Abstract
The offspring of rats with experimental hyperhomocysteinemia caused by alimentary loading with dietary methionine within pregnancy has been studied. Using pinealon (Glu-Asp-Arg) under these conditions was found to result in the offspring cognitive function being improved significantly and their cerebellum neurons becoming more resistant to oxidative stress. This may be proved by the fact that the administration of pinealon to pregnant rats loaded with methionine improved their offspring spatial orientation and learning ability and decreased both reactive oxygen species accumulation and the number of necrotic cells in the population of the neurons isolated from the cerebellum of the offspring developed under the prenatal hyperhomocysteinemia. Our experiments allowed confirming the neuroprotective properties of pinealon, which is in agreement with the previous data obtained by us in vitro.
Keywords: Prenatal hyperhomocysteinemia, rat offspring, oxidative stress, neuroprotection, pinealon
Introduction
The metabolic imbalance of homocysteine (НС) and its accumulation in blood (hyperhomocysteinemia) result in the development of severe pathologies that are accompanied by endothelial dysfunction and blood clottage. An essential aspect of hyperhomocysteinemia is its aggravating effect in pregnancy. Hyperhomocysteinemia is associated with an approximately two to three-fold increased risk for pregnancy-induced hypertension, placenta abruptio, thromboembolic events, neural tube defects and intrauterine growth restriction [1]. Placentation abnormality and placentofetal blood flow disturbance may cause subfertility, e.g. miscarriage and even infertility as a result of implantation defects [2]. Also, hyperhomocysteinemia causes chronic fetoplacental insufficiency and chronic intrauterine hypoxia at the late stage of gestation. This leads to the delivery of infants with low birth weight, and to the depletion of all life-supporting systems functional backup of the new-born, and to the enlargement of a number of complications in the neonatal period.
Hyperhomocysteinemia may be a one cause of generalized microangiopathy at the second half of gestation, which can manifest itself as late toxicosis (gestosis) – nephropathy, preeclampsia and eclampsia [3]. Immature delivery in such cases is accompanied by high infant mortality and a large ratio of neonatal complications.
The infants born by mothers under hyperhomocysteinemia have mental and physical retardation, the mechanisms of which have not been elucidated as yet. Forasmuch as HC is a toxic structural analog of glutamic acid, glutamate receptors are plausible targets for its toxic effect. Under physiological conditions, the HC blood plasma level (as well as its concentration in the brain) is very low; therefore HC cannot compete for ligand-binding sites of the receptors. When the concentration of HC is significantly increased, however, it starts to compete with glutamate for the binding sites, and to hyperactivate subsequent receptors, which causes a number of toxic effects [4].
The most dangerous effect of this competition is the interacting of HC with ionotropic NMDA glutamate receptors, which are involved in the processes of long-term potentiation and are therefore responsible for learning and memorizing, as well as other cognitive functions of the brain. The hyperactivation of the receptors in neurons leads to reactive oxygen species (ROS) accumulation and signal transduction mechanisms disorder [5].
It has been shown recently that carnosine, which is able to regulate the intracellular ROS level and to maintain the viability and functional activity of neurons, can effectively protect neuronal cells from the HC toxic effects [6]. Subsequently, using various cell models (the cerebellum granule cells, neutrophils, and РС-12 pheochromocytoma cells), we showed that the synthetic tripeptide pinealon (Glu-Asp-Arg) could restrict ROS accumulation and prevent apoptosis induced by НС or Н2О2; effective pinealon concentrations being much lower than those of carnosine [7].
The aim of this study was to investigate a possible neuroprotective effect of pinealon on the rat offspring developed under conditions of experimental prenatal hyperhomocysteinemia in vivo.
Materials and methods
Experimental protocol
Female Wistar rats weighing 180-200 g were used in this study. Experimental hyperhomocysteinemia was created with the alimentary loading of the pregnant rats with methionine starting from second trimester of pregnancy and continuing till the end of experiment. Methionine was added to the drinking water in the concentration providing the daily dose of 1±0.01 g/kg b.w. This procedure led to a stable increase in total HC levels in the blood plasma of the pregnant rats (33.0±3.9 μM, as compared to the control value of 5.9±1.8), as had been shown earlier [8]. The enhancement of HC established in this experimental model corresponds to moderate hyperhomocysteinemia at complicated pregnancy in women [3].
The offspring obtained from the intact pregnant rats with the physiological HC blood plasma level was used as a control (intact) group and that obtained from the rats with hyperhomocysteinemia as an experimental (methionine loaded) group. The latter was developed under constant oxidative stress induced by an increased HC level in the parental blood plasma. Another experimental group of animals was treated with methionine and pinealon simultaneously (“methionine+pinealone” loaded group). Pinealon (Garmonika, Russia) was dissolved in 0.9% NaCl and administered i.p. during 5 days prior to the methionine loading in a daily dose of 10 μg/kg b.w. This treatment mode is based on the pinealone antihypoxic effect in vivo described earlier [9,10].
All the offspring obtained was registered with regard to their quantity and weight, and was then used for cytometric analysis of cerebellum neurons (isolated from 10-day-old pups) and for behavioral experiments (using 45-day-old pups). The protocol for this study was approved by Ethics Committee of D.O. Ott Institute of Obstetrics and Gynecology, the North-Western Branch of the Russian Academy of Medical Sciences.
Determination of total homocysteine in the blood plasma of 45-day-old pups was performed by the immunofluorescent method, using the AxisTM kit (Axis-Shield, UK). This method is based on the reducing of all the chemical forms of HC in the blood plasma to free HC followed by its enzymatic conversion to S-adenosyl-L-homocysteine [8].
Spatial orientation and learning ability of animals were assessed by the Morris water maze test, which is widely used to study the cognitive function of the animals [11-13]. A water pool of 1.7 m in diameter was filled with water (22°C) and 2 L milk to make water cloudy. A platform of 15 cm in diameter was placed 0.5-1 cm under the water level and 30 cm away from the pool edge. All the movements of the animals in the pool were being shot on the video tape throughout the experiment, which enabled to determine an average rate of the platform search and the time, which the rat had spent to find the platform (latency) [8,14].
45-day-old pups normally fed were used in the experiment. On the first day, the average rate of the platform search was detected, and it tentatively correlated with the animal orientation in the water pool [12,14]. On the second day, the latency was measured in 5 successive trials to characterize the learning ability [11]. The latency is known to correlate well with the spatial orientation and learning processes [13,14]. Each experimental group consisted of 23 animals (intact, “methionine loaded” and “methionine+pinealon loaded”).
Cerebellum granule cells were isolated from 10-day-old pups after treatment of cerebellar slices with dispase (2 mg/ml collagenase 400 U, Wako, Japan) followed by one washing with standard Tyrode’s solution (NaCl 148 mM, KCl 5 mM, MgCl2 mM, glucose 10 mM, HEPES 10 mM, pH 7.4) and filtration through a 53 μm nylon filter [15]. Oxidative stress was induced by an incubation of the cell suspension with 5 mM H2O2 over 30 min at 37°C. Each sample was analyzed in triplicate based on the results of 10,000 events, and all the experimental series were reproduced using the pups obtained from at least two different families.
Flow cytometry technique
The measurements were performed on a BD FACSCalibur flow cytometer (Becton, Dickinson, USA) after the cell population had been gated matching neuronal cells in size (≈ 10 mμ) [13]. To determine the intracellular ROS level, the cerebellum granule cells were pre-loaded with the fluorescent dye DCF-DA (2,7-dichlorodihydrofluoresceine diacetate, Molecular probes, USA) at the final concentration of 100 μM. The DCF-DA pre-loaded cells were exposed to 5 mM H2O2 (Sigma, Germany) over 30 min and 1 min prior to the measurement, 10 μM propidium iodide (Sigma, Germany) being added to the samples as well. Propidium iodide (PI; excitation wavelength 485 nm, emission wavelength 610 nm) is unable to penetrate a native cell membrane, so it is only accumulated in the cells with membrane defects, where it reacts with nucleic acids to produce stable colored complexes. From this analysis, the mean DCF fluorescence corresponded to the stationary ROS level of viable cells and PI fluorescence allowed to calculate the ratio of neсrotic cells in the neuronal population.
Statistics
The data was expressed as mean ± S.D. Statistical analyses were performed using Statistica software (version 6.0), with the Kruskal-Wallis, Fisher and Duncan tests applied. Results were considered significant when P values were less than 0.05. The results obtained with use of the flow cytometry method were analyzed by WIN MDI software.
Results
Table 1 shows the characteristics of the offspring on the tenth day after delivery brought by all of the three groups of the studied animals – intact ones, methionine loaded ones, and those loaded with pinealon along with methionine. It has been found that the average number of infant animals in the brood of all the studied groups was the same. Their weight (17.86±3.05 g) was significantly lower than that in the control group (23.05±1.45 g). Also, the average weight standard deviation of the methionine loaded animals, as well as of the animals that were administered pinealon along with methionine, was more profound, which proximately testified to the different sensitivity of particular families in each experimental group to methionine. Besides, it has been shown that the weight of the animals that were administered pinealon under the conditions of experimental hyperhomocysteinemia did not decrease. The analysis of the development rate, outward appearance and activity of the animals from the different experimental groups, however, has shown that the animals that developed under the conditions of prenatal hyperhomocysteinemia were exceeded in many test results by the control animals. It could be seen from the weight gain hysteresis, the absence of vibrissae growth and the behavioral activity of the pups being disturbed during further development.
Table 1.
The characteristics of the offspring obtained from the experimental animals
| Experimental group | Quantity of families | Average quantity of animals per brood | Weight (for the offspring aged 10 days), g |
|---|---|---|---|
| Control | 6 | 9±3 | 23.05±1.45 |
| Methionine | 6 | 9±2 | 17.86±3.05 (*) |
| Methionine+pinealon | 6 | 8±3 | 25.03±4.86 (#) |
– p<0.05 (as compared to the control group);
– p<0.05 (as compared to the methionine-loaded group).
It has been shown in our experiments that the offspring brought by the methionine loaded animals had the elevated HC blood plasma level, too, though it was less profound than that found earlier in the adult animals treated with methionine (Table 2). It should be noted that the HC level stayed high in the blood plasma of the offspring brought by the animals loaded with pinealon along with methionine.
Table 2.
The total homocysteine content in the blood plasma of the offspring obtained from the rats of different experimental groups
| Experimental group | Total homocysteine content, μM |
|---|---|
| Control, n=5 | 5.6±0.6 |
| Methionine, n=4 | 9.8±0.2* |
| Мethionine+pinealon, n=4 | 10.5±0.2* |
p<0.05 (as compared to the control group).
When assessing the cognitive abilities (training and learning processes) of the offspring brought by the animals from all of the three experimental groups, with use of the Morris water maze test, it has been found that the young animals reared under the conditions of hyperhomocysteinemia (the second group) had a lower swimming rate than the intact ones, whereas the swimming rate of the animals from the “methionine+pinealon” loaded group did not differ from that of the intact ones (Figure 1).
Figure 1.
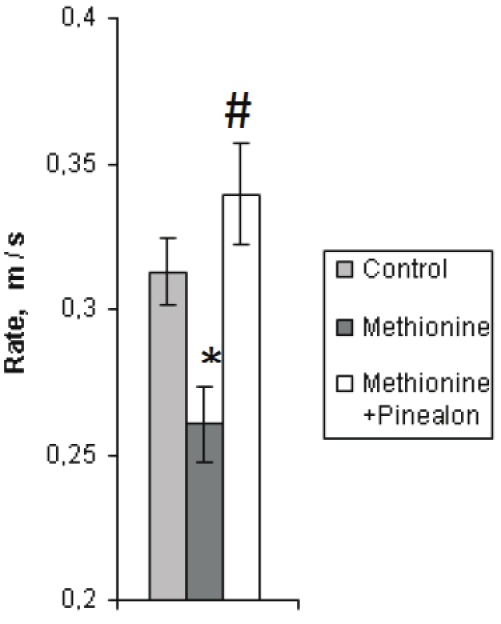
Average swimming rate (m/s) during learning to find the platform by the animals from the three experimental groups (the first day of the experiment). Sign “*” corresponds to the statistically significant difference from intact group (p<0.05), “#” – to the statistically significant difference from methionine loaded group (p<0.05).
During the first trial of the platform finding on the second day of our experiments, the studied groups of the animals differed very much from each other. The time of the platform search was 112 sec for the intact animals, and 170 sec for the methionine-loaded ones (p<0.01). For the “methionine+pinealon” loaded group, the time was 137 sec, which was statistically significant, when compared to the methionine-loaded group (p<0.05) and the control group (p<0.05). The further trials did not reveal any significant differences in the time of the platform finding by the intact animals and the animals from the “methionine+pinealon” loaded group, even though the difference between the methionine loaded group and the “methionine+pinealon” loaded group still could be observed during the second, third and fourth trial (p<0.05). By the fifth trial, the differences in the performance of the platform finding between all of the studied experimental groups had annihilated completely, and all the animals reached the platform in the Morris water pool over the same time (Figure 2).
Figure 2.
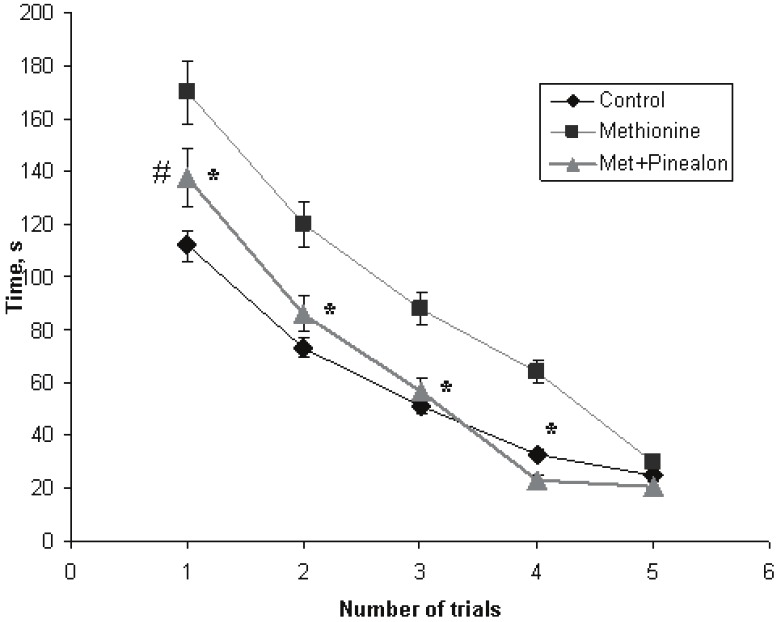
Time of the platform searching (latency) by the animals of the three experimental groups during training (Morris water maze test, abscissa – a number of trials, ordinate – time, sec; the second day of the experiment). Sign “*” corresponds to the statistically significant difference from “methionine+pinealon” group (p<0.05), “#” – to the statistically significant difference from intact group (p<0.05).
Subsequently, in the further experiments performed with use of flow cytometry, we compared the properties of the neurons isolated from the cerebellum of the animals from the two experimental groups – the methionine-loaded and “methionine+pinealon” ones. As Figure 3 shows, in the population of the cells obtained from the methionine-loaded animals, a certain quantity of necrotic cells was found, while they were practically non-existent in the “methionine+pinealon” loaded group. The subpopulation of living cells is highly diffused in the first instance and is conglomerated in the second.
Figure 3.
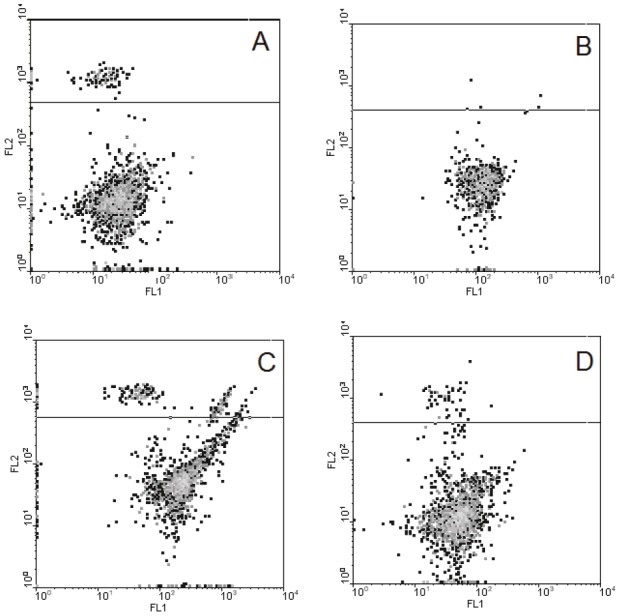
The characteristic of cerebellum neurons isolated from the brain of animals after prenatal hyperhomocysteinemia not treated and treated with pinealon (typical record). Isolated cerebellum granule cells were loaded with DCFH2 and PI and then analyzed by flow cytometry technique as described in Materials and methods. Axis FL1 corresponds to DCF fluorescence, marker of free radical levels, abscissa; FL2 – to PI fluorescence, marker of necrotic cells, ordinate). A and B correspond to the intact cells isolated from the pups of methionine loaded rats not treated (A) or treated (B) with pinealon, C and D – the same as A and B after the 30 min incubation of the isolated cells with 5 mM H202.
The oxidative stress induced by the incubation of the cells with H2O2 has revealed some more differences in the properties of the neurons obtained from the experimental animals. The ratio of necrotic cells increased significantly in the population of the neurons isolated from the cerebellum of the methionine loaded animals, while the population of living cells drifted strongly rightwards along the X-axis, i.e. to the area of the greater intracellular ROS level. In the population of the neurons isolated from the cerebellum of the “methionine+pinealon” loaded animals, however, both of these parameters were less profound (Table 3).
Table 3.
The effect of Н202 on DCF mean fluorescence (Mean Flu) and the ratio of necrotic cells in populations of neurons isolated from cerebellum of pups subjected to prenatal hyperhomocysteinemia±pinealon (* – statistically significant difference from I, # – statistically significant difference from III)
| Animal group | Mean Flu, Arb.U. | % of PI labelled cells |
|---|---|---|
| I. Methionine loaded | 51.6±2.3 | 12.6±1.2 |
| II. “Methionine+pinealon” loaded | 54.2±2.8 | 3.4±0.7 |
| III. Methionine loaded + Н202 | 158.7±3.8 (*, p<0.01) | 17.7±2.1 (*, p<0.05) |
| IV. “Methionine+pinealon” loaded + Н202 | 108.6±4.1 (#, p<0.05) | 7.0±0.9 (#, p<0.05) |
As Table 3 shows, the population of the neurons isolated from the cerebellum of the animals that were administered pinealon contains less necrotic cells and possesses a definite resistance to H2O2, as the incubation with that caused the less pronounced cell necrosis and ROS accumulation. Thus, as can be seen from the above, pinealon has a strong protective effect in the experiments in vivo.
Discussion
As had been stated earlier, the total HC level in the blood plasma of the mature animals that consumed methionine with the drinking water increased 5 times on the average, when compared to the reference value [8]. This testifies to hyperhomocysteinemia being evidently developed in the rat females as a response to the addition of an excessive amount of methionine to the drinking water. According to the data presented here, the offspring of these animals had the elevated HC blood plasma level, too, though less profound than in the mature animals. At the same time, it has been shown that pinealon did not abolish the development of hyperhomocysteinemia in our experiments. It may be suggested that the protective action of pinealon is directed to the decreasing of the toxic effect of HC but does not stimulate the metabolizing of this compound in the tissues.
This conclusion has been confirmed by the investigation of cognitive function of the animals which underwent prenatal hyperhomocysteinemia. It has been established that pinealon induced a significant improvement of the spatial orientation and learning ability in the Morris water maze test. This was revealed in the swimming rate being enhanced and the platform search time shortened of these infant pups compared to those brought by the animals loaded with methionine without pinealon treatment (see Figures 1 and 2). The effect of pinealon may be due to the increased resistance of the cells to the oxidative stress, as was shown earlier in our in vitrо study [7]. This suggestion was confirmed in the cytometric study, which allowed concluding that pinealon decreased the stationary ROS level and contributed to the neuronal cell survival in the culture of the cerebellum granule cells isolated from the animals that had developed under the conditions of prenatal hyperhomocysteinemia (see Figure 3).
The data obtained by us testifies that pinealon, which has geroprotective properties [10,17], is able to protect the developing brain under the conditions of prenatal hyperhomocysteinemia. The neuroprotective effect of pinealon on the fetus was confirmed in our previous studies, in which we explored the influence of it, along with some other short peptides, upon the resistance of the cerebellum neurons and the cognition ability of the rats that had undergone chronic intrauterine hypoxia [9]. The data presented allow concluding that the protective effect of pinealon is due to the lessened neurotoxic influence of HC on the developing brain, rather than its decreased level in blood. Recently, the protective effect of carnosine has also been reported in the study dedicated to the experimental brain ischemia aggravated by hyperhomocysteinemia [18]. It can be suggested that the protective effect of pinealon is due to the fact that it suppresses the ROS accumulation in the neuronal cells, making them more resistant to the oxidative stress, and prevents HC and its derivatives from the interacting with glutamate receptors. However, one may not exclude some other mechanisms of neuroprotection that are still unknown and associated with the effect of pinealon on the offspring that underwent prenatal hyperhomocysteinemia. Thus, it has been shown recently that the neurotoxicity of HC may be due to the prenatal hyperhomocysteinemia-caused suppression of the expression of the neurospecific proteins S-100, GFAP and N-CAM, which deal with neurogenesis and the plasticity of the developing brain, with those being accompanied by the worsening of the cognition ability of the offspring [19,20]. This process is reversible and can be prevented by the administration of melatonin to pregnant female rats [20].
Recently, hyperhomocysteinemia has widely been discussed as one of crucial factors associated with the progeria syndrome [21]. A great number of facts has been reported to prove that the dysmetabolism of methionine and, as a consequence, the age-caused depression of methylation and the elevation of HC and its derivatives with age are among the factors of premature aging [21,22]. In this regard, it is overwhelmingly important to bring it into focus that the toxic effects of hyperhomocysteinemia being weakened may be one plausible constituent of the geroprotective effect of pinealon.
Acknowledgements
The study was partially supported by the RFBR (#10-04-00749а, Russia). The authors are very grateful to Prof. А.А. Boldyrev for useful discussion and to Dr. М.С. Belyayev and A.V. Makhro for their help in the experimental part of the study.
References
- 1.Aubard Y, Darodes N, Cantaloube M. Hyperhomocysteinemia and pregnancy review in our present understanding and therapeutic implications. Eur J Obstet Gynec and Reprod Biol. 2000;93:157–165. doi: 10.1016/s0301-2115(00)00282-7. [DOI] [PubMed] [Google Scholar]
- 2.Verspyck E, Marpeau L. Thrombophilias and vascular placental pathology. A survey of the literature. Rev Med Int. 2005;26:103–108. doi: 10.1016/j.revmed.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Vollset SE, Refsum H, Irgens LM, Emblem BM, Tverdal A, Gjessing HK, Monsen AL, Ueland PM. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine Study. Am J Clin Nutr. 2000;71:962–968. doi: 10.1093/ajcn/71.4.962. [DOI] [PubMed] [Google Scholar]
- 4.Boldyrev A, Johnson P. Homocysteine and its derivatives as possible modulators of neuronal and non-neuronal glutamate receptors in Alzheimer’s disease. J Alzheimer's disease. 2007;11:219–228. doi: 10.3233/jad-2007-11209. [DOI] [PubMed] [Google Scholar]
- 5.Boldyrev AA. Molecular mechanisms of homocysteine toxicity. Biochemistry (Moscow) 2009;74:589–598. doi: 10.1134/s0006297909060017. [DOI] [PubMed] [Google Scholar]
- 6.Boldyrev AA, Bulygina EP, Trunova ОА, Тjulina ОV, Маkhro АV, Маshkinа АP, Solenaya ОА. Carnosine protects from oxidative stress induced by hyperhomocysteinaemia. Neurochem J. 2008;25:233–240. [Google Scholar]
- 7.Khavinson V, Ribakova Y, Kulebiakin K, Vladychenskaya E, Kozina L, Arutjunyan A, Boldyrev A. Pinealon increases cell viability by suppression of free radical levels and activating proliferative processes. Rejuvenation Res. 2011;14:535–541. doi: 10.1089/rej.2011.1172. [DOI] [PubMed] [Google Scholar]
- 8.Makhro AV, Mashkina AP, Solenaya OA, Trunova OA, Kozina LS, Arutjunyan AV, Bulygina ER. Prenatal hyperhomocysteinemia as a model of oxidative stress in the brain. Bull Exp Biol Med. 2008;146:33–35. doi: 10.1007/s10517-008-0233-0. [DOI] [PubMed] [Google Scholar]
- 9.Kozina LS, Arutjunyan AV, Stvolinski SL, Stepanova MS, Makletsova MG, Khavinson VK. Regulatory peptides protect brain neurons from hypoxia in vivo. Doklady Biol Sci. 2008;418:419–422. doi: 10.1134/S0012496608010031. [DOI] [PubMed] [Google Scholar]
- 10.Kozina LS, Arutjunyan AV, Stvolinski SL, Khavinson VK. Biological activity of regulatory peptides in model experiments in vitro. Adv Gerontol. 2008;21:68–73. [PubMed] [Google Scholar]
- 11.Morris RGM, Garrud P, Rawlins JN, O’Keefe J. Place-navigation impaired in rats with hyppocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 12.Fukunaga A, Kawase T, Uchida K. Functional recovery after simultaneous transplantation with neuro-epithelial stem cells and adjacent mesenchimal tissues into infracted rat brain. Acta Neurochir. 2003;145:517–522. doi: 10.1007/s00701-003-0038-x. [DOI] [PubMed] [Google Scholar]
- 13.Gao J, Prough DS, McAdoo DJ, Grady JJ, Parsley MO, Ma L, Tarensenko YI, Wu P. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Experim Neurol. 2006;201:281–292. doi: 10.1016/j.expneurol.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 14.Whishaw IQ. Formation of a place-learning set by the rat. A new paradigm for neurobehavioral studies. Physiol Behav. 1984;5:139–143. doi: 10.1016/0031-9384(85)90186-6. [DOI] [PubMed] [Google Scholar]
- 15.Boldyrev AA, Carpenter DO, Huentelman M, Peters C, Johnson P. Sources of reactive oxygen species production in excitotoxin-stimulated cerebellar granule cells. Biochem Biophys Res Commun. 1999;256:320–324. doi: 10.1006/bbrc.1999.0325. [DOI] [PubMed] [Google Scholar]
- 16.Маkhrо АV, Bulyginа ЕR, Boldyrev АА. Effects of homocysteine and homocysteinic acid on cerebellar granule cells. Neurochem J. 2008;1:127–132. [Google Scholar]
- 17.Khavinson VKh, Grigoriev EI, Malinin VV, Ryzhak GA., inventors. Peptide substance stimulating regeneration of central nervous system neurons, pharmaceutical composition on its base, and the method of its application. ER 2 024 388 B1. European patent. international publication number WO 2007/139431, 06.12.2007. Gazette 2007/49.
- 18.Dobrotvorskaya IS, Fedorova TN, Dobrota D, Berezov TT. Charactristics of oxidative stress in experimental rat brain ischemia aggravated hyperhomocysteinemiea. Neurochem J. 2011;28:49–54. [Google Scholar]
- 19.Baydas G, Koz ST, Tuzcu M, Nedzvetsky VS, Etem E. Effects of maternal hyperhomocysteinemia induced by high methionine diet on the learning and memory performance in offspring. Int J Dev Neurosci. 2007;25:133–139. doi: 10.1016/j.ijdevneu.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Baydas G, Koz ST, Tuzcu M, Nedzvetsky VS. Melatonin prevents gestational hyperhomocysteinemia- associated alterations in neurobehavioral developments in brain. J Pineal Res. 2008;44:181–188. doi: 10.1111/j.1600-079X.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 21.Perez FP, Ilie JI, Zhou X, Feinstein D, Jurvich DA. Pathomolecular effects of homocysteine on the aging process: A new theory of aging. Med Hypothesis. 2007;69:149–160. doi: 10.1016/j.mehy.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 22.Roach H, Yamada N, Cheung K, Tilley S, Clarke N, Oreffo R. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52:3110–3124. doi: 10.1002/art.21300. [DOI] [PubMed] [Google Scholar]


