Abstract
A labeled ON parasol ganglion cell from a macaque retina was analyzed in serial, ultrathin sections. It received 13% of its input from diffuse bipolar cells. These directed a large proportion of their output to amacrine cells but received a relatively small proportion of their amacrine cell input via feedback synapses. In these respects, they were similar to the DB3 bipolar cells that make synapses onto OFF parasol cells. Bipolar cell axons that contacted the ON parasol cell in stratum 4 of the inner plexiform layer always made synapses onto the dendrite, and therefore, the number of bipolar cell synapses onto these ganglion cells could be estimated reliably by light microscopy in the future. Amacrine cells provided the majority of inputs to the ON parasol cell. Only a few of the presynaptic amacrine cell processes received inputs from the same bipolar cells as the parasol cells, and most of the presynaptic amacrine cell processes did not receive any inputs at all within the series. These findings suggest that most of the inhibitory input to the ON parasol cell originates from other areas of the retina. Amacrine cells presynaptic to the parasol ganglion cell interacted very infrequently with other neurons in the circuit, and therefore, they would be expected to act independently, for the most part.
Keywords: Magnocellular, Amacrine, Bipolar, Electron microscopy, Intracellular injection
Introduction
Parasol cells are one of the major types of ganglion cells in the primate retina (Watanabe & Rodieck, 1989). Parasol cells with dendrites in the proximal half of the inner plexiform layer (IPL) have ON responses, excited by increments in light intensity in the centers of their receptive fields and inhibited by that stimulus in the surrounding area; parasol cells branching in the distal half of the IPL have the opposite, or OFF, responses (Dacey & Lee, 1994). Parasol cells are more sensitive to luminance contrast than the other major types of ganglion cells, and they respond more transiently at a given ambient light intensity (Kaplan et al., 1990). They also have a greater absolute sensitivity to light than midget cells because they receive more input from the rod pathway (Lee et al., 1997).
Many of the differences between the light responses of parasol cells and the other major types of primate ganglion cells are attributable to the local circuit neurons that provide their input. There are no detectable differences in the responses of midget and parasol ganglion cells to bath applied amino acid neurotransmitters (Zhou et al., 1994). The distributions of glycine and GABAA receptors on midget and parasol cells are also very similar (Grünert & Ghosh, 1999; Grünert, 2000; Macri et al., 2000). Like midget ganglion cells, midget bipolar cells have red–green color-opponent responses, and they have relatively small receptive-field centers (Dacey, 1999; Martin et al., 2001). Their receptive-field surrounds are the same size as those of horizontal cells, a finding suggesting that the surrounds might originate in the outer plexiform layer (OPL). Diffuse bipolar cells have larger receptive-field surrounds, and this finding suggests that they receive a contribution from amacrine cells in the IPL (Dacey et al., 2000). DB3 diffuse bipolar cells provide a major, excitatory input to OFF parasol cells (Calkins, 1999; Jacoby et al., 2000). Their homologues in the ground squirrel retina have glutamate receptors that recover rapidly from desensitization and reliably signal transient components of the cone responses (DeVries, 2000). If the same were true in primate retinas, bipolar cell input would account for some of the temporal characteristics of the responses of OFF parasol cells to light. The light responses of blue cone bipolar cells have never been recorded, but there is indirect evidence that they are depolarized by short-wavelength stimuli and make excitatory synapses onto small bistratified ganglion cells (Dacey, 2000; Vardi et al., 2000; Calkins, 2001).
The pathways providing input to the three types of ganglion cells also differ in the prevalence of gap junctions. The DB3 bipolar cells make extensive, homologous gap junctions, which may increase the ratio of signal to noise in the pathway (Jacoby & Marshak, 2000). ON parasol cells make gap junctions with amacrine cells (Jacoby et al., 1996), and these may enhance their responses to luminance contrast and promote synchronous firing (Kenyon & Marshak, 1998). Small bistratified ganglion cells are also tracer coupled to amacrine cells (Dacey, 1993). However, midget bipolar cells are not likely to be electrically coupled because their axons do not contact one another, and their dendrites receive inputs from different cones (Wässle et al., 1994). Midget ganglion cells are not tracer coupled to any other cells (Dacey & Brace, 1992).
The bipolar cells that provide excitatory input to the ON parasol cells have not been described, however. The amacrine cells presynaptic to midget ganglion cells engage in many local synaptic interactions (Calkins & Sterling, 1996), but it is uncertain whether the amacrine cells presynaptic to parasol ganglion cells have a similar pattern of connections. This is important because more than 80% of the input to ON parasol cells in peripheral retina comes from amacrine cells (Jacoby et al., 1996). To address these questions, we analyzed serial, ultrathin sections through a Neurobiotin injected ON parasol cell from a macaque retina.
Methods
A 20-year-old female macaque (Macaca mulatta) was overdosed with sodium pentobarbital (50–100 mg/kg, IV) by another investigator at the conclusion of experiments that did not involve the eyes. The University of Texas Health Science Center Animal Care and Use Committee approved this protocol. An enucleated eye was transported to the laboratory on ice, hemisected, and put into oxygenated Ames medium (Sigma, St. Louis, MO) at room temperature. The vitreous humor was removed with fine forceps, and the procedure for intracellular injection was the same as described previously (Jacoby et al., 2000). The retina was then mounted on a Zeiss Standard upright, fixed-stage microscope with a 30× long-working-distance objective and treated with acridine orange (4 min, 10 µM). Microelectrodes made from thin-walled borosilicate glass (50–100 MΩ) were filled with 2.5% Lucifer yellow (Molecular Probes, Eugene, OR) and 5% Neurobiotin (Vector Laboratories, Burlingame, CA) in 20 mM 3-[N-morpholino] propanesulfonic acid (MOPS, Sigma), pH 7.6.Alarge retinal ganglion cell located approximately 8 mm from the fovea was injected with Lucifer yellow for 1–2 min with 2 Hz, 5–10 nA of negative current until it was confirmed to be a parasol cell, and then it was filled with Neurobiotin using positive current of the same amplitude, frequency, and duration for 5 min.
The tissue was immersion fixed in 0.5% glutaraldehyde and 2% parformaldehyde in 0.1 M sodium phosphate buffer (PB), pH 7.4 for 1 h at 37°C. After rinsing with phosphate buffered saline (PBS), the tissue was treated with 1% sodium borohydride in PBS for 1 h followed by PBS rinses and an ascending and descending series of graded ethanol solutions in PBS (10 min each in 10, 25, and 40%; 30 min in 50%; 10 min each in 40, 25, and 10%). The tissue was treated with avidin-biotin peroxidase (1:100 overnight at 4°C, Vector), and the labeled cells were visualized using the silver-enhanced diaminobenzidine sulfide procedure (Smiley & Goldman-Rakic, 1993), The tissue was then treated with 1% OsO4 for 1 h rinsed in PBS, embedded in Epon, and mounted on a glass slide.
The Neurobiotin injected cell was identified as a parasol cell based on the sizes of its soma and dendritic tree and by its relatively dense arborization pattern (Fig. 1). It was photographed, reembedded, and sectioned at approximately 100 nm with a Reichert Ultracut E ultramicrotome. Sections were collected on single hole, formvar-coated grids and then stained with uranyl acetate (2% in 50% methanol) and lead citrate (2% aqueous, 1 min). Labeled parasol cell dendrites were photographed using a rotating goniometer stage on a JEOL 100 CX electron microscope. Labeled dendrites were photographed at 2000× and photomontages were constructed to measure the depth at which the dendrites ramified in the IPL. A total of 458 serial sections through 28 segments of labeled dendrites were photographed at 10,000×. Two segments were from primary dendrites, and the rest were from distal dendrites located at a depth of 65% in the IPL. Selected areas were rephotographed at 20,000× to produce some of the figures.
Fig. 1.
Photomicrographs of the labeled ON parasol cell after processing for electron microscopy. Because the retina was not perfectly flat after embedding, the dendrites on the left (A) are in a slightly different plane of focus than the dendrites on the right (B). The boxes indicate an estimate of the area sampled in this study. The axon is shown in C. Calibration bar is 50 µm.
Results and discussion
In all, 134 chemical synapses onto the labeled ON parasol ganglion cell dendrites were analyzed. Of these, 18 (13.9%) were from bipolar cell axon terminals like the synapse onto a labeled dendrite shown in Fig. 2. The majority of inputs, 116 (86.5%), were from amacrine cell processes. This is the same as the average value reported in an earlier study of five ON parasol cells from peripheral baboon retinas (Jacoby et al., 1996). However, the proportion of input from amacrine cells to an OFF parasol cell reconstructed from serial sections of the macaque parafovea is much lower, only 45% (Calkins, 1999). Although it is not possible to rule out differences between the ON and OFF pathways, the most plausible explanation is that this is due to a difference between central and peripheral retina. The other two types of primate ganglion cells studied to date also have higher proportions of amacrine cell input in the peripheral retina. Small bistratified cells receive only 30% of their input from amacrine cells in the parafovea (Calkins et al., 1998), but in the peripheral retina they receive approximately 80% of their input from amacrine cells (Ghosh & Grünert, 1999). Midget ganglion cells receive approximately half of their input bipolar cells in the parafovea (Kolb & DeKorver, 1991; Calkins et al., 1994), but a much larger proportion of their input is from amacrine cells in the peripheral retina (Kolb et al., 1998). Although the proportion of bipolar cell input decreases in periphery, the same types of bipolar cells are presynaptic to each type of ganglion cell throughout the retina.
Fig. 2.
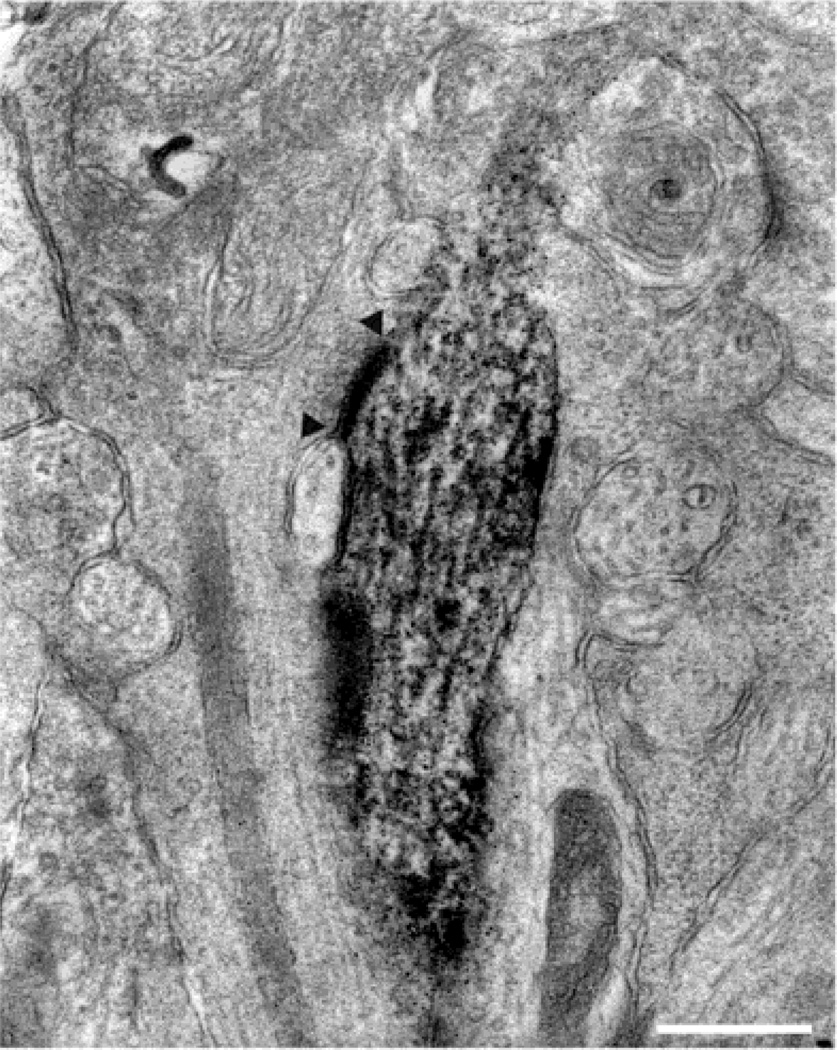
A ribbon synapse (arrow) from a diffuse bipolar cell axon onto a labeled ON parasol ganglion cell dendrite and an amacrine cell process. This was the most common type of dyad synapse made by these bipolar cells. The amacrine cell process also makes a feedback synapse onto the bipolar cell axon (arrowheads). Calibration bar is 0.5 µm.
All of the bipolar cell axons that contacted the distal ON parasol cell dendrites made synapses on those dendrites, but only two of the four bipolar cell axons in contact with the primary dendrite made synapses. Therefore, it would be possible to reliably estimate the numbers of synapses from bipolar cells onto ON parasol cell dendrites in stratum 4 of the IPL by light microscopy using double-labeled material. The same is true of amacrine cells presynaptic to midget ganglion cells in the macaque parafovea. There, all the bipolar cells in contact with these amacrine cells make synapses onto them (Calkins & Sterling, 1996). On the other hand, the blue cone bipolar cells in the parafovea are highly selective, contacting only small bistratified and not other types of ganglion cells (Calkins et al., 1998). Only 25% of the amacrine cell processes that contacted the distal parasol cell dendrites made synapses within the volume of tissue analyzed. The percentage of contacts from amacrine cell processes onto primary dendrites that resulted in synapses was even lower, 16%.
Sixteen axon terminals of diffuse bipolar cells presynaptic to the labeled ganglion cell dendrites were followed through serial sections. In all, they made 25 ribbon synapses, with two postsynaptic processes, or dyads. Of these, 18 (72%) consisted of an amacrine cell and a ganglion cell (Fig. 1) and 4 (16%) of two amacrine cells. There were six monads, 3 with the ganglion cell dendrite and three with amacrine cell processes. Thus, 52% of their output was directed to amacrine cells and 48% to ganglion cells. The bipolar cells received four synapses from amacrine cells that received input from the bipolar cells and also from nine amacrine cells that did not receive input from the bipolar cell within the series. Thus, feedback synapses accounted for 31% of the amacrine cell input and feedforward synapses 69%. Based on their level of stratification, these inputs to the ON parasol cell are expected to be from diffuse bipolar cells DB4, DB5, or a combination of the two (Boycott & Wässle, 1991). The synaptic connections of the axon terminals of these bipolar cells have not yet been described, however. For comparison, the values of these parameters for the other types of primate bipolar cells are listed in Table 1.
Table 1.
Synaptic connections of primate bipolar cellsa
| This study | DB3 | Midget | Blue cone | |
|---|---|---|---|---|
| Dyad composition | ||||
| AG % | 72 | 61 | 88 | 65 |
| AA % | 16 | 31 | 9 | 0 |
| GG % | 12 | 8 | 3 | 35 |
| % Output to amacrine cells | 52 | 61 | 50 | 40 |
| % Input via feedback | 31 | 25 | 73 | 35 |
Dyads are ribbon synapses with two postsynaptic elements, either amacrine cell processes (A) ganglion cell dendrites (G) or both. Output synapses include 25 dyads and 6 monads, ribbon synapses with a single postsynaptic element. Feedback synapses are those made by amacrine cell processes onto the bipolar cells that provided their input. Data on DB3 diffuse bipolar cells are from Jacoby and Marshak, 2000. Data on midget bipolar cells are from Calkins and Sterling, 1996. Data on blue cone bipolar cells are from Calkins et al., 1998 and Calkins, personal communication.
The bipolar cells presynaptic to the labeled parasol ganglion cell dendrites were similar in some respects to the DB3 bipolar cells, which provide the major bipolar cell input to OFF parasol cells (Calkins, 1999; Jacoby et al., 2000). Both types directed the majority of their output to amacrine cells, and both had a relatively small amount of their input from amacrine cells via feedback synapses. The composition of the dyads was also very similar (Jacoby & Marshak, 2000). The midget bipolar cells directed 50% of their output to amacrine cells, and they received the majority of their amacrine cell input via feedback synapses (Calkins et al., 1994; Calkins & Sterling, 1996). These findings suggest that many of the inhibitory inputs from amacrine cells to midget bipolar cells originate from the same area of the retina as their excitatory inputs, but the majority of inhibitory inputs to diffuse bipolar cells are from other areas. The same is true of blue cone bipolar cells, but they are different from the diffuse bipolar cells in dyad composition and in the percentage of their output directed to amacrine cells (Calkins et al., 1998).
Twelve amacrine cell processes that shared dyad synapses with the labeled parasol ganglion cell dendrites were analyzed. Of these, two (17%) made feedback synapses onto the bipolar cell, and one (8.3%) inhibited the ganglion cell, as well. In all, four (33%) made synapses onto the ganglion cell. One (8.3%) of the amacrine cell processes at a dyad with the parasol cell made synapses onto other amacrine cells, but most did not make or receive synapses within the series. The proportion of amacrine cell processes at dyads with midget ganglion cells that make feedforward synapses, 63%, is higher than that for parasol cells, and so is the percentage that also feed back to midget ganglion cells, 52% (Calkins & Sterling, 1996). At dyads with small bistratified ganglion cells, only 15% of amacrine cell processes make feedforward synapses. Feedback synapses are more common, however; 60% of the amacrine cell processes at dyads of blue cone bipolar cells make feedback synapses (Calkins et al., 1998).
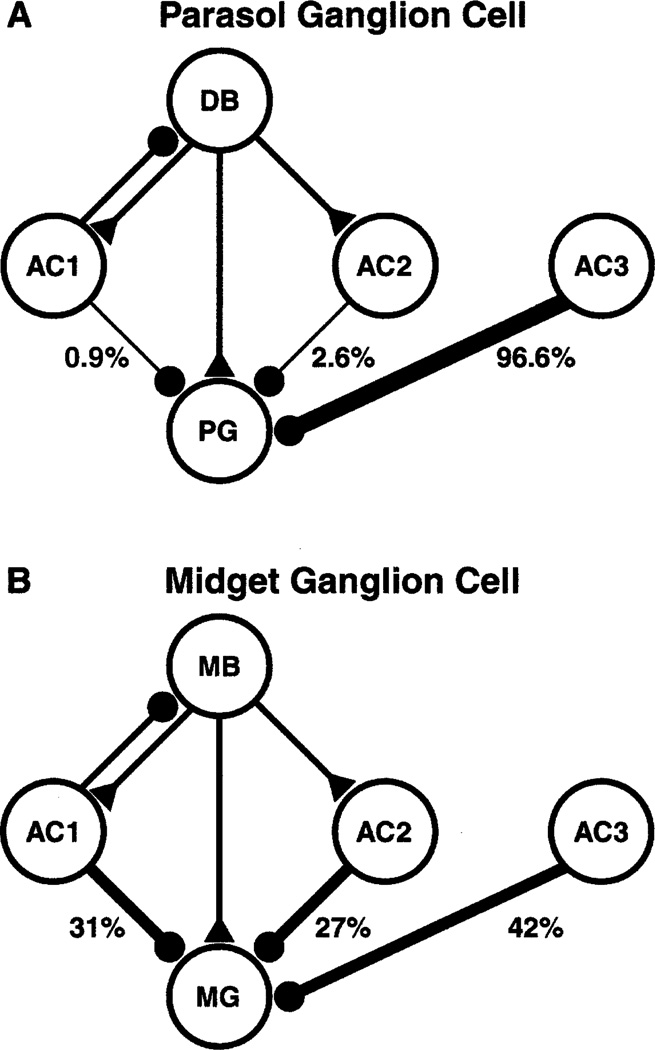
The amacrine cell processes at dyads provided only a small proportion of the input to the parasol cell. The vast majority of the amacrine cell processes that made synapses onto the ON parasol cell, 111 of 116 or 96%, did not receive input from the same bipolar cell. Thus, the ON parasol ganglion cell was similar to the diffuse bipolar cells that provided its input, receiving most of its inhibitory input from other areas of the retina. In this respect, the ON parasol cell was quite different from midget ganglion cells (Fig. 3). Most of the amacrine cells presynaptic to a midget ganglion cell get input from the same midget bipolar cell, and all the presynaptic amacrine cells get input from every bipolar cell within their dendritic fields (Calkins & Sterling, 1996).
Fig. 3.
Inputs to parasol (A) and midget (B) ganglion cells. A: The parasol ganglion cell (PG) receives excitatory input (triangle) from a diffuse bipolar cell (DB) and inhibitory input (circles) from three types of amacrine cells (AC). The frequencies of each type of input are indicated below each amacrine cell. AC1 receives input from the same diffuse bipolar cell as the parasol cell and it also makes an inhibitory feedback synapse onto that bipolar cell. AC2 receives input from the same diffuse bipolar cell but does not feed back. AC3 receives input from a different bipolar cell; the major inhibitory input to the parasol cell, by far, is from this type of amacrine cell. B: The midget ganglion cell (MG) receives input from a midget bipolar cell (MB); otherwise the abbreviations are the same as in A. Note that the majority of the inhibitory input to the midget ganglion cell comes from AC1 and AC2, the amacrine cells that receive input from the same midget bipolar cell providing its excitatory input. Data in A are from this study, and the data in B are from Calkins and Sterling, 1996.
Some of the inputs from amacrine cells in the sample were located on or near the bases of dendritic spines, as illustrated in Fig. 4. However, there was only one synapse on the spine, itself. Most of the amacrine cell processes that made synapses onto the labeled ON parasol cell, 92 of 116 (79%), did not receive any synapses within the series. Although 20 of these made synapses onto other processes within the series, none of the 92 contacted the bipolar cells presynaptic to the labeled parasol cell, and only one made a synapse onto an amacrine cell that, in turn, made a synapse onto the labeled parasol ganglion cell.
Fig. 4.
Synapse from an amacrine cell process onto a labeled ON parasol ganglion cell dendrite with a spine. There was only one synapse onto a spine in the sample, but synapses like this one at the base of the spine were common. Calibration bar is 1.0 µm.
In some instances, the amacrine cells presynaptic to the labeled ON parasol cell could be further subdivided according to their ultrastructure. Some were relatively electron lucent, filled with microtubules and had very few synaptic vesicles (Fig. 5). A longitudinal section through this type of amacrine cell process is illustrated in Fig. 6. This ultrastructure and pattern of synaptic connections suggests that these processes originate from widefield amacrine cells (Kolb & Nelson, 1993). These amacrine cells have large perikarya, and GABAergic amacrine cell perikarya are larger than the other types (Koontz et al., 1993). Synapses from GABAergic amacrine cells to ganglion cells have their highest density at the same level in the IPL that ON parasol cells ramify (Koontz & Hendrickson, 1990). These wide-field amacrine cells are expected to fire action potentials and make an important contribution to the receptive-field surrounds, as they do in other mammalian retinas (Taylor, 1999; Demb et al., 1999, 2001; Flores-Herr et al., 2001). One candidate is the large, tracer-coupled amacrine cell that contains cholecystokinin and costratifies with the ON parasol cell (Jacoby et al., 1996).
Fig. 5.
Synapses (arrowheads) from two different types of amacrine cell processes onto a labeled ON parasol ganglion cell dendrite. The process on the left is relatively electron lucent; it has numerous microtubules but very few vesicles. The process at the right is larger, more electron dense, and filled with synaptic vesicles. Calibration bar is 1.0 µm.
Fig. 6.
Synapse (arrowheads) from a microtubule filled amacrine cell process onto a labeled ON parasol ganglion cell dendrite. Based on its ultrastructure, this process is likely to originate from a wide-field amacrine cell. Calibration bar is 0.5 µm.
Other presynaptic amacrine cell processes were filled with synaptic vesicles and mitochondria, and they were concave at the point where they contacted the labeled ganglion cell dendrite. An example of this type of amacrine cell process is illustrated in Fig. 7. This ultrastructure is characteristic of the processes of cholinergic displaced amacrine cells (Yamada et al., 1998), which co-stratify with the ON parasol cells and make extensive contacts. Based on physiological experiments in other mammals, these cholinergic cells are expected to excite ON parasol cells and enhance their responses to rapidly changing stimuli (Jacoby et al., 1996). Other amacrine cell processes were also filled with synaptic vesicles, but they were more electron dense and made flat synapses (Fig. 8). These may have originated from a smaller, bistratified amacrine cell containing cholecystokinin that also co-stratifies with parasol cells (Marshak et al., 1990). These resemble the DAPI-3 amacrine cells of rabbit retinas, which have ON responses to light (Bloomfield, 1992) and use the neurotransmitter glycine (Wright et al., 1997). It was not possible to unequivocally classify all the presynaptic amacrine cells into one of these three types based on ultrastructural criteria.
Fig. 7.
Synapse (arrowheads) from a vesicle filled amacrine cell process onto a labeled ON parasol ganglion cell dendrite. Processes like these that are indented by the postsynaptic ganglion cell dendrite are typical of those of cholinergic amacrine cells. Calibration bar is 0.5 µm.
Fig. 8.
Synapse from an electron dense amacrine cell process onto a labeled ON parasol ganglion cell dendrite. This amacrine cell process was more electron dense than those of cholinergic amacrine cells and not indented at the synapse with the ganglion cell dendrite. Calibration bar is 0.5 µm.
Interactions between the amacrine cells presynaptic to the ON parasol cell dendrite were quite rare. There was only one instance of synapses between two amacrine cell processes presynaptic to the ganglion cell dendrite. This finding suggests that the amacrine cells presynaptic to ON parasol ganglion cells do not engage in many local synaptic interactions and, therefore, would be expected to act independently, for the most part. In contrast, the amacrine cells presynaptic to midget ganglion cells frequently contact one another (Calkins & Sterling, 1996). Taken together, these results suggest that the surrounds of midget cells would be strongly influenced by central stimulation, but the surrounds of parasol cells would be relatively unaffected.
Acknowledgments
We wish to thank Lillemor Krosby, Jerry Ebalunode, and Sadia Saleem for excellent technical assistance and Dr. David Calkins for permission to cite his unpublished data in Table 1. This research was supported by Grant EY 06472 and Core Grant EY10608 from the National Eye Institute.
References
- Bloomfield SA. Relationship between receptive and dendritic field size of amacrine cells in the rabbit retina. Journal of Neurophysiology. 1992;68:711–725. doi: 10.1152/jn.1992.68.3.711. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Wässle H. Morphological classification of bipolar cells of the primate retina. European Journal of Neuroscience. 1991;3:1069–1088. doi: 10.1111/j.1460-9568.1991.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Calkins DJ. Synaptic organization of cone pathways in the primate retina. In: Gegenfurtner KR, Sharpe LT, editors. Color Vision: From Genes to Perception. Cambridge: United Kingdom; 1999. pp. 163–179. [Google Scholar]
- Calkins DJ. Seeing with S cones. Progress in Retinal and Eye Research. 2001;20:255–287. doi: 10.1016/s1350-9462(00)00026-4. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Sterling P. Absence of spectrally specific lateral inputs to midget ganglion cells in primate retina. Nature. 1996;381:613–615. doi: 10.1038/381613a0. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Schein SJ, Tsukamoto Y, Sterling P. M and L cones macaque fovea connect to midget ganglion cells by different numbers of excitatory synapses. Nature. 1994;371:70–72. doi: 10.1038/371070a0. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Tsukamoto Y, Sterling P. Microcircuitry and mosaic of a blue–yellow ganglion cell in the primate retina. Journal of Neuroscience. 1998;18:3373–3385. doi: 10.1523/JNEUROSCI.18-09-03373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM. Morphology of a small-field bistratified ganglion cell type in the macaque and human retina. Visual Neuroscience. 1993;10:1081–1098. doi: 10.1017/s0952523800010191. [DOI] [PubMed] [Google Scholar]
- Dacey DM. Primate retina: Cell types, circuits and color opponency. Progress in Retinal and Eye Research. 1999;18:737–763. doi: 10.1016/s1350-9462(98)00013-5. [DOI] [PubMed] [Google Scholar]
- Dacey DM. Parallel pathways for spectral coding in primate retina. Annual Review of Neuroscience. 2000;23:743–775. doi: 10.1146/annurev.neuro.23.1.743. [DOI] [PubMed] [Google Scholar]
- Dacey D, Packer OS, Diller L, Brainard D, Peterson B, Lee B. Center surround receptive field structure of cone bipolar cells in primate retina. Vision Research. 2000;40:1801–1811. doi: 10.1016/s0042-6989(00)00039-0. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB. The “blue-on” opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Brace S. A coupled network for parasol but not midget ganglion cells in the female retina. Visual Neuroscience. 1992;9:279–290. doi: 10.1017/s0952523800010695. [DOI] [PubMed] [Google Scholar]
- Demb JB, Haarsma L, Freed MA, Sterling P. Functional circuitry of the retinal ganglion cell’s nonlinear receptive field. Journal of Neuroscience. 1999;19:9756–9767. doi: 10.1523/JNEUROSCI.19-22-09756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Zaghloul K, Haarsma L, Sterling P. Bipolar cells contribute to nonlinear spatial summation in the brisk-transient (Y) ganglion cell in mammalian retina. Journal of Neuroscience. 2001;21:7447–7454. doi: 10.1523/JNEUROSCI.21-19-07447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- Flores-Herr N, Protti DA, Wässle H. Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina. Journal of Neuroscience. 2001;21:4852–4863. doi: 10.1523/JNEUROSCI.21-13-04852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Grünert U. Synaptic input to small bistratified (blue-on) ganglion cells in the retina of a new world monkey, the marmoset callithrix jacchus. Journal of Comparative Neurology. 1999;413:417–428. doi: 10.1002/(sici)1096-9861(19991025)413:3<417::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Grünert U, Ghosh KK. Midget parasol ganglion cells of the primate retina express the α1 subunit of the glycine receptor. Visual Neuroscience. 1999;16:957–966. doi: 10.1017/s0952523899165155. [DOI] [PubMed] [Google Scholar]
- Grünert U. Distribution of GABA and glycine receptors on bipolar and ganglion cells in the mammalian retina. Microscopy Research and Technique. 2000;50:130–140. doi: 10.1002/1097-0029(20000715)50:2<130::AID-JEMT5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Jacoby RA, Marshak DW. Synaptic connections of DB3 diffuse bipolar cell axons in macaque retina. Journal of Comparative Neurology. 2000;416:19–29. doi: 10.1002/(sici)1096-9861(20000103)416:1<19::aid-cne3>3.0.co;2-h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby RA, Stafford D, Kouyama N, Marshak D. Synaptic inputs to ON parasol ganglion cells in the primate retina. Journal of Neuroscience. 1996;16:8041–8056. doi: 10.1523/JNEUROSCI.16-24-08041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby RA, Wiechmann AF, Amara SG, Leighton BH, Marshak DW. Diffuse bipolar cells provide input to OFF parasol ganglion cells in the macaque retina. Journal of Comparative Neurology. 2000;416:6–18. doi: 10.1002/(sici)1096-9861(20000103)416:1<6::aid-cne2>3.0.co;2-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Lee BB, Shapley RM. New views of primate retinal function. Progress in Retinal Research. 1990;9:273–336. [Google Scholar]
- Kenyon GT, Marshak DW. Gap junctions with amacrine cells provide a feedback pathway for ganglion cells within the retina. Proceedings of the Royal Society B (London) 1998;265:919–925. doi: 10.1098/rspb.1998.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H, Dekorver L. Midget ganglion cells of the parafovea of the human retina: A study by electron microscopy and serial section reconstruction. Journal of Comparative Neurology. 1991;303:617–636. doi: 10.1002/cne.903030408. [DOI] [PubMed] [Google Scholar]
- Kolb H, Nelson R. OFF-alpha and OFF-beta ganglion cells in cat retina: II. Neural circuit as revealed by electron microscopy of HRP stains. Journal of Comparative Neurology. 1993;329:85–110. doi: 10.1002/cne.903290107. [DOI] [PubMed] [Google Scholar]
- Kolb H, Dekorver L, Church J, Crooks J, Jacoby R, Marshak D. P cells of the primate retina. Investigative Ophthalmology and Visual Sciences. 1998;39:S565. [Google Scholar]
- Koontz MA, Hendrickson AE. Distribution of GABA-immunoreactive amacrine cell synapses in the inner plexiform layer of macaque monkey retina. Visual Neuroscience. 1990;5:17–28. doi: 10.1017/s0952523800000043. [DOI] [PubMed] [Google Scholar]
- Koontz MA, Hendrickson LE, Brace ST, Hendrickson AE. Immunocytochemical localization of GABA and glycine in amacrine and displaced amacrine cells of macaque monkey retina. Vision Research. 1993;18:2617–2628. doi: 10.1016/0042-6989(93)90220-q. [DOI] [PubMed] [Google Scholar]
- Lee BB, Smith VC, Pokorny J, Kremers J. Rod inputs to macaque ganglion cells. Vision Research. 1997;37:2813–2828. doi: 10.1016/s0042-6989(97)00108-9. [DOI] [PubMed] [Google Scholar]
- Macri J, Martin PR, Grünert U. Distribution of the α1 subunit of the GABAA receptor on midget and parasol ganglion cells in the retina of the common marmoset Callithrix jacchus. Visual Neuroscience. 2000;17:437–448. doi: 10.1017/s0952523800173109. [DOI] [PubMed] [Google Scholar]
- Marshak DW, Aldrich LB, Del Valle J, Yamada T. Localization of immunoreactive cholecystokinin precursor to amacrine cells and bipolar cells of the macaque monkey retina. Journal of Neuroscience. 1990;10:3045–3055. doi: 10.1523/JNEUROSCI.10-09-03045.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR, Lee BB, White AJR, Solomon SG, Ruttiger L. Chromatic sensitivity of ganglion cells in the peripheral primate retina. Nature. 2001;410:933–936. doi: 10.1038/35073587. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Goldman-Rakic PS. Silver-enhanced diaminobenzidine-sulfide (SEDS): A technique for high-resolution immunoelectron microscopy demonstrated with monoamine immunoreactivity in monkey cerebral cortex and caudate. Journal of Histochemistry and Cytochemistry. 1993;41:1393–1404. doi: 10.1177/41.9.8354879. [DOI] [PubMed] [Google Scholar]
- Taylor WR. TTX attenuates surround inhibition in rabbit retinal ganglion cells. Visual Neuroscience. 1999;16:285–290. doi: 10.1017/s0952523899162096. [DOI] [PubMed] [Google Scholar]
- Vardi N, Duvoisin R, Wu G, Sterling P. Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. Journal of Comparative Neurology. 2000;423:402–412. doi: 10.1002/1096-9861(20000731)423:3<402::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Martin PR, Boycott BB. Immunocytochemical characterization and spatial distribution of midget bipolar cells in the macaque monkey retina. Vision Research. 1994;34:561–579. doi: 10.1016/0042-6989(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Rodieck RW. Parasol and midget ganglion cells of the primate retina. Journal of Comparative Neurology. 1989;289:434–454. doi: 10.1002/cne.902890308. [DOI] [PubMed] [Google Scholar]
- Wright LL, Macqueen CL, Elston GN, Young HM, Pow DV, Vaney DI. The DAPI-3 amacrine cells of the rabbit retina. Visual Neuroscience. 1997;14:473–492. doi: 10.1017/s0952523800012141. [DOI] [PubMed] [Google Scholar]
- Yamada E, Keyser K, Dimitryeva N, Lindstrom J, Marshak D. Cholinergic input to bipolar cell axon terminals in the macaque retina. Society for Neuroscience Abstracts. 1998;24:520. [Google Scholar]
- Zhou ZJ, Marshak DW, Fain GL. Amino acid receptors of midget and parasol ganglion cells in primate retina. Proceedings of the National Academy of Sciences of the USA. 1994;91:4907–4911. doi: 10.1073/pnas.91.11.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]