Abstract
Cancer patients without evidence of brain metastases often exhibit constitutional symptoms, cognitive dysfunction and mood changes at the time of clinical diagnosis, i.e. prior to surgical and/or chemotherapy treatment. At present however, there is limited information on brain metabolic and functional status in patients with systemic cancers such as lung cancer prior to initiation of treatment. Therefore, a prospective, observational study was conducted on patients with a clinical diagnosis of lung cancer to assess the cerebral metabolic status before treatment using proton magnetic resonance spectroscopy (1HMRS). Together with neurocognitive testing, 1HMRS was performed in the parietal and occipital cortices of patients diagnosed with a lung mass (N=17) and an age-matched control group (N=15). Glutamate concentrations in the occipital cortex were found to be lower in the patients compared to controls and the concentrations of creatine and phosphocreatine were significantly lower in the parietal cortex of the patients. The lung cancer patients were also characterized by greater fatigue scores (but not depression) prior to treatment when compared to controls. In addition, the serum concentration of interleukin-6 (proinflammatory cytokine) was higher in patients compared to controls; and the concentration of tumor-necrosis factor alpha ([TNF-α]) was positively correlated to the metabolic activity of the lung tumor as defined by the 2-deoxy-2-(18F)fluoro-D-glucose (18FDG) positron emission tomography (PET) derived maximal standardized uptake values (SUVmax). Finally, multivariate statistical modeling revealed that the concentration of N-acetyl-aspartate [NAA] in the occipital cortex was negatively associated with [TNF-α]. In conclusion, our data demonstrate that the cerebral metabolic status of patients with lung cancer is changed even prior to treatment. In addition, the association between inflammatory cytokines, SUVmax and [NAA] points towards interactions between the cancer’s inherent metabolic activity, systemic subclinical inflammation and brain function.
Keywords: Lung cancer, proton magnetic resonance spectroscopy, brain, glutamate, proinflammatory cytokines, fatigue
Introduction
It is undisputed that cancer patients exhibit constitutional symptoms (e.g. weight loss, loss of appetite, fatigue and malaise) at the time of clinical diagnosis [1-3]. However, whether or not molecular signatures exist for these symptoms is far less clear. Even less information is available as to how these symptoms and their molecular equivalents (if existent) relate to brain metabolic function and ultimately, cancer outcomes. The importance of a cancer’s direct effects on brain function is evidenced by recent prospective studies showing that as many as 20% of cancer patients can experience cognitive dysfunction and mood changes prior to treatment [4-6].
Brain imaging techniques such as positron emission tomography (PET) have documented significant changes in the resting cerebral metabolic rate of glucose (reflecting brain functional activity) in patients with primary brain cancer or brain metastases and in breast cancer patients although after chemotherapy treatment [7-9]. However, there is very limited information in the literature on brain metabolic and functional status in patients with systemic cancers such as breast, prostate or lung cancer prior to treatment. This gap in knowledge is likely related to the complexity of conducting such studies in newly diagnosed cancer patients (without evidence of brain metastases), already overwhelmed by what is perceived as a time sensitive medical emergency with life threatening implications, requiring diagnostic testing procedures; and the often urgent need for surgical intervention.
To our knowledge, no imaging studies have documented brain metabolic status or neurochemical profiles prior to treatment in patients with lung cancer, although these patients typically present with significant constitutional symptoms and often advanced disease. We therefore conducted a study to investigate the feasibility of characterizing brain metabolic status by non-invasive proton magnetic resonance spectroscopy (1HMRS) in parallel with brief assessment of neurocognitive and mood status as well as systemic inflammatory status in patients with a clinical diagnosis of lung cancer before treatment. Non-invasive 1HMRS allows for an evaluation of the metabolic status of the brain in real time by tracking levels of metabolites involved in ‘energetics’ [10]. We hypothesized that patients with malignant lung cancers would display cerebral metabolic profile changes in comparison to non-cancer controls.
Materials and methods
Subjects
Eligible patients for this prospective, observational study included those who were referred to our surgical oncology clinic with a lung mass. Age-matched controls were recruited from the local community. We excluded subjects with severe psychiatric and neurological illness, vision or hearing impairment, liver or kidney failure, addiction to drugs of abuse and those who had been on any type of chemotherapy 6 months prior to study participation. All participants were subjected to the Mini-Cog and/or Mini Mental Status Exam for dementia screening. A score of 24 or higher on the Mini Mental Status Exam was required for eligibility. The verbal IQ of the subjects was also assessed by the Wechsler Test of Adult Reading. The subjects gave written informed consent and the study was approved by the local institutional review board.
Experimental design and data collection
All patients underwent 1) anatomical magnetic resonance imaging (MRI) followed by 1HMRS, 2) neurocognitive testing and 3) blood sampling for analysis of two cytokines, TNF-α and interleukin- 6 (IL-6) prior to treatment; in parallel with control subjects. Additional data collection for all subjects included laboratory screening tests, medical history and physical exam. For the patients the whole-body [18F]fluoro-2-deoxyglucose positron emission tomography (18FDG PET) scans and corresponding maximum standardized uptake values (SUVmax) of the lung mass obtained during the clinical work up were also acquired for analysis; as well as diagnosis of the lung mass by pathology attained in conjunction with surgical resection and/or biopsy.
1HMRS scanning
MRI procedures for all patients and controls were conducted on a 3.0T Philips whole body scanner (Achieva system) equipped with a 12 channel phase array head coil; and included an initial T1 weighted 3D anatomical scan acquired with the following parameters: field of view =240mm, repetition time =8.5 ms, echo time=4 ms, Flip Angle=8°, 1.0 mm slice thickness with an acquisition matrix of 240×240, yielding a reconstructed isotropic voxel dimension of 1.00mm3. 1HMRS was performed by using a point-resolved spectroscopy sequence acquired in the parietal (15 x 15 x 15 mm3) and in the occipital lobe (15 x 15 x 27 mm3) with short echo time (32ms), repetition time of 2 seconds, receiver bandwidth=2000Hz, number of points=2048, and number of excitations = 256. Both shimming and water suppression routines were performed with automatic adjustments. A water unsuppressed scan was used to perform eddy current correction and to serve as a concentration reference for absolute quantification of metabolite concentrations.
Spectral data analysis
Data analysis of 1HMRS spectra was performed using linear combination modeling (LCModel [11]) with prior knowledge of simulated spectral signatures for the following brain metabolites: Alanine, Aspartate, Creatine (Cr), Phosphocreatine (PCr), γ-aminobutyric acid, Glucose, Glutamine (Gln), Glutamate (Glu), Glycerophosphocholine (GPC), Phosphocholine (PCh), myo-Inositol, Lactate, N-Acetyl-Aspartate (NAA), N-acetyl-aspartyl-glutamate, Scyllo-inositol, Taurine and Guanidoacetate; in addition to lipid and macromolecules. No baseline correction, zero-filling or apodization functions were applied to the data prior to the analysis. Figure 1 shows a typical processed 1HMRS spectra from the parietal and occipital cortices and the respective voxel positions from a control subject. A quality analysis of all spectra was performed and included evaluation of the signal-to-noise ratio, spectral width (full-width half maximum), baseline and residual tracings derived from the LCModel analysis. All spectra with signal-to-noise ratio <8 and a full-width half maximum >0.080 ppm were considered of poor quality and excluded from data analysis.
Figure 1.
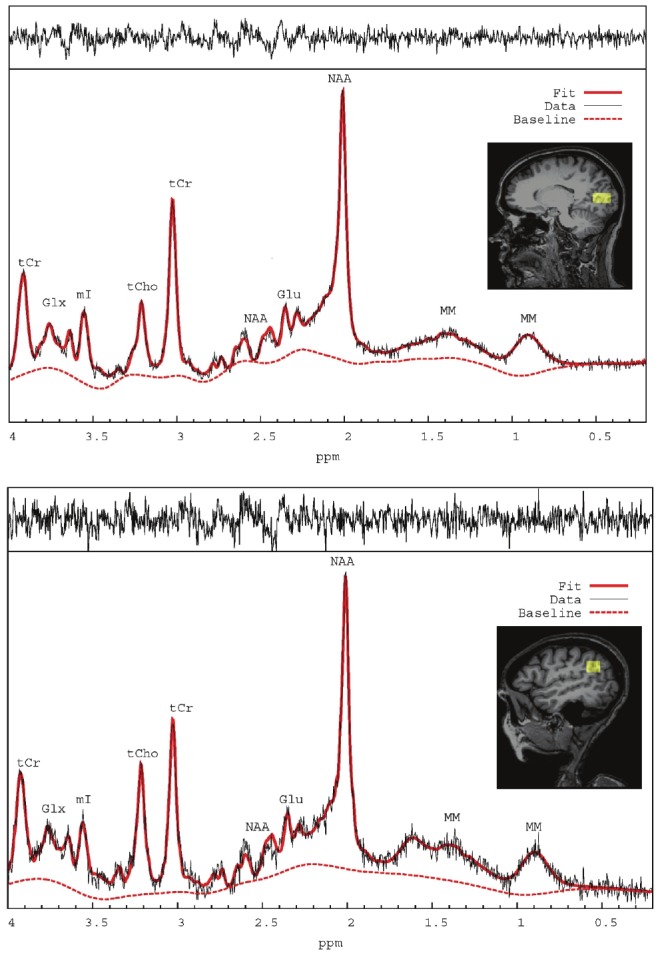
Localized 1HMRS spectra processed by LCModel software from the occipital cortex (top) and parietal cortex (bottom) with the appropriate locations in parietal and occipital cortex shown on corresponding T1-weighted MR images. Labeling of the spectral signatures for glutamate (Glu), N-Acetyl-Asparate (NAA), Glutamate + Glutamine = GLX, total choline (tCho), total creatine (tCr), myo-inositol (mI) and macromolecules (MM). The raw (black) and fitted (solid red line) spectrum as well as the remaining baseline (stippled red line) are shown in each of the spectra.
Neurocognitive testing
A brief test battery was administered by an experienced psychologist to all subjects. The domains assessed were the following: Fatigue (Profile of Mood States (POMS)) [12]; Depression (Profile of Mood States (POMS)) [12]; Long-term memory (Hopkins Verbal Learning Test- Revised); Short term memory (Digit Span) and Psychomotor Learning (Digit Symbol Coding) (WAIS-III) [13]; Verbal Fluency (semantic generation and response inhibition).
Analysis of blood for cytokines
Serum samples were prepared from freshly drawn blood that had been permitted to clot for 25-30 min. The samples were flash frozen in liquid N2 and stored at -80 °C until analysis. (Quantikine and Quantikine HS immunoassay kits, R&D Systems, Inc, MN) were used to measure the serum levels of each cytokine in the samples according to the manufacturer’s instructions. The color intensities were read at 450 nm (IL-6) or 490 nm (TNF-α), corrected at 540 nm, or 640 nm, respectively, corrected to the appropriate background absorbencies and compared to the corresponding cytokine standard curve. The cytokine concentration in each sample, expressed in pg/ml, was calculated from the standard curve equation derived from the linear fit to the standards. The assays had confirmed sensitivities of 1 and 0.2 pg/ml for IL-6 and TNF-α, respectively.
Statistical analysis
A two-sided independent t-test (Mann-Whitney-U test without normality) or Fisher’s exact test, where appropriate were used to examine group differences in demographic and co-morbidity parameters at baseline. The neurocognitive and mood test scores between the two groups were compared using the non-parametric Mann-Whitney two-tailed test. Differences in cytokine concentrations between the two groups; and metabolite concentrations for each brain region calculated by LCModel for the two groups were analyzed by an independent t-test, and statistical significance was determined using a Type I error threshold of 0.05. Differences in metabolite concentrations between occipital and parietal cortices within groups were assessed using a paired, two-sided t-test. A multiple regression analysis was performed on the metabolic activity of lung tumors (SUVmax) and the corresponding levels of proinflammatory cytokines. Finally, the relation between inflammatory markers and LCModel quantified metabolites concentrations was explored using multivariate approach with a stepwise selection to select the significant metabolites in relation to the concentration of proinflammatory markers. Analysis was conducted using SAS software and XLSTAT (Version 2011.4.03).
Results
Subjects
A total of 37 subjects were assessed for eligibility; 17 controls (recruited from the local community) and 20 patients referred to the surgical oncology clinic with a lung mass. Two subjects in the control group and three in the patient group were unable to undergo MRI. One of the patients was found to have two metastases in the cerebellum. The demographics of the two groups are listed in Table 1; and show no differences, in age, gender, educational level, frequency of employment and verbal IQ. Differences in smoking history were significantly different between the groups. The average body mass index of the patients was slightly higher compared to controls at a significance level of 0.048 (Table 1). Table 2 shows comorbidity data for the two groups and demonstrates that the frequency of chronic obstructive lung disease (COPD) and diabetes mellitus was significantly higher in the patient group when compared to controls. All of the control subjects and patients had hematocrits ≥ 35%, however the average hematocrits of the patients was slightly higher compared to controls (patient hematocrits: 41.8% ± 2.9% versus control hematocrits: 38.9% ± 3.8%, p=0.02) probably secondary to the higher frequency of tobacco use in the former group.
Table 1.
Basic demographics and social history
| Parameter | Controls (N=15) | Patients (N=17) | P-value | |
|---|---|---|---|---|
| Age (Mean ± SD) | 61.9 ± 9.0 | 59.4 ± 11.3 | 0.49 | |
| Gender | Male N, (%) | 8, (53%) | 8, (47%) | 1.0 |
| Female N, (%) | 7, (47%) | 9, (53%) | ||
| BMI | 26.8 ± 3.1 | 30.1 ± 5.4 | 0.048 | |
| Smoking history | Active Smoker N, (%) | 3, (20%) | 10, (59%) | |
| Former Smoker N, (%) | 2, (13%) | 6, (35%) | 0.001** | |
| Never Smoked N, (%) | 10, (67%) | 1, (6%) | ||
| Employed N, (%) | 8, (53.3%) | 8, (47%) | 1.0 | |
| Education years (Mean ± SD) | 15.6 ± 3.8 | 13.7 ± 2.7 | 0.18 | |
| Verbal IQ (estimated by WTAR) | 108.9 ± 10.3 | 101.8 ± 11.9 | 0.09 | |
Table 2.
Co-Morbidity
| Disease | Controls (N=15) | Pathints (N=16) | P-value |
|---|---|---|---|
| COPD N, (%) | 0, (0%) | 6, (35.3%) | 0.019* |
| Hypertension N, (%) | 6, (40%) | 11, (64.7%) | 0.287 |
| Cardiac Disease N, (%) | 2, (13.3%) | 6, (37.5%) | 0.229 |
| Thyroid Disease N, (%) | 4, (26.7%) | 4, (23.5%) | 1.0 |
| Diabetes Mellitus N, (%) | 0, (0%) | 7, (41.1%) | 0.008** |
| Prior Cancer N, (%) | 1, (6.7%) | 3, (18.7%) | 0.603 |
Pathology of lung tumors
Pathology revealed malignant lesions in 13 patients and benign lesions in 4 (Table 3). Seventy-seven percent of the patients with malignant lung lesions received a histological diagnosis of adenocarcinoma and 46% were classified as stage I, 31% stage II and 15% stage III according to the International Association for the Study of Lung Cancer staging system [14,15]. Only one of the patients with a malignant lung cancer had evidence of metastases in the brain. Table 3 also shows the corresponding 18FDG PET SUVmax characterizing the metabolic activity of the lung lesion, used to assess potential malignancy of the lung lesion prior to treatment.
Table 3.
Surgical pathology of lung tumors and corresponding SUVmax values
| Patient ID | Surgical Pathology | Staging | 18FDG SUVmax |
|---|---|---|---|
| P-001 | Adenocarcinoma | IIIa | 9.0 |
| P-011 | Aspergilloma | N/A | 4.9 |
| P-012 | Carcinoid tumor | N/A | 2.7 |
| P-013 | Squamous cell carcinoma | IIa | 6.4 |
| P-017 | Squamous cell carcinoma | IIb | 7.7 |
| P-018 | Adenocarcinoma | Ia | 2.5 |
| P-019 | Adenocarcinoma | Ib | 1.0 |
| P-020 | Nodule of chronic inflammation | N/A | 3.6 |
| P-021 | Adenocarcinoma | Ia | 7.6 |
| P-023* | Adenocarcinoma | IV | 15.7 |
| P-024 | Adenocarcinoma | IIb | 6.0 |
| P-026 | Hamartoma | N/A | 0.0 |
| P-027 | Adenocarcinoma | Ib | 18.8 |
| P-029 | Adenocarcinoma | IIIa | 8.4 |
| P-030 | Adenocarcinoma | Ia | 1.6 |
| P-036 | Adenocarcinoma | IIb | 6.0 |
| P-037 | Squamous cell carcinoma | Ib | 13.6 |
P-023 was found to have metastases in the cerebellum
Mood status and cognitive performance
Analysis of POMS fatigue scores were higher in patients compared to controls (Patients: 7.3 ± 4.2 versus Controls: 3.5 ± 3.3, p=0.009). However, there were no group differences in POMS depression scores (p=0.18). Table 4 shows neurocognitive performance in patients and controls and demonstrates no differences in regards to memory, learning and psychomotor functioning between the two groups.
Table 4.
Mood status and cognitive performance
| Measure | Domain | Control (N=15) | Patient (N=17) | P-value |
|---|---|---|---|---|
| Hopkins Verbal Learning Test-Revised (% Retained) | Long-Term Memory | 92.2 (20.3) | 90.0 (19.5) | 0.716 |
| Digit Symbol Coding (Age adjusted) | Learning & Psychomotor function | 11.7 (3.2) | 10.7 (3.2) | 0.477 |
| Digit Span (Age adjusted) | Working Memory | 11.1 (2.8) | 10.3 (2.7) | 0.434 |
| Verbal Fluency | Semantic Generation, Response Inhibition | 36.2 (9.7) | 39.2 (12.8) | 0.582 |
Data are presented as mean ± (SD)
Brain metabolites
The average signal-to-noise ratio calculated based on the NAA peak was ~13 and ~20 for parietal and occipital spectra, respectively; and the average full-width half maximum ~0.055 ppm. The average Cramer-Rao-Lower-Bounds (CRLB), reflective of reliability of measurements, were consistently within acceptable range (<20%) across subjects for analyzing the concentrations of the following metabolites: Glutamate [Glu] (CRLB ~ 14%), N-acetyl-aspartate [NAA] (CRLB ~ 5%), Glutamate + Glutamine [Glu+Gln] (CRLB ~ 15%), Creatine + Phosphocreatine [Cr + PCr] (CRLB ~3%) and the total choline containing compounds Glycerophosphocholine and Phosphocholine [GPC + PCh] (CRLB ~5).
In control subjects the concentrations of [Glu], [NAA], [Cr+PCr] and [Glu+Gln] of the occipital cortex was similar to that measured in the parietal cortex; however [GPC+PCh] was higher in parietal compared to occipital cortex (Table 5). In the patient group [GPC+PCh] was also higher in the parietal cortex when compared to occipital cortex; and in addition [NAA] was lower in the parietal compared to occipital cortex (Table 5). The quantitative analysis further demonstrated that the occipital cortex [Glu] was significantly lower in the patients when compared to controls at baseline (Patients: 5.99 mM ± 0.78 versus Controls: 7.00 ± 1.04 mM, p=0.011, Figure 2 and Table 5). This difference in [Glu] was slightly more significant if patients with benign lesions were excluded from the patient group (p=0.003); and also remained significant if the patient with brain metastases was excluded (p=0.005). There were no differences in the concentrations of other occipital metabolites ([Cr+PCr], [NAA] and/or choline containing compounds) between the two groups. Since it has been documented that [Glu] in the brain is age-dependent [16] we also performed an one-way ANCOVA for [Glu] with age as a covariate including all subjects. This analysis demonstrated no age effect (p=0.92) but a significant group difference in [Glu] (p=0.02). As shown in Table 5, in the parietal cortex, the concentrations of [Cr+PCr] were lower in the patients compared to controls (p=0.035); and [NAA] also trended to be ~10% lower in the patients (p=0.09).
Table 5.
Neurochemical profile of controls and patients
| Metabolite | Control | Patient | Control | Patient |
|---|---|---|---|---|
| Occipital Cortex (N=12) | Occipital Cortex (N=13) | Parietal Cortex (N=11) | Parietal Cortex (N=13) | |
| [Glu] | 7.00 (1.04) | 5.99 (0.78)** | 7.07 (1.13) | 6.72 (1.52) |
| [NAA] | 8.80 (0.72) | 8.52 (0.37) | 8.51 (0.86) | 7.87 (0.97)# |
| [GPC + CPh] | 1.04 (0.15) | 1.09 (0.23) | 1.47 (0.24)₮ | 1.36 (0.25)# |
| [Cr + PCr] | 6.89 (0.55) | 6.76 (0.54) | 6.79 (0.66) | 6.28 (0.46)* |
| [Glu + Gln] | 7.40 (2.22) | 6.38 (0.92) | 7.50 (1.28) | 7.17 (1.59) |
Data are presented as mean and (SD).
P=0.011(comparison of occipital [Glu] between controls and patients);
p=0.035 (comparison of parietal [Cr + PCr] between controls and patients);
p < 0.001 (comparison of occipital versus parietal metabolites of control subjects);
p < 0.02 (for comparison of occipital and parietal metabolites of patients)
Figure 2.
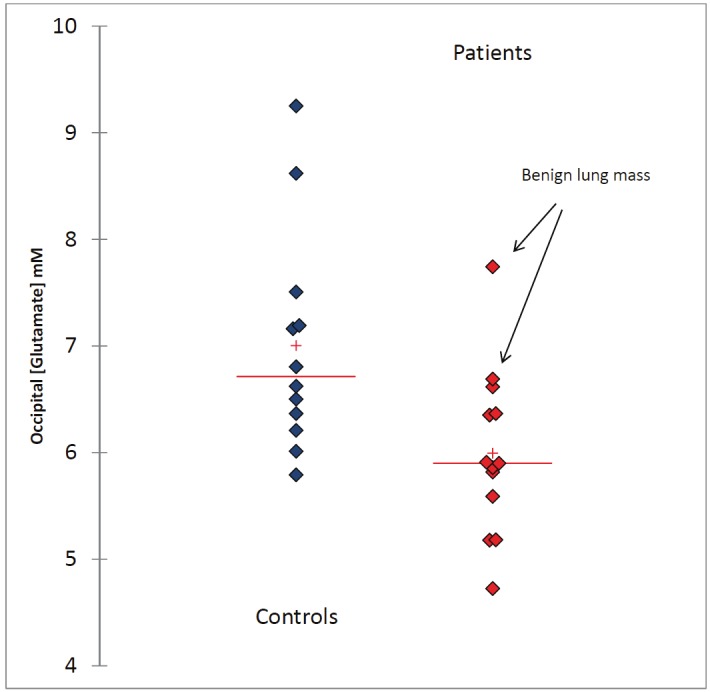
The scattergram shows occipital [Glu] from individual subjects in the control and patient groups and demonstrates that the mean concentration of [Glu] in the patients is lower compared to controls. The arrows point to two patients with the highest levels of [Glu] who were both found to have benign lung lesions.
Proinflammatory markers, SUVmax of the lung mass and relation to brain metabolites
The proinflammatory cytokines TNF-α and IL-6 from the patients with malignancy were compared with the controls without cancer. The average [TNF-α] of all patients was within normal range reported in the literature (< 15 pg/ml, [17]) and not different from controls. The serum concentration of IL-6 ([IL-6], was very variable among the patients but the average [IL-6] was significantly higher compared to control subjects (7.24 ± 6.83 pg/ml versus 2.23 ± 2.68 pg/ml, p=0.038).
A linear regression analysis revealed a trend (though not statistically significant) towards a positive relationship between [TNF-α] and SUVmax (R2=0.299, p=0.066) and [IL-6] and SUVmax (R2=0.24, p=0.12) suggesting that the greater the metabolic activity of the lung mass the greater the inflammatory response.
To examine the potential interaction between inflammatory markers and brain metabolites we performed a multiple regression analysis on [IL-6] and [TNF-α] of all subjects (both patients and controls) using all metabolites. A stepwise selection was used to select the significant metabolites in relation to the magnitude of the inflammatory response. Interestingly, in the occipital cortex, after stepwise selection a significant relation between [NAA] and [TNF-α] was found. The model derived was as follows: [TNF-α] = 15 - 1.5*[NAA] (p=0.036); indicating that an elevated [TNF-α] will decrease occipital [NAA] (Figure 3). No statistically significant relationship between inflammatory markers and parietal cortex metabolites were observed.
Figure 3.
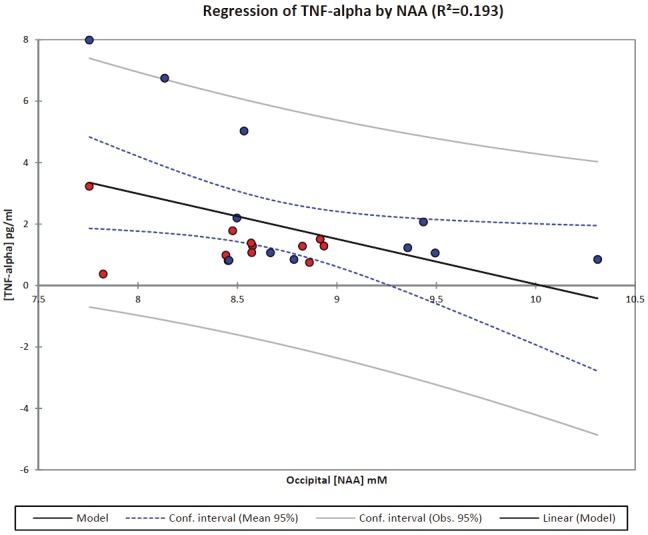
A multiple regression analysis was performed on [TNF-α] of all subjects (both patients and controls) using all metabolites in occipital cortex. A stepwise selection was used to select the significant metabolites in relation to the magnitude of the inflammatory response. After stepwise selection a significant relation between [NAA] and [TNF-α] was found. The model derived was as follows: [TNF-α] = 15 - 1.5*[NAA] (R2=0.193; p=0.036); indicating that an elevated [TNF-α] will decrease occipital [NAA]. The patients and controls are marked by a red and blue circle, respectively.
Discussion
Our data provide evidence that the cerebral metabolic status of lung cancer patients is altered prior to treatment when compared to an age-matched control group. Specifically we showed that [Glu] in the occipital cortex of the lung cancer patients was approximately 10% lower than controls; even in patients without evidence of brain metastases. The lung cancer patients were also characterized by more fatigue and higher levels of IL-6 when compared to controls.
To our knowledge, the finding of cerebral metabolic status changes in lung cancer patients prior to treatment is novel and not previously documented. However, several prospective studies have documented the effects of chemotherapy or hormone treatment on cognition and revealed significant deterioration in neuropsychological test scores when compared to baseline performance in women with breast cancer [18] and in men with prostate cancer [19,20]. Functional MRI studies have also supported evidence of cognitive dysfunction by demonstrating that treatment results in differences of task-related neural activation patterns in patients with prostate cancer in comparison to controls [20]. Further, women treated with tamoxifen to reduce the risk of breast cancer display changes in brain metabolites including choline containing compounds [21]. Measurements of the cerebral metabolic rate of glucose using 18FDG PET have also shown that patients with breast cancer (but no metastases) can display abnormalities following treatment [22]. Another recent study demonstrated that 23% of patients with breast cancer exhibit cognitive impairment prior to treatment [23] suggesting that having a diagnosis of cancer affects brain function. In our patients the documented changes in occipital [Glu] were not associated with changes in cognitive performance in comparison to controls as evaluated by the brief battery of neurocognitive testing, possibly due to the fact that our groups were well matched at baseline in terms of estimated premorbid IQ (Wechsler Test of Adult Reading test done at screening). However, the lung cancer patients were characterized by higher fatigue scores which have also previously been demonstrated in cancer patients including lung cancer [24-27].
Spectroscopy studies using combined proton and 13C-labeled precursors have shown that [Glu] is directly related to neuronal mitochondrial metabolism [neuronal tricarboxylic acid cycle (TCA) cycle rate (VTCAn)] in normal brain [28,29]. Further, a decrease in VTCAn in the elderly has been shown to correlate with decreases in [Glu] and [NAA], suggesting that mitochondria lose oxidative capacity with normal aging [10,30,31]. If one accepts, that [Glu] represents brain 'energy metabolism' and thereby indirectly brain function, the decrease in [Glu] observed in the lung cancer patients suggests that the presence of lung cancer itself reduces brain function prior to treatment. However, it is important to point out that the patients included in this study were heterogeneous with respect to their final lung mass diagnosis and included early as well as more advanced stages of lung cancer. Due to the small sample size it was not possible to correlate the cerebral metabolomic status specifically with tumor staging; and since our sample was dominated by early stage lung cancer patients it is likely that the overall impact of ‘lung cancer’ on [Glu] and potentially other brain metabolites may have been underestimated and might prove more significant in patients with more advanced disease. In support of this statement, Figure 2 shows that the highest levels of [Glu] of the patient group belonged to two of the subjects with non-cancer. Future studies focused on characterizing a larger group of patients with lung cancer at various stages of progression will help address this issue.
It is important to also consider how the patient’s other comorbidities might have interacted with metabolism and influenced [Glu]. For example, in contrast to controls a larger proportion of the lung cancer patients were diagnosed with COPD and it is possible therefore that this chronic condition is responsible for the change in [Glu] at baseline. However, none of the COPD patients were oxygen dependent and all had normal hematocrit and oxygen saturation at baseline. Further, a recent 1HMRS study on oxygen-dependent and oxygen-independent COPD patients did not reveal metabolic differences in the brain when compared to controls [32], supporting our main hypothesis that the metabolic changes we observed in the cancer patients are caused by the cancer and not by COPD. Nonetheless, to further evaluate our preliminary findings and ascertain their independence of comorbidities such as COPD, it will be essential to enlarge our sample size and also include other cancer types.
Previous quantitative 1HMRS studies of the normal human brain have documented a heterogeneous distribution of [NAA] and [GPC+PCh] in the brain with higher [NAA] in the occipital cortex when compared to the parietal and frontal cortices; and higher [GPC+PCh] in the parietal compared to occipital cortex [33]. In contrast, [Glu] in grey matter is not region-dependent at least in normal, young human brain [33]. In our study, the quantitative profile of metabolites of control subjects also revealed higher concentrations of choline-containing compounds in the parietal compared to the occipital cortex (Table 5); but we did not observe higher [NAA] in the occipital compared to the parietal cortex as previously reported, which might be related to age-differences of the two control populations [33]. We also did not observe a [Glu] decrease in the parietal cortex of the patient group in comparison to controls, however, [NAA] trended to be lower in the patients (p=0.09). The relatively small sample size prohibits further interpretation and conclusions as to whether the effect of ‘lung mass’ or lung cancer exert a region-specific or global effect on cerebral metabolic status.
The measurements of proinflammatory cytokines revealed higher [IL-6] in the patients with a malignant lung mass compared to controls which is in agreement with previous reports [17,34]. The normal range of [IL-6] in human serum is reported in the literature to be <15-20pg/ml [34]; and has been shown to increase in patients with lung cancer, although the increase varies greatly and is also dependent on tumor type and stage [17,34,35]. In our study the patient’s average [IL-6] was still within the reported normal range probably because the majority of the patients were sampled at an early diagnostic stage (i.e. stage I-II). In the patient group, [TNF-α] was also within normal range and no different from controls; which also indicates the early stage of the cancer. When exploring the potential relation between the metabolic activity of the lung mass as evaluated by the SUVmax and inflammation, we found a positive association (p=0.066) between [TNF-α] and SUVmax. This finding indirectly supports previous data reporting that 1) TNF-α and other proinflammatory cytokines are produced locally in lung cancers [35,36] and 2) the metabolic activity of the lung cancer is related to proliferative tumor activity [37] and increased tumor cell glycolysis secondary to local hypoxia [38].
It was intriguing that [TNF-α] was found to be negatively associated with [NAA] in the occipital cortex suggesting that ‘inflammation’(regardless of cancer state) influences cerebral metabolic status, in agreement with previous reports. Thus, proinflammatory cytokines can cause cognitive dysfunction [39-41]; and elevations of cytokines have been found to co-occur with a reduction of the metabolic rate of glucose utilization in the brain [42-44].
Limitations of the study
The major limitation of the current study is the small sample size and as such the data presented are preliminary. The enrollment of patients for the study was difficult due to the often urgent need for extensive clinical work-up and the patient’s emotional stress associated with the diagnosis and imminent need for surgery. The ability to perform 1HMRS in conjunction with 18FDG-PET would be ideal for this patient population since the study protocols could be carried out with less of a time-burden for the patient. This approach may be possible in the future with implementation of a combined MRI-PET imaging modality the clinical arena. It would also be important to compare 1HMRS results with corresponding regional cerebral metabolic rate of glucose data in order to obtain more accurate spatial information. The latter will enable a better understanding of the neuronal networks involved in cerebral effects associated with cancer.
Acknowledgements
We would like to thank Sunday Campolo, RN and April Frank, RN for their superb assistance in recruiting the lung cancer patients from the Surgical Oncology Clinic. We would also like to thank all the patients who participated in our study and who sacrificed their time prior to treatment for the various study protocol procedures including the neurocognitive test battery as well as the MRI/1HMRS scans. Finally, we would also like to acknowledge Dr. Sachin Jambawalikar’s expertise in regard to 1HMRS data acquisitions as well as Dr. Joseph Conrad for helping out with data collection. This work was supported by funding from a Translational Research Opportunity Grant from the School of Medicine, Stony Brook University.
References
- 1.Puccio M, Nathanson L. The cancer cachexia syndrome. Semin Oncol. 1997;24:277–287. [PubMed] [Google Scholar]
- 2.Kotler DP. Cachexia. Ann Intern Med. 2000;133:622–634. doi: 10.7326/0003-4819-133-8-200010170-00015. [DOI] [PubMed] [Google Scholar]
- 3.Palesty JA, Dudrick SJ. What we have learned about cachexia in gastrointestinal cancer. Dig Dis. 2003;21:198–213. doi: 10.1159/000073337. [DOI] [PubMed] [Google Scholar]
- 4.Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahles TA. Do systemic cancer treatments affect cognitive function? Lancet Oncol. 2004;5:270–271. doi: 10.1016/S1470-2045(04)01463-9. [DOI] [PubMed] [Google Scholar]
- 6.Wefel JS, Vidrine DJ, Veramonti TL, Meyers CA, Marani SK, Hoekstra HJ, Hoekstra-Weebers JE, Shahani L, Gritz ER. Cognitive impairment in men with testicular cancer prior to adjuvant therapy. Cancer. 2011;117:190–196. doi: 10.1002/cncr.25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverman DH, Alavi A. PET imaging in the assessment of normal and impaired cognitive function. Radiol Clin North Am. 2005;43:67–77. doi: 10.1016/j.rcl.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Wang GJ, Volkow ND, Lau YH, Fowler JS, Meek AG, Park TL, Wong C, Roque CT, Adler AJ, Wolf AP. Glucose metabolic changes in nontumoral brain tissue of patients with brain tumor following radiotherapy: a preliminary study. J Comput Assist Tomogr. 1996;20:709–714. doi: 10.1097/00004728-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Saykin AJ, Ahles TA, McDonald BC. Mechanisms of chemotherapy-induced cognitive disorders: neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry. 2003;8:201–216. [PubMed] [Google Scholar]
- 10.Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24:943–957. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 12.Cella DF, Jacobsen PB, Orav EJ, Holland JC, Silberfarb PM, Rafla S. A brief POMS measure of distress for cancer patients. J Chronic Dis. 1987;40:939–942. doi: 10.1016/0021-9681(87)90143-3. [DOI] [PubMed] [Google Scholar]
- 13.Wechsler D. Administration and Scoring Manual. In: , editor. Wechsler Adult Intelligence Scale. Third edition. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 14.Detterbeck FC, Boffa DJ, Tanoue LT, Wilson LD. Details and difficulties regarding the new lung cancer staging system. Chest. 2010;137:1172–1180. doi: 10.1378/chest.09-2626. [DOI] [PubMed] [Google Scholar]
- 15.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 16.Chang L, Jiang CS, Ernst T. Effects of age and sex on brain glutamate and other metabolites. Magn Reson Imaging. 2009;27:142–145. doi: 10.1016/j.mri.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atwell DM, Grichnik KP, Newman MF, Reves JG, McBride WT. Balance of proinflammatory and antiinflammatory cytokines at thoracic cancer operation. Ann Thorac Surg. 1998;66:1145–1150. doi: 10.1016/s0003-4975(98)00592-x. [DOI] [PubMed] [Google Scholar]
- 18.Jansen CE, Dodd MJ, Miaskowski CA, Dowling GA, Kramer J. Preliminary results of a longitudinal study of changes in cognitive function in breast cancer patients undergoing chemotherapy with doxorubicin and cyclophosphamide. Psychooncology. 2008;17:1189–1195. doi: 10.1002/pon.1342. [DOI] [PubMed] [Google Scholar]
- 19.Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology. 2009;18:237–247. doi: 10.1002/pon.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherrier MM, Borghesani PR, Shelton AL, Higano CS. Changes in neuronal activation patterns in response to androgen deprivation therapy: a pilot study. BMC Cancer. 2010;10:1. doi: 10.1186/1471-2407-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst T, Chang L, Cooray D, Salvador C, Jovicich J, Walot I, Boone K, Chlebowski R. The effects of tamoxifen and estrogen on brain metabolism in elderly women. J Natl Cancer Inst. 2002;94:592–597. doi: 10.1093/jnci/94.8.592. [DOI] [PubMed] [Google Scholar]
- 22.Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ. Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage. 2004;21:364–371. doi: 10.1016/j.neuroimage.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 23.Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy- induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19:1647–1656. doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- 24.Stone P, Richards M, Hardy J. Fatigue in patients with cancer. Eur J Cancer. 1998;34:1670–1676. doi: 10.1016/s0959-8049(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 25.Nail LM. Fatigue in patients with cancer. Oncol Nurs Forum. 2002;29:537. doi: 10.1188/onf.537-546. [DOI] [PubMed] [Google Scholar]
- 26.Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S. Cancer-related fatigue: evolving concepts in evaluation and treatment. Cancer. 2003;98:1786–1801. doi: 10.1002/cncr.11742. [DOI] [PubMed] [Google Scholar]
- 27.Campos MP, Hassan BJ, Riechelmann R, Del Giglio A. Cancer-related fatigue: a review. Rev Assoc Med Bras. 2011;57:211–219. doi: 10.1590/s0104-42302011000200021. [DOI] [PubMed] [Google Scholar]
- 28.de Graaf RA, Chowdhury GM, Brown PB, Rothman DL, Behar KL. In situ 3D magnetic resonance metabolic imaging of microwave-irradiated rodent brain: a new tool for metabolomics research. J Neurochem. 2009;109:494–501. doi: 10.1111/j.1471-4159.2009.05967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Graaf RA, Mason GF, Patel AB, Behar KL, Rothman DL. In vivo 1H-[13C] -NMR spectroscopy of cerebral metabolism. NMR Biomed. 2003;16:339–357. doi: 10.1002/nbm.847. [DOI] [PubMed] [Google Scholar]
- 30.Boumezbeur F, Mason GF, de Graaf RA, Behar KL, Cline GW, Shulman GI, Rothman DL, Petersen KF. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2010;30:211–221. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, Rothman DL. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2010;30:13983–13991. doi: 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borson S, Scanlan J, Friedman S, Zuhr E, Fields J, Aylward E, Mahurin R, Richards T, Anzai Y, Yukawa M, Yeh S. Modeling the impact of COPD on the brain. Int J Chron Obstruct Pulmon Dis. 2008;3:429–434. doi: 10.2147/copd.s2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pouwels PJ, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med. 1998;39:53–60. doi: 10.1002/mrm.1910390110. [DOI] [PubMed] [Google Scholar]
- 34.Yanagawa H, Sone S, Takahashi Y, Haku T, Yano S, Shinohara T, Ogura T. Serum levels of interleukin 6 in patients with lung cancer. Br J Cancer. 1995;71:1095–1098. doi: 10.1038/bjc.1995.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tonnesen E, Wanscher M, Hohndorf K, Bendtzen K, Hansen MB, Diamant M, Hansen GL, Toft P. Effect of methylprednisolone on the cytokine response in patients undergoing lung surgery. Acta Anaesthesiol Scand. 1993;37:410–414. doi: 10.1111/j.1399-6576.1993.tb03738.x. [DOI] [PubMed] [Google Scholar]
- 36.Martinet N, Charles T, Vaillant P, Vignaud JM, Lambert J, Martinet Y. Characterization of a tumor necrosis factor-alpha inhibitor activity in cancer patients. Am J Respir Cell Mol Biol. 1992;6:510–515. doi: 10.1165/ajrcmb/6.5.510. [DOI] [PubMed] [Google Scholar]
- 37.Dooms C, van Baardwijk A, Verbeken E, van Suylen RJ, Stroobants S, De Ruysscher D, Vansteenkiste J. Association between 18F-fluoro -2-deoxy-D-glucose uptake values and tumor vitality: prognostic value of positron emission tomography in early-stage non-small cell lung cancer. J Thorac Oncol. 2009;4:822–828. doi: 10.1097/JTO.0b013e3181a97df7. [DOI] [PubMed] [Google Scholar]
- 38.van Baardwijk A, Dooms C, van Suylen RJ, Verbeken E, Hochstenbag M, Dehing-Oberije C, Rupa D, Pastorekova S, Stroobants S, Buell U, Lambin P, Vansteenkiste J, De Ruysscher D. The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and GLUT-1 in non-small cell lung cancer. Eur J Cancer. 2007;43:1392–1398. doi: 10.1016/j.ejca.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 39.Scheibel RS, Valentine AD, O'Brien S, Meyers CA. Cognitive dysfunction and depression during treatment with interferon-alpha and chemotherapy. J Neuropsychiatry Clin Neurosci. 2004;16:185–191. doi: 10.1176/jnp.16.2.185. [DOI] [PubMed] [Google Scholar]
- 40.Malik UR, Makower DF, Wadler S. Interferon-mediated fatigue. Cancer. 2001;92:1664–1668. doi: 10.1002/1097-0142(20010915)92:6+<1664::aid-cncr1494>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Meyers CA. Mood and cognitive disorders in cancer patients receiving cytokine therapy. Adv Exp Med Biol. 1999;461:75–81. doi: 10.1007/978-0-585-37970-8_5. [DOI] [PubMed] [Google Scholar]
- 42.Loftis JM, Socherman RE, Howell CD, Whitehead AJ, Hill JA, Dominitz JA, Hauser P. Association of interferon-alpha-induced depression and improved treatment response in patients with hepatitis C. Neurosci Lett. 2004;365:87–91. doi: 10.1016/j.neulet.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 43.Reichenberg A, Gorman JM, Dieterich DT. Interferon-induced depression and cognitive impairment in hepatitis C virus patients: a 72 week prospective study. AIDS. 2005;19(Suppl 3):S174–178. doi: 10.1097/01.aids.0000192087.64432.ae. [DOI] [PubMed] [Google Scholar]
- 44.Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22:870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


