Abstract
Burn and toll-like receptors (TLR) are associated with innate immune system activation, but the impact of burn on TLR-induced inflammation responses by circulating leukocytes is unknown. To study this, C57BL/6 mice were subjected to burn (3rd degree, 25% TBSA) or sham procedure and 1-7 days later blood was collected. Whole blood cell suspensions were incubated for 24 hr in the presence of zymosan (TLR-2 agonist) or LPS (TLR-4 agonist). The blood cultures were responsive to TLR2 and TLR4-mediated activation, resulting in the production of IL-6, IL-10, IL-17, TNF-α, MIP-1α, MIP-1β, KC and RANTES. TLR2-induced KC and MIP-1β production was greater in the burn group at 3-7 days post-injury, whereas IL-6, IL-10, KC and MIP-1β were greater for TLR4-induced activation after burn. In conclusion, circulating leukocytes were responsive to TLR-induced activation and TLR-mediated inflammatory responses were enhanced 3-7 days post-injury, as evidenced by increased production of these inflammatory mediators.
Keywords: Cytokines, burn, blood, toll-like receptors, inflammation
Introduction
Major burn is associated with a local and systemic activation of the innate immune system resulting in a profound inflammatory response, immunosuppression and an increased susceptibility to subsequent septic complications and multiple organ failure [1-4]. The causative factors for immune dysfunction appear to be multiple including T-cell dysfunction, macrophage hyperactivation and prolonged neutrophil accumulation [5].
Important sensors of the innate immune system, are pattern recognition receptors (PRRs). These receptors detect phylogenetically conserved molecular patterns found in a wide range of microorganisms [6]. Of these PRRs, toll-like receptors (TLRs) are the most studied [7]. TLRs are type I transmembrane proteins with an extracellular domain containing leucine-rich repeats and cytoplasmic Toll/IL-1 receptor domain. TLRs are expressed mainly by immune cells, including peripheral blood leukocytes. Currently, 10 human and 12 murine TLRs have been characterized. Among them, TLR2 recognizes multiple components in Gram-positive bacteria and TLR4 is predominantly activated by LPS. TLR activation induces a complex signaling cascade that leads to the induction of inflammatory genes, resulting in the production of chemokines and cytokines and other inflammatory events [8,9].
Experimental models of burn have shown enhanced TLR-mediated reactivity in the spleen [10], microvasculature vessels [11], heart [12] lung [13] and intestines [14], supporting the concept that immunoinflammatory responses after burn are associated with post-injury complications. Previous studies have shown that responses after burn differ between fixed-tissue immune cells and circulating immune cells [15]. In the current study, a mouse burn model was used to determine how injury alters responses by circulating leukocytes to well-defined TLR2 and TLR4 agonists.
Materials and methods
Animals
C57BL/6 male mice (18 to 22 gm; 8 to 10 wk, Charles River Laboratories, Wilmington, MA) were used for all experiments. The mice were allowed to acclimatize in the animal facility for at least 1 week prior to experimentation. Animals were randomly assigned into either a thermal injury group or a sham treatment group and for the 3 different time points of the experiment. The experiments in this study were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio, and were performed in accordance with the National Institutes of Health guidelines for the care and handling of laboratory animals.
Burn procedure
Mice received a scald burn as described previously [16]. Briefly, the mice were anesthetized by i.m. injection of ketamine/xylazine, and the dorsal surface was shaved. The anesthetized mouse was placed in a custom insulated mold exposing 12.5% of their total body surface area (TBSA) along the right dorsum. The mold was immersed in 70°C water for 10 sec to produce a 3rd degree burn [17,18]. The burn procedure was repeated on the left dorsum yielding a total burn size of 25% TBSA. The mice were then resuscitated with 1 ml of Ringer's lactate solution administered by intraperitoneal injection and returned to their cages. The cages were placed on a heating pad for 2 hr until the mice were fully awake, at which time they were returned to the animal facility. Sham treatment consisted of anesthesia, dorsal surface shaving and resuscitation with Ringer's lactate solution only.
Blood collection and murine whole blood assay
At 1, 3 or 7 days after injury heparinized whole blood was collected by cardiac puncture. The whole blood stimulation assay used with human samples [19] was modified as follows for use with murine blood. In brief, heparinized blood was diluted (1:3) with PBS and then added to a 96-well tissue culture plate (200 μl/well). The diluted blood was stimulated with either zymosan (33 μg/ml) or LPS (10 ng/ml) to activate TLR2 or TLR4, respectively. Zymosan was obtained from Sigma Chemical (Zymosan A from Saccharomyces cerrevisiae) and LPS was obtained from Calbiochem (Lipopolysaccharide, Ultra Pure, Salmonella minnesota R595). These concentrations were determined to be optimal for the induction of cytokine production in preliminary experiments (data not shown). The resulting whole blood cultures were incubated for 24 hrs at 37°C, 5% CO2. Cell-free supernatants were collected and stored at -80°C prior to cytokine/ chemokine determination.
Cytokine and chemokine determinations
Cell culture supernatants were analyzed for cytokine/ chemokine levels (IL-6, IL-10, IL-17, TNF-α, KC, MIP-1α, MIP-1β and RANTES) by Bioplex according to the manufacturer's recommendations.
Statistical analysis
Data are expressed as mean ± SE. Comparisons were analyzed using ANOVA. Student’s t-test was used for comparisons between 2 groups and Tukey's test was used for multiple comparisons. Data comparing the TLR2/TLR4 ratios were log transformed prior to analysis due to the lack of a normal distribution. A p-value of < 0.05 was considered to be statistically significant for all analyses.
Results
Impact of burn on harvested blood volume
There were no animal deaths after burn or sham procedures. The blood volumes obtained by cardiac puncture are shown in Table 1. Burn did not significantly alter harvested blood volume as compared with respective sham groups at 1 day or 7 days after injury. In contrast, the harvested blood volume was significantly reduced (p<0.05) by approximately 12% in the burn group at 3 days after injury. The blood volume harvested at 3 days after injury was significantly greater (p<0.05) than that harvested at 1 day after injury in the sham group.
Table 1.
Harvested blood volume (μl) after burn or sham procedure.
Data are expressed as mean ± SE for 4-10 mice/group;
p<0.05 as compared with respective sham group;
p<0.05 as compared with 1 day blood harvest
TLR2-induced responses by circulating leukocytes
Whole blood cultures were prepared at 1 day, 3 days and 7 days after injury and stimulated with zymosan to invoke TLR2-mediated responses. The results in Figure 1 show the cytokine responses by the cultures after TLR2 activation. TLR2-induced cytokine production was not different between whole blood cultures from sham and burn mice at any time points evaluated. However, IL-6 production increased approximately 4-fold at 7 days post-injury as compared with day 1 values (p<0.05).
Figure 1.
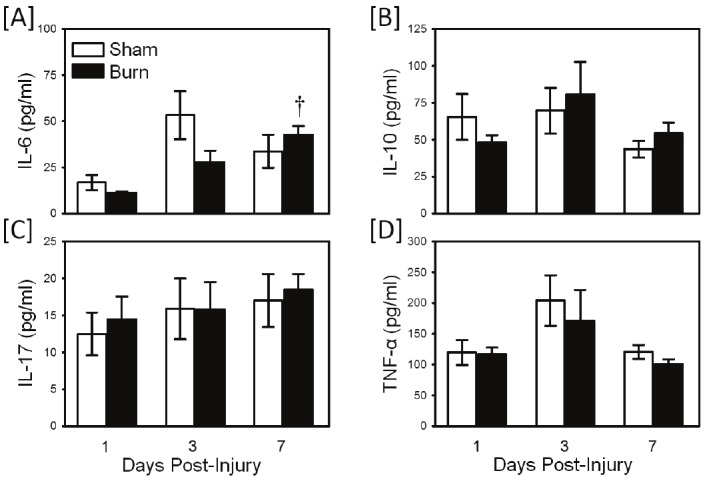
TLR2-induced cytokine production. Whole blood was collected from mice at 1, 3 and 7 days after sham procedure or burn and cultured for 24 hrs with the TLR2 agonist, zymosan as described in the materials and methods. Supernatants were assayed for IL-6 [A], IL-10 [B], IL-17 [C] and TNF-α [D] by Bioplex assay. Data are the mean ± SEM for n=4-10/ group. † p<0.05 as compared with day 1 values.
In contrast to TLR2-induced cytokine production, TLR2-induced chemokine responses were altered after burn injury (Figure 2). KC production was significantly elevated at 1-3 days after burn as compared with sham values (Figure 2A; p<0.05). At 3 days post-injury values were maximally elevated with approximately a 3-fold increase over sham values. In contrast, MIP-1α production was suppressed at 1 day after injury as compared with sham values (Figure 2B; p<0.05). MIP-1β production was elevated in the burn group at 3-7 days after injury, with the largest difference at 7 days after burn (Figure 2C; p<0.05). RANTES production was not different between the sham and burn groups (Figure 2D).
Figure 2.
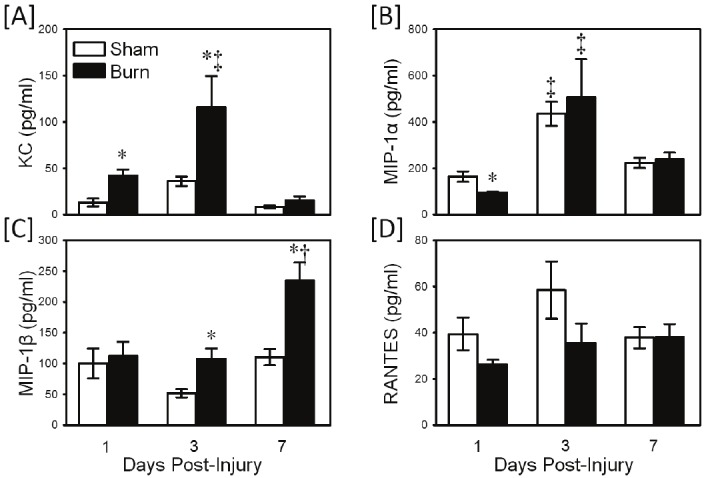
TLR2-induced chemokine production. Whole blood was collected from mice at 1, 3 and 7 days after sham procedure or burn and cultured for 24 hrs with the TLR2 agonist, zymosan as described in the materials and methods. Supernatants were assayed for KC [A], MIP-1α [B], MIP-1β [C] and RANTES [D] by Bioplex assay. Data are the mean ± SEM for n=4-10/group. * p<0.05 vs. respective sham group. † p<0.05 as compared with day 1 value. ‡ p<0.05 as compared with day 1 and day 7 values.
TLR-4 induced responses by circulating leukocytes
Whole blood cultures were prepared as described above, but stimulated with LPS to induce TLR4-mediated responses. The results in Figure 3 show the cytokine responses by the cultures after TLR4 activation. A significantly greater (p<0.05) production of IL-6 (Figure 3A) and IL-10 (Figure 3B) was observed in the burn group as compared with shams. IL-6 responses were elevated at 7 days post-injury, whereas IL-10 responses were increased at 3 days after injury. Neither IL-17 nor TNF-α production was different between the sham and burn groups.
Figure 3.
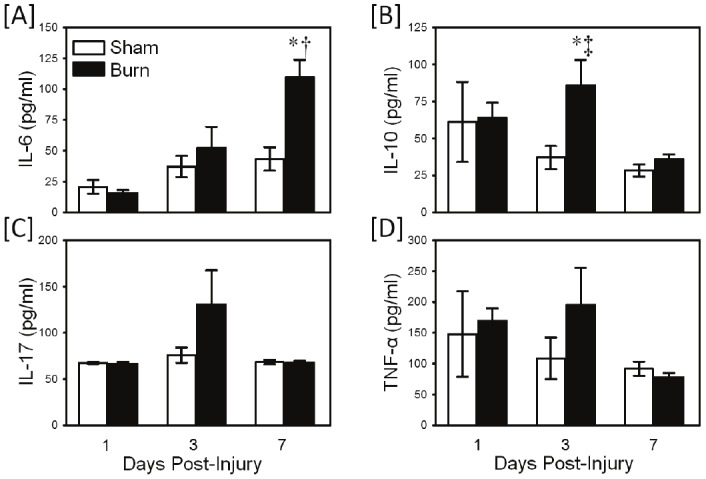
TLR4-induced cytokine production. Whole blood was collected from mice at 1, 3 and 7 days after sham procedure or burn and cultured for 24 hrs with the TLR4 agonist, LPS as described in the materials and methods. Supernatants were assayed for IL-6 [A], IL-10 [B], IL-17 [C] and TNF-α [D] by Bioplex assay. Data are the mean ± SEM for n=4-10/group. * p<0.05 vs. respective sham group. † p<0.05 as compared with day 1 value. ‡ p<0.05 as compared with day 7 values.
TLR4 activation with LPS induced a strong chemokine response (Figure 4). KC production was significantly elevated at 1-7 days after burn as compared with sham values (Figure 4A; p<0.05). At 3 days post-injury values were maximally elevated with approximately a 7-fold increase over sham values. In contrast, MIP-1α production was suppressed at 1 day after injury as compared with sham values (Figure 4B; p<0.05). MIP-1β production was elevated in the burn group at 3-7 days after injury, with a 2-4 fold increase over sham values (Figure 4C; p<0.05). Similar to TLR2-induced responses, LPS stimulated RANTES production was not different between the sham and burn groups (Figure 4D).
Figure 4.
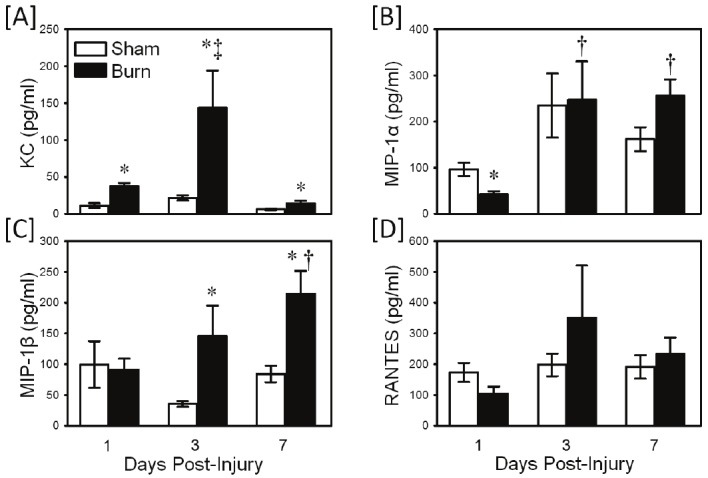
TLR4-induced chemokine production. Whole blood was collected from mice at 1, 3 and 7 days after sham procedure or burn and cultured for 24 hrs with the TLR4 agonist, LPS as described in the materials and methods. Supernatants were assayed for KC [A], MIP-1α [B], MIP-1β [C] and RANTES [D] by Bioplex assay. Data are the mean ± SEM for n=4-10/group. * p<0.05 vs. respective sham group. † p<0.05 as compared with day 1 value. ‡ p<0.05 as compared with day 1 (panel A and B) and day 7 (panel A) values.
Comparison of TLR2 and TLR4 responses by circulating leukocytes
In order to evaluate the relative strength of the TLR2 and TLR4 responses, the ratio of the TLR2 response to that of the TLR4 response was determined for cytokine and chemokine production. The data in Table 2 shows the response for cytokines. A shift towards a stronger TLR-4 response after burn was observed as evidenced by the significant (P<0.05) decrease in the TLR2/TLR4 ratio for IL-6 at day 7 and the decreased ratio for IL-10 and TNF-α at day 3 in the burn group as compared with shams. In contrast, TLR2/TLR4 ratios for IL-17 were similar for burn and sham cultures and primarily induced by TLR4 activation with ratio values that were significantly (p<0.05) lower than 1.0, irrespective of the time post-injury.
Table 2.
Ratio of TLR2/TLR4 cytokine responsesa.
| Sham | Burn | ||||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 7 | Day 1 | Day 3 | Day 7 | ||
| IL-6 | 1.04±0.43b | 1.45±0.19 | 0.84±0.11 | 0.80±0.16 | 0.84±0.23 | 0.46±0.09c,d | |
| IL-10 | 1.35±0.26 | 2.05±0.30c | 1.59±0.10 | 0.81±0.16 | 1.09±0.23d | 1.53±0.19 | |
| IL-17 | 0.19±0.05c | 0.21±0.05c | 0.25±0.05c | 0.22±0.05c | 0.17±0.04c | 0.28±0.04c | |
| TNF-α | 1.12±0.25 | 2.25±0.36c | 1.39±0.15 | 0.72±0.11 | 1.27±0.24d | 1.37±0.12 | |
The ratio of the TLR2 (zymosan) cytokine response to that of the TLR4 (LPS) response was determined for each culture as an index of the relative TLR response for each cytokine. A ratio of 1.0 indicates comparable TLR2 and TLR4 responses; a ratio < 1.0 indicates a stronger TLR4 response and; a ratio > 1.0 indicates a stronger TLR-2 response. All data was log transformed prior to analysis due to non-normal distribution;
Data are expressed as mean ± SE for 4-10 mice/group;
p<0.05 as compared with 1.0;
p<0.05 as compared with respective sham value
Chemokine responses (Table 3) also showed a shift towards stronger TLR4 responses after burn as demonstrated by the significantly (p<0.05) lower ratio values for KC and MIP-1α at 3-7 days post-injury than those observed in shams. Responses for MIP-1β were comparable for TLR2 and TLR4, whereas RANTES responses were primarily TLR4 driven. MIP-1β and RANTES ratios were unchanged after burn.
Table 3.
Ratio of TLR2/TLR4 chemokine responsesa.
| Sham | Burn | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 7 | Day 1 | Day 3 | Day 7 | |
| KC | 1.19±0.11b | 1.76±0.17c | 1.60±0.23 | 1.11±0.53 | 1.15±0.18d | 1.08±0.08d |
| MIP-1α | 1.93±0.57 | 2.66±0.44 | 1.59±0.10 | 2.47±0.43 | 2.30±0.73 | 0.97±0.08d |
| MIP-1β | 1.19±0.22 | 1.55±0.15 | 1.44±0.17 | 1.26±0.14 | 1.37±0.30 | 1.19±0.91 |
| RANTES | 0.23±0.03c | 0.30±0.05c | 0.26±0.05c | 0.30±0.07c | 0.30±0.11c | 0.21±0.04c |
The ratio of the TLR2 (zymosan) chemokine response to that of the TLR4 (LPS) response was determined for each culture as an index of the relative TLR response for each cytokine. A ratio of 1.0 indicates comparable TLR2 and TLR4 responses; a ratio < 1.0 indicates a stronger TLR4 response and; a ratio > 1.0 indicates a stronger TLR2 response. All data was log transformed prior to analysis due to non-normal distribution;
Data are expressed as mean ± SE for 4-10 mice/group;
p<0.05 as compared with 1.0;
p<0.05 as compared with respective sham value
Discussion
The field of innate immunity has been significantly rewritten since the discovery of mammalian TLRs, a family of pattern-recognition receptors (PRRs). TLRs have been implicated in immunoregulation in a wide range of disease states including atherosclerosis, allergies, autoimmunity, burn and sepsis [20]. Because TLRs recognize pathogen associated conserved molecules shared among members of a particular class of microbes (e.g., LPS from Gram-negative pathogens and ssRNA from RNA viruses), a wide range of pathogens can be recognized by a small group of receptors [21]. Studies also indicate that TLRs can regulate responses to endogenous stimuli, such as necrotic cells, heat-shock proteins and extracellular matrix breakdown products, these stimuli have come to be known as “alarmins” or damage associated molecular patterns (DAMPs). In this regard, necrotic cell death may be a primary cytotoxic mechanism following tissue injury. Necrotic cells can release their intracellular contents, which might contribute to inflammation following injury [22]. Matzinger has proposed a concept involving danger signaling [23]. In the Matzinger model, initiation of the immunoinflammatory response is the result of the recognition of molecular patterns by cellular receptors in response to molecular patterns that can be associated with either pathogens or specific normal cellular components released by damaged cells. This concept reconciles the paradox of the immunoinflammatory response in sterile and nonsterile conditions. Iwasaki and Medzhithov have discussed that exogenous TLR agonists (pathogen associated molecular patterns, PAMPS) activate genes involved in inflammation, tissue repair and adaptive immune response. In contrast, endogenous TLR ligands (DAMPS) only activate genes involved in inflammation and tissue repair [24]. DAMPS associated with TLR activation include HMGB1, components of the extracellular matrix (hyaluronic acid and heparin sulfate), and heat shock proteins (HSP60 and HSP72) [25]. All these DAMPs are associated with cellular injury or stress responses common to burn. Recent findings by Zhang et al. [26] have also shown that injury releases mitochondrial DAMPs into the circulation that can activate neutrophils through TLRs and elicit neutrophil-mediated organ injury. Due to the nature of burns extensive tissue damage and tissue necrosis, rather than apoptosis, is commonplace. Thus, burn provides a fertile environment for the activation of the immunoinflammatory response by DAMPs via TLR activation.
Activation of an inflammatory cascade after burn injury is important in the development of subsequent immune dysfunction. In this regard, previous findings have shown that macrophage productive capacity for these mediators is markedly enhanced after burn and thereby contributing to immune dysfunction [27]. Our study shows that the TLR2 and TLR4-mediated inflammatory responses by circulating leukocytes after burn occurs primarily at 3-7 days post-injury, as evidenced by the significantly increased production of IL-6, IL-10, KC and MIP-1β. IL-6, in general, is considered a marker of injury severity and levels correlate with burn severity in both animal models and humans [28,29]. In addition, elevated levels of IL-6 early after sepsis (6 hrs) are predictive of death [30]. In contrast, IL-10 is counter-inflammatory and has been shown to be important in the down regulation of inflammation after injury [31,32]. While the current study and most of these referenced studies involve mice, rather than human subjects, studies by Finnerty et al, have validated the applicability of the mouse model of burn in terms of its similarity in magnitude and duration of the inflammatory response to that seen in humans [33,34].
Cairns et al, have also shown that mouse splenocytes activated by TLR2 and TLR4 ligands 14 days after burn resulted in an increased inflammatory response [35]. Similarly, Paterson et al have also shown increased splenocyte TLR responses up to 7 days post-burn [36]. Recent findings from our laboratory have shown a marked increase in TLR expression on circulating γδ T-cells, but early (i.e., 24 hr) after burn [37]. Our findings here extend those of other investigators by examining the impact of burn on TLR reactivity in the circulating immune cells, rather than in fixed tissues. Our previous findings have shown that the post-burn immune derangements differ between fixed tissues (ie, spleen) and circulating immune cells, indicating the importance of examining multiple tissues [38]. Blood also represents the tissue that is predominately studied in humans, therefore understanding the responses in this tissue compartment is paramount in applying findings between animal models and the human condition. Moreover, our study examined a much wider array of cytokines and chemokines than the previously cited studies and showed that the burn-induced enhancements in TLR responses were specific for given cytokines rather than global in nature.
In general, the current study demonstrates a stronger shift towards enhanced TLR4 responses, rather than TLR2 responses after burn. Moreover, these responses were delayed till 3-7 days post-injury. Previous studies have shown, primarily via LPS-induced activation, that TLR4 responses are enhanced after burn, but are not immediate [39,40]. The enhanced TLR4 reactivity after burn is mediated, at least in part, by enhanced activation of the p38 signaling pathway [41,42]. The mechanism for the enhanced TLR2 responses is unknown, but may also be related to the p38 signaling pathway [43].
The current study did not examine TLR expression on the circulating leukocytes, however; we have previously shown upregulation of TLR2 and TLR4 expression on circulating γδ T-cells, but not αβ T-cells early after burn [44]. Nonetheless, while the regulation of TLR expression is not clearly understood [45], under “normal conditions”, TLR expression is most likely limited to avoid excessive activation. In this regard, studies have shown that TLR expression is transiently upregulated on monocytes in response to LPS or TNF-α [46,47]. Whether burn induces a dysregulated expression of TLRs by circulating leukocytes which contributes to the enhanced response to TLR agonists remains to be determined.
In conclusion, our findings show that at 3-7days post-burn TLR responses are enhanced in circulating leukocytes. This TLR hyperresponsiveness likely contributes to inflammatory complications after burn. An improved understanding of how injury modulates innate immune responses will reveal new insights into ways in which normal immune function could be restored after critical injury.
Acknowledgements
Support was provided by National Institutes of Health grant GM079122. These findings were presented in part at the 34th annual meeting of the Shock Society in Norfolk, VA. Author contributions: MGS was responsible for scientific conception, design and drafted the manuscript. QZ was responsible for the, cell culture experiments, Bioplex and data analysis. MR was responsible for animal experiments Bioplex, data analysis and scientific interpretation. TC was responsible for animal experimentation and cell culture experiments. RFO was responsible for blood collection and assisted in the preparation of the initial draft of the manuscript. All authors read and approved the final manuscript.
References
- 1.Schwacha MG, Chaudry IH. The cellular basis of post-burn immunosuppression: Macrophages and mediators (Review) Int J Mol Med. 2002;10:239–243. [PubMed] [Google Scholar]
- 2.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, Branski LK, Gauglitz GG, Mlcak RP, Herndon DN. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11:153–159. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Valenti L, Mathieu J, Chancerelle Y, Levacher M, Chanaud B, De Sousa M, Strzalko S, Dinh-Xuan AT, Giroud JP, Florentin I. Nitric oxide inhibits spleen cell proliferative response after burn injury by inducing cytostasis, apoptosis, and necrosis of activated T lymphocytes: role of the guanylate cyclase. Cell Immunol. 2003;221:50–63. doi: 10.1016/s0008-8749(03)00064-9. [DOI] [PubMed] [Google Scholar]
- 5.Schwacha MG, Chaudry IH. The cellular basis of post-burn immunosuppression: Macrophages and mediators (Review) Int J Mol Med. 2002;10:239–243. [PubMed] [Google Scholar]
- 6.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manicassamy S, Pulendran B. Modulation of adaptive immunity with Toll-like receptors. Semin Immunol. 2009;21:185–193. doi: 10.1016/j.smim.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 9.Drage MG, Pecora ND, Hise AG, Febbraio M, Silverstein RL, Golenbock DT, Boom WH, Harding CV. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell Immunol. 2009;258:29–37. doi: 10.1016/j.cellimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cairns B, Maile R, Barnes CM, Frelinger JA, Meyer AA. Increased Toll-like receptor 4 expression on T cells may be a mechanism for enhanced T cell response late after burn injury. J Trauma. 2006;61:293–298. doi: 10.1097/01.ta.0000228969.46633.bb. [DOI] [PubMed] [Google Scholar]
- 11.Breslin JW, Wu MH, Guo M, Reynoso R, Yuan SY. Toll-like receptor 4 contributes to microvascular inflammation and barrier dysfunction in thermal injury. Shock. 2008;29:349–355. doi: 10.1097/shk.0b013e3181454975. [DOI] [PubMed] [Google Scholar]
- 12.Bruns B, Maass D, Barber R, Horton J, Carlson D. Alterations in the cardiac inflammatory response to burn trauma in mice lacking a functional Toll-like receptor 4 gene. Shock. 2008;30:740–746. doi: 10.1097/SHK.0b013e318173f329. [DOI] [PubMed] [Google Scholar]
- 13.Oppeltz RF, Rani M, Zhang Q, Schwacha MG. Burn-induced alterations in toll-like receptor-mediated responses by bronchoalveolar lavage cells. Cytokine. 2011;55:396–401. doi: 10.1016/j.cyto.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber NL, Bailey SR, Schuster RM, Ogle CK, Lentsch AB, Pritts TA. Remote thermal injury increases LPS-induced intestinal IL-6 production. J Surg Res. 2010;160:190–195. doi: 10.1016/j.jss.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwacha MG, Schneider CP, Chaudry IH. Differential expression and tissue compartmentalization of the inflammatory response following thermal injury. Cytokine. 2002;17:266–274. doi: 10.1006/cyto.2001.1003. [DOI] [PubMed] [Google Scholar]
- 16.Alexander M, Chaudry IH, Schwacha MG. Relationships between burn size, immunosuppression, and macrophage hyperactivity in a murine model of thermal injury. Cell Immunol. 2002;220:63–69. doi: 10.1016/s0008-8749(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 17.Schwacha MG, Knoferl MW, Chaudry IH. Does burn wound excision after thermal injury attenuate subsequent macrophage hyperactivity and immunosuppression? Shock. 2000;14:623–628. doi: 10.1097/00024382-200014060-00009. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki JR, Zhang Q, Schwacha MG. Burn induces a Th-17 inflammatory response at the injury site. Burns. 2011;37:646–651. doi: 10.1016/j.burns.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heagy W, Nieman K, Hansen C, Cohen M, Danielson D, West MA. Lower levels of whole blood LPS-stimulated cytokine release are associated with poorer clinical outcomes in surgical ICU patients. Surg Infect (Larchmt) 2003;4:171–180. doi: 10.1089/109629603766956960. [DOI] [PubMed] [Google Scholar]
- 20.Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 21.Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 22.Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509–512. doi: 10.1016/s1471-4906(02)02317-7. [DOI] [PubMed] [Google Scholar]
- 23.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 24.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prince LR, Whyte MK, Sabroe I, Parker LC. The role of TLRs in neutrophil activation. Curr Opin Pharmacol. 2011;11:397–403. doi: 10.1016/j.coph.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwacha MG, Chaudry IH. The cellular basis of post-burn immunosuppression: Macrophages and mediators (Review) Int J Mol Med. 2002;10:239–243. [PubMed] [Google Scholar]
- 28.Agay D, Andriollo-Sanchez M, Claeyssen R, Touvard L, Denis J, Roussel AM, Chancerelle Y. Interleukin-6, TNF-alpha and interleukin-1 beta levels in blood and tissue in severely burned rats. Eur Cytokine Netw. 2008;19:1–7. doi: 10.1684/ecn.2008.0113. [DOI] [PubMed] [Google Scholar]
- 29.Jeschke MG, Mlcak RP, Finnerty CC, Norbury WB, Gauglitz GG, Kulp GA, Herndon DN. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11:R90. doi: 10.1186/cc6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Schwacha MG, Schneider CP, Bland KI, Chaudry IH. Resistance of macrophages to the suppressive effect of interleukin-10 following thermal injury. Am J Physiol- Cell Physiol. 2001;281:C1180–C1187. doi: 10.1152/ajpcell.2001.281.4.C1180. [DOI] [PubMed] [Google Scholar]
- 32.Schneider CP, Schwacha MG, Chaudry IH. The role of interleukin-10 in the regulation of the systemic inflammatory response following trauma-hemorrhage. Biochim Biophys Acta. 2004;1689:22–32. doi: 10.1016/j.bbadis.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Finnerty CC, Przkora R, Herndon DN, Jeschke MG. Cytokine expression profile over time in burned mice. Cytokine. 2009;45:20–25. doi: 10.1016/j.cyto.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, Rocha AM, Jeschke MG. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–19. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 35.Cairns B, Maile R, Barnes CM, Frelinger JA, Meyer AA. Increased Toll-like receptor 4 expression on T cells may be a mechanism for enhanced T cell response late after burn injury. J Trauma. 2006;61:293–298. doi: 10.1097/01.ta.0000228969.46633.bb. [DOI] [PubMed] [Google Scholar]
- 36.Paterson HM, Murphy TJ, Purcell EJ, Shelley O, Kriynovich SJ, Lien E, Mannick JA, Lederer JA. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171:1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 37.Schwacha MG, Daniel T. Up-regulation of cell surface Toll-like receptors on circulating gammadelta T-cells following burn injury. Cytokine. 2008;44:328–334. doi: 10.1016/j.cyto.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwacha MG, Schneider CP, Chaudry IH. Differential expression and tissue compartmentalization of the inflammatory response following thermal injury. Cytokine. 2002;17:266–274. doi: 10.1006/cyto.2001.1003. [DOI] [PubMed] [Google Scholar]
- 39.Schwacha MG, Chaudry IH. The cellular basis of post-burn immunosuppression: Macrophages and mediators (Review) Int J Mol Med. 2002;10:239–243. [PubMed] [Google Scholar]
- 40.Maung AA, Fujimi S, Miller L, Macconmara MP, Mannick JA, Lederer JA. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78:568–573. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]
- 41.Maung AA, Fujimi S, Miller L, Macconmara MP, Mannick JA, Lederer JA. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78:568–573. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]
- 42.Alexander M, Daniel T, Chaudry IH, Schwacha MG. MAP kinases differentially regulate the expression of macrophage hyperactivity after thermal injury. J Cell Physiol. 2004;201:35–44. doi: 10.1002/jcp.20050. [DOI] [PubMed] [Google Scholar]
- 43.Liu G, Yang Y, Yang S, Banerjee S, De Freitas A, Friggeri A, Davis KI, Abraham E. The Receptor for Urokinase Regulates TLR2 Mediated Inflammatory Responses in Neutrophils. PLoS One. 2011;6:e25843. doi: 10.1371/journal.pone.0025843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwacha MG, Daniel T. Up-regulation of cell surface Toll-like receptors on circulating gammadelta T-cells following burn injury. Cytokine. 2008;44:328–334. doi: 10.1016/j.cyto.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 47.Muzio M, Bosisio D, Polentarutti N, D'amico G, Stoppacciaro A, Mancinelli R, van't Veer C, Penton- Rol G, Ruco LP, Allavena P, Mantovani A. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]


