Abstract
High levels of endogenous cholecystokinin (CCK) are present in the rat retina (Eskay & Beinfeld, 1982), but the cellular localization and physiological actions of CCK in the rat retina are uncertain. The goals of this study were to characterize the cells containing CCK, identify cell types that interact with CCK cells, and investigate the effects of CCK on rod bipolar cells. Rat retinas were labeled with antibody to gastrin-CCK (gCCK) using standard immunofluorescence techniques. Patch-clamp methods were used to record from dissociated rod bipolar cells from rats and mice. Gastrin-CCK immunoreactive (-IR) axons were evenly distributed throughout the retina in stratum 5 of the inner plexiform layer of the rat retina. However, the gCCK-IR somata were only detected in the ganglion cell layer in the peripheral retina. The gCCK-IR cells contained glutamate decarboxylase, and some of them also contained immunoreactive substance P. Labeled axons contacted PKC-IR rod bipolar cells, and recoverin-IR ON-cone bipolar cells. CCK-octapeptide inhibits GABAC but not GABAA mediated currents in dissociated rod bipolar cells.
Keywords: Neuropeptides, GABAC receptors, Substance P, Bipolar cells, Displaced amacrine cells
Introduction
Cholecystokinin (CCK) is one of the most abundant neuropeptides in the central nervous system (Beinfeld, 1998), and CCK has been detected in retinal extracts from rats, rabbits, cows, monkeys, and humans by radioimmunoassay (Osborne et al., 1981; Eskay & Beinfeld, 1982). The levels of immunoreactive CCK in rat retinal extracts are higher than in other mammalian species, and the sulfated octapeptide is the predominant molecular form (Eskay & Beinfeld, 1982). However, the cellular localization of CCK in rat retina is uncertain. Eriksen and Larsson (1981) described very sparse labeling in the innermost stratum of the inner plexiform layer (IPL) that resembled amacrine cell processes, but they did not label the cell bodies from which they originated. Another group reported negative results in the rat retina (Osborne et al., 1982). CCK is known to have physiological effects in mammalian retinas, however. In the cat retina, iontophoretically applied CCK octapeptide inhibits both light-evoked and spontaneous activity of all briskly responding types of ganglion cells, without altering their receptive-field organization (Thier & Bolz, 1985). In the rat retina, CCK activates intracellular signaling cascades leading to rapid tyrosine phosphorylation (de la Villa et al., 2000).
The first goal of these experiments was to identify the cells that contain CCK in the rat retina. We show that gastrin-CCK-like immunoreactive (gCCK-IR) neurons are amacrine cells with somata displaced to the ganglion cell layer (GCL) of the peripheral retina and processes in the innermost stratum of the IPL. CCKcontaining neurons elsewhere in the CNS also contain conventional neurotransmitters (Beinfeld, 1998). For example, many neurons contain both dopamine and CCK (Hokfelt et al., 1980; Seroogy et al., 1989) or gamma-aminobutyric acid (GABA) and CCK (Freund et al., 1986; McDonald & Pearson, 1989; Gulyas et al., 1991). In the rat retina, dopaminergic cells are occasionally displaced to the GCL (Nguyen-Legros et al., 1981). These dopaminergic cells can be labeled with antisera to tyrosine hydroxylase (TH). Most amacrine cells are GABAergic or glycinergic and can be labeled with antisera to glutamate decarboxylase (GAD) or glycine transporter-1 (Glyt-1), respectively (Brandon, 1985; Nguyen-Legros, et al. 1997; Menger et al., 1998). The highest density of GABAergic processes is in the innermost stratum of the IPL in the rat retina (Brandon 1985; Mosinger et al., 1986). We show that gCCK-IR amacrine cells contain GAD immunoreactivity. The second goal was to identify cell types that might interact with the gCCK-IR cells. Based on their stratification, the rod bipolar cells and other neurons in the rod pathway are good candidates. In the rat retina, rod bipolar cells can be labeled with antisera to protein kinase C (PKC, Wood et al., 1988; Greferath et al., 1990), and AII amacrine cells can be labeled with antisera to parvalbumin (Sanna et al., 1990; Wässle et al., 1993). A17-like amacrine cells are not likely targets of gCCK-IR amacrine cells because the A17 amacrine cells do not contact amacrine cells in stratum 5 in cats (Nelson & Kolb, 1985) or rabbits (Sandell et al., 1989). ON-cone bipolar cells also terminate in the inner strata of the IPL; in rats, these are types 8 and 9 ON-cone bipolar cells (Euler & Wässle, 1995). The type 8 bipolar cells can be labeled with antisera to recoverin (Milam et al., 1993; Euler & Wässle, 1995). We show that gCCK-IR processes contact both rod bipolar cells and type 8 cone bipolar cells. Amacrine and ganglion cells that contain substance P are particularly good candidates to interact with the gCCK-IR cells because their processes are most abundant in the stratum where the gCCK-IR processes ramify (Eriksen & Larsson, 1981; Osborne et al., 1982; Caruso et al., 1990; Zhang & Yeh, 1992). We show that some gCCK-IR cells contact substance P-IR cells and that some gCCK-IR cells also contain substance P. The third goal was to investigate physiological actions of CCK in the retina. GABAA and GABAC receptors have been described on the rod bipolar cells of the rat (Feigenspan et al., 1993) and mouse retina (Vaquero & de la Villa, 1999). We show that CCK-8 partially inhibits GABAC-mediated currents, but not GABAA-mediated currents in dissociated rod bipolar cells.
Methods
Anatomy
Eyes from rats (Spague-Dawley or Long-Evans) were donated by other investigators following experiments that did not affect the eyes. The eyes were immersion fixed with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH = 7.4) for half an hour and then rinsed in phosphate buffered saline (PBS). For whole mounts, the retina was then isolated, and the vitreous was removed. For sections, the eyes were cryoprotected with 30% sucrose in PBS overnight. This tissue was freeze-mounted in OCT medium, 32-µm vertical sections were cut using a cryostat, and the sections were collected onto gelatin-coated glass slides.
Sections were preincubated in 1–2% normal donkey serum with 0.3% Triton X-100 for 1 h at room temperature and then incubated with primary antibody for 12–48 h in PBS with 0.3% Triton X-100 at 4°C. Primary antibodies included mouse monoclonal anti-human gastrin-CCK 1:2000 (gCCK, 9303) donated by H. Wong, University of California Los Angeles; rabbit anti-human PKC 1:1000 (AB1610, Chemicon, Temecula, CA); rabbit anti-human recoverin (320-4) 1:500 donated by A.F. Wiechmann, University of Oklahoma (Wiechmann, 1996), rabbit anti-Glyt-1 1:1000 donated by D.V. Pow, University of Queensland (Pow & Hendrickson, 1999); goat anti-parvalbumin 1:1000 (PVG-214, Swant, Bellinzona, Switzerland); rabbit anti-human GAD 65 1:1000 (AB5082, Chemicon); rabbit anti-TH 1:1000 donated by J.W. Haycock, Louisiana State University, rabbit anti-substance P 1:1000 (AB1566, Chemicon); and rat monoclonal anti-substance P 1:50 (MAS035, Accurate Chemical & Scientific Corp., Westbury, NY).
Following several rinses with PBS, the sections were incubated in affinity purified biotinylated donkey secondary antibody 1:100 (Jackson Immunoresearch Laboratories, Westgrove, PA) in PBS for 1–2 h at room temperature. Secondary antibodies labeled with indocarbocyanine (Cy-3)-streptavidin 1:100 (Jackson Immunoresearch Laboratories) or Alexa 488-streptavidin 1:2000 (Molecular Probes, Eugene, OR) in PBS were applied for 1 h at room temperature. For double labeling, the second primary antibodies (raised in different species) were incubated as before. The primary antibodies were then labeled directly with affinity-purified secondary antibodies conjugated to different fluorophores including Cy-3, indodicarbocyanine (Cy-5, Jackson Immunoresearch Laboratories) at 1:100, or Alexa 488 (Molecular Probes) at 1:500 in PBS for 1 h at room temperature. The sections were then rinsed in PBS and mounted in Vector Shield (Vector Labs, Burlingame, CA). For whole mounts, a similar procedure was used; however, incubation periods were longer. The tissue was incubated in both primary antibodies for 5–8 days and incubated in the secondary antibodies overnight.
Images were acquired using a confocal microscope (Zeiss LSM410, Thornwood, NY) with a krypton-argon laser using an oil immersion lens (63×, numerical aperture 1.4). Excitation was at 568 nm for Cy-3, 647 nm for Cy-5, and 488 nm for Alexa 488 with emission filters of 590–610 nm, 670–810 nm, and 515–540 nm, respectively. For double-labeled tissue, single optical sections (0.5-µm) were analyzed. However, stacks of 0.5 µm optical sections are illustrated whenever superposition does not create ambiguity. These digital images were processed in Adobe Photoshop (V 5.5, Adobe Systems, San Jose, CA) to enhance the color and contrast.
The statistical analysis was done on single optical sections. The labeled puncta were considered to contact the bipolar cell terminal when pixels overlapped. The number of puncta contacting the bipolar cell terminals was then expressed as a percentage of the total number of puncta counted on that section. The number of contacts expected by chance was estimated by calculating the percentage of gCCK-IR puncta contacting the bipolar cell terminals after the gCCK label was rotated 90 deg. All results are expressed as mean ± S.E.M. Significance was calculated using a generalized linear model with repeated measures, with the assumption that the distribution is binomial (SAS system, version 8.1).
No labeling of the tissue was seen when the primary antibodies were omitted. In addition, synthetic peptide (CCK-8 nonsuphated 26–33, 10 µM, H-2085, Bachem, Torrance, CA) was preincubated with the gCCK antibody for 2 h at 4°C, and the amacrine cells were unlabeled.
Physiology
Bipolar cells were isolated from rat and mouse retinas by a method described previously (Kaneko et al., 1989; de la Villa et al., 1995). Briefly, adult animals were sacrificed by cervical dislocation following the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research. Eyes were enucleated and hemisected, and the retinas were detached from the pigment epithelium. The standard solution contained (in mM) NaCl 135, KCl 5, MgCl2 1, CaCl2 2, glucose 10, and Hepes 10. It also contained 0.001% phenol red, and the pH was adjusted to 7.4 with NaOH. For enzymatic dissociation, the isolated retina was incubated in the standard solution containing 10–20 U/ml papain and 0.1 mg/ml cysteine for 20–30 min at 30°C. After rinsing with a standard solution containing 0.01% bovine serum albumin (BSA), the retina was triturated using a glass pipette. One to two drops of the cell suspension were dispersed in a plastic culture dish containing ca. 2 ml BSA-containing standard solution. The bottom of the plastic dish was replaced by a cover-glass coated with concanavalin A, and the cells were maintained at 5°C for 2–12 h. All experiments were done at room temperature.
This dissociation of the retina produced a mixture of cells. Rod bipolar cells were easily distinguished from other retinal cells by their characteristic morphology. In previous experiments, these cells were labeled with antiserum to PKC and confirmed to be rod bipolar cells (Vaquero et al., 1996).
For recording, the culture dish was mounted on the stage of an inverted microscope with phase-contrast optics (Nikon, Garden City, NY; TMD). A stainless-steel ring was put into the dish to reduce the volume to about 0.15 ml. Cells were perfused continuously at a rate of 0.6 ml/min, initially with standard solution containing BSA. Membrane currents were recorded by patch pipettes connected to a current–voltage converter (Axoclamp IIA, Axon Instruments, Foster City, CA) in the whole-cell configuration (Hamill et al., 1981). The Ag–AgCl indifferent electrode was connected via an agarose-bridge to the superfusate. Holding (Vh) and command (Vp) voltages were generated by a personal computer with a CED plus interface (Cambridge Electromedical Devices, Cambridge, England). The time and voltage resolution of the pulse generator were 0.5 ms and 1 mV, respectively. Data were sampled and digitized using a 12-bit A/D converter after passing through an 8-pole Bessel filter. The sampling rate was a set value between 0.2 and 20 ms, depending on the type of analysis. Data analysis was done using software described previously (Blanco et al., 1996).
Patch pipettes were made of Pyrex tubing (1.2 mm o.d.) pulled in two steps on a pipette puller (Narishige Scientific Instruments, P- 83, Greenvale, NY). After heat polishing, the inner diameter of the pipette was 0.5–1 µm. They were filled with a solution that contained (in mM) CsCl 120, MgCl2 1, EGTA 5, CaCl2 0.5, HEPES 10, GTP 1, cGMP 0.1, ATP 1, and cAMP 0.01 (pH adjusted to 7.2 with CsOH). The capacitance of the pipette was compensated electronically. The resistance of the pipette as measured in the bath was typically 5–20 MΩ. After obtaining the Giga-seal, the membrane patch under the pipette was ruptured by gentle suction, and the voltage clamp was established. Electrode series resistance ranged from 10 MΩ to 50 MΩ, and it was compensated up to 80%. The leak currents were not subtracted from the data.
Peptides were applied by bath perfusion, and the GABAergic drugs were applied by pressure, from a puff pipette positioned near the recorded cell (de la Villa et al., 1995). In most experiments, a puff pipette with a fine tip (ca. 1 µm) was used. All drugs and chemicals were purchased from Sigma Chemical Co., St. Louis, MO. The data were collected from 37 rod bipolar cells, and each result presented here was obtained from at least five different cells. Averaged data are expressed as mean ± SD.
Results
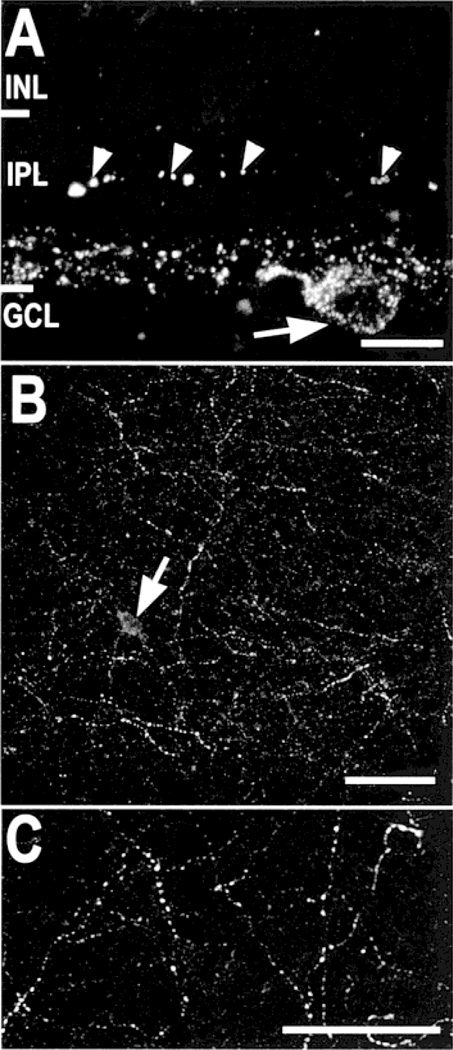
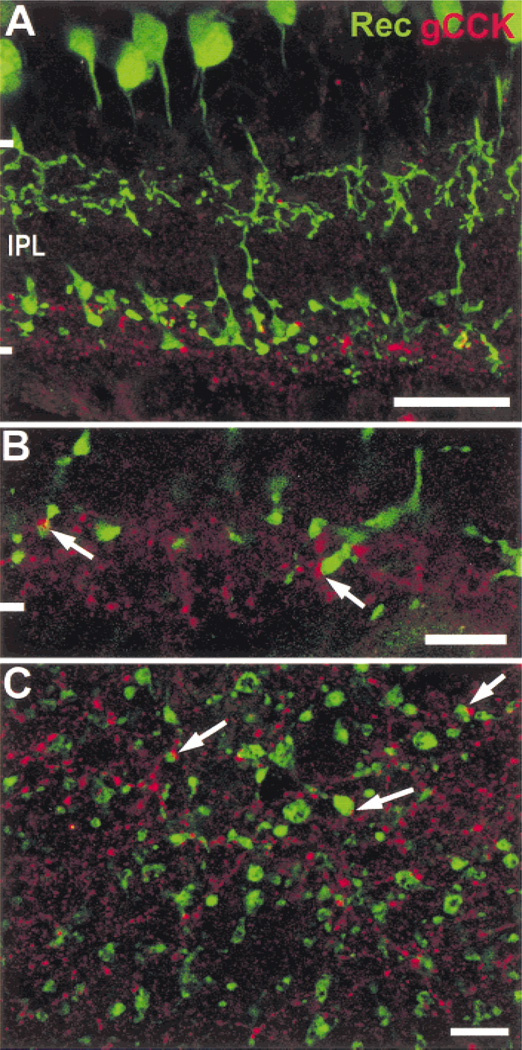
We saw varicose gCCK-IR processes in the innermost 15–25% (stratum 5) of the IPL (Fig. 1A). They were found throughout the whole mounts in both peripheral (Fig. 1B) and central retina (Fig. 1C). These processes were incompletely labeled, and individual processes could only be followed, for 50–100 µm, at the most. The labeled varicosities were generally small, less than 0.8 µm in diameter. Larger labeled varicosities approximately 1.5–2 µm were also seen, but these were much less common.
Fig. 1. Gastrin CCK-IR amacrine cells in the rat retina.
A: Varicose gCCK-IR axons are seen in a vertical section of the inner 20–25% of the inner plexiform layer (IPL). There is punctate labeling in a cell body (arrow) in the ganglion cell layer (GCL). Larger varicosities (arrowheads), probably on dendrites, are also seen in the middle of the IPL. Stack = 8 × 0.5 µm. (INL, inner nuclear layer). B: A meshwork of varicose gCCK-IR processes is seen in the far periphery of a whole mount. A single gCCK-IR cell body (arrow) is seen. Stack = 25 × 0.5 µm. C: The gCCK-IR axons are seen at higher magnification in central retina from a whole mount preparation. Stack = 8 × 0.5 µm. Scale bars = 10 µm (A) and 50 µm (B & C).
We saw gCCK-IR somata exclusively in the ganglion cell layer of the far peripheral retina. These somata were approximately 10 µm in diameter. Somata were not reliably labeled in whole-mounted retina; therefore, the gCCK-IR cells were not counted. In 32-µm sections through the whole eye, 1–3 labeled somata were typically seen. In the vicinity of the somata, a few processes ran into the outer layers of the IPL (Fig. 1A) and, very rarely, into the inner nuclear layer (INL). These processes were found only in the peripheral retina and not in the central retina. Gastrin-CCK-IR processes were not detected in the GCL or optic fiber layer near the optic disc.
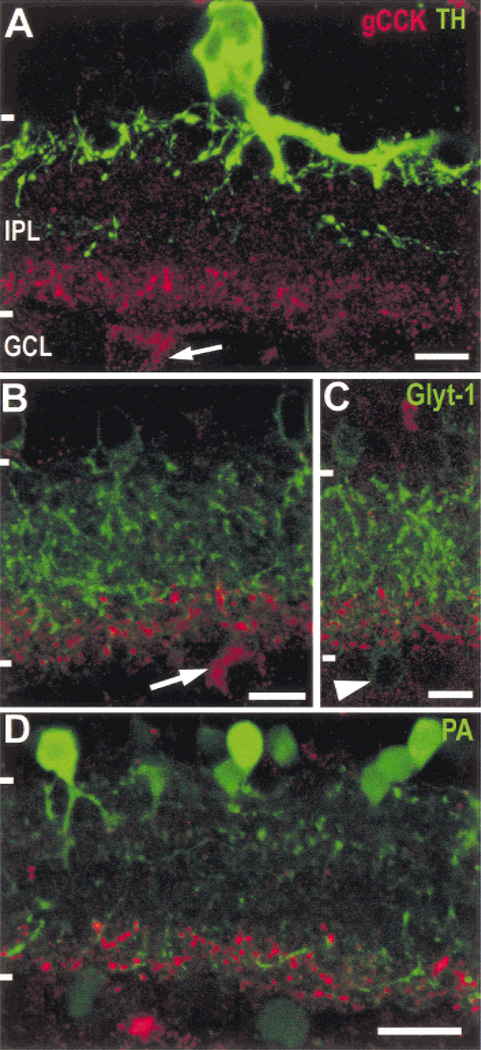
TH-IR processes were not commonly detected in the inner strata of the IPL, and when present, they did not contain gCCK immunoreactivity (Fig. 2A). In the peripheral retina, where the gCCK-IR perikarya are found, we did not detect TH-IR perikarya displaced to the ganglion cell layer, and the gCCK-IR perikarya did not contain TH immunoreactivity. Perikarya of glycinergic amacrine cells are also occasionally found in the GCL in the rat retina (Menger et al., 1998; Pow & Hendrickson, 1999). We confirmed this, but immunoreactive gCCK and immunoreactive Glyt-1 were not colocalized in cell bodies or in processes (Figs. 2B & 2C).
Fig. 2. Gastrin CCK-IR amacrine cells do not contain tyrosine hydroxylase (TH) or glycine transporter-1 (Glyt-1), and the gCCK-IR varicosities costratify with parvalbumin immunoreactivity.
A: The TH-IR (green) processes are dense in the outer stratum of the IPL; there is also a sparse plexus of TH-IR processes in the center of the IPL. The large TH-IR amacrine cell soma is seen in the typical position in the inner row of somata in the INL. A gCCK-IR (red) amacrine cell soma (arrow) is shown in the GCL in peripheral retina. Neither the varicose gCCK-IR processes nor the perikarya contain TH-IR. Stack = 13 × 0.5 µm. B,C: Glyt-1 immunoreactivity (green) is present in processes throughout the IPL, as well as in a population of amacrine cells in the INL. The gCCK-IR (red) processes are associated with some of the Glyt-1 processes in the inner stratum of the IPL. Neither the perikaryon (B, arrow) nor the gCCK-IR processes contain Glyt-1 immunoreactivity. Glyt-1-IR perikaryon (C, arrowhead) in the ganglion cell layer did not contain gCCK immunoreactivity. Stack = 3 × 0.5 µm (B) and 7 × 0.5 µm (C). D: The parvalbumin-IR (green) perikarya in the inner nuclear layer (INL) are mostly AII-like amacrine cells, with dendrites in the inner and outer strata of the inner plexiform layer (IPL). There are also fainter parvalbumin-IR somata in the ganglion cell layer (GCL). Many of the gCCK-IR (red) varicosities are found in the vicinity of parvalbumin-IR processes in the inner stratum of the IPL. Stack = 5 × 0.5 µm. Scale bars = 10 µm (A, B, & C) and 20 µm (D).
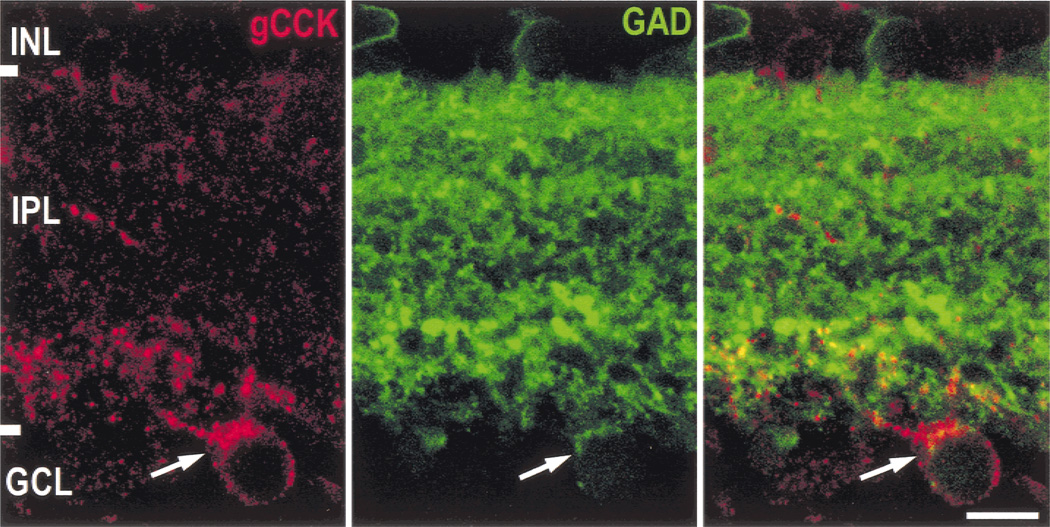
Gastrin CCK-IR somata and most of the gCCK-IR varicosities also contained GAD immunoreactivity, and we concluded that the gCCK-IR amacrine cell is GABAergic (Fig. 3). However, GAD immunoreactivity was not always detectable in the gCCK-IR dendrites in the outer layers of the IPL in the peripheral retina. We had difficulty resolving the individual processes labeled with GAD antiserum, and therefore, we were unable to determine whether they contacted the gCCK-IR processes.
Fig. 3.
Gastrin CCK-IR amacrine cells are GABAergic. In a single optical section (0.5 µm), glutamate decarboxylase 65 (GAD) immunoreactivity (green) is seen throughout the IPL, with the highest levels in stratum 1, a narrow band at stratum 203 and a wider, more densely labeled band in stratum 5. There are also faintly GAD-IR somata in both the INL and GCL. The gCCK-IR (red) soma (arrow) clearly contains GAD immunoreactivity (green); the channels are combined on the right. The majority of the gCCK-IR processes appear to also contain GAD immunoreactivity. Scale bar = 10 µm.
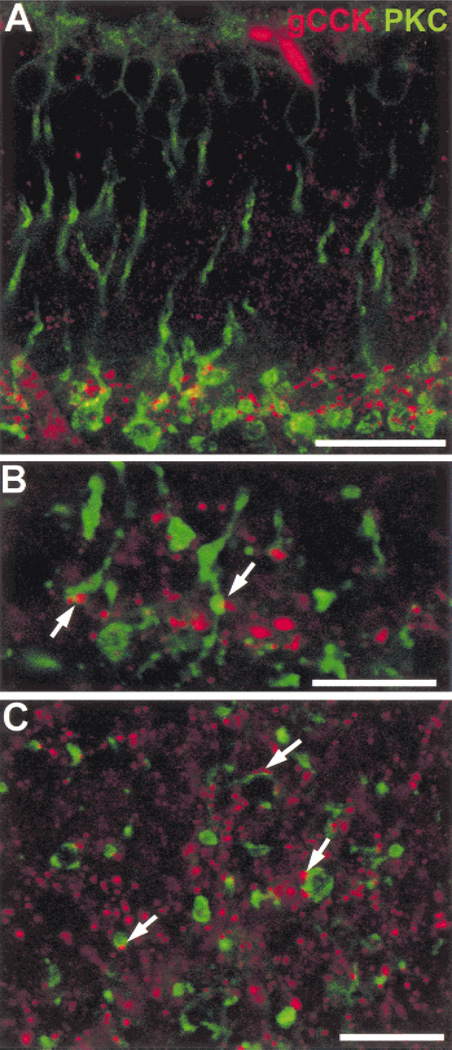
The gCCK-IR amacrine cells have processes ramifying in stratum 5 of the IPL, where the axons of rod bipolar cells terminate. We saw contacts of gCCK-IR varicosities with the PKC-IR rod bipolar cell terminals, a finding suggesting that there are likely to be synaptic interactions between rod bipolar cells and gCCK-IR amacrine cells (Fig. 4). There were also many gCCK-IR varicosities that did not appear to contact rod bipolar cell terminals. When contacts were counted on single optical sections from flat-mounted retina, 22.7 ± 2.8% of the gCCK-IR varicosities contacted rod bipolar cell terminals, which was significantly different from the number expected by chance, 16.6 ± 2.3% (n = 10, P < 0.001).
Fig. 4. Gastrin CCK-IR processes contact rod bipolar axon terminals.
A: Gastrin CCK-IR (red) varicose processes are found in stratum 5 in the vicinity of the protein kinase C-IR (green, PKC-IR) axon terminals in vertical section. Stack = 10 × 0.5 µm. B: At a higher magnification of a vertical section, the gCCK-IR varicosities contact the PKC-IR axon terminals (arrows). Single optical section = 0.5 µm. C: The contacts (arrows) between the gCCK-IR varicosities and the PKC-IR rod bipolar cell terminals are seen more clearly in a single optical section (0.5 µm) from whole-mount preparation. Scale bars = 25 µm (A), 5 µm (B), and 10 µm (C).
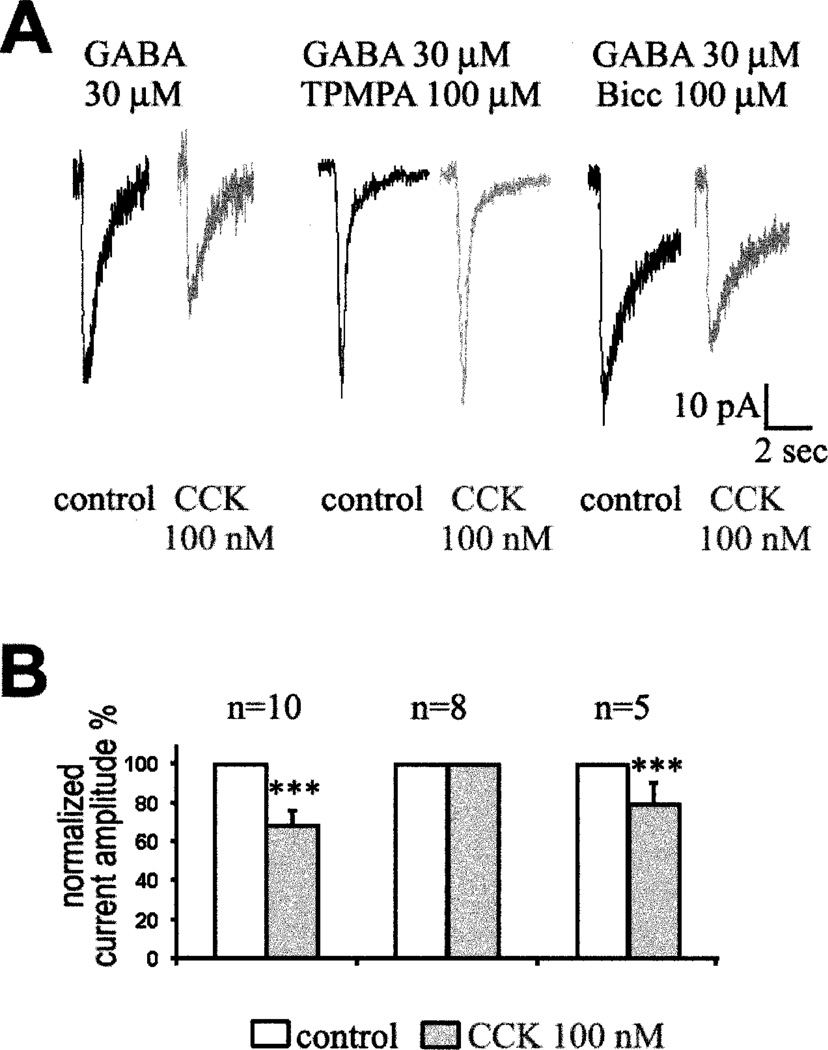
In electrophysiological experiments, CCK caused a clear decrease in the GABA-induced currents recorded from dissociated rod bipolar cells. Bicuculline was used to block the GABAA-mediated currents, and 1,2,5,6-tetrahydropyridine-4yl methylphosphinic acid (TPMPA) was used to block the GABAC-mediated currents. Fig. 5A shows the effect of 100 nM CCK on the currents elicited by 30 µM GABA in presence or absence of specific blockers of the GABA receptors. CCK decreased the current induced by GABA in mouse rod bipolar cells (Fig. 5A, left). However, CCK did not affect the GABA-induced current when 100 µM TPMPA was included in the puff pipette to block GABAC receptors (Fig. 5A, center). CCK did not affect GABAA-mediated currents in any of the eight cells tested. When 100 µM bicuculline was included with the GABA to block GABAA-mediated currents (Fig. 5A, right), CCK reduced the amplitude of the remaining current by 20%. The response of individual dissociated bipolar cells to GABA was variable in amplitude; therefore we have analyzed normalized data (Fig. 5B). Several experiments were done on rat bipolar cells and the results (not illustrated) were consistent with the data from mouse bipolar cells. We concluded that CCK partially inhibits GABAC- but not GABAA-mediated currents in rod bipolar cells.
Fig. 5. CCK effect on GABA-induced currents in rod bipolar cells from mouse retina.
A: Currents induced by 100-ms puff application of GABA in absence (black traces) or presence (grey traces) of 100 nM CCK with a sample rate of 10 ms. For these experiments, GABA(30 µM) was included in the puff-pipette alone (left pair of current traces), or together with TPMPA (100 µM, middle pair of current traces) or Bicuculine (100 µM, right pair of current traces). This data was obtained from three different cells. B: Histogram representation of the effect of CCK on the GABAC-mediated current. Current amplitudes were normalized and the number of recorded cells is indicated above each bar representation. *** indicates statistical significance, P < 0.001.
The gCCK-IR amacrine cells might also interact with other neurons in the rod pathway. In rabbits, the vitreal dendrites of AII amacrine cells receive synapses from other amacrine cells (Strettoi et al., 1992). These AII dendrites comprise the majority of the Glyt-1-IR processes in stratum 5. Many gCCK-IR varicosities were found in the same stratum as the Glyt-1-IR (Figs. 2B & 2C). Gastrin-CCK-IR varicosities also costratified with parvalbumin-IR processes, which are mainly AII amacrine cell dendrites in stratum 5 (Fig. 2D). However, the labeling with Glyt-1 and parvalbumin antisera was not intense enough to determine whether the gCCK-IR amacrine cells contact the AII amacrine cell dendrites directly. It is also possible that both contact the same rod bipolar cell or that the gCCK-IR varicosities are merely costratified with the AII dendrites in this layer.
Some contacts between the gCCK-IR varicosities and the type 8, recoverin-IR cone bipolar cell terminals were also detected in stratum 5 of the rat retina (Fig. 6), a finding suggesting there are synaptic interactions between these ON-cone bipolar cells and gCCK-IR varicosities. When contacts were counted on a single optical section from flat-mounted retina, 11.3 ± 2.2% of the gCCK-IR varicosities contacted rod bipolar cell terminals, which was significantly different from the number expected by chance, 7.5 ± 1.5% (n = 8, P = 0.0023). It is also possible that gCCK-IR processes contact the rarer, type 9 cone bipolar axon terminals, but it was not possible to test this hypothesis because there is no specific marker available for these bipolar cells.
Fig. 6. Gastrin CCK-IR processes contact ON-cone bipolar cell axon terminals.
A: Type 2 and 8 cone bipolar cells are labeled with antisera to recoverin (green) in a vertical section. The ON-cone bipolar cells (type 8) have axon terminals in the vicinity of the gCCK-IR (red) varicosities in stratum 5. Stack = 7 × 0.5 µm. B: Contacts between the gCCK-IR varicosities and recoverin-IR ON-cone bipolar cells are seen at a higher magnification of a vertical section (arrows). Single optical section = 0.5 µm. C: The contacts (arrows) between gCCK-IR varicosities and recoverin-IR ON-cone bipolar cell terminal are seen more clearly in a single optical section (0.5 µm) in stratum 5 of the IPL. Scale bars=25 µm (A) and 10 µm (B & C).
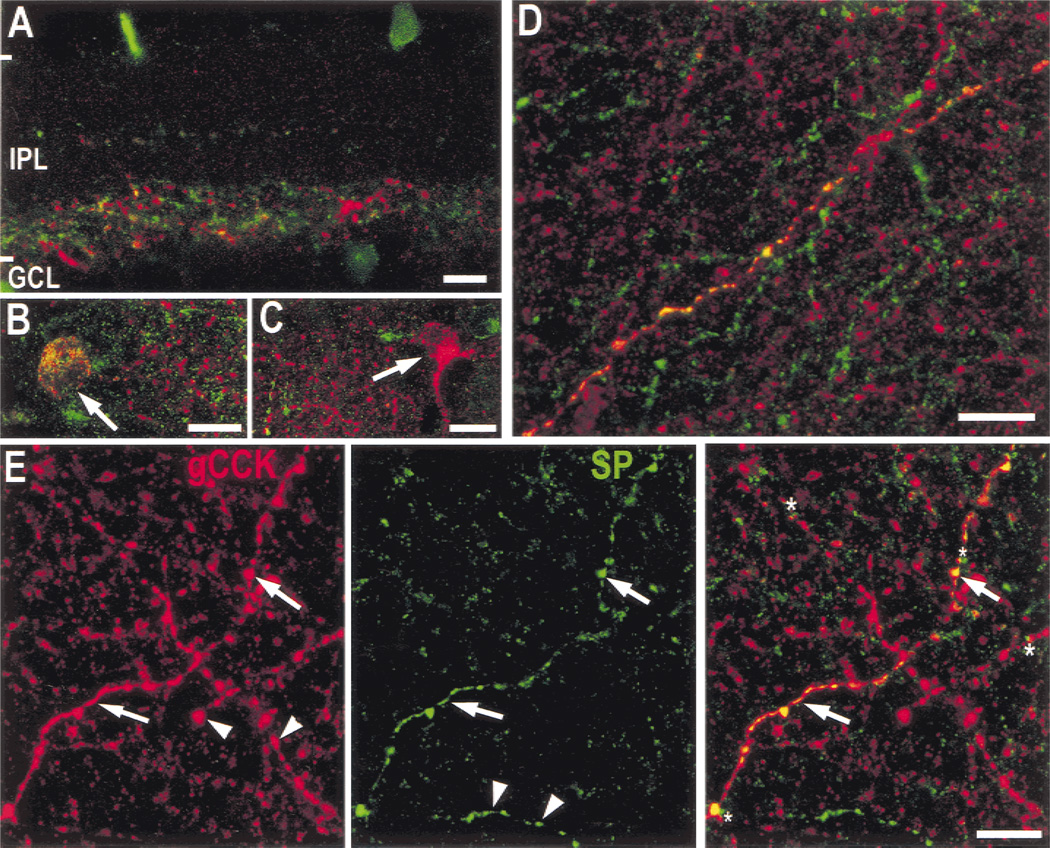
The gCCK-IR and substance P-IR processes were costratified, and some of the gCCK-IR perikarya and processes also contained substance P-like immunoreactivity. However, many of the substance P-IR somata in the GCL do not contain immunoreactive gCCK (Fig. 7). Approximately half of the gCCK-IR somata were not double labeled, but this maybe an underestimate because there were no obvious morphological differences between the double-labeled cells and the cells that contained only immunoreactive gCCK. The double-labeled axons frequently have large varicosities and are found at the outer borders of the gCCK-IR plexus; however, there were also single-labeled axons with similar characteristics. We concluded that at least some of the gCCK-IR amacrine cells contain substance P, as well as GABA. There was no effect of substance P on GABA-induced currents recorded from rod bipolar cells (not illustrated).
Fig. 7. Some gCCK-IR (red) processes contain substance P-like immunoreactivity (green).
A: Substance P-IR processes in a vertical section are in narrow bands in strata 1 and 3, as well as a much denser band throughout stratum 5 of the inner plexiform layer (IPL). There are cell bodies in both the inner nuclear (INL) and ganglion cell layer (GCL). Some of the gCCK-IR processes in stratum 5 contain substance P immunoreactivity (yellow) or contact the substance P-IR processes. However, there are substance P-IR processes without immunoreactive gCCK and gCCK-IR processes without immunoreactive substance P. Stack = 5 × 0.5 µm. B,C: In peripheral retina, the colocalization of immunoreactive gCCK and substance P in somata was investigated in the GCL in a whole-mount preparation. Some of the gCCK-IR somata contained substance P-IR (B, arrow) and others did not (C, arrow). Stacks 8 × 0.5 µm. D: Stratum 5 of the IPL at a higher magnification is shown in a whole mount; only a subset of the gCCK-IR processes contain substance P immunoreactivity. Stack = 6 × 0.5 µm. E: In this single optical section (1 µm), the gCCK (red, left) and substance P immunoreactivity (green, middle) are shown separately and combined on the right. This clearly shows gCCK-IR processes that contain substance P immunoreactivity (arrows) and many processes that contain only immunoreactive gCCK or only immunoreactive substance P (arrowheads). Contacts (asterisks) between these processes were also detected. All scale bars = 10 µm.
Discussion
Gastrin-CCK immunoreactive processes are found throughout the retina in stratum 5 of the IPL, but their somata are found only in the GCL in the peripheral retina. The labeling we saw in stratum 5 is consistent with the earlier description by Eriksen and Larsson (1981). Presumably, these authors did not see the perikarya and dendrites because they did not study the far peripheral retina. These gCCK-IR cells are also GABAergic, and some of them contained immunoreactive substance P. Some labeled varicosities contacted rod bipolar cells and ON-cone bipolar cells. CCK-octapeptide inhibits GABAC- but not GABAA-mediated currents in dissociated rod bipolar cells.
Perry and Walker (1980) have described rat amacrine cells labeled using the Golgi method. The displaced subtype of their type a, wide-field, unistratified amacrine cell was similar in some respects to the gCCK-IR amacrine cells. It has a soma comparable in size, 8.8–12.5 mm, and its processes are found in the inner strata of the IPL (Perry & Walker, 1980). However, the gCCK-IR amacrine cell also has some dendrites that extend into other strata of the IPL. The gCCK-IR neurons most closely resemble the polyaxonal type 2 amacrine (PA2) cells described previously in the rabbit retina (Famiglietti, 1992). These cells have somata displaced to the GCL, with most of their dendrites at the border between strata 4 and 5 and a few short dendritic branches that ascend to stratum 1. These cells have 1–3 thin axons that arise from the soma or proximal dendrites and extend beyond the dendritic tree. The major difference between the PA2 cells in rabbits and the gCCK-IR cells in rats is that the perikarya of the PA2 cells are found in the central retina, but the gCCK-IR perikarya are only found in the periphery. We are confident that somata are absent from the central rat retina because we used cryostat sections for the analysis of their distribution. Therefore, we conclude that the gCCK-IR cell is an axon-bearing, wide-field amacrine cell, and we classify the processes in the central retina as axons. Further description of the gCCK-IR amacrine cell would require a method that labels individual gCCK-IR cells more completely.
The gCCK-IR somata and their sparse dendritic processes that extend into the outer strata of the IPL are only found in the peripheral retina. This is not surprising because peptide-containing amacrine cell somata are not always distributed uniformly throughout the retina. Perikarya of one type of neuropeptide Y-IR amacrine cell and one type of glucagon-IR amacrine cell in turtles are both restricted to the far peripheral retina, like the gCCK-IR cells we describe in the rat (Wetzel & Eldred, 1997). Somatostatin-IR amacrine cells are distributed nonuniformly in rats (Sagar et al., 1985), humans (Sagar & Marshall, 1988), and rabbits (Sagar, 1987). In rabbits, their perikarya are found in the inferior retina and in the extreme periphery of all quadrants of the retina. However, the entire retina contains a meshwork of somatostatin-IR processes (Sagar, 1987).
A CCK precursor was localized previously in macaque retina (Marshak et al., 1990). The antisera used in that study were directed against the glycine extended CCK precursor (G6-gly) rather than CCK itself. We were not able to label the macaque retina with the gCCK antibody, and the G6-gly antisera did not label the rat retina (not illustrated). In the monkey, G6-gly antibodies labeled blue cone bipolar cells and two types of amacrine cells. Neither type of amacrine cell labeled in the monkey retina resembled the amacrine cells we labeled in the rat retina.
We show that there are contacts between the gCCK amacrine cells and rod bipolar cells in the rat retina. In rabbits, there are numerous amacrine cell processes that are presynaptic to the rod bipolar cells in stratum 5, including small ones less than 1 µm in diameter (Strettoi et al., 1990). Based on their diameter, the gCCK-IR axons we observed could be the rat homologues of these small processes. This anatomical result is consistent with the physiological effect of CCK-8 we observed on rod bipolar cells in rats and mice. The gCCK-IR amacrine cells might also be postsynaptic to rod bipolar cells. In dyad synapses of rod bipolar cells in rats, one postsynaptic amacrine cell is typically an AII and the other has not yet been identified (Chun et al., 1993).
Our electrophysiological experiments demonstrate that CCK inhibits, at least partially, GABAC-mediated currents in rod bipolar cells. A number of neuromodulators are known to act on GABA receptors in retinal neurons (Feigenspan & Bormann, 1998). For example, another neuropeptide has an opposite effect on rod bipolar cells in the rat; vasoactive intestinal peptide potentiates the GABA-induced currents (Veruki & Yeh, 1992). It is uncertain how inhibition of GABAC receptors would influence the light responses of rod bipolar cells. However, there have been two studies of the contributions of GABAC receptors to the b-wave of the electroretinogram (ERG) in mammals. In rabbits, blocking GABAC receptors decreased the b-wave amplitude and prolonged its decay (Dong & Hare, 2002). In GABACρ1 null mice, the time to peak of the b-wave was faster, and there was an increase in both the number and the amplitude of the oscillatory potentials compared to wild type mice (McCall et al., 2002). The decrease of the GABAergic feedback onto rod bipolar cells mediated by CCK could explain, at least partially, the decrease in spontaneous activity of retinal ganglion cells observed in the cat retina (Thier & Bolz, 1985). In the presence of CCK, the rate of glutamate release at the axon terminal of rod bipolar cells would increase, thus depolarizing the AII amacrine cells. The AII amacrine cells are inhibitory to OFF-cone bipolar cells, and this would explain the decrease in the spontaneous activity of retinal ganglion cells. However, the spontaneous activity of ON ganglion cells would be expected to increase, and the opposite was observed in the cat retina.
CCK might also have effects on the cone bipolar cells that are contacted by gCCK-IR processes. The majority of outputs from type 8 cone bipolar cells are onto amacrine cells, and the axons of these type 8 cone bipolar cells also receive input from amacrine cells (Chun et al., 1999). GABAC receptors make a significant contribution to the inhibitory synaptic currents of type 8 bipolar cells (Euler & Wässle, 1998). Inhibition of GABAC receptors on bipolar cells would be expected to have significant effects on the light responses of the postsynaptic amacrine and ganglion cells. In the mouse retina, inhibition of GABAC receptors enhances both the sustained and transient components of the light responses of amacrine cells (Matsui et al., 2001). In the rabbit retina, blockade of GABAC receptors has two effects on the light responses of ganglion cells. An OFF component of ON cell responses becomes apparent, and a component of the lateral inhibition mediated by amacrine cell feedback onto bipolar cells is also blocked (Roska & Werblin, 2001).
Some of the gCCK-IR cells contained immunoreactive substance P, but there was no effect of substance P on GABA-induced currents of rod bipolar cells. Neurokinin (NK) receptors have not been detected on rod bipolar cells (Oyamada et al., 1999; Casini et al., 2000). However, there may be other types of NK receptors that have not yet been localized. Substance P may also have other physiological effects. For example, substance P inhibits the voltagedependent calcium currents in goldfish bipolar cells (Ayoub & Matthews, 1992). Furthermore, if CCK and substance P are coreleased, the effect of substance P might only be apparent when it is applied with CCK. Physiological experiments using a combination of the two peptides would be required to test this hypothesis.
The processes of gCCK-IR amacrine cells and those containing immunoreactive substance P were costratified. In rats, most substance P-IR cells are amacrine cells, but a few are ganglion cells (Osborne et al., 1982; Caruso et al., 1990; Zhang & Yeh, 1992). The processes of these two types of substance P-IR cells cannot be distinguished reliably in our material. Gastrin-CCK-IR cells might have NK receptors. The NK 1 receptor, for which substance P is the preferred ligand, has been localized to amacrine cells and cells in the GCL of rats (Casini et al., 1997). NK 3 receptors have also been localized in rat amacrine cells (Oyamada et al., 1999; Casini et al., 2000). In addition, it is possible that CCK acts on substance P-IR cells. Receptors for CCK have been characterized in membrane preparation from other vertebrate retinas (Osborne, 1985; Bone & Rosenzweig, 1988), but these receptors have not been localized anatomically.
In conclusion, the anatomical evidence suggests that gCCK-IR amacrine cells make contacts with rod bipolar cells, ON-cone bipolar cells, and possibly, other amacrine cells. We have not been able to determine whether these were, in fact, synapses because the gCCK antibody required a brief paraformaldehyde fixation that would not be adequate for electron microscopy. We also provide evidence that the gCCK-IR amacrine cells are GABAergic and that some also contain substance P. CCK inhibits the sustained GABACinduced currents, but not the more transient GABAA-induced currents in rod bipolar cells from rats and mice. Further physiological studies using other target neurons of the gCCK-IR amacrine cells are required for a more complete understanding of the role of CCK in retinal information processing.
Acknowledgments
We wish to thank Dr. Aice Chuang for help with the statistical analysis. This work was supported by a grant from the Robert J. Kleberg Jr. & Helen C. Kleberg Foundation, grant EY06472 from the National Eye Institute to D.W. Marshak and also by grants from the Spanish Ministry of Science and Technology (SAF-98-1038-C02-01), Comunidad Autónoma de Madrid (08.500069.1), and Fondo de Investigaciones Sanitarias (FISS-01-0050-02) to P. de la Villa. C. Varela was supported by a fellowship from the MCYT and S.I. Firth by postdoctoral fellowship PD01032 from Fight for Sight, Inc.
References
- Ayoub GS, Matthews G. Substance P modulates calcium current in retinal bipolar neurons. Visual Neuroscience. 1992;8:539–544. doi: 10.1017/s0952523800005630. [DOI] [PubMed] [Google Scholar]
- Beinfeld MC. Cholecystokinin/Gastrin. In: Watson SJ, editor. Psychopharmacology. New York: Lippincott-Raven Press; 1998. CD/ROM Edition http://www.acnp.org/citations/ GN401000056: Chapter 56. [Google Scholar]
- Blanco R, Vaquero CF, De La Villa P. Action potentials in axonless horizontal cells isolated from the rabbit retina. Neuroscience Letters. 1996;203:57–60. doi: 10.1016/0304-3940(95)12263-x. [DOI] [PubMed] [Google Scholar]
- Bone EA, Rosenzweig SA. Characterization of cholecystokinin receptors in toad retina. Peptides. 1988;9:373–381. doi: 10.1016/0196-9781(88)90273-2. [DOI] [PubMed] [Google Scholar]
- Brandon C. Retinal GABA neurons: Localization in vertebrate species using an antiserum to rabbit brain glutamate decarboxylase. Brain Research. 1985;344:286–295. doi: 10.1016/0006-8993(85)90806-6. [DOI] [PubMed] [Google Scholar]
- Caruso DM, Owczarzak MT, Pourcho RG. Colocalization of substance P and GABA in retinal ganglion cells: A computer-assisted visualization. Visual Neuroscience. 1990;5:389–394. doi: 10.1017/s095252380000047x. [DOI] [PubMed] [Google Scholar]
- Casini G, Rickman DW, Sternini C, Brecha NC. Neurokinin 1 receptor expression in the rat retina. Journal of Comparative Neurology. 1997;389:496–507. [PMC free article] [PubMed] [Google Scholar]
- Casini G, Brecha NC, Bosco L, Rickman DW. Developmental expression of neurokinin-1 and neurokinin-3 receptors in the rat retina. Journal of Comparative Neurology. 2000;421:275–287. [PubMed] [Google Scholar]
- Chun M-H, Han SH, Chung JW, Wässle H. Electron microscopic analysis of the red pathway of the rat retina. Journal of Comparative Neurology. 1993;332:421–432. doi: 10.1002/cne.903320404. [DOI] [PubMed] [Google Scholar]
- Chun M-H, Kim I-B, Oh S-J, Chung J-W. Synaptic connectivity of two types of recoverin-labeled bipolar cells and glutamic acid decarboxylase immunoreactive amacrine cells in the inner plexiform layer of the rat retina. Visual Neuroscience. 1999;16:791–800. doi: 10.1017/s0952523899164174. [DOI] [PubMed] [Google Scholar]
- De La Villa P, Kurahashi T, Kaneko A. L-Glutamate-induced responses and cGMP-activated channels in three subtypes of retinal bipolar cells dissociated from cat. Journal of Neuroscience. 1995;15:3571–3582. doi: 10.1523/JNEUROSCI.15-05-03571.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Villa P, Garcia-Marin LJ, Bragado MJ. Regulation of tyrosine phosphorylation in rat retina following cholecystokinin stimulation. Investigative Ophthalmology and Visual Science. 2000;41:S246, 1290. [Google Scholar]
- Dong C-J, Hare WA. GABAC feedback pathway modulates the amplitude and kinetics of ERG b-wave in mammalian retina in vivo. Vision Research. 2002;42:1081–1087. doi: 10.1016/s0042-6989(02)00032-9. [DOI] [PubMed] [Google Scholar]
- Eriksen EF, Larsson LI. Neuropeptides in the retina: Evidence for differential topographical localization. Peptides. 1981;2:153–157. doi: 10.1016/s0196-9781(81)80028-9. [DOI] [PubMed] [Google Scholar]
- Eskay RL, Beinfeld MC. HPLC and RIA of cholecystokinin peptides in the vertebrate neural retina. Brain Research. 1982;246:315–318. doi: 10.1016/0006-8993(82)91183-0. [DOI] [PubMed] [Google Scholar]
- Euler T, Wässle H. Immunocytochemical identification of cone bipolar cells in the rat retina. Journal of Comparative Neurology. 1995;361:461–478. doi: 10.1002/cne.903610310. [DOI] [PubMed] [Google Scholar]
- Euler T, Wässle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in rat retinal slice preparation. Journal of Neurophysiology. 1998;79:1384–1395. doi: 10.1152/jn.1998.79.3.1384. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. Polyaxonal amacrine cells of the rabbit retina: PA2, PA3 and PA4 cells. Light and electron microscopic studies with functional interpretation. Journal of Comparative Neurology. 1992;316:422–446. doi: 10.1002/cne.903160404. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. GABA-gated Cl− channels in the rat retina. Progress in Retinal Eye Research. 1998;17:99–126. doi: 10.1016/s1350-9462(97)00008-6. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Wässle H, Bormann J. Pharmacology of GABA receptor Cl− channels in rat retinal bipolar cells. Nature. 1993;361:159–163. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- Freund TF, Magloczky Z, Soltesz I, Soymogyi P. Synaptic connections, axonal and dendritic patterns of neurons immunoreactive for cholecystokinin in the visual cortex of the cat. Neuroscience. 1986;19:1133–1159. doi: 10.1016/0306-4522(86)90129-6. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Wässle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. Journal of Comparative Neurology. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Toth K, Danos P, Freund TF. GABAergic neurons containing parvalbumin, calbindin D28k, and cholecystokinin in rat hippocampus. Journal of Comparative Neurology. 1991;312:371–378. doi: 10.1002/cne.903120305. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archivs. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Rehfeld JF, Skirboll L, Ivemark B, Goldstein B, Markey K. Evidence for coexistence of dopamine and CCK in meso-limbic neurons. Nature. 1980;285:476–478. doi: 10.1038/285476a0. [DOI] [PubMed] [Google Scholar]
- Kaneko A, Pinto LH, Tachibana M. Transient calcium current of retinal bipolar cells in mouse. Journal of Physiology (London) 1989;410:613–629. doi: 10.1113/jphysiol.1989.sp017551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak DW, Aldrich LB, Del Valle J, Yamada T. Localization of immunoreactive cholecystokinin precursor to amacrine cells and bipolar cells of the macaque monkey retina. Journal of Neuroscience. 1990;10:3045–3055. doi: 10.1523/JNEUROSCI.10-09-03045.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Haesegawa J, Tachibana M. Modulation of excitatory synaptic transmission by GABAC receptor-mediated feedback in the mouse inner retina. Journal of Neurophysiology. 2001;86:2285–2298. doi: 10.1152/jn.2001.86.5.2285. [DOI] [PubMed] [Google Scholar]
- McCall MA, Lukasiewicz PD, Gregg RG, Peachy NS. Elimination of the ρ1 subunit abolishes GABAC receptor expression and alters visual processing in the mouse retina. Journal of Neuroscience. 2002;22:4163–4174. doi: 10.1523/JNEUROSCI.22-10-04163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basal lateral amygdala. Neuroscience Letters. 1989;100:53–58. doi: 10.1016/0304-3940(89)90659-9. [DOI] [PubMed] [Google Scholar]
- Menger N, Pow DV, Wässle H. Glycinergic amacrine cells of the rat retina. Journal of Comparative Neurology. 1998;401:34–46. doi: 10.1002/(sici)1096-9861(19981109)401:1<34::aid-cne3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Milam AH, Dacey DM, Dizhoor AM. Recoverin immunoreactivity in mammalian cone bipolar cells. Visual Neuroscience. 1993;10:1–12. doi: 10.1017/s0952523800003175. [DOI] [PubMed] [Google Scholar]
- Mosinger JL, Yazulla S, Studholme KM. GABA-like immunoreactivity in the vertebrate retina: A species comparison. Experimental Eye Research. 1986;42:631–644. doi: 10.1016/0014-4835(86)90052-7. [DOI] [PubMed] [Google Scholar]
- Nelson R, Kolb H. A17:Abroad-field amacrine cell in the rod system of the cat retina. Journal of Neurophysiology. 1985;54:592–614. doi: 10.1152/jn.1985.54.3.592. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Berger B, Vigny A, Alvarez C. Tyrosine hydroxylase-like immunoreactive interplexiform cells in rat retina. Neuroscience Letters. 1981;27:255–259. doi: 10.1016/0304-3940(81)90439-0. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Versaux-Botteri C, Savy S. Dopaminergic and GABAergic retinal cell populations in mammals. Microscopy Research and Technique. 1997;36:26–42. doi: 10.1002/(SICI)1097-0029(19970101)36:1<26::AID-JEMT3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Osborne NN. Cholecystokinin in the retina of vertebrates. Annals of the New York Academy of Science. 1985;448:157–166. doi: 10.1111/j.1749-6632.1985.tb29916.x. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Nicholas DA, Cuello AC, Dockray GJ. Localization of cholecystokinin immunoreactivity in amacrine cells of the retina. Neuroscience Letters. 1981;26:31–35. doi: 10.1016/0304-3940(81)90421-3. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Nicholas DA, Dockray GJ, Cuello AC. Cholecystokinin and substance P immunoreactivity in retinas of rats, frogs, lizards and chicks. Experimental Eye Research. 1982;34:639–649. doi: 10.1016/0014-4835(82)90038-0. [DOI] [PubMed] [Google Scholar]
- Oyamada H, Takatsuji K, Senba E, Mantyh PW, Tohyama M. Postnatal development of NK1, NK2, and NK3 neurokinin receptors expression in the rat retina. Developmental Brain Research. 1999;117:59–70. doi: 10.1016/s0165-3806(99)00099-1. [DOI] [PubMed] [Google Scholar]
- Perry VH, Walker M. Amacrine cells, displaced amacrine cells and interplexiform cells in the retina of rat. Proceedings of the Royal Society B (London) 1980;208:415–431. doi: 10.1098/rspb.1980.0060. [DOI] [PubMed] [Google Scholar]
- Pow DV, Hendrickson AE. Distribution of the glycine transporter glyt-1 in mammalian and nonmammalian retinae. Visual Neuroscience. 1999;16:231–239. doi: 10.1017/s0952523899162047. [DOI] [PubMed] [Google Scholar]
- Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature. 2001;410:583–587. doi: 10.1038/35069068. [DOI] [PubMed] [Google Scholar]
- Sagar SM. Somatostatin-like immunoreactive material in the rabbit retina: Immunohistochemical staining using monoclonal antibodies. Journal of Comparative Neurology. 1987;266:291–299. doi: 10.1002/cne.902660212. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Marshall PE. Somatostatin-like immunoreactive material in associational ganglion cells of human retina. Neuroscience. 1988;27:507–516. doi: 10.1016/0306-4522(88)90284-9. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Marshall PE, Landis DMD. Immunoreactive somatostatin in the rat retina: Light microscopic immunocytochemistry and chromatographic characterization. Brain Research. 1985;336:325–342. doi: 10.1016/0006-8993(85)90650-x. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Masland RH, Raviola E, Dacheux RF. Connections of indoleamine-accumulating cells in the rabbit retina. Journal of Comparative Neurology. 1989;283:303–313. doi: 10.1002/cne.902830210. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Keyser KT, Battenberg E, Bloom FE. Parvalbumin in the rat retina. Neuroscience Letters. 1990;118:136–139. doi: 10.1016/0304-3940(90)90267-d. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Dangaram K, Lim S, Haycock JW, Fallon JH. Ventral mesencephalic neurons containing both cholecystokinin- and tyrosine hydroxylase-like immunoreactivities project to forebrain regions. Journal of Comparative Neurology. 1989;279:397–414. doi: 10.1002/cne.902790306. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Dacheux RF, Raviola E. Synaptic connections of the rod bipolar cells in the inner plexiform layer of rabbit retina. Journal of Comparative Neurology. 1990;295:449–466. doi: 10.1002/cne.902950309. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in rabbit retina. Journal of Comparative Neurology. 1992;325:152–168. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- Thier P, Bolz J. Cholecystokinin in the cat retina. Action of exogenous CCK8 and localization of cholecystokinin-like immunoreactivity. Investigative Ophthalmology and Visual Science. 1985;26:266–272. [PubMed] [Google Scholar]
- Vaquero CF, De La Villa P. Localisation of the GABA(C) receptors at the axon terminal of the rod bipolar cells of the mouse retina. Neuroscience Research. 1999;35:1–7. doi: 10.1016/s0168-0102(99)00050-4. [DOI] [PubMed] [Google Scholar]
- Vaquero CF, Velasco A, De La Villa P. Protein Kinase C localization in the synaptic terminal of rod bipolar cells. Neuroreport. 1996;7:2176–2180. doi: 10.1097/00001756-199609020-00024. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Yeh HH. Vasoactive intestinal polypeptide modulates GABAA receptor function in bipolar cells and ganglion cells of the rat retina. Journal of Neurophysiology. 1992;67:791–797. doi: 10.1152/jn.1992.67.4.791. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grunert U, Rohrenbeck J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. Journal of Comparative Neurology. 1993;332:407–420. doi: 10.1002/cne.903320403. [DOI] [PubMed] [Google Scholar]
- Wetzel RK, Eldred WD. Specialized neuropeptide y-glucagon-like immunoreactive amacrine cells in the peripheral retina of turtle. Visual Neuroscience. 1997;14:867–877. doi: 10.1017/s0952523800011603. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF. Recoverin in cultured human retinablastoma cells: Enhanced expression during morphological differentiation. Journal of Neurochemistry. 1996;67:105–110. doi: 10.1046/j.1471-4159.1996.67010105.x. [DOI] [PubMed] [Google Scholar]
- Wood JG, Hart CE, Mazzei GJ, Girard PR, Kuo JF. Distribution of protein kinase C immunoreactivity in rat retina. Histochemistry Journal. 1988;20:63–68. doi: 10.1007/BF01746605. [DOI] [PubMed] [Google Scholar]
- Zhang D, Yeh HH. Substance P-like immunoreactive amacrine cells in the adult and developing rat retina. Developmental Brain Research. 1992;68:55–65. doi: 10.1016/0165-3806(92)90247-t. [DOI] [PubMed] [Google Scholar]