Abstract
Acute infection with equine infectious anemia virus (EIAV), a lentivirus of horses, results in a persistent high-level viremia in Arabian foals affected with severe combined immunodeficiency (SCID). This observation argues against the idea that the transient nature of acute lentiviral viremia is solely a function of viral population dynamics. To extend these studies, EIAV-specific immune reconstitution was attempted prior to EIAV challenge in 2 SCID foals, using adoptively transferred virus-stimulated lymphocytes derived from persistently EIAV-infected half sibling donors. Following transfer, lymphocyte engraftment occurred in 1 foal, and EIAV-specific cytotoxic T lymphocytes as well as neutralizing antibody activity developed. Following a brief period of plasma viremia in this foal, EIAV replication was controlled and plasma virus could not be detected by RT-PCR or culture. These results provide further direct evidence that a specific immune response is required for termination of plasma viremia in acute lentiviral infections.
Keywords: EIAV, lentivirus, equine, adoptive transfer, SCID, CTL, lymphocyte engraftment
Introduction
Specific immune responses, especially cytotoxic T lymphocytes (CTL), appear important in the control of acute lentiviral infections, including human immunodeficiency virus-1 (HIV-1), simian immunodeficiency virus (SIV), and equine infectious anemia virus (EIAV) (1-3). However, it has been reported that the decline of acute HIV viremia could occur in the absence of a specific immune response, due to exhaustion of target cells (4). Arguing against this possibility for other lentiviruses are the observations that rhesus monkeys depleted of CD8+ T lymphocytes during primary SIV infection fail to control viral replication (5), and that viral load is decreased as compared to controls in acutely HIV-1-infected (hu)-PBL-SCID mice injected with Gag-specific CTL clones (6). In contrast to normal foals, EIAV-infected severe combined immunodeficient (SCID) foals develop a fatal high-titer viremia that is not terminated (7, 8). SCID in Arabian foals is caused by a frame-shift mutation in the gene encoding the catalytic subunit of DNA-dependent protein kinase (DNA-PK(CS)) (9), and has an autosomal recessive mode of inheritance (10). The equine SCID defect is more severe than its murine counterpart in that SCID foals are incapable of forming either coding or signal joints (9). Interestingly, EIAV titers reach a plateau in SCID foals (8), suggesting that the number of monocyte/macrophage target cells (11, 12) may eventually become limiting.
Memory CTL (CTLm) from EIAV-infected horses recognize epitopes in Gag, Pol, Env, and Rev proteins, and in the protein encoded by the S2 open reading frame (13-15, R.H. Mealey, unpublished observation). Candidate vaccines designed to induce protective CTL responses in horses with a diverse MHC class I repertoire will likely contain constructs expressing several or all of the above epitope-containing regions. However, if viral population dynamics alone can explain the decline in lentiviral load following acute infection, then the assignment of protective effects to candidate CTL-inducing vaccines against primary lentiviral challenge might be in error. The purpose of this study was to determine the contribution of viral-specific immunity to the control of primary EIAV plasma viremia and clinical disease.
Material and Methods
Horses
Two SCID foals (SCID1 and SCID2) were obtained by selective breeding of Arabian mares and stallions heterozygous for the SCID trait (9, 10). Initial diagnosis of SCID was based on persistent lymphopenia (< 1,000 peripheral blood lymphocytes (PBL)/μl; normal Arabian foals: 4,119 ± 1649 PBL/μl) (16), and confirmed by identification of the homozygous mutation in the DNA-PK(CS) gene sequence (9). For the first 2 days of life, both SCID foals were housed in individual box stalls with their dams to allow ingestion of colostrum. Adequate passive transfer of maternal IgG was confirmed using a Cite Test (Idexx Laboratories, Inc., USA). The SCID foals were then removed from their dams, housed in individual isolation stalls, and fed a commercial mare’s milk replacer. In an effort to prevent bacterial infection as well as opportunistic lung infection by Pneumocystis carinii (17), SCID1 and SCID2 were administered systemic antibiotics, including erythromycin (25 mg/kg, PO, q 8 hrs), enrofloxacin (5 mg/kg, PO, q 12 hrs), and trimethoprim-sulfamethoxazole (20 mg/kg, PO, q 12 hrs).
Half-siblings to SCID1 and SCID2 (A2140 and A2141) were infected with 107 TCID50 EIAVWSU5 (18), for 1 year and 4 months, respectively, before use as lymphocyte donors. The equine leukocyte alloantigen (ELA)-A types of A2140 and A2141 (Table 1) were determined serologically (19). The ELA-A locus is the best defined polymorphic MHC class I locus in the horse (20), but at least 4 loci likely exist (21). Because SCID foals are profoundly lymphopenic, ELA-A types could not be directly determined using established lymphocytotoxicity-based serological techniques. MHC class II DRA and DQA typing was performed (22, 23) for both SCID foals and both lymphocyte donors (Table 1). Since little recombination between MHC class I and class II genes has been observed in the horse (D.G. Fraser, unpublished observation), the ELA-A haplotypes for SCID1 and SCID2 were inferred from their determined DRA and DQA haplotypes, using known inheritance patterns for these loci for each dam and sire. The ELA-A haplotypes of SCID1 and SCID2 were later confirmed with CTL assays, using EIAV-infected equine kidney (EK) cell targets (3, 14) from these foals, and EIAV-stimulated CTL (14) from the lymphocyte donors (A2140 and A2141, respectively).
Table 1.
ELA-A class I and ELA class II (DRA and DQA) haplotypes of lymphocyte donors and SCID recipients.
| Class I | Class II | |
|---|---|---|
| Donors* | ||
| A2140 | A1/w11 | DRA 1/2; DQA 5/9 |
| A2141 | A5/A4 | DRA 2/3; DQA 5/9 |
| Recipients | ||
| SCID1 | A1/w11 | DRA 1/2; DQA 5/9 |
| SCID2 | A5/w11 | DRA 1/2; DQA 5/11 |
Stimulated lymphocytes from A2140 were infused into SCID1, while stimulated lymphocytes from A2141 were infused into SCID2.
Preparation of Viral-Specific Lymphocytes
To generate viral-specific donor lymphocytes, peripheral blood mononuclear cells (PBMC) from A2140 and A2141 were isolated (15) and stimulated in vitro twice for a 2 week period with EIAVWSU5-infected autologous monocytes. Recombinant human IL-2 and additional irradiated EIAVWSU5-infected autologous PBMC were added in the second week as described (14, 15) with modifications. PBMC were suspended at 5 × 106/ml RPMI 1640 medium with 10% fetal bovine serum (FBS), 20 mM HEPES, 10 μg/ml gentamicin, and 10 μM 2-mercaptoethanol. Tissue culture flasks (175 cm2) were seeded with 40 ml/flask, and incubated at 37°C with 5% CO2. After 2 weeks in culture (immediately before adoptive transfer), effector CTL (CTLe) activity and frequency were determined using published methods (3, 24) for CTL assays and limiting dilution analysis (LDA), except that no stimulation of PBMC was performed prior to each assay, and in LDA there were 80,000, 40,000, 20,000, and 10,000 PBMC effectors/well. Homologous and heterologous target cells for these assays were EIAVWSU5-infected and noninfected EK cells (3). Fluorescent flow cytometry was performed (25) using the following murine anti-equine monoclonal antibodies: ETC91A (25) and HT14A (anti-CD8); HB61A (anti-CD4); and F66 (anti-CD3) (26).
Lymphocyte Adoptive Transfer and EIAV Challenge
Beginning at 30 days of age, SCID1 and SCID2 received an intravenous (IV) infusion of EIAVWSU5-stimulated donor lymphocytes on 2 consecutive days, and a third infusion 14 days later (Fig. 1). Before the experiment, CTL assays confirmed EIAV-specific, MHC class I-restricted killing of SCID1 and SCID2 EK cell targets by CTLm present in EIAVWSU5-stimulated PBMC from A2140 and A2141, respectively. Prior to each infusion, the lymphocytes were harvested from the stimulation culture flasks, pelleted by centrifugation at 250 × g for 10 minutes, and resuspended in 20 ml RPMI 1640 with 20% FBS. The cells were counted, and a sufficient number retained for functional and phenotypic analyses (performed the same day). The remaining cells were again pelleted, resuspended in 500ml Hank’s balanced salt solution, and kept on ice until infusion. Complete blood counts (CBC) for both SCID1 and SCID2 revealed < 100 PBL/μl prior to the infusions.
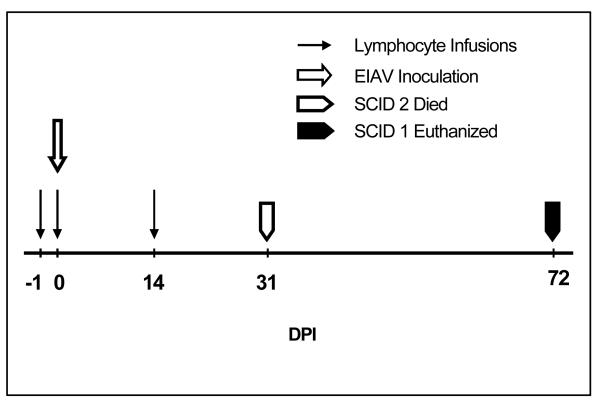
Fig. 1.
Experimental time line. DPI = days post-EIAV inoculation
Both foals were inoculated with 106 TCID50 EIAVWSU5, IV, immediately prior to the second lymphocyte infusion (Fig. 1). Of 17 untreated SCID foals inoculated with the same dose of EIAVWSU5, all died or were euthanized due to severe EIAV-induced disease (characterized by profound anemia and thrombocytopenia) within 35 days post-EIAV inoculation (DPI; mean = 32 ± 3 DPI) (7, 8, R.H. Mealey, unpublished observation). Survival greater than 41 DPI for foals in the present experiment was considered significant (3 S.D. above the mean historical survival).
Clinical Disease Monitoring, Sample Collection, and Virus Detection Following Adoptive Transfers and EIAV Challenge
Physical examinations were performed daily, and body temperature was obtained daily from both SCID foals. CBC and platelet counts were performed 2 - 3 times weekly. Plasma separated from whole blood collected separately in EDTA and heparin on the same days was stored at −80°C. After PBL were detected by CBC (SCID1 only), PBMC were isolated every 1 - 2 weeks.
To detect virus in SCID1 and SCID2, viral RNA was isolated from frozen EDTA plasma (27), using a QIAamp Viral RNA kit (Qiagen Inc., USA), and treated with DNAse I. RT-PCR was performed using a SuperScript One-Step RT-PCR kit (Life Technologies, USA). Outside forward (5′-GACAGCAGAGGAGAACTTAC-3′) and reverse (5′-CCTCTCTTTCTTGTCCTG-3′) primers, and nested forward (5′-AAGATGGGAGACCCTTTGAC-3′) and reverse (5′-TGGAATGACATCCCTCAGC-3′) primers were designed to amplify 289 bp and 177 bp segments, respectively, of EIAV gag p15. Reaction conditions for RT-PCR were 30 min at 45°C, 2 min at 94°C, followed by 40 cycles of 30 sec at 94°C, 30 sec at 54°C, 30 sec at 72°C, and then 7 min at 72°C. Reaction conditions for nested PCR were 2 min at 94°C, followed by 35 cycles of 30 sec at 94°C, 30 sec at 55°C, 30 sec at 72°C, and then 7 min at 72°C. DNA was extracted from post mortem splenic tissue (27), and proviral DNA amplified by PCR using the same primers and conditions as for RT-PCR but without the RT step. The specificity of PCR and RT-PCR products was confirmed by sequencing. In addition to RT-PCR for virus detection, virus in frozen heparinized plasma was titrated in cell culture as described (18).
Immunological Studies Following Adoptive Transfer and EIAV Challenge
PBMC obtained from SCID1 were phenotyped using fluorescent flow cytometry as described above. In addition, EIAV-specific CTLe activity and frequency were determined in SCID1 PBMC as described above, while neutralizing antibody activity in frozen heparinized plasma from both SCID1 and SCID2 was determined using a virus reduction method (28) with modifications. Briefly, 300 μl heparinized plasma was mixed with 300 μl EIAVWSU5 (approximate titer 104 TCID50/ml) stock, and incubated for 1 hr at 37°C. The same was performed using negative control plasma from 4 horses not infected with EIAV. For each plasma-virus mixture, 3-fold serial dilutions were made, and titration performed in EK cell culture (18). Percent virus reduction was calculated using the formula:
Immunohistochemistry
Splenic tissue obtained post mortem from both SCID foals was frozen and labeled by immunohistochemistry as described, using the murine monoclonal antibody DH59B, which identifies macrophages in equine tissue sections (29).
Results
Adoptively Transferred Lymphocytes Engrafted in SCID1 but not in SCID2
SCID1
SCID1 received a total of 1.6 × 1010 stimulated lymphocytes (all 3 infusions combined), including 9.2 × 109 CD4+ lymphocytes (58.5%), 4.5 × 109 CD3+CD8+ lymphocytes (28.6%), 1.7 × 108 B lymphocytes (1.1%), and 6.1 × 106 (0.04%) EIAV-specific MHC class I-restricted CTLe. Engraftment and proliferation of the infused lymphocytes occurred, and were first detected in peripheral blood 5 days post-infusion. The PBL count ranged from 1,001 - 11,400 cells/μl, remaining above 1,000/μl throughout the study period (Table 2). PBMC obtained from SCID1 on 14, 20, 29, 41, 49, 57, and 70 DPI were predominantly CD3+ T lymphocytes (range: 47 - 79%) and included CD4+ lymphocytes (range: 21 - 46%) and CD8+ lymphocytes (range: 33 - 48%).
Table 2.
Lymphocyte, neutrophil, and band neutrophil counts (/ml peripheral blood) for SCID1 and SCID2.
| SCID1 | SCID2 | |||||
|---|---|---|---|---|---|---|
| DPI | Lymphocytes/ul | Neutrophils/ul | Band Neutrophils/ul | Lymphocytes/ul | Neutrophils/ul | Band Neutrophils/ul |
| Pre | 56 | 5,376 | 0 | 72 | 3,420 | 0 |
| 1 | 22 | 880 | 110 | 96 | 1,008 | 0 |
| 5 | 2,010 | 690 | 30 | ND | ND | ND |
| 6 | ND | ND | ND | 0 | 2,392 | 0 |
| 8 | 3,838 | 5,757 | 0 | 46 | 4,370 | 0 |
| 11 | 11,400 | 18,000 | 0 | 102 | 3,264 | 0 |
| 15 | 2,904 | 5,456 | 0 | 80 | 1,800 | 0 |
| 18 | 4,212 | 6,552 | 0 | 0 | 3,267 | 0 |
| 22 | 3,275 | 8,515 | 0 | 104 | 2,288 | 52 |
| 25 | 3,872 | 7,623 | 0 | 0 | 1,520 | 16 |
| 29 | 2,640 | 8,640 | 0 | 40 | 1,703 | 178 |
| 32 | 1,936 | 6,336 | 0 | SCID2 Died 31 DPI | ||
| 36 | 1,806 | 2,021 | 0 | |||
| 39 | 1,840 | 115 | 0 | |||
| 40 | 1,092 | 84 | 0 | |||
| 43 | 1,679 | 23 | 0 | |||
| 46 | 2,772 | 0 | 0 | |||
| 48 | 1,736 | 0 | 0 | |||
| 50 | 2,250 | 0 | 0 | |||
| 53 | 2,592 | 72 | 0 | |||
| 55 | 3,128 | 0 | 0 | |||
| 57 | 2,552 | 0 | 0 | |||
| 60 | 1,458 | 0 | 0 | |||
| 62 | 1,462 | 0 | 0 | |||
| 64 | 1,092 | 0 | 0 | |||
| 69 | 1,001 | 0 | 0 | |||
| 71 | 1,100 | 0 | 0 | |||
DPI = days post-EIAV inoculation. Normal Arabian foal peripheral blood lymphocyte count = 4,119 ± 1649 μl (mean ± S.D.). Normal Arabian foal peripheral blood neutrophil count = 5,007 ± 2,101 μl. Band neutrophils are normally < 100 μl. ND = not done.
SCID2
SCID2 received a total of 6.5 × 109 stimulated lymphocytes (all 3 infusions combined), including 4.5 × 109 CD4+ lymphocytes (69.9%), 1.5 × 109 CD3+CD8+ lymphocytes (23.5%), 3.5 × 107 B lymphocytes (0.53%), and 1.9 × 107 (0.29%) EIAV-specific MHC class I-restricted CTLe. Engraftment of the infused lymphocytes did not occur, as the PBL count never exceeded 104 cells/μl (Table 2).
EIAV Replication was Controlled in SCID1 but not in SCID2
SCID1
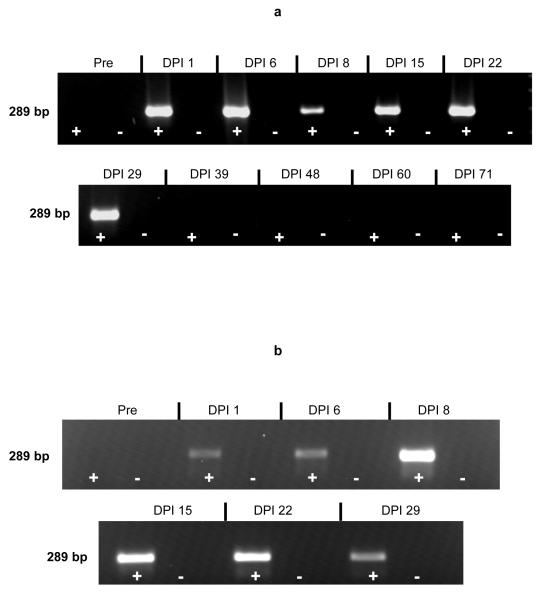
EIAV was detected in SCID1 plasma by cell culture (101.3 TCID50/ml) 15 DPI, but could not be detected 1, 8, 29, 50, 60, or 71 DPI. EIAV RNA was detected in plasma 1, 6, 8, 15, 22, and 29 DPI by RT-PCR (Fig. 2a), but could not be detected 39, 48, 60, or 71 DPI. Nested PCR detected viral RNA in the 39 DPI plasma sample, but the 48, 60, and 71 DPI samples remained negative. Despite control of plasma virus, proviral DNA was detected by PCR from post mortem splenic tissue at the end of the study, indicating that even though virus was controlled, it had not been eliminated.
Fig. 2.
EIAV-specific RT-PCR products derived from SCID1 (a) and SCID2 (b) plasma, and analyzed by electrophoresis through a 2% agarose gel. Pre = pre-EIAV inoculation. Lanes designated “+” indicate products from reactions containing reverse transcriptase, while lanes designated “-” indicate products from reactions without reverse transcriptase. Gel image obtained using the AlphaImager 2000 digital imaging system (Alpha Innotech Corp., USA)
SCID2
EIAV was detected in SCID2 plasma by cell culture 8, 15, 22, and 29 DPI (range: 101.3 - 103.2 TCID50/ml), but was not detected 1 DPI. EIAV RNA was detected in plasma 1, 6, 8, 15, 22, and 29 DPI by RT-PCR (Fig. 2b), and proviral DNA was detected by PCR from post mortem splenic tissue.
EIAV-Specific Immunity Developed in SCID1 but not in SCID2
SCID1
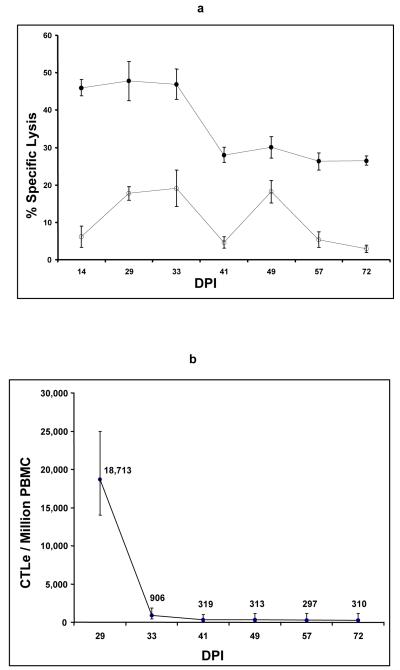
PBMC obtained between 14 and 72 DPI from SCID1 contained EIAV-specific MHC class I-restricted CTLe, with specific lysis of EIAV-infected homologous EK target cells ranging from 26 - 48% (Fig. 3a). These CTLe occurred in frequencies ranging from 297-18,700/106 PBMC (Fig. 3b). In addition, EIAV-neutralizing antibody was detected in SCID1 plasma 22 and 71 DPI (100% virus reduction, both days).
Fig. 3.
(a) CTLe activity in SCID1 PBMC against EIAV-infected homologous (closed circles) and heterologous (open circles) equine kidney (EK) cell targets. Effector:target cell ratio was 20:1. Error bars are standard error. (b) Frequency of EIAV-specific CTLe in SCID1 PBMC, as determined by limiting dilution analysis. Error bars indicate 95% confidence interval.
SCID2
Since lymphocyte engraftment did not occur in SCID2, PBMC for CTLe studies could not be obtained. EIAV-neutralizing antibody was not detected (0% virus reduction) in plasma collected 29 DPI.
EIAV-Induced Disease was Transient in SCID1 but Severe in SCID2
SCID1
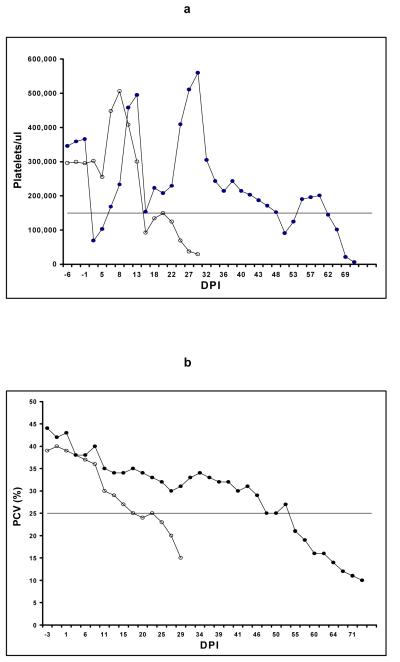
Thrombocytopenia (platelets < 151,000/μl) (7) occurred 1 DPI in SCID1, but rapidly resolved and was unlikely related to EIAV infection since infectious virus was not detected in DPI 1 plasma. Platelet numbers transiently decreased 15 DPI to 153,000/μl (Fig. 4a), associated with plasma viremia as detected by both cell culture and RT-PCR (Fig. 2a). Anemia (packed cell volume < 25%) (8) associated with plasma viremia did not occur (Figs. 4b, 2a). Clinically, the foal was bright, alert and responsive throughout the study, maintained a normal appetite, and survived to 72 DPI (Fig. 1).
Fig. 4.
(a) Platelet counts/μl for SCID1 (closed circles) and SCID2 (open circles). The horizontal line indicates the low-range normal platelet count for uninfected Arabian SCID foals (151,000/μl). DPI = days post-EIAV inoculation. (b) Packed cell volume (PCV) % for SCID1 (closed circles) and SCID2 (open circles). The horizontal line indicates the low-range normal PCV for 2-month-old uninfected Arabian SCID foals (25%).
SCID2
Persistent thrombocytopenia and anemia associated with plasma viremia occurred 15 and 18 DPI, respectively (Figs. 4a, 4b, 2b), in SCID2. The foal became listless and inappetent, and died 31 DPI (Fig. 1).
Sequelae to Lymphocyte Infusions in SCID1
Transient fever (body temperature = 40°C; normal < 38.6°C), neutropenia (normal Arabian foals: 5,007 ± 2,101 neutrophils/μl) (16) and thrombocytopenia (Table 2, Figs. 4a, 4b) occurred the day after the second lymphocyte infusion (Fig. 1) in SCID1, and were thought to be the result of an acute inflammatory response to the infusion. Persistent agranulocytosis, anemia and thrombocytopenia (Table 2, Figs. 4a, 4b) occurred in the absence of plasma viremia (Fig. 2a) after 46 DPI in SCID1. In contrast, the neutrophil count ranged from 1008 - 4370/μl in SCID2, with band neutrophils present after 22 DPI (Table 2). Evaluation of SCID1 bone marrow biopsy samples revealed a lack of hematopoietic cells, and SCID1 was euthanized 72 DPI due to presumed severe aplastic anemia. It was concluded that the aplastic anemia was the result of lymphocyte engraftment since in previous studies, aplastic anemia was not observed in 17 SCID foals inoculated with the same dose of EIAVWSU5 (7, 8, R.H. Mealey, unpublished observation).
Post mortem Examinations
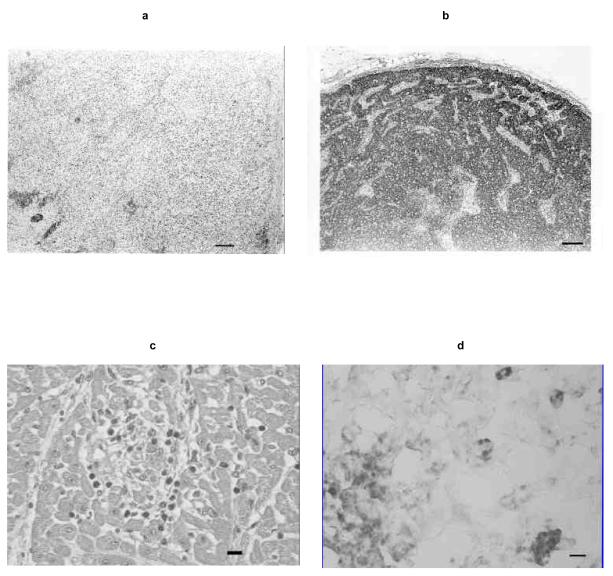
Gross and histologic findings in SCID2 were typical, including suppurative bronchopneumonia, thymic hypoplasia, absence of periarteriolar lymphocytic sheaths in the spleen, and lymph nodes devoid of follicles and lymphocytes (16) (Fig. 5a). In contrast, the lymphoid tissues of SCID1 were fully populated with lymphocytes and plasma cells (Fig. 5b), and bronchopneumonia was absent. Except for scattered lymphocytes and plasma cells, SCID1 bone marrow was hypocellular with a paucity of hematopoietic cells, supporting the ante mortem diagnosis of aplastic anemia (30). Scattered foci of lymphocytes and necrosis in SCID1 liver, kidney, and heart (Fig. 5c) suggested mild graft-versus-host disease (GVHD). Immunohistochemical labeling showed many splenic macrophages in SCID1, indicating that EIAV target cells had not been eliminated (Fig. 5d).
Fig. 5.
(a) Photomicrograph of a SCID2 lymph node section, lacking follicles and lymphocytes. Bar, 100 μm. (b) Photomicrograph of a SCID1 lymph node section, fully populated with lymphocytes. Bar, 100 μm. (c) Photomicrograph of a SCID1 heart section containing a focus of myocardial necrosis with lymphocyte infiltrates. Bar, 10μm. (d) Photomicrograph of macrophages labeled by immunohistochemistry (dark-staining cells) in a SCID1 spleen section. Bar, 10μm.
Discussion
Sustained and complete immune reconstitution in a SCID foal following histocompatible bone marrow transplant has been reported (31), as has temporary functional lymphocyte engraftment in SCID foals following transplant with equine fetal liver and thymus cells (32). This report extends previous reconstitution experiments by documenting functional lymphocyte engraftment in SCID1 that resulted in control of virus replication following adoptive transfer of virus-stimulated lymphocytes. The reason for failure of lymphocyte engraftment in SCID2 is not known. It is possible that the MHC class II mismatch between donor and recipient could have prevented CD4+ antigen recognition and lack of helper support, resulting in apoptosis of the infused lymphocyte effectors. Alternatively, endogenous NK activity in the recipient could have prevented engraftment of the allogeneic cells. A third possibility is that not enough lymphocytes were transferred to SCID2. Less than 108 cells/kg body weight were infused, a dose which seems necessary for successful engraftment of histocompatible bone marrow cells in SCID foals (31-34).
The control of plasma EIAV in SCID1 could have been mediated by CTLe, neutralizing antibody from transferred B lymphocytes, or both. Passive transfer of serum antibody was not involved since the donor PBMC were washed extensively during the initial isolation, and the lymphocytes were washed again prior to infusion. The presence of numerous macrophages ruled out the possibility that viral control was the result of target cell elimination. Interestingly, the level of EIAV-specific killing exhibited by CTLe from SCID1 following adoptive transfer decreased over time, as did the CTLe frequency (Figs. 2a, 2b), similar to that observed following adoptive transfer of HIV-1-specific autologous CTL clones in HIV-1-infected patients and in (hu)-PBL-SCID mice (6, 35). Whether this was due to CTLe apoptosis secondary to decreased viral load or elimination of virus infectivity by neutralizing antibody, or alternatively, CTLe elimination due to encounter with infected target cells (6), is not known. Despite the decrease, the CTLe frequency remained consistent with that observed for CTLm in inapparent EIAV carrier horses (24).
Although EIAV RNA was unexpectedly detected in DPI 1 plasma by RT-PCR in both SCID foals, this probably reflected the presence of non-infectious virus particles administered in the inoculum. Plasma levels of HIV-1 determined by PCR methods are 60,000-fold higher than virus titers determined by endpoint dilution culture (36), and the discrepancy is likely due to substantial proportions of defective or otherwise non-infectious virus (37).
The initial 16% decline and subsequent dramatic elevation in platelet counts in SCID2 shortly after virus inoculation was consistent with previous observations (7), and was not likely related to EIAV infection, since infectious virus was not present in plasma at that time. As hypothesized in the previous study, the unexplained fluctuations and bizarre elevations in platelet numbers observed in both foals could have been due to stress (7). The initial thrombocytopenia in SCID1 was also not likely related to EIAV infection, but might have been due to sequestration secondary to a systemic inflammatory response to the infused lymphocytes or to other proteins present in the infusion or in the cell culture-derived virus inoculum. However, the decline in platelet counts in both foals 15 DPI associated with plasma virus detected by both RT-PCR and cell culture was also consistent with the previous study (7) and was considered a result of EIAV infection. The subsequent resolution of thrombocytopenia and decline in plasma viremia observed in SCID1 was similar to that observed in EIAV-infected immunocompetent foals (7).
Based on the absence of plasma viremia, it is unlikely that the thrombocytopenia, anemia, and agranulocytosis observed in SCID1 after 46 DPI were due to EIAV. Agranulocytosis is not typical of EIAV infection (38-40), and it is not a feature of equine SCID (16, 41). Furthermore, agranulocytosis did not occur in 17 SCID foals infected with the same dose and strain of EIAV used in this study (7, 8, R.H. Mealey, unpublished observation) and bone marrow evaluations in EIAV-infected SCID foals do not show evidence of aplastic anemia (7). Although SCID2 was neutropenic during the current study, the likely cause was neutrophil consumption secondary to septic bronchopneumonia. The presence of band neutrophils indicated that SCID2 bone marrow remained functional. In SCID1, the neutropenia and subsequent neutrophilia observed early in the experiment was consistent with systemic inflammation, and reflected a normal bone marrow response. After agranulocytosis developed however, bone marrow evaluation supported a diagnosis of aplastic anemia, a possible manifestation of GVHD. Although SCID1 shared ELA-A, DRA, and DQA haplotypes with its lymphocyte donor, mismatches at other MHC class I and II loci were likely. In humans, autoreactive T lymphocytes have been implicated in the pathogenesis of aplastic anemia (42, 43), and aplastic anemia has been reported following allogeneic lymphocyte infusion (44-46).
When viewed in the context of previous work utilizing EIAV-infected SCID foals, this study provides clear evidence that viral-specific immunity is required for EIAV control. Additionally, these results show that adoptive transfer experiments in SCID foals are feasible. This model should allow further dissection of the relative contributions of neutralizing antibody and CTL to EIAV control, providing useful information for future vaccine strategies.
Acknowledgments
The authors appreciate the technical assistance of Steve Leib, Emma Karel, Andi Parsons, Alison Brendel, Susan Simon, and Shirley Elias. This research was supported by U.S. Public Health Service, National Institutes of Health, grants AI01575, AI24291, and AI44638.
References
- 1.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J.Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Letvin NL, Schmitz JE, Jordan HL, Seth A, Hirsch VM, Reimann KA, Kuroda MJ. Cytotoxic T lymphocytes specific for the simian immunodeficiency virus. Immunological Reviews. 1999;170:127–34. 127–134. doi: 10.1111/j.1600-065x.1999.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 3.McGuire TC, Tumas DB, Byrne KM, Hines MT, Leib SR, Brassfield AL, O’Rourke KI, Perryman LE. Major histocompatibility complex-restricted CD8+ cytotoxic T lymphocytes from horses with equine infectious anemia virus recognize Env and Gag/PR proteins. J.Virol. 1994;68:1459–1467. doi: 10.1128/jvi.68.3.1459-1467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips AN. Reduction of HIV concentration during acute infection: independence from a specific immune response. Science. 1996;271:497–499. doi: 10.1126/science.271.5248.497. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 6.McKinney DM, Lewinsohn DA, Riddell SR, Greenberg PD, Mosier DE. The antiviral activity of HIV-specific CD8+ CTL clones is limited by elimination due to encounter with HIV-infected targets. J.Immunol. 1999;163:861–867. [PubMed] [Google Scholar]
- 7.Crawford TB, Wardrop KJ, Tornquist SJ, Reilich E, Meyers KM, McGuire TC. A primary production deficit in the thrombocytopenia of equine infectious anemia. J.Virol. 1996;70:7842–7850. doi: 10.1128/jvi.70.11.7842-7850.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perryman LE, O’Rourke KI, McGuire TC. Immune responses are required to terminate viremia in equine infectious anemia lentivirus infection. J.Virol. 1988;62:3073–3076. doi: 10.1128/jvi.62.8.3073-3076.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin EK, Perryman LE, Meek K. A kinase-negative mutation of DNA-PK(CS) in equine SCID results in defective coding and signal joint formation. J.Immunol. 1997;158:3565–3569. [PubMed] [Google Scholar]
- 10.Perryman LE, Torbeck RL. Combined immunodeficiency of Arabian horses: confirmation of autosomal recessive mode of inheritance. Journal Of The American Veterinary Medical Association. 1980;176:1250–1251. [PubMed] [Google Scholar]
- 11.McGuire TC, Crawford TB, Henson JB. Immunofluorescent localization of equine infectious anemia virus in tissue. Am.J.Pathol. 1971;62:283–294. [PMC free article] [PubMed] [Google Scholar]
- 12.Sellon DC, Perry ST, Coggins L, Fuller FJ. Wild-type equine infectious anemia virus replicates in vivo predominantly in tissue macrophages, not in peripheral blood monocytes. J.Virol. 1992;66:5906–5913. doi: 10.1128/jvi.66.10.5906-5913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lonning SM, Zhang W, Leib SR, McGuire TC. Detection and induction of equine infectious anemia virus-specific cytotoxic T-lymphocyte responses by use of recombinant retroviral vectors. J.Virol. 1999;73:2762–2769. doi: 10.1128/jvi.73.4.2762-2769.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuire TC, Leib SR, Lonning SM, Zhang W, Byrne KM, Mealey RH. Equine infectious anaemia virus proteins with epitopes most frequently recognized by cytotoxic T lymphocytes from infected horses. J.Gen.Virol. 2000;81:2735–2739. doi: 10.1099/0022-1317-81-11-2735. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Lonning SM, McGuire TC. Gag protein epitopes recognized by ELA-A-restricted cytotoxic T lymphocytes from horses with long-term equine infectious anemia virus infection. J.Virol. 1998;72:9612–9620. doi: 10.1128/jvi.72.12.9612-9620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuire TC, Poppie MJ, Banks KL. Combined (B- and T-lymphocyte) immunodeficiency: a fatal genetic disease in Arabian foals. Journal Of The American Veterinary Medical Association. 1974;164:70–76. [PubMed] [Google Scholar]
- 17.Perryman LE, McGuire TC, Crawford TB. Maintenance of foals with combined immunodeficiency: causes and control of secondary infections. Am.J.Vet.Res. 1978;39:1043–1047. [PubMed] [Google Scholar]
- 18.O’Rourke K, Perryman LE, McGuire TC. Antiviral, anti-glycoprotein and neutralizing antibodies in foals with equine infectious anaemia virus. J.Gen.Virol. 1988;69:667–674. doi: 10.1099/0022-1317-69-3-667. [DOI] [PubMed] [Google Scholar]
- 19.Bailey E. Identification and genetics of horse lymphocyte alloantigens. Immunogenetics. 1980;11:499–506. doi: 10.1007/BF01567818. [DOI] [PubMed] [Google Scholar]
- 20.Antczak DF, Bailey E, Barger B, Guerin G, Lazary S, McClure J, Mottironi VD, Symons R, Templeton J, Varewyck H. Joint report of the Third International Workshop on Lymphocyte Alloantigens of the Horse, Kennett Square, Pennsylvania, 25-27 April 1984. Anim.Genet. 1986;17:363–373. doi: 10.1111/j.1365-2052.1986.tb00730.x. [DOI] [PubMed] [Google Scholar]
- 21.Ellis SA, Martin AJ, Holmes EC, Morrison WI. At least four MHC class I genes are transcribed in the horse: phylogenetic analysis suggests an unusual evolutionary history for the MHC in this species. Eur.J.Immunogenet. 1995;22:249–260. doi: 10.1111/j.1744-313x.1995.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 22.Albright-Fraser DG, Reid R, Gerber V, Bailey E. Polymorphism of DRA among equids. Immunogenetics. 1996;43:315–317. [PubMed] [Google Scholar]
- 23.Fraser DG, Bailey E. Polymorphism and multiple loci for the horse DQA gene. Immunogenetics. 1998;47:487–490. doi: 10.1007/s002510050387. [DOI] [PubMed] [Google Scholar]
- 24.McGuire TC, Zhang W, Hines MT, Henney PJ, Byrne KM. Frequency of memory cytotoxic T lymphocytes to equine infectious anemia virus proteins in blood from carrier horses. Virology. 1997;238:85–93. doi: 10.1006/viro.1997.8795. [DOI] [PubMed] [Google Scholar]
- 25.Tschetter JR, Davis WC, Perryman LE, McGuire TC. CD8 dimer usage on alpha beta and gama delta T lymphocytes from equine lymphoid tissues. Immunobiology. 1998;198:424–438. doi: 10.1016/s0171-2985(98)80050-8. [DOI] [PubMed] [Google Scholar]
- 26.Kydd J, Antczak DF, Allen WR, Barbis D, Butcher G, Davis W, Duffus WP, Edington N, Grunig G, Holmes MA, et al. Report of the First International Workshop on Equine Leucocyte Antigens, Cambridge, UK, July 1991. Vet.Immunol.Immunopathol. 1994;42:3–60. doi: 10.1016/0165-2427(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Auyong DB, Oaks JL, McGuire TC. Natural variation of equine infectious anemia virus Gag protein cytotoxic T lymphocyte epitopes. Virology. 1999;261:242–252. doi: 10.1006/viro.1999.9862. [DOI] [PubMed] [Google Scholar]
- 28.McGuire TC, Norton LK, O’Rourke KI, Cheevers WP. Antigenic variation of neutralization-sensitive epitopes of caprine arthritis-encephalitis lentivirus during persistent arthritis. J.Virol. 1988;62:3488–3492. doi: 10.1128/jvi.62.9.3488-3492.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tumas DB, Brassfield AL, Travenor AS, Hines MT, Davis WC, McGuire TC. Monoclonal antibodies to the equine CD2 T lymphocyte marker, to a pan-granulocyte/monocyte marker and to a unique pan-B lymphocyte marker. Immunobiology. 1994;192:48–64. doi: 10.1016/S0171-2985(11)80407-9. [DOI] [PubMed] [Google Scholar]
- 30.Jandl JH. Blood: Textbook of Hematology. Little, Brown and Company; New York: 1996. Aplastic Anemia; pp. 201–250. [Google Scholar]
- 31.Bue CM, Davis WC, Magnuson NS, Mottironi VD, Ochs HD, Wyatt CR, Perryman LE. Correction of equine severe combined immunodeficiency by bone marrow transplantation. Transplantation. 1986;42:14–19. doi: 10.1097/00007890-198607000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Perryman LE, Bue CM, Magnuson NS, Mottironi VD, Ochs HS, Wyatt CR. Immunologic reconstitution of foals with combined immunodeficiency. Vet.Immunol.Immunopathol. 1987;17:495–508. doi: 10.1016/0165-2427(87)90165-6. [DOI] [PubMed] [Google Scholar]
- 33.Ardans AA, Trommershausen-Smith A, Osburn BI, Mayhew IG, Trees C, Park MI, Sawyer M, Stabenfeldt GH. Immunotherapy in two foals with combined immunodeficiency, resulting in graft versus host reaction. Journal Of The American Veterinary Medical Association. 1977;170:167–175. [PubMed] [Google Scholar]
- 34.Campbell TM, Studdert MJ, Ellis WM, Paton CM. Attempted reconstitution of a foal with primary severe combined immunodeficiency. Equine Veterinary Journal. 1983;15:233–237. doi: 10.1111/j.2042-3306.1983.tb01776.x. [DOI] [PubMed] [Google Scholar]
- 35.Brodie SJ, Lewinsohn DA, Patterson BK, Jiyamapa D, Krieger J, Corey L, Greenberg PD, Riddell SR. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat.Med. 1999;5:34–41. doi: 10.1038/4716. [DOI] [PubMed] [Google Scholar]
- 36.Piatak MJ, Saag MS, Yang LC, Clark SJ, Kappes JC, Luk KC, Hahn BH, Shaw GM, Lifson JD. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993;259:1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- 37.Wei X, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, Lifson JD, Bonhoeffer S, Nowak MA, Hahn BH. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 38.McGuire TC, O’Rourke KI, Perryman LE. Immunopathogenesis of equine infectious anemia lentivirus disease. Dev.Biol.Stand. 1990;72:31–37. [PubMed] [Google Scholar]
- 39.Montelaro RC, Ball JM, Rushlow KE. Equine Retroviruses. In: Levy JA, editor. The Retroviridae. Plenum Press; New York and London: 1993. pp. 257–360. [Google Scholar]
- 40.Sellon DC, Fuller FJ, McGuire TC. The immunopathogenesis of equine infectious anemia virus. Virus Res. 1994;32:111–138. doi: 10.1016/0168-1702(94)90038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banks KL, McGuire TC. Surface receptors on neutrophils and monocytes from immunodeficient and normal horses. Immunology. 1975;28:581–588. [PMC free article] [PubMed] [Google Scholar]
- 42.Maciejewski JP, Hibbs JR, Anderson S, Katevas P, Young NS. Bone marrow and peripheral blood lymphocyte phenotype in patients with bone marrow failure. Exp.Hematol. 1994;22:1102–1110. [PubMed] [Google Scholar]
- 43.Melenhorst JJ, van KJ, Dreef E, Landegent JE, Willemze R, Fibbe WE. T cells selectively infiltrate bone marrow areas with residual haemopoiesis of patients with acquired aplastic anaemia. Br.J.Haematol. 1997;99:517–519. doi: 10.1046/j.1365-2141.1997.4353245.x. [DOI] [PubMed] [Google Scholar]
- 44.Garicochea B, van Rhee F, Spencer A, Chase A, Lin F, Cross NC, Goldman JM. Aplasia after donor lymphocyte infusion (DLI) for CML in relapse after sex-mismatched BMT: recovery of donor-type haemopoiesis predicted by non-isotopic in situ hybridization (ISH) Br.J.Haematol. 1994;88:400–402. doi: 10.1111/j.1365-2141.1994.tb05039.x. [DOI] [PubMed] [Google Scholar]
- 45.Hathaway WE, Fulginiti VA, Pierce CW, Githens JH, Pearlman DS, Muschenheim F, Kempe CH. Graft-vs-host reaction following a single blood transfusion. JAMA. 1967;201:1015–1020. [PubMed] [Google Scholar]
- 46.Porter DL, Roth MS, McGarigle C, Ferrara JL, Antin JH. Induction of graft-versus-host disease as immunotherapy for relapsed chronic myeloid leukemia. N.Engl.J.Med. 1994;330:100–106. doi: 10.1056/NEJM199401133300204. [DOI] [PubMed] [Google Scholar]