Abstract
The number of end stage of renal disease patients that need dialysis or renal transplantation increased in the world. Insertion and maintenance functional vascular access remain the challenging problem. Arteriovenous fistula is the common access for dialysis but complication and its failure is the main problem. The aim of this study is to evaluate patients with arteriovenous fistula during 4 years and describe the probable influenced factors on fistula patency. In this analytical descriptive study, we fallowed 245 patients during 4 years and evaluated them for primary failures and effective factors on vascular patency. The patients were asked about demographic data, how to caring condition arteriovenous fistula, dialysis and complications. The mean age of the patients was 47.77 years. The underline diseases were hypertension (43.3%), hypertension and diabetes mellitus (21.2%) and diabetes mellitus (4.5%). According Log rank test there were meaningful results between arteriovenous patency with sex and dialysis (P < 0.05). Our result of primary patency at 6 months, 1, 2, 3 and 4 years for all patients were 79.5%, 70%, 65%, 60.5% and 48%. Our study showed dialysis could increase the fistula patency rate. Other factors were not associated with primary patency. It seems ESRD patients undergoing dialysis have better fistula patency, may be due to homeostasis abnormalities induced by their particular conditions.
Keywords: Dialysis, arteriovenous fistula, primary failure, end stage renal disease, patency
Introduction
In patients suffering from renal failure, we can use hemodialysis or transplantation. This disorder has significantly increased from 209,000 in 1991 to 472,000 cases in 2004 in the USA [1]. The incidence of end stage renal disease (ESRD) has been increased 43 percent based on age, gender, and race around the world since 1991 [2]. The patient physical state and other factors determine choice treatment. Although, creation of vascular access is a necessary maneuver for hemodialysis, creation and maintenance of a well-functioning vascular access are remained the most challenging problems for hemodialysis therapy [3]. The first access method was Brescia-Cimino fistula which was introduced in 1966. In the first years, only young and healthy patients were candidates for AVF creation [4]. Nowadays, creation of arteriovenous fistula (AVF) is feasible in most cases including diabetics and old patients. Thrombosis and/or lack of maturation are the reasons of primary failure [5], but the risk factor for primary failures is not limited to these like the site and diameter of vessels are thought to fulfill an important role [6]. One study which was done from 1997 to 1999 showed the primary failure rate between 10-20% [7]. A recent meta-analysis has demonstrated 15.3% primary failure rate for native AVF [8]. Proper access-site selection, improved surgical techniques and appropriate management of complications are the main factors for long-term success. However, other factors such as regular vascular laboratory surveillance, post-operative and central coordination by a dedicated access coordinator are equally important in ensuring a functional and cost-effective access [9]. There are different reports for the radiocephalic AV fistula patency in previous reported article: 85% survival in the first year and 80% in the second year [10]. Kalman and his colleagues showed a total patency including primary, assisted primary and secondary success rate of 66% for 2 years and primary patency rate of 36% for 2 years [9]. The aim of this study was to finding the possible factors influencing on the fistula patency.
Methods
Study design
This study is a descriptive-analytic single-center prospective study based on referral patients to vascular surgery clinic of a University Hospital, whom underwent primary arteriovenous fistula.
Patient’s selection
All of 245 patients were included in a program for the first time AVF access from 22 November 2005 to 22 November 2006. Exact analysis of the type and reason of the previously reported complications was used to design a new questionnaire for this study.
The necessary information including demographic data, the primary renal disease, type and length of anastomosis, surgical technique, type and diameter of the artery and vein, the immediate result of surgery and complementary supports gathered from patients’ recorded data. All patients were followed by face to face visit or phone after 1 week, 1 month, 3 months, 6 months and then 1, 2, 3 and 4 years of surgery. The patients were asked about functional AVF, complications, AVF caring, the time they started dialysis, the number of dialysis per week and dialysis center. In addition, personal factors such as smoking and training about AVF caring were questioned.
Any complications or AVF loss was recorded and finally the questionnaire was filled in to define the reasons. Questionnaire included questions about hard jobs, wearing tight clothes, blood Pressure of the same limb, sleeping on anastomosed arm, frequency of puncture, and using garo. Additional complications such as Steal syndrome, venous hypertension (HTN), infection, aneurism, heart failure and neuropathy were recorded. In this study from the total number of 245 patients, 197 ones remained available until the end of the study.
Statistical analysis
The collected data entered into SPSS software version 11.5. Cox models and Kaplan-Meier curves were used to analyze the primary patency of vascular access.
Results
The mean age of patients was 47.77 years (6 to 85 years). Considering gender, 148 (60.7%) were males and 96 (39.3%) were females (Table 1).
Table 1.
Demographic data and history of 245 patients
| Variables | Value | % | |
|---|---|---|---|
| Men/women | 148/96 | 60.7/39.3 | |
| Mean age (year) | 47.77 | ||
| Smoking | 36 | 18.75 | |
| Underline disease | HTN | 106 | 43.3 |
| HTN + DM | 52 | 21.2 | |
| DM | 11 | 4.5 | |
| No disease | 26 | 10.6 | |
| missing | 16 | 6.5 | |
| Limb of AVF | Dominant | 36 | 14.7 |
| Not dominant | 155 | 63.3 | |
| missing | 54 | 22 | |
| Limb of AVF | Right | 202 | 82.4 |
| Left | 32 | 13.1 | |
| missing | 11 | 4.5 | |
| Place of AVF | Elbow | 95 | 38.8 |
| Snuff box | 89 | 36.3 | |
| Forearm | 24 | 9.8 | |
| Wrist | 16 | 6.5 | |
| missing | 21 | 8.6 | |
| Kind of anastomosis | S-S | 131 | 53.6 |
| E-S | 92 | 37.6 | |
| E-E | 3 | 1.2 | |
| missing | 19 | 7.8 | |
| Meand length of anastomosis (mm) | 9.99 (4-18) | ||
| Vein | Cephalic | 145 | 59.2 |
| Cubital | 75 | 30.6 | |
| Basilic | 1 | 0.4 | |
| missing | 24 | 9.8 | |
| Atherosclerosis | No | 182 | 74.3 |
| Mild | 30 | 12.2 | |
| Severe | 8 | 3.3 | |
| missing | 25 | 10.2 | |
| AVF function | Good | 205 | 83.7 |
| Poor | 14 | 5.7 | |
| No | 3 | 1.2 | |
| missing | 23 | 9.4 | |
AVF: Arteriovenous fistula, HTN: Hypertension, DM: Diabetes Mellitus, S-S: side to side, E-S: End to Side, E-E: End to End.
Most of our patients were under-educated (36.5%) and was non smokers (81.25%). The underling diseases in our patients included 43.3% HTN, 21.2% HTN and diabetes mellitus and 4.5% only diabetes mellitus. Other diseases detected in 10.6% of patients were included glomerulonephritis, polycystic kidney, uropathy, pyelonephritis and lupus erythematous
Frequency of AVF creation of in non-dominant, dominant, right, upper and left limbs was 63.3%, 14.7%, 13.1% and 82.4%, respectively. The AVF creation sites were snuff box, wrist, forearm and elbow: 36.3%, 6.5%, 9.8% and 38.8%, respectively.
Anastomosis type included side to side fistulas (53.6%), end to side (37.6%) and end to end (1.2%). The mean length of the used anastomosis was 9.9 mm (with minimum and maximum length of 4 mm and 18 mm, respectively).
Cephalic vein was in 59.2% of cases whereas antecubital vein and Basilic vein was in 30.6% and 0.4% of cases respectively. Radial, Brachial and Ulnar arteries used in 53.1%, 35.9% and 1.2% of patients, respectively. Patients with mild atherosclerotic arteries (12.2% of cases) encountered 18% loss of fistula, and severe atherosclerotic arteries (3.3% of cases) suffered 50% loss in 6 months. Reversely, in non-atherosclerotic arteries (74.3% of cases), 17% of AVF break down reported in 6 months.
Most of fistulas had appropriate functions immediately after the operation (83.7%), 5.7% had poor function and 1.2% showed no function. Patients monitoring revealed that 134 cases (69.8%) required immediate dialysis. Among the other 55 patients (28.1%), 20 of the live cases were not dialyzed till the end of follow up period. Thirty five of cases were not dialyzed at all due to death or kidney transplantation.
The minimum time starting to use access after surgery was 2 weeks. Large group of the patients (31.1%) had 4 weeks interval, while 14% after 6 and 9.8% after 8 weeks began dialysis. Long interval rate in starting dialysis (between 13 to 22 weeks after fistula creation) was 1.5%. Most of the patients were dialyzed 4 hours per day and 3 times a week. Considering the patients follow up, 28 (11.5%) died, 7 (2.9%) were transplanted and 31 (12.7%) encountered fistula loss.
Access survival
Primary patency for AVF is illustrated in Figure 1. Our result of primary patency at 6 months, 1, 2, 3 and 4 years for 245 patients were 79.5%, 70%, 65%, 60.5% and 48% respectively. We evaluated variables data by Log rank test, as well. According to the analysis, just significant relation between arteriovenous patency and sex and conduction of dialysis was found. As Table 2 shows, there is no other significant relationship between access survival and the other variables including: education, underlying disease, smoking, location of AVF, surgical techniques, surgeon experience, complications, type of vein and artery, diameter of vein and artery, degree of arteriosclerosis and age (P > 0.05).
Figure 1.
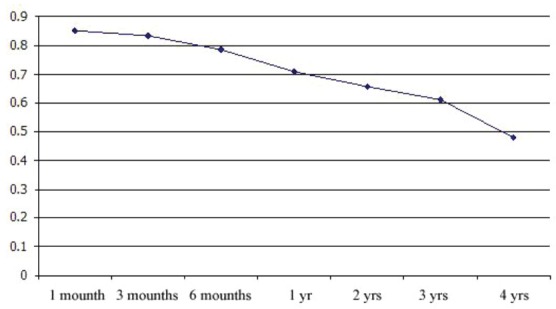
Cumulative primary patency for AVF (4 years).
Table 2.
Analytic results of access survival.
| Variable | Access survival | Average of survival (days) | Log rank testresult | |||
|---|---|---|---|---|---|---|
| 6 months | 1 yr | 3 yrs | ||||
| Sex | Male | %87 | %77 | %66 | 140.98 | 0.023 |
| Female | %72 | %63 | %55 | 116.71 | ||
| Underline disease | No | %85 | %85 | %85 | 166.04 | 0.3 |
| Yes | %81 | %70 | %59 | 126.32 | ||
| Place of AVF | Snuff box | %82 | %78 | %62 | 130.91 | 0.273 |
| Wrist | %58 | %46 | %35 | 91.217 | ||
| Forearm | %75 | %75 | %60 | 121.85 | ||
| Cubital | %84 | %67 | %67 | 131.48 | ||
| Limb of AVF | Dominant | %88 | %82 | %70 | 158.07 | 0.122 |
| Non dominant | %81 | %70 | %60 | 124.28 | ||
| Kind of anastomosis | S-S | %82 | %76 | %64 | 135.9 | 0.463 |
| E-S | %84 | %66 | %59 | 128.7 | ||
| E-E | %33 | %33 | %33 | 68.66 | ||
| Surgeon | A | %79 | %71 | %56 | 120.05 | 0.7 |
| B | %84 | %71 | %63 | 136.52 | ||
| C | %83 | %83 | %83 | 131.16 | ||
| Dialysis | Yes | %93 | %82 | %70 | 147.05 | < 0.001 |
| No | %48 | %43 | %43 | 86.97 | ||
| Vein | Cephalic | %85 | %73 | %59 | 130.21 | 0.584 |
| Cubital | %81 | %67 | %67 | 130.70 | ||
| Artery | Radial | %80 | %72 | %59 | 129.52 | 0.19 |
| Ulnar | %66 | 44.33 | ||||
| Brachial | %86 | %79 | %69 | 134.98 | ||
| Degree of AS | Nothing | %83 | %69 | %61 | 134.39 | 0.115 |
| Mild | %82 | %82 | %57 | 119.75 | ||
| Severe | %50 | 20.33 | ||||
| Quality of surgery | Easy | %84 | %73 | %62 | 134.84 | 0.154 |
| Difficult | %67 | %53 | %53 | 105.47 | ||
AVF: Arteriovenous fistula, S-S: side to side, E-S: End to Side, E-E: End to End. Multivariate model (Cox regression) shows use of Av fistula by dialysis had meaningful affect on access survival.
Discussion
In current study, the patients were followed up for 4 years and the primary patency of AVF was estimated. In Kalman et al [11] study the primary success rate of 466 patients for 2 years was about 54% ± 4, while in our study the primary patency rate for two and four years were 65% and 48%. In our study investigators concerned about finding predisposing factor of primary patency of AVF. Monroy-Cuadros and colleagues studied the associating factors in the first 6 months of using AVF and the primary failure. They measured the primary failure rate 10% in the initial 6 months. In their study, the initial flow of each access measured lower than 500 ml/min, reported as a risk factor. The other risk factors like age, history of smoking, diabetes mellitus and forearm site fistula did not change the patency rate [12].
Diehm et al studied on the access outcome on diabetic female patients, subsequently they found out that being female gender and diabetic are risk factors in patency outcome [13]. However, Huijbregts et al showed that decrement in the primary patency and function of AVF in diabetic patients was as long as non-diabetic patients [14]. Wand and colleagues expressed that co-morbidities did not have meaningful association with primary failure. The primary success rate was 64% in 2 years [15]. Our study showed that the underlying diseases, like diabetes mellitus, hypertension, glomerulonephritis, uropathy and lupus had no considerable statistical relationship with primary patency. We could not show that smoking could affect on the AVF outcome. Similar results were revealed in atherosclerosis: the degree of atherosclerosis had no considerable role on outcome. Nguyen and colleagues worked on type of AV fistula and examined the outcome of brachycephalic and radiocephalic fistula. Their results showed primary patency and maturity was higher in brachiocephalic than radiocephalic [16] but in other study the outcome of this two type of access were similar [15]. In radiocephalic we can made fistula by two procedures that include proximal and distal radiocephalic fistula (pRCF and dRCF). Bhalodia et al reported pRCF had a lower primary failure than dRCF [17]. In current study examined the patency of location (Dominant or non dominant limb and right or left limb) and site (elbow, forearm, snuff box and wrist) and our result showed no meaningful statistical relationship between site or type of fistula and primary patency (P > 0.05).
Saran et al worked on surgeons’ skills and outcome of their fistula patency [18]. They reported when fistula is placed by surgeons who tried equal or more than 25 fistulas during training courses, had lower primary failure than others [18]. Our Hospital is referral center and annually 1100 AVF surgeries are conducted by surgeons collaborated in current trial and we didn’t find any relation between surgeon and primary patency. Moreover, we examined the different techniques used for anastomosis (side to side, end to side and end to end) and these variables were not important on outcome rate.
Our result showed the primary patency rate was 70% in hemodialysis patients in comparison with 43% in non-dialysis patients. Most of the patients were dialyzing 3 times a week. The mechanism of hemodialysis affect on AVF patency is unknown. But it seems that hemostasis and blood flow changes in hemodialytic patient are role-players. Platelet dysfunction is one of the hemostasis changes in ESRD patients [19]. Several factors influence the platelet dysfunction including impaired function of platelet glycoproteins, changes in ADP and serotonin from platelet granules, arachidonic acid and prostaglandin [19]. Also several factors such as increased tissue factors, protein C, factor XIIa, factor VIIa and platelet hyperactivity lead to thrombosis and increase the atherosclerosis risk [20-24]. So several changes in hemostasis, among mild stage of renal failure, cause cardiovascular complications and vascular access thrombosis, but the main changes in severe stage of renal disease is bleeding and coagulopathy. It is likely to happen due to platelet dysfunction and uremic condition in these patients. On the other hand, hemodialysis and using the access could advocate some hemodynamic changes on the access site. This condition may prevent thrombosis in vulnerable fistulas. So it seems lower risk of thrombosis and access loss in severe stage of disease, may be the consequence of these changes.
Conclusion
Several factors could affect the AVF patency. Our study showed hemodialysis may increase fistula patency while the other mentioned factors do not have any significant association with primary patency of AVFs. Maybe hemostasis abnormalities in ESRD patients and blood flow changes in hemodialytic patients maybe the causes of theses results, so more controlled studies is recommended.
References
- 1.US Renal Data Systems. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of Chronic Kidney Disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 3.Konner K, Hulbert-Shearon TE, Roys EC, Port FK. Tailoring the initial vascular access for dialysis patients. Kidney Int. 2002;62:329–338. doi: 10.1046/j.1523-1755.2002.00436.x. [DOI] [PubMed] [Google Scholar]
- 4.Brescia MJ, Cimino JE, Appel K, Hurwich BJ. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med. 1966;275:1089–1092. doi: 10.1056/NEJM196611172752002. [DOI] [PubMed] [Google Scholar]
- 5.Ravani P, Spergel LM, Asif A, Roy-Chaudhury P, Besarab A. Clinical Epidemiology of arteriovenous fistula in 2007. J Nephrol. 2007;20:141–149. C Surg 1999; 30: 727-733. [PubMed] [Google Scholar]
- 6.Juan A, Armadans L, Eugenio F. The function of permanent vascular access. Nephrol Dial Transplant. 2000;15:402–408. doi: 10.1093/ndt/15.3.402. [DOI] [PubMed] [Google Scholar]
- 7.Rutherford RB. Vascular Surgery. 6th ed. Philadelphia, Pennsylvania. ELSEVIER SAUNDERS. 2005 Monroy-Cuadros M, Yilmaz S, Salazar- Bañuelos A, Doig Marko Malovrh. Strategy for the Maximal Use of Native Arteriovenous Fistulae for Hemodialysis. The Scientific World JOURNAL. 2006;6:808–81. doi: 10.1100/tsw.2006.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irish A, Dogra G, Mori T, Beller E, Heritier S, Hawley C, Kerr P, Robertson A, Rosman J, Paul- Brent PA, Starfield M, Polkinghorne K, Cass A. Preventing AVF thrombosis: the rationale and design of the Omega-3 fatty acids (Fish Oils) and Aspirin in Vascular access outcomes in renal Disease (FAVOURED) study. BMC nephrol. 2009;10:1. doi: 10.1186/1471-2369-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rooijens PP, Tordoir JH, Stijnen T, Burgmans JP, Smet de AA, Yo TI. Radiocephalic wrist arteriovenous fistula for hemodialysis: metaanalysis indicates a high primary failure rate. Eur J Vasc Endovasc Surg. 2004;28:583–589. doi: 10.1016/j.ejvs.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Kalman PG, Pope M, Bhola C, Richardson R, Sniderman KW. A practical approach to vascular access for hemodialysis and predictors of success. J Vasc Surg. 1999;30:727–733. doi: 10.1016/s0741-5214(99)70112-6. [DOI] [PubMed] [Google Scholar]
- 11.Diehm N, van den Berg JC, Schnyder V, Bühler J, Willenberg T, Widmer M, Mohaupt MG, Baumgartner I. Determinants of haemodialysis access survival. Vasa. 2010;39:133–139. doi: 10.1024/0301-1526/a000018. [DOI] [PubMed] [Google Scholar]
- 12.Huijbregts HJ, Bots ML, Wittens CH, Schrama YC, Moll FL, Blankestijn PJ CIMINO study group. Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative. Clin J Am Soc Nephrol. 2008;3:714–719. doi: 10.2215/CJN.02950707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Murphy B, Yilmaz S, Tonelli M, Macrae J, Manns BJ. Comorbidities do not influence primary fistula success in incident hemodialysis patients: a prospective study. Clin J Am Soc Nephrol. 2008;3:78–84. doi: 10.2215/CJN.00370107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen TH, Bui TD, Gordon IL, Wilson SE. Functional patency of autogenous AV fistulas for hemodialysis. J Vasc Access. 2007;8:275–280. [PubMed] [Google Scholar]
- 15.Bhalodia R, Allon M, Hawxby AM, Maya ID. Comparison of Radiocephalic Fistulas Placed in the Proximal Forearm and in the Wrist. Semin Dial. 2011;24:355–357. doi: 10.1111/j.1525-139X.2010.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saran R, Elder SJ, Goodkin DA, Akiba T, Ethier J, Rayner HC, Saito A, Young EW, Gillespie BW, Merion RM, Pisoni RL. Enhanced training in vascular access creation predicts arteriovenous fistula placement and patency in hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study. Ann Surg. 2008;247:885–891. doi: 10.1097/SLA.0b013e31816c4044. [DOI] [PubMed] [Google Scholar]
- 17.Jalal DI, Chonchol M, Targher G. Disorders of hemostasis associated with chronic kidney disease. Semin Thromb Hemost. 2010;36:34–40. doi: 10.1055/s-0030-1248722. [DOI] [PubMed] [Google Scholar]
- 18.Saran R, Elder SJ, Goodkin DA, Akiba T, Ethier J, Rayner HC, Saito A, Young EW, Gillespie BW, Merion RM, Pisoni RL. Enhanced training in vascular access creation predicts arteriovenous fistula placement and patency in hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study. Ann Surg. 2008;247:885–891. doi: 10.1097/SLA.0b013e31816c4044. [DOI] [PubMed] [Google Scholar]
- 19.Jalal DI, Chonchol M, Targher G. Disorders of hemostasis associated with chronic kidney disease. Semin Thromb Hemost. 2010;36:34–40. doi: 10.1055/s-0030-1248722. [DOI] [PubMed] [Google Scholar]
- 20.Rios DR, Carvalho MG, Lwaleed BA, Simões e Silva AC, Borges KB, Dusse LM. Hemostatic changes in patients with end stage renal disease undergoing hemodialysis. Clin Chim Acta. 2010;411:135–139. doi: 10.1016/j.cca.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Małyszko J, Małyszko JS, Myliwiec M. Endothelial cell injury markers in chronic renal failure on conservative treatment and continuous ambulatory peritoneal dialysis. Kidney Blood Press Res. 2004;27:71–77. doi: 10.1159/000075810. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo T, Koide M, Kario K, Suzuki S, Matsuo M. Extrinsic coagulation factors and tissue factor pathway inhibitor in end-stage chronic renal failure. Haemostasis. 1997;27:163–167. doi: 10.1159/000217449. [DOI] [PubMed] [Google Scholar]
- 23.Segarra A, Chaco´n P, Martinez-Eyarre C, Argelaguer X, Vila J, Ruiz P, Fort J, Bartolomé J, Camps J, Moliner E, Pelegrí A, Marco F, Olmos A, Piera L. Circulating levels of plasminogen activator inhibitor type-1, tissue plasminogen activator, and thrombomodulin in hemodialysis patients: biochemical correlations and role as independent predictors of coronary artery stenosis. J Am Soc Nephro. 2001;12:1255–1263. doi: 10.1681/ASN.V1261255. [DOI] [PubMed] [Google Scholar]
- 24.Takagi M, Wada H, Mukai K, Minamikawa K, Wakita Y, Deguchi K, Junji N, Hayashi T, Suzuki K, Shiku H. Increased activated protein C: protein C inhibitor complex and decreased protein C inhibitor levels in patients with chronic renal failure on maintenance hemodialysis. Clin Appl Thromb Hemost. 1999;5:113–116. doi: 10.1177/107602969900500207. [DOI] [PubMed] [Google Scholar]


