erectile dysfunction. Methods Mol Biol. 2010;610:213–227. doi: 10.1007/978-1-60327-029-8_13. [DOI] [PubMed] [Google Scholar]
- 45.Denisov ET. Afanas'ev IB. Oxidation and Antioxidants in Organic Chemistry and Biology. Boca Ratón, LA: 2005. [Google Scholar]
- 46.Denizalti M. Bozkurt TE. Akpulat U. Sahin-Erdemli I. Abacioglu N. The vasorelaxant effect of hydrogen sulfide is enhanced in streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:509–517. doi: 10.1007/s00210-011-0601-6. [DOI] [PubMed] [Google Scholar]
- 47.Derwall M. Francis RC. Kida K. Bougaki M. Crimi E. Adrie C. Zapol WM. Ichinose F. Administration of hydrogen sulfide via extracorporeal membrane lung ventilation in sheep with partial cardiopulmonary bypass perfusion: A proof of concept study on metabolic and vasomotor effects. Crit Care. 2011;15:R51. doi: 10.1186/cc10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desai KM. Chang T. Untereiner A. Wu L. Hydrogen sulfide and the metabolic syndrome. Expert Rev Clin Pharmacol. 2011;4:63–73. doi: 10.1586/ecp.10.133. [DOI] [PubMed] [Google Scholar]
- 49.Dombkowski RA. Russell MJ. Schulman AA. Doellman MM. Olson KR. Vertebrate phylogeny of hydrogen sulfide vasoactivity. Am J Physiol Regul Integr Comp Physiol. 2005;288:R243–52. doi: 10.1152/ajpregu.00324.2004. [DOI] [PubMed] [Google Scholar]
- 50.Drabek T. Kochanek PM. Stezoski J. Wu XR. Bayir H. Morhard RC. Stezoski SW. Tisherman SA. Intravenous hydrogen sulfide does not induce hypothermia or improve survival from hemorrhagic shock in pigs. Shock. 2011;35:67–73. doi: 10.1097/SHK.0b013e3181e86f49. [DOI] [PubMed] [Google Scholar]
- 51.Droge W. Oxidative stress and ageing: Is ageing a cysteine deficiency syndrome? Philos Trans R Soc Lond B Biol Sci. 2005;360:2355–2372. doi: 10.1098/rstb.2005.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Droge W. Kinscherf R. Aberrant insulin receptor signaling and amino acid homeostasis as a major cause of oxidative stress in aging. Antioxid Redox Signal. 2008;10:661–678. doi: 10.1089/ars.2007.1953. [DOI] [PubMed] [Google Scholar]
- 53.Droge W. Schulze-Osthoff K. Mihm S. Galter D. Schenk H. Eck HP. Roth S. Gmunder H. Functions of glutathione and glutathione disulfide in immunology and immunopathology. FASEB J. 1994;8:1131–1138. [PubMed] [Google Scholar]
- 54.Duan Y. Wang B. Influence of allitridi on oxidative stress reaction in rats with type 2 diabetes mellitus. Mod J Integ Trad Chinese Western Med. 2010 [Google Scholar]
- 55.Elrod JW. Calvert JW. Morrison J. Doeller JE. Kraus DW. Tao L. Jiao X. Scalia R. Kiss L. Szabo C. Kimura H. Chow CW. Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filomeni G. Aquilano K. Rotilio G. Ciriolo MR. Reactive oxygen species-dependent c-Jun NH2-terminal kinase/c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfide. Cancer Res. 2003;63:5940–5949. [PubMed] [Google Scholar]
- 57.Fiorucci S. Antonelli E. Distrutti E. Rizzo G. Mencarelli A. Orlandi S. Zanardo R. Renga B. Di Sante M. Morelli A. Cirino G. Wallace JL. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 58.Fiorucci S. Distrutti E. Cirino G. Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 59.Giustarini D. Del Soldato P. Sparatore A. Rossi R. Modulation of thiol homeostasis induced by H2S-releasing aspirin. Free Radic Biol Med. 2010;48:1263–1272. doi: 10.1016/j.freeradbiomed.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 60.Grimble R. Sulfur amino acids, glutathione, and immune function. In: Roberta M, editor; Guiseppe M, editor. Glutathione and Sulfur Amino Acids in Human Health and Disease. Hoboken, NJ: John Wiley and Sons, Inc.; 2009. pp. 273–288. [Google Scholar]
- 61.Guayerbas N. Puerto M. Hernanz A. Miquel J. De la Fuente M. Thiolic antioxidant supplementation of the diet reverses age-related behavioural dysfunction in prematurely ageing mice. Pharmacol Biochem Behav. 2005;80:45–51. doi: 10.1016/j.pbb.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 62.Gupta S. Kuhnisch J. Mustafa A. Lhotak S. Schlachterman A. Slifker MJ. Klein-Szanto A. High KA. Austin RC. Kruger WD. Mouse models of cystathionine beta-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia. FASEB J. 2009;23:883–893. doi: 10.1096/fj.08-120584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ha MW. Ma R. Shun LP. Gong YH. Yuan Y. Effects of allitridi on cell cycle arrest of human gastric cancer cells. World J Gastroenterol. 2005;11:5433–5437. doi: 10.3748/wjg.v11.i35.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hao L. Hui-quing L. Yun W. Hai-xiu X. Wan-teng F. Mei-ling W. Pei-hong S. Xio-yan X. An intervention study to prevent gastric cancer by micro-selenium and large dose of allitridum. Chin Med J (Engl) 2004;117:1155–1160. [PubMed] [Google Scholar]
- 65.Henderson PW. Singh SP. Weinstein AL. Nagineni V. Rafii DC. Kadouch D. Krijgh DD. Spector JA. Therapeutic metabolic inhibition: Hydrogen sulfide significantly mitigates skeletal muscle ischemia reperfusion injury in vitro and in vivo. Plast Reconstr Surg. 2010;126:1890–1898. doi: 10.1097/PRS.0b013e3181f446bc. [DOI] [PubMed] [Google Scholar]
- 66.Herman-Antosiewicz A. Powolny AA. Singh SV. Molecular targets of cancer chemoprevention by garlic-derived organosulfides. Acta Pharmacol Sin. 2007;28:1355–1364. doi: 10.1111/j.1745-7254.2007.00682.x. [DOI] [PubMed] [Google Scholar]
- 67.Herman-Antosiewicz A. Singh SV. Checkpoint kinase 1 regulates diallyl trisulfide-induced mitotic arrest in human prostate cancer cells. J Biol Chem. 2005;280:28519–28528. doi: 10.1074/jbc.M501443200. [DOI] [PubMed] [Google Scholar]
- 68.Herman-Antosiewicz A. Stan SD. Hahm ER. Xiao D. Singh SV. Activation of a novel ataxia-telangiectasia mutated and Rad3 related/checkpoint kinase 1-dependent prometaphase checkpoint in cancer cells by diallyl trisulfide, a promising cancer chemopreventive constituent of processed garlic. Mol Cancer Ther. 2007;6:1249–1261. doi: 10.1158/1535-7163.MCT-06-0477. [DOI] [PubMed] [Google Scholar]
- 69.Higuchi K. Umegaki E. Watanabe T. Yoda Y. Morita E. Murano M. Tokioka S. Arakawa T. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44:879–888. doi: 10.1007/s00535-009-0102-2. [DOI] [PubMed] [Google Scholar]
- 70.Hildebrandt TM. Grieshaber MK. Redox regulation of mitochondrial sulfide oxidation in the lugworm, Arenicola marina. J Exp Biol. 2008;211:2617–2623. doi: 10.1242/jeb.019729. [DOI] [PubMed] [Google Scholar]
- 71.Hildebrandt TM. Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008;275:3352–3361. doi: 10.1111/j.1742-4658.2008.06482.x. [DOI] [PubMed] [Google Scholar]
- 72.Hosono T. Fukao T. Ogihara J. Ito Y. Shiba H. Seki T. Ariga T. Diallyl trisulfide suppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of beta-tubulin. J Biol Chem. 2005;280:41487–41493. doi: 10.1074/jbc.M507127200. [DOI] [PubMed] [Google Scholar]
- 73.Hosono T. Hosono-Fukao T. Inada K. Tanaka R. Yamada H. Iitsuka Y. Seki T. Hasegawa I. Ariga T. Alkenyl group is responsible for the disruption of microtubule network formation in human colon cancer cell line HT-29 cells. Carcinogenesis. 2008;29:1400–1406. doi: 10.1093/carcin/bgn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Howard EW. Ling MT. Chua CW. Cheung HW. Wang X. Wong YC. Garlic-derived S-allylmercaptocysteine is a novel in vivo antimetastatic agent for androgen-independent prostate cancer. Clin Cancer Res. 2007;13:1847–1856. doi: 10.1158/1078-0432.CCR-06-2074. [DOI] [PubMed] [Google Scholar]
- 75.Hsu CC. Huang CN. Hung YC. Yin MC. Five cysteine-containing compounds have antioxidative activity in Balb/cA mice. J Nutr. 2004;134:149–152. doi: 10.1093/jn/134.1.149. [DOI] [PubMed] [Google Scholar]
- 76.Huovinen JA. Gustafsson BE. Inorganic sulphate, sulphite and sulphide as sulphur donors in the biosynthesis of sulphur amino acids in germ-free and conventional rats. Biochim Biophys Acta. 1967;136:441–447. doi: 10.1016/0304-4165(67)90003-7. [DOI] [PubMed] [Google Scholar]
- 77.Huseby N-E. Sundkvist E. Svineng G. Glutathione and sulfur containing amino acids: antioxidant and conjugation activities. In: Masella R, editor; Mazza G, editor. Glutathione and Sulfur Amino Acids in Human Health and Disease. Hoboken, NJ: John Wiley and Sons, Inc; 2009. pp. 93–120. [Google Scholar]
- 78.Ikeno Y. Bronson RT. Hubbard GB. Lee S. Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: Correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 79.Isenberg JS. Jia Y. Field L. Ridnour LA. Sparatore A. Del Soldato P. Sowers AL. Yeh GC. Moody TW. Wink DA. Ramchandran R. Roberts DD. Modulation of angiogenesis by dithiolethione-modified NSAIDs and valproic acid. Br J Pharmacol. 2007;151:63–72. doi: 10.1038/sj.bjp.0707194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishigami M. Hiraki K. Umemura K. Ogasawara Y. Ishii K. Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 81.Jacob C. Anwar A. Burkholz T. Perspective on recent developments on sulfur-containing agents and hydrogen sulfide signaling. Planta Med. 2008;74:1580–1592. doi: 10.1055/s-0028-1088299. [DOI] [PubMed] [Google Scholar]
- 82.Jha S. Calvert JW. Duranski MR. Ramachandran A. Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: Role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295:H801–806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jung KA. Kwak MK. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15:7266–7291. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jurkowska H. Wrobel M. N-acetyl-L-cysteine as a source of sulfane sulfur in astrocytoma and astrocyte cultures: Correlations with cell proliferation. Amino Acids. 2008;34:231–237. doi: 10.1007/s00726-007-0471-2. [DOI] [PubMed] [Google Scholar]
- 86.Kabil O. Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26:243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- 88.Kamyshny A. Ekeltchik I. Gun J. Lev O. Method for the determination of inorganic polysulfide distribution in aquatic systems. Anal Chem. 2006;78:2631–2639. doi: 10.1021/ac051854a. [DOI] [PubMed] [Google Scholar]
- 89.Kamyshny A. Zilberbrand M. Ekeltchik I. Voitsekovski T. Gun J. Lev O. Speciation of polysulfides and zerovalent sulfur in sulfide-rich water wells in southern and central Israel. Aqua Geochem. 2008;14:171–192. [Google Scholar]
- 90.Kaneko Y. Kimura T. Taniguchi S. Souma M. Kojima Y. Kimura Y. Kimura H. Niki I. Glucose-induced production of hydrogen sulfide may protect the pancreatic beta-cells from apoptotic cell death by high glucose. FEBS Lett. 2009;583:377–382. doi: 10.1016/j.febslet.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 91.Kida K. Yamada M. Tokuda K. Marutani E. Kakinohana M. Kaneki M. Ichinose F. Inhaled hydrogen sulfide prevents neurodegeneration and movement disorder in a mouse model of Parkinson's disease. Antioxid Redox Signal. 2011;15:343–352. doi: 10.1089/ars.2010.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim YA. Xiao D. Xiao H. Powolny AA. Lew KL. Reilly ML. Zeng Y. Wang Z. Singh SV. Mitochondria-mediated apoptosis by diallyl trisulfide in human prostate cancer cells is associated with generation of reactive oxygen species and regulated by Bax/Bak. Mol Cancer Ther. 2007;6:1599–1609. doi: 10.1158/1535-7163.MCT-06-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kimura Y. Goto Y. Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 94.Kimura Y. Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 95.Kitteringham NR. Abdullah A. Walsh J. Randle L. Jenkins RE. Sison R. Goldring CE. Powell H. Sanderson C. Williams S. Higgins L. Yamamoto M. Hayes J. Park BK. Proteomic analysis of Nrf2 deficient transgenic mice reveals cellular defence and lipid metabolism as primary Nrf2-dependent pathways in the liver. J Proteom. 2010;73:1612–1631. doi: 10.1016/j.jprot.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klaassen CD. Reisman SA. Nrf2 the rescue: Effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol. 2010;244:57–65. doi: 10.1016/j.taap.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klatt P. Molina EP. De Lacoba MG. Padilla CA. Martinez-Galesteo E. Barcena JA. Lamas S. Redox regulation of c-Jun DNA binding by reversible S-glutathiolation. FASEB J. 1999;13:1481–1490. doi: 10.1096/fasebj.13.12.1481. [DOI] [PubMed] [Google Scholar]
- 98.Kloesch B. Liszt M. Steiner G. Broll J. Inhibitors of p38 and ERK1/2 MAPkinase and hydrogen sulphide block constitutive and IL-1beta-induced IL-6 and IL-8 expression in the human chondrocyte cell line C-28/I2. Rheumatol Int. 2012;32:729–736. doi: 10.1007/s00296-010-1682-0. [DOI] [PubMed] [Google Scholar]
- 99.Kraus J. Packman S. Fowler B. Rosenberg LE. Purification and properties of cystathionine beta-synthase from human liver. Evidence for identical subunits. J Biol Chem. 1978;253:6523–6528. [PubMed] [Google Scholar]
- 100.Laggner H. Muellner MK. Schreier S. Sturm B. Hermann M. Exner M. Gmeiner BM. Kapiotis S. Hydrogen sulphide: A novel physiological inhibitor of LDL atherogenic modification by HOCl. Free Radic Res. 2007;41:741–747. doi: 10.1080/10715760701263265. [DOI] [PubMed] [Google Scholar]
- 101.Lan H. Lu YY. [Effect of allitridi on cyclin D1 and p27(Kip1) protein expression in gastric carcinoma BGC823 cells] Ai Zheng. 2003;22:1268–1271. [PubMed] [Google Scholar]
- 102.Lan H. Lu YY. Allitridi induces apoptosis by affecting Bcl-2 expression and caspase-3 activity in human gastric cancer cells. Acta Pharmacol Sin. 2004;25:219–225. [PubMed] [Google Scholar]
- 103.Lands LC. Grey VL. Smountas AA. Effect of supplementation with a cysteine donor on muscular performance. J Appl Physiol. 1999;87:1381–1385. doi: 10.1152/jappl.1999.87.4.1381. [DOI] [PubMed] [Google Scholar]
- 104.Lee BC. Park BH. Kim SY. Lee YJ. Role of Bim in diallyl trisulfide-induced cytotoxicity in human cancer cells. J Cell Biochem. 2011;112:118–127. doi: 10.1002/jcb.22896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee M. Sparatore A. Del Soldato P. McGeer E. McGeer PL. Hydrogen sulfide-releasing NSAIDs attenuate neuroinflammation induced by microglial and astrocytic activation. Glia. 2010;58:103–113. doi: 10.1002/glia.20905. [DOI] [PubMed] [Google Scholar]
- 106.Lee M. Tazzari V. Giustarini D. Rossi R. Sparatore A. Del Soldato P. McGeer E. McGeer PL. Effects of hydrogen sulfide-releasing L-DOPA derivatives on glial activation: Potential for treating Parkinson disease. J Biol Chem. 2010;285:17318–17328. doi: 10.1074/jbc.M110.115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee S. Park Y. Zuidema MY. Hannink M. Zhang C. Effects of interventions on oxidative stress and inflammation of cardiovascular diseases. World J Cardiol. 2011;3:18–24. doi: 10.4330/wjc.v3.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee ZW. Zhou J. Chen CS. Zhao Y. Tan CH. Li L. Moore PK. Deng LW. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One. 2011;6:e21077. doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lefer DJ. Potential importance of alterations in hydrogen sulphide (H2S) bioavailability in diabetes. Br J Pharmacol. 2008;155:617–619. doi: 10.1038/bjp.2008.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lefer DJ. Scalia R. Campbell B. Nossuli T. Hayward R. Salamon M. Grayson J. Lefer AM. Peroxynitrite inhibits leukocyte-endothelial cell interactions and protects against ischemia-reperfusion injury in rats. J Clin Invest. 1997;99:684–691. doi: 10.1172/JCI119212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leiser SF. Miller RA. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol Cell Biol. 2010;30:871–884. doi: 10.1128/MCB.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li L. Rose P. Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 113.Li L. Salto-Tellez M. Tan CH. Whiteman M. Moore PK. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic Biol Med. 2009;47:103–113. doi: 10.1016/j.freeradbiomed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 114.Limon-Pacheco JH. Hernandez NA. Fanjul-Moles ML. Gonsebatt ME. Glutathione depletion activates mitogen-activated protein kinase (MAPK) pathways that display organ-specific responses and brain protection in mice. Free Radic Biol Med. 2007;43:1335–1347. doi: 10.1016/j.freeradbiomed.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 115.Lin CC. Yin MC. Antiglycative and anti-VEGF effects of S-ethyl cysteine and S-propyl cysteine in kidney of diabetic mice. Mol Nutr Food Res. 2008;52:1358–1364. doi: 10.1002/mnfr.200800007. [DOI] [PubMed] [Google Scholar]
- 116.Loffredo L. Marcoccia A. Pignatelli P. Andreozzi P. Borgia MC. Cangemi R. Chiarotti F. Violi F. Oxidative-stress-mediated arterial dysfunction in patients with peripheral arterial disease. Eur Heart J. 2007;28:608–612. doi: 10.1093/eurheartj/ehl533. [DOI] [PubMed] [Google Scholar]
- 117.Luther GW. Findlay AJ. MacDonald DJ. Owings SM. Hanson TE. Beinart RA. Girguis PR. Thermodynamics and kinetics of sulfide oxidation by oxygen: A look at inorganically controlled reactions and biologically mediated processes in the environment. Front Microbiol. 2011;2:62. doi: 10.3389/fmicb.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lykakis IN. Ferreri C. Chatgilialoglu C. The sulfhydryl radical (HS(.)/S(.-)): A contender for the isomerization of double bonds in membrane lipids. Angew Chem Int Ed Engl. 2007;46:1914–1916. doi: 10.1002/anie.200604525. [DOI] [PubMed] [Google Scholar]
- 119.Lynn EG. Austin RC. Hydrogen sulfide in the pathogenesis of atherosclerosis and its therapeutic potential. Expert Rev Clin Pharmacol. 2010;4:97–108. doi: 10.1586/ecp.10.130. [DOI] [PubMed] [Google Scholar]
- 120.Ma K. Liu Y. Zhu Q. Liu CH. Duan JL. Tan BK. Zhu YZ. H2S donor, S-propargyl-cysteine, increases CSE in SGC-7901 and cancer-induced mice: Evidence for a novel anti-cancer effect of endogenous H2S? PLoS One. 2011;6:e20525. doi: 10.1371/journal.pone.0020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maher J. Yamamoto M. The rise of antioxidant signaling—The evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol. 2010;244:4–15. doi: 10.1016/j.taap.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 122.Mantovani G. Madeddu C. Maccio A. Gramignano G. Lusso MR. Massa E. Astara G. Serpe R. Cancer-related anorexia/cachexia syndrome and oxidative stress: An innovative approach beyond current treatment. Cancer Epidemiol Biomarkers Prev. 2004;13:1651–1659. [PubMed] [Google Scholar]
- 123.Mari M. Morales A. Colell A. Garcia-Ruiz C. Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marsden PA. Low-molecular-weight S-nitrosothiols and blood vessel injury. J Clin Invest. 2007;117:2377–2380. doi: 10.1172/JCI33136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Matteucci E. Giampietro O. Thiol signalling network with an eye to diabetes. Molecules. 2010;15:8890–8903. doi: 10.3390/molecules15128890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Medeiros JV. Bezerra VH. Gomes AS. Barbosa AL. Lima-Junior RC. Soares PM. Brito GA. Ribeiro RA. Cunha FQ. Souza MH. Hydrogen sulfide prevents ethanol-induced gastric damage in mice: Role of ATP-sensitive potassium channels and capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther. 2009;330:764–770. doi: 10.1124/jpet.109.152801. [DOI] [PubMed] [Google Scholar]
- 127.Meng JL. Mei WY. Dong YF. Wang JH. Zhao CM. Lan AP. Yang CT. Chen PX. Feng JQ. Hu CH. Heat shock protein 90 mediates cytoprotection by HS against chemical hypoxia-induced injury in PC12 cells. Clin Exp Pharmacol Physiol. 2011;38:42–49. doi: 10.1111/j.1440-1681.2010.05462.x. [DOI] [PubMed] [Google Scholar]
- 128.Mikami Y. Shibuya N. Kimura Y. Nagahara N. Ogasawara Y. Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J. 2011;439:479–485. doi: 10.1042/BJ20110841. [DOI] [PubMed] [Google Scholar]
- 129.Mikami Y. Shibuya N. Kimura Y. Nagahara N. Yamada M. Kimura H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J Biol Chem. 2011;286:39379–39386. doi: 10.1074/jbc.M111.298208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miller DL. Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2007;104:20618–20622. doi: 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Minamishima S. Bougaki M. Sips PY. Yu JD. Minamishima YA. Elrod JW. Lefer DJ. Bloch KD. Ichinose F. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation. 2009;120:888–896. doi: 10.1161/CIRCULATIONAHA.108.833491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mitsuhashi H. Yamashita S. Ikeuchi H. Kuroiwa T. Kaneko Y. Hiromura K. Ueki K. Nojima Y. Oxidative stress-dependent conversion of hydrogen sulfide to sulfite by activated neutrophils. Shock. 2005;24:529–534. doi: 10.1097/01.shk.0000183393.83272.de. [DOI] [PubMed] [Google Scholar]
- 133.Miyoshi N. Takabayashi S. Osawa T. Nakamura Y. Benzyl isothiocyanate inhibits excessive superoxide generation in inflammatory leukocytes: Implication for prevention against inflammation-related carcinogenesis. Carcinogenesis. 2004;25:567–575. doi: 10.1093/carcin/bgh051. [DOI] [PubMed] [Google Scholar]
- 134.Morrison ML. Blackwood JE. Lockett SL. Iwata A. Winn RK. Roth MB. Surviving blood loss using hydrogen sulfide. J Trauma. 2008;65:183–188. doi: 10.1097/TA.0b013e3181507579. [DOI] [PubMed] [Google Scholar]
- 135.Morsy MA. Ibrahim SA. Abdelwahab SA. Zedan MZ. Elbitar HI. Curative effects of hydrogen sulfide against acetaminophen-induced hepatotoxicity in mice. Life Sci. 2010;87:692–698. doi: 10.1016/j.lfs.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 136.Mosharov E. Cranford MR. Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry. 2000;39:13005–13011. doi: 10.1021/bi001088w. [DOI] [PubMed] [Google Scholar]
- 137.Munchberg U. Anwar A. Mecklenburg S. Jacob C. Polysulfides as biologically active ingredients of garlic. Organic Biomol Chem. 2007;5:1505–1518. doi: 10.1039/b703832a. [DOI] [PubMed] [Google Scholar]
- 138.Mustafa AK. Gadalla MM. Sen N. Kim S. Mu W. Gazi SK. Barrow RK. Yang G. Wang R. Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nagy P. Winterbourn CC. Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides. Chem Res Toxicol. 2010;23:1541–1543. doi: 10.1021/tx100266a. [DOI] [PubMed] [Google Scholar]
- 140.Nagy P. Winterbourn CC. Chapter 6: Redox chemistry of biological thiols. In: James CF, editor. Advances in Molecular Toxicology. Elsevier; 2010. pp. 183–222. [Google Scholar]
- 141.Nelson KC. Armstrong JS. Moriarty S. Cai J. Wu MW. Sternberg P., Jr. Jones DP. Protection of retinal pigment epithelial cells from oxidative damage by oltipraz, a cancer chemopreventive agent. Invest Ophthalmol Vis Sci. 2002;43:3550–3554. [PubMed] [Google Scholar]
- 142.Nian H. Delage B. Ho E. Dashwood RH. Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: Studies with sulforaphane and garlic organosulfur compounds. Environ Mol Mutagen. 2009;50:213–221. doi: 10.1002/em.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nimni ME. Han B. Cordoba F. Are we getting enough sulfur in our diet? Nutr Metab (Lond) 2007;4:24. doi: 10.1186/1743-7075-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Oh GS. Pae HO. Lee BS. Kim BN. Kim JM. Kim HR. Jeon SB. Jeon WK. Chae HJ. Chung HT. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 145.Olson KR. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim Biophys Acta. 2009;1787:856–863. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 146.Olson KR. Whitfield NL. Hydrogen sulfide and oxygen sensing in the cardiovascular system. Antioxid Redox Signal. 2010;12:1219–1234. doi: 10.1089/ars.2009.2921. [DOI] [PubMed] [Google Scholar]
- 147.Osborne NN. Ji D. Abdul Majid AS. Fawcett RJ. Sparatore A. Del Soldato P. ACS67, a hydrogen sulfide-releasing derivative of latanoprost acid, attenuates retinal ischemia and oxidative stress to RGC-5 cells in culture. Invest Ophthalmol Vis Sci. 2010;51:284–294. doi: 10.1167/iovs.09-3999. [DOI] [PubMed] [Google Scholar]
- 148.Otani H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxid Redox Signal. 2011;15:1911–1926. doi: 10.1089/ars.2010.3739. [DOI] [PubMed] [Google Scholar]
- 149.Pallardo FV. Markovich J. Garcia JL. Viña F. Role of nuclear glutathione as a key regulator of cell proliferation. Molecular Aspects of Medicine. 2009;30:77–85. doi: 10.1016/j.mam.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 150.Palmer LA. Doctor A. Chhabra P. Sheram ML. Laubach VE. Karlinsey MZ. Forbes MS. Macdonald T. Gaston B. S-nitrosothiols signal hypoxia-mimetic vascular pathology. J Clin Invest. 2007;117:2592–2601. doi: 10.1172/JCI29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pan LL. Liu XH. Gong QH. Wu D. Zhu YZ. Hydrogen sulfide attenuated tumor necrosis factor-alpha-induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS One. 2011;6:e19766. doi: 10.1371/journal.pone.0019766. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 152.Pinto JT. Krasnikov BF. Cooper AJ. Redox-sensitive proteins are potential targets of garlic-derived mercaptocysteine derivatives. J Nutr. 2006;136:835S–841S. doi: 10.1093/jn/136.3.835S. [DOI] [PubMed] [Google Scholar]
- 153.Porter PN. Grishaver MS. Jones OW. Characterization of human cystathionine beta-synthase. Evidence for the identity of human L-serine dehydratase and cystathionine beta-synthase. Biochim Biophys Acta. 1974;364:128–139. doi: 10.1016/0005-2744(74)90140-5. [DOI] [PubMed] [Google Scholar]
- 154.Powolny AA. Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305–314. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Predmore BL. Lefer DJ. Development of hydrogen sulfide-based therapeutics for cardiovascular disease. J Cardiovasc Transl Res. 2010;3:487–498. doi: 10.1007/s12265-010-9201-y. [DOI] [PubMed] [Google Scholar]
- 156.Predmore BL. Lefer DJ. Hydrogen sulfide-mediated myocardial pre- and post-conditioning. Expert Rev Clin Pharmacol. 2011;4:83–96. doi: 10.1586/ecp.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pryor WA. Gojon G. Church DF. Relative rate constants for hydrogen atom abstraction by the cyclohexanethiyl and benzenethiyl radicals. J Organic Chem. 1978;43:793–800. [Google Scholar]
- 158.Pryor WA. Houk KN. Foote CS. Fukuto JM. Ignarro LJ. Squadrito GL. Davies KJ. Free radical biology and medicine: It's a gas, man! Am J Physiol Regul Integr Comp Physiol. 2006;291:R491–511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- 159.Qiu X. Villalta J. Lin G. Lue TF. Role of hydrogen sulfide in the physiology of penile erection. J Androl. 2011 doi: 10.2164/jandrol.111.014936. [Epub ahead of Print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rabai G. Orban M. Epstein IR. Systematic design of chemical oscillators. A model for the pH-regulated oscillatory reaction between hydrogen peroxide and sulfide ion. J Phys Chem. 1992;96:5414–5419. [Google Scholar]
- 161.Rahangdale S. Yeh SY. Malhotra A. Veves A. Therapeutic interventions and oxidative stress in diabetes. Front Biosci. 2009;14:192–209. doi: 10.2741/3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reisman SA. Buckley DB. Tanaka Y. Klaassen CD. CDDO-Im protects from acetaminophen hepatotoxicity through induction of Nrf2-dependent genes. Toxicol Appl Pharmacol. 2009;236:109–114. doi: 10.1016/j.taap.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rosado JO. Salvador M. Bonatto D. Importance of the trans-sulfuration pathway in cancer prevention and promotion. Mol Cell Biochem. 2007;301:1–12. doi: 10.1007/s11010-006-9389-y. [DOI] [PubMed] [Google Scholar]
- 164.Rossoni G. Sparatore A. Tazzari V. Manfredi B. Del Soldato P. Berti F. The hydrogen sulphide-releasing derivative of diclofenac protects against ischaemia-reperfusion injury in the isolated rabbit heart. Br J Pharmacol. 2008;153:100–109. doi: 10.1038/sj.bjp.0707540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ryter SW. Alam J. Choi AMK. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 166.Sabelli R. Iorio E. De Martino A. Podo F. Ricci A. Viticchie G. Rotilio G. Paci M. Melino S. Rhodanese-thioredoxin system and allyl sulfur compounds. FEBS J. 2008;275:3884–3899. doi: 10.1111/j.1742-4658.2008.06535.x. [DOI] [PubMed] [Google Scholar]
- 167.Saez G. Thornalley PJ. Hill HA. Hems R. Bannister JV. The production of free radicals during the autoxidation of cysteine and their effect on isolated rat hepatocytes. Biochim Biophys Acta. 1982;719:24–31. doi: 10.1016/0304-4165(82)90302-6. [DOI] [PubMed] [Google Scholar]
- 168.Sahu RP. Zhang R. Batra S. Shi Y. Srivastava SK. Benzyl isothiocyanate-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis. 2009;30:1744–1753. doi: 10.1093/carcin/bgp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schalinske KL. Hepatic sulfur amino acid metabolism. In: Masella R, editor; Mazza G, editor. Glutathione and Sulfur Amino Acids in Human Health and Disease. Hoboken, NJ: John Wiley and Sons, Inc.; 2009. pp. 73–90. [Google Scholar]
- 170.Seki T. Hosono T. Hosono-Fukao T. Inada K. Tanaka R. Ogihara J. Ariga T. Anticancer effects of diallyl trisulfide derived from garlic. Asia Pac J Clin Nutr. 2008;17:249–252. [PubMed] [Google Scholar]
- 171.Sen N. Paul BD. Gadalla MM. Mustafa AK. Sen T. Xu R. Kim S. Snyder SH. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sen N. Snyder SH. Protein modifications involved in neurotransmitter and gasotransmitter signaling. Trends Neurosci. 2010;33:493–502. doi: 10.1016/j.tins.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sen U. Basu P. Abe OA. Givvimani S. Tyagi N. Metreveli N. Shah KS. Passmore JC. Tyagi SC. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am J Physiol Renal Physiol. 2009;297:F410–419. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shin S. Wakabayashi J. Yates MS. Wakabayashi N. Dolan PM. Aja S. Liby KT. Sporn MB. Yamamoto M. Kensler TW. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur J Pharmacol. 2009;620:138–144. doi: 10.1016/j.ejphar.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shukla N. Rossoni G. Hotston M. Sparatore A. Del Soldato P. Tazzari V. Persad R. Angelini GD. Jeremy JY. Effect of hydrogen sulphide-donating sildenafil (ACS6) on erectile function and oxidative stress in rabbit isolated corpus cavernosum and in hypertensive rats. Bju Int. 2009;103:1522–1529. doi: 10.1111/j.1464-410X.2009.08415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sidhu R. Singh M. Samir G. Carson RJ. L-cysteine and sodium hydrosulphide inhibit spontaneous contractility in isolated pregnant rat uterine strips in vitro. Pharmacol Toxicol. 2001;88:198–203. doi: 10.1034/j.1600-0773.2001.d01-104.x. [DOI] [PubMed] [Google Scholar]
- 177.Sies H. Preface. In: Sies H, editor. Antioxidants in Disease Mechanisms and Therapy. San Diego, CA: Academic Press; 1996. p. xxiii. [Google Scholar]
- 178.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 179.Simon F. Giudici R. Duy CN. Schelzig H. Oter S. Groger M. Wachter U. Vogt J. Speit G. Szabo C. Radermacher P. Calzia E. Hemodynamic and metabolic effects of hydrogen sulfide during porcine ischemia/reperfusion injury. Shock. 2008;30:359–364. doi: 10.1097/SHK.0b013e3181674185. [DOI] [PubMed] [Google Scholar]
- 180.Singh SV. Srivastava SK. Choi S. Lew KL. Antosiewicz J. Xiao D. Zeng Y. Watkins SC. Johnson CS. Trump DL. Lee YJ. Xiao H. Herman-Antosiewicz A. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 181.Sodha NR. Clements RT. Feng J. Liu Y. Bianchi C. Horvath EM. Szabo C. Stahl GL. Sellke FW. Hydrogen sulfide therapy attenuates the inflammatory response in a porcine model of myocardial ischemia/reperfusion injury. J Thorac Cardiovasc Surg. 2009;138:977–984. doi: 10.1016/j.jtcvs.2008.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sommer F. Klotz T. Steinritz D. Bloch W. Evaluation of tetrahydrobiopterin (BH4) as a potential therapeutic agent to treat erectile dysfunction. Asian J Androl. 2006;8:159–167. doi: 10.1111/j.1745-7262.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- 183.Sparatore A. Perrino E. Tazzari V. Giustarini D. Rossi R. Rossoni G. Erdman K. Schroder H. Soldato PD. Pharmacological profile of a novel H2S-releasing aspirin. Free Radical Biol Med. 2009;46:586–592. doi: 10.1016/j.freeradbiomed.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 184.Sparatore A. Santus G. Giustarini D. Rossi R. Del Soldato P. Therapeutic potential of new hydrogen sulfide-releasing hybrids. Expert Rev Clin Pharmacol. 2011;4:109–121. doi: 10.1586/ecp.10.122. [DOI] [PubMed] [Google Scholar]
- 185.Srilatha B. Adaikan PG. Li L. Moore PK. Hydrogen sulphide: A novel endogenous gasotransmitter facilitates erectile function. J Sex Med. 2007;4:1304–1311. doi: 10.1111/j.1743-6109.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 186.Srilatha B. Adaikan PG. Moore PK. Possible role for the novel gasotransmitter hydrogen sulphide in erectile dysfunction—A pilot study. Eur J Pharmacol. 2006;535:280–282. doi: 10.1016/j.ejphar.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 187.Srilatha B. Hu L. Adaikan GP. Moore PK. Initial characterization of hydrogen sulfide effects in female sexual function. J Sex Med. 2009;6:1875–1884. doi: 10.1111/j.1743-6109.2009.01291.x. [DOI] [PubMed] [Google Scholar]
- 188.Srivastava SK. Singh SV. Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis. 2004;25:1701–1709. doi: 10.1093/carcin/bgh179. [DOI] [PubMed] [Google Scholar]
- 189.Stasko A. Brezova V. Zalibera M. Biskupic S. Ondrias K. Electron transfer: A primary step in the reactions of sodium hydrosulphide, an H2S/HS(-) donor. Free Radic Res. 2009;43:581–593. doi: 10.1080/10715760902977416. [DOI] [PubMed] [Google Scholar]
- 190.Stipanuk MH. Sulfur amino acid metabolism: Pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 191.Stoyanovsky DA. Maeda A. Atkins JL. Kagan VE. Assessments of thiyl radicals in biosystems: Difficulties and new applications. Anal Chem. 2011;83:6432–6438. doi: 10.1021/ac200418s. [DOI] [PubMed] [Google Scholar]
- 192.Sykiotis GP. Habeos IG. Samuelson AV. Bohmann D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr Opin Clin Nutr Metab Care. 2011;14:41–48. doi: 10.1097/MCO.0b013e32834136f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Takeuchi K. Kita K. Hayashi S. Aihara E. Regulatory mechanism of duodenal bicarbonate secretion. Roles of endogenous prostaglandins and nitric oxide. Pharmacol Therapeut. 2011;130:59–70. doi: 10.1016/j.pharmthera.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 194.Tan BH. Wong PT. Bian JS. Hydrogen sulfide: A novel signaling molecule in the central nervous system. Neurochem Int. 2010;56:3–10. doi: 10.1016/j.neuint.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 195.Tan Y. Ichikawa T. Li J. Si Q. Yang H. Chen X. Goldblatt CS. Meyer CJ. Li X. Cai L. Cui T. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 2011;60:625–633. doi: 10.2337/db10-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tang G. Wu L. Wang R. Interaction of hydrogen sulfide with ion channels. Clin Exp Pharmacol Physiol. 2010;37:753–763. doi: 10.1111/j.1440-1681.2010.05351.x. [DOI] [PubMed] [Google Scholar]
- 197.Taniguchi S. Kang L. Kimura T. Niki I. Hydrogen sulphide protects mouse pancreatic beta-cells from cell death induced by oxidative stress, but not by endoplasmic reticulum stress. Br J Pharmacol. 2011;162:1171–1178. doi: 10.1111/j.1476-5381.2010.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Taniguchi S. Niki I. Significance of hydrogen sulfide production in the pancreatic beta-cell. J Pharmacol Sci. 2011;116:1–5. doi: 10.1254/jphs.11r01cp. [DOI] [PubMed] [Google Scholar]
- 199.Tay AS. Hu LF. Lu M. Wong PT. Bian JS. Hydrogen sulfide protects neurons against hypoxic injury via stimulation of ATP-sensitive potassium channel/protein kinase C/extracellular signal-regulated kinase/heat shock protein 90 pathway. Neuroscience. 2010;167:277–286. doi: 10.1016/j.neuroscience.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 200.Tiranti V. Viscomi C. Hildebrandt T. Di Meo I. Mineri R. Tiveron C. Levitt MD. Prelle A. Fagiolari G. Rimoldi M. Zeviani M. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med. 2009;15:200–205. doi: 10.1038/nm.1907. [DOI] [PubMed] [Google Scholar]
- 201.Tirouvanziam R. Conrad CK. Bottiglieri T. Herzenberg LA. and Moss RB. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc Natl Acad Sci USA. 2006;103:4628–4633. doi: 10.1073/pnas.0511304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tomaskova Z. Cacanyiova S. Benco A. Kristek F. Dugovicova L. Hrbac J. Ondrias K. Lipids modulate H(2)S/HS(-) induced NO release from S-nitrosoglutathione. Biochem Biophys Res Commun. 2009;390:1241–1244. doi: 10.1016/j.bbrc.2009.10.128. [DOI] [PubMed] [Google Scholar]
- 203.Trachootham D. Lu WQ. Ogasawara MA. Valle NRD. Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Trelle S. Reichenbach S. Wandel S. Hildebrand P. Tschannen B. Villiger PM. Egger M. Juni P. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. Br Med J. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tyagi N. Givvimani S. Qipshidze N. Kundu S. Kapoor S. Vacek JC. Tyagi SC. Hydrogen sulfide mitigates matrix metalloproteinase-9 activity and neurovascular permeability in hyperhomocysteinemic mice. Neurochem Int. 2010;56:301–307. doi: 10.1016/j.neuint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ubuka T. Assay methods and biological roles of labile sulfur in animal tissues. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:227–249. doi: 10.1016/s1570-0232(02)00623-2. [DOI] [PubMed] [Google Scholar]
- 207.Uthus EO. Brown-Borg HM. Methionine flux to transsulfuration is enhanced in the long living Ames dwarf mouse. Mech Ageing Dev. 2006;127:444–450. doi: 10.1016/j.mad.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Viscomi C. Burlina AB. Dweikat I. Savoiardo M. Lamperti C. Hildebrandt T. Tiranti V. Zeviani M. Combined treatment with oral metronidazole and N-acetylcysteine is effective in ethylmalonic encephalopathy. Nat Med. 2010;16:869–871. doi: 10.1038/nm.2188. [DOI] [PubMed] [Google Scholar]
- 209.Wakabayashi N. Slocum SL. Skoko JJ. Shin S. Kensler TW. When NRF2 talks, who's listening? Antioxid Redox Signal. 2010;13:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wallace JL. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol Sci. 2007;28:501–505. doi: 10.1016/j.tips.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 211.Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: Why doesn't the stomach digest itself? Physiol Rev. 2008;88:1547–1565. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]
- 212.Wallace JL. Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract. Antioxid Redox Signal. 2010;12:1125–1133. doi: 10.1089/ars.2009.2900. [DOI] [PubMed] [Google Scholar]
- 213.Wallace JL. Vong L. McKnight W. Dicay M. Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137:569–578. doi: 10.1053/j.gastro.2009.04.012. 578 e1. [DOI] [PubMed] [Google Scholar]
- 214.Wang M. Li K. Zhu RR. Cheng LL. Wu QS. Wang SL. The protective function of hydrogen sulfide for lysozyme against riboflavin-sensitized photo-oxidation. J Photochem Photobiol B-Biol. 2011;103:186–191. doi: 10.1016/j.jphotobiol.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 215.Wang MJ. Cai WJ. Li N. Ding YJ. Chen Y. Zhu YC. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal. 2010;12:1065–1077. doi: 10.1089/ars.2009.2945. [DOI] [PubMed] [Google Scholar]
- 216.Wang R. Two's company, three's a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 217.Watanabe A. Okada K. Shimizu Y. Wakabayashi H. Higuchi K. Niiya K. Kuwabara Y. Yasuyama T. Ito H. Tsukishiro T. Kondoh Y. Emi N. Kohri H. Nutritional therapy of chronic hepatitis by whey protein (non-heated) J Med. 2000;31:283–302. [PubMed] [Google Scholar]
- 218.Westrop GD. Georg I. Coombs GH. The mercaptopyruvate sulfurtransferase of Trichomonas vaginalis links cysteine catabolism to the production of thioredoxin persulfide. J Biol Chem. 2009;284:33485–33494. doi: 10.1074/jbc.M109.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Whiteman M. Armstrong JS. Chu SH. Jia-Ling S. Wong BS. Cheung NS. Halliwell B. Moore PK. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite 'scavenger'? J Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 220.Whiteman M. Cheung NS. Zhu YZ. Chu SH. Siau JL. Wong BS. Armstrong JS. Moore PK. Hydrogen sulphide: A novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys Res Commun. 2005;326:794–798. doi: 10.1016/j.bbrc.2004.11.110. [DOI] [PubMed] [Google Scholar]
- 221.Whiteman M. Gooding KM. Whatmore JL. Ball CI. Mawson D. Skinner K. Tooke JE. Shore AC. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53:1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]
- 222.Whiteman M. Li L. Rose P. Tan CH. Parkinson DB. Moore PK. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid Redox Signal. 2010;12:1147–1154. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wondrak GT. Redox-directed cancer therapeutics: Molecular mechanisms and opportunities. Antioxid Redox Signal. 2009;11:3013–3069. doi: 10.1089/ars.2009.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wood JL. Sulfane sulfur. Methods Enzymol. 1987;143:25–29. doi: 10.1016/0076-6879(87)43009-7. [DOI] [PubMed] [Google Scholar]
- 225.Wrobel M. Lewandowska I. Bronowicka-Adamska P. Paszewski A. The level of sulfane sulfur in the fungus Aspergillus nidulans wild type and mutant strains. Amino Acids. 2009;37:565–571. doi: 10.1007/s00726-008-0175-x. [DOI] [PubMed] [Google Scholar]
- 226.Wu CC. Lii CK. Tsai SJ. Sheen LY. Diallyl trisulfide modulates cell viability and the antioxidation and detoxification systems of rat primary hepatocytes. J Nutr. 2004;134:724–728. doi: 10.1093/jn/134.4.724. [DOI] [PubMed] [Google Scholar]
- 227.Wu PP. Liu KC. Huang WW. Chueh FS. Ko YC. Chiu TH. Lin JP. Kuo JH. Yang JS. Chung JG. Diallyl trisulfide (DATS) inhibits mouse colon tumor in mouse CT-26 cells allograft model in vivo. Phytomedicine. 2011;18:672–676. doi: 10.1016/j.phymed.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 228.Wu XJ. Hu Y. Lamy E. Mersch-Sundermann V. Apoptosis induction in human lung adenocarcinoma cells by oil-soluble allyl sulfides: Triggers, pathways, and modulators. Environ Mol Mutagen. 2009;50:266–275. doi: 10.1002/em.20467. [DOI] [PubMed] [Google Scholar]
- 229.Wu XJ. Hua X. Targeting ROS: Selective killing of cancer cells by a cruciferous vegetable derived pro-oxidant compound. Cancer Biol Ther. 2007;6:646–647. doi: 10.4161/cbt.6.5.4092. [DOI] [PubMed] [Google Scholar]
- 230.Xia M. Chen L. Muh RW. Li PL. Li NJ. Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J Pharmacol Exp Therap. 2009;329:1056–1062. doi: 10.1124/jpet.108.149963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Xiao D. Choi S. Johnson DE. Vogel VG. Johnson CS. Trump DL. Lee YJ. Singh SV. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–5606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 232.Xiao D. Lew KL. Kim YA. Zeng Y. Hahm ER. Dhir R. Singh SV. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res. 2006;12:6836–6843. doi: 10.1158/1078-0432.CCR-06-1273. [DOI] [PubMed] [Google Scholar]
- 233.Xiao D. Pinto JT. Gundersen GG. Weinstein IB. Effects of a series of organosulfur compounds on mitotic arrest and induction of apoptosis in colon cancer cells. Mol Cancer Ther. 2005;4:1388–1398. doi: 10.1158/1535-7163.MCT-05-0152. [DOI] [PubMed] [Google Scholar]
- 234.Xiao D. Pinto JT. Soh JW. Deguchi A. Gundersen GG. Palazzo AF. Yoon JT. Shirin H. Weinstein IB. Induction of apoptosis by the garlic-derived compound S-allylmercaptocysteine (SAMC) is associated with microtubule depolymerization and c-Jun NH(2)-terminal kinase 1 activation. Cancer Res. 2003;63:6825–6837. [PubMed] [Google Scholar]
- 235.Xiao D. Powolny AA. Antosiewicz J. Hahm ER. Bommareddy A. Zeng Y. Desai D. Amin S. Herman-Antosiewicz A. Singh SV. Cellular responses to cancer chemopreventive agent D,L-sulforaphane in human prostate cancer cells are initiated by mitochondrial reactive oxygen species. Pharm Res. 2009;26:1729–1738. doi: 10.1007/s11095-009-9883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Xiao D. Singh SV. Diallyl trisulfide, a constituent of processed garlic, inactivates Akt to trigger mitochondrial translocation of BAD and caspase-mediated apoptosis in human prostate cancer cells. Carcinogenesis. 2006;27:533–540. doi: 10.1093/carcin/bgi228. [DOI] [PubMed] [Google Scholar]
- 237.Xiao D. Zeng Y. Hahm ER. Kim YA. Ramalingam S. Singh SV. Diallyl trisulfide selectively causes Bax- and Bak-mediated apoptosis in human lung cancer cells. Environ Mol Mutagen. 2009;50:201–212. doi: 10.1002/em.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yang G. Hydrogen sulfide in cell survival: A double-edged sword. Expert Rev Clin Pharmacol. 2011;4:33–47. doi: 10.1586/ecp.10.131. [DOI] [PubMed] [Google Scholar]
- 239.Yang G. Wu L. Jiang B. Yang W. Qi J. Cao K. Meng Q. Mustafa AK. Mu W. Zhang S. Snyder SH. Wang R. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yonezawa D. Sekiguchi F. Miyamoto M. Taniguchi E. Honjo M. Masuko T. Nishikawa H. Kawabata A. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology. 2007;241:11–18. doi: 10.1016/j.tox.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 241.Yong QC. Lee SW. Foo CS. Neo KL. Chen X. Bian JS. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am J Physiol Heart Circ Physiol. 2008;295:H1330–H1340. doi: 10.1152/ajpheart.00244.2008. [DOI] [PubMed] [Google Scholar]
- 242.Yusof M. Kamada K. Kalogeris T. Gaskin FS. Korthuis RJ. Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NO- and p38 MAPK-dependent mechanism. Am J Physiol Heart Circ Physiol. 2009;296:H868–876. doi: 10.1152/ajpheart.01111.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zanardo RC. Brancaleone V. Distrutti E. Fiorucci S. Cirino G. Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 244.Zeng T. Zhang CL. Zhu ZP. Yu LH. Zhao XL. Xie KQ. Diallyl trisulfide (DATS) effectively attenuated oxidative stress-mediated liver injury and hepatic mitochondrial dysfunction in acute ethanol-exposed mice. Toxicology. 2008;252:86–91. doi: 10.1016/j.tox.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 245.Zhang DD. The Nrf2-Keap1-ARE signaling pathway: The regulation and dual function of Nrf2 in cancer. Antioxid Redox Signal. 2010;13:1623–1626. doi: 10.1089/ars.2010.3301. [DOI] [PubMed] [Google Scholar]
- 246.Zhang G. Feng Z. Hao T. Zhang H. Jiang Z. Effect of allitridum on the activation of T-lymphocytes. Pharmacol Clinics Chinese Materia Medica. 1995 [Google Scholar]
- 247.Zhang G. Feng Z. Hao T. Zhang H. Jiang Z. Effect of allitridum on macrophage-mediated cytotoxicity. China J Chinese Materia Medica. 1996;21:45. [PubMed] [Google Scholar]
- 248.Zhang H. Gao Y. Zhao F. Dai Z. Meng T. Tu S. Yan Y. Hydrogen sulfide reduces mRNA and protein levels of beta-site amyloid precursor protein cleaving enzyme 1 in PC12 cells. Neurochem Int. 2011;58:169–175. doi: 10.1016/j.neuint.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 249.Zhang R. Loganathan S. Humphreys I. Srivastava SK. Benzyl isothiocyanate-induced DNA damage causes G2/M cell cycle arrest and apoptosis in human pancreatic cancer cells. J Nutr. 2006;136:2728–2734. doi: 10.1093/jn/136.11.2728. [DOI] [PubMed] [Google Scholar]
- 250.Zhao ZZ. Wang Z. Li GH. Wang R. Tan JM. Cao X. Suo R. Jiang ZS. Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp Biol Med (Maywood) 2011;236:169–176. doi: 10.1258/ebm.2010.010308. [DOI] [PubMed] [Google Scholar]
- 251.Zheng GH. Li H. Fan WT. Li HQ. [Study on the long-time effect on allitridum and selenium in prevention of digestive system cancers] Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26:110–112. [PubMed] [Google Scholar]
- 252.Zhu X. Gu H. Ni X. Hydrogen sulfide in the endocrine and reproductive systems. Expert Rev Clin Pharmacol. 2011;4:75–82. doi: 10.1586/ecp.10.125. [DOI] [PubMed] [Google Scholar]