Abstract
Objectives
Adenosine deaminase (ADA) is an enzyme being involved in purine metabolism and plays a significant role in the immune system. The aim of this study was to investigate the use of adenosine deaminase levels in differentiating between rheumatoid arthritis and osteoarthritis.
Material and methods
Thirty patients with rheumatoid arthritis and 30 osteoarthritis patients enrolled the study. They were matched in sex and age. Using the ROC curve, we determined the optimal serum and synovial cutoff for rheumatoid effusion.
Results
The results showed a statistically significant difference between ADA levels in joint effusion and serum of patients with rheumatoid arthritis and osteoarthritis (p<0.001). Synovial fluid cutoff value for diagnosing rheumatoid arthritis was 20 with a sensitivity of 90% and specificity of 80% and the serum cutoff value was 15 with a sensitivity of 93% and specificity of 53.3%. Area under ROC curve for synovial ADA, ESR and CRP co-linearity was 99%.
Conclusion
We concluded that synovial total ADA assay can be a sensitive and specific test, being suitable for rapid diagnosis of rheumatoid effusions.
Keywords: Adenosine deaminase (ADA), enzyme, purine metabolism, immune system, rheumatoid arthritis, serum, synovial fluid, osteoarthritis, human
Introduction
Rheumatiod arthritis (RA) is a chronic multisystem disease of unknown cause. The characteristic feature of RA is persistent inflammatory synovitis, usually involving peripheral joints in a symmetric distribution and is characterized by presentation of secreted products of activated lymphocytes, macrophages, fibroblasts and leukocytes in joints [1,2]. Proteolytic enzymes and cytokines like IL1B, IL6 and TNFα cause joint inflammation. Arthritis is often progressive and coordinates with joint destruction [1,3]. Inflammatory mediators especially free radicals release oxygen that is toxic. Endogenous adenosine suppresses this process and actually mediates inflammatory system [4].
Adenosine is metabolized by adenosine deaminase (ADA), getting converted to inozine [5,6]. During inflammatory process, this enzyme is released in extra cellular and serosal fluids and produces different levels of ADA. The levels depend on the numbers of nuclear cells, especially T cells and macrophages [5]. Researchers have shown that plural effusion in patients with RA and tuberculosis produce higher levels of ADA in contrast to Lupus Erythematosis and malignancies. Therefore, determination of ADA activity can be an appropriate technique for diagnosis, especially when the cause is unknown [7].
Osteoarthritis (OA) actually means dysfunction of diarthrodial (moveable, with synovial coverage) joints. According to National Health and Nutrition Examination studies, the prevalence of OA is approximately 0.1% among people aged between 25 and 34 years old and increases to 80% among those aged over 55 [8]. Many studies have shown greater risk of OA in females. The approximate risk of OA in women is 2.6 times more than men after equalization of age, weight and smoking [9].
According to different studies, increases in ADA activity in synovial fluid have a close relationship with activity of underlying disease and can show the degree of inflammation in joints. Therefore, ADA, as a marker of cellular immunity activation, may help in better understanding some pathophysiologic aspects of the disease and may help in relieving the triggering factors of inflammation and promoting new therapeutic approaches [10]. Thus, we designed a study to evaluate ADA levels in both specimens of serum and synovial fluids in patients with RA or OA.
Materials and methods
Patients
Thirty patients with rheumatoid arthritis and 30 patients with osteoarthritis randomly enrolled the study. The 30 patients with RA had definite diagnosis according to the criteria of the American College of Rheumatology in 1987. All patients were monitored at Rheumatology Clinics of Ali Ebne Abitaleb University Hospital of Zahedan over a period of 1 year. The same physician visited all patients with RA in their active phases of the disease. Active disease was defined by the presence of swelling and or limitation of movement or tenderness in at least one joint, and elevated C-reactive protein (CRP) level and erythrocyte sedimentation rate (ESR). The diagnosis of OA was based on the criteria of the American College of Rheumatology 1987.
Project method
Synovial fluid specimens were collected from knee joint effusion. 10cc of synovial fluid and concurrently 10cc of patient’s blood in clot form were collected using 10cc syringes. Synovial fluid was examined for infectious causes according to its cell count and smear results. After disproving the infection, ADA test was performed. In RA patients, serum ADA, ESR and CRP were also checked, but in OA patients, serum was checked only for ADA.
Method of ADA measurement
In laboratory, after centrifuging, the samples were kept in -20 degrees centigrade for one night. Next day, enzyme assays were performed in two steps on an indirect method. In the first step, Adenosine was deaminated and released ammonia. In the second step, Glutamate Dehydroxygenase catalyzed it inside inherent eluestric activator. Therefore, the speed of reduction in absorption at 240 nanometer (modifying NADPH to NADP) had a direct relation with ADA activity [10].
Reagents
Number one: tampon about 1×45ml, Number two: ADP, NADPH 1×5ml, Number three: enzyme solution GDH. To prepare the fresh solution, we used reagent 1, 2 and 3(volume ratio was 9:1:0/5). We used Chem enzyme, lot number co22044 [10]. The values of ADA were reported as IU/l at 37ċ [10]. Normal values of ADA activity in synovial fluid was considered as <10 IU/L [11].
Deliberation limit
By using commended volume ratio for sponsor and volume in devices of parameters, ADA activity was accountable at least up to 100 IU/L (international unite per liter). In higher activation (concentration), which occurs rarely, we used the diluted samples. In case of low level of this enzyme in biologic liquids especially in CSF, if the parameter of device permitted, we could elevate the sample volume up to one third of prepared solution volume [10].
Statistical analysis
Descriptive statistical exams, Kay Square, Fisher test and independent t-test for binary and continuous variants were used and also odds ratio was estimated as appropriate (using SPSS software, version 11.5). Standard deviation and mean values were also calculated for each group. For all statistical parameters, the confidence interval of 95% and p-values less than 0.05 were considered significant. ROC curve was drawn to determine cut-off value and STATA (Ver.8) was used for analysis.
Results
The mean age of patients in RA group was 52 ± 8.7 years (ranging from 44-60 years) and in OA group was 56 ± 11.7 years (ranging from 44-67).
Cell counts of all synovial samples were examined to rule out the presence of infectious causes. Cell counts of synovial fluids were 100-1700 WBC/ml and 1500-33000 WBC/ml in OA an RA groups, respectively. But none of them were in boundary of infected arthritis. The average level of synovial ADA in RA patients was 28.1 ± 6.8 IU/L and in OA group was 16.5 ± 5.8 IU/L. Also, the average levels of serum ADA were 18.9 ± 2.5 IU/L and 14.9 ± 4.5 IU/L in RA and OA groups, respectively.
According to simple variant analysis with independent sample t-test, the average levels of serum and synovial fluid ADA between RA and OA groups showed a statistically significant difference (p- value < 0.05). The average of ADA level in synovial fluid was significantly higher than serum (11.6 IU/L vs. 4.0 IU/L, p-value < 0.05) (Table 1).
Table 1.
The average levels of serum and synovial fluid ADA between RA and OA group with independent sample T test
| Sample | Group | N | Average (IU / L) | SD | p-value |
|---|---|---|---|---|---|
| Synovial ADA | RA | 30 | 28.1 | 6.8 | |
| OA | 30 | 16.5 | 5.8 | <0.001 | |
| Serum ADA | RA | 30 | 18.9 | 2.5 | |
| OA | 30 | 14.9 | 4.5 | <0.001 |
Average ESR in RA patients was 48.8 ± 20.6 mm/h and in OA group was 23 ± 13.3 mm/h. Also divergence of ESR average in two groups was significant (P-value <0.001).
Considering CRP as a ranking variant, to evaluate the difference between the two groups in their CRP levels, the Mann-whitney test was used. The average was 42 in RA and 17 in OA group (p<0.001). CRP divergence in two groups was evaluated using interval by interval test, which was significant (p<0.001). According to this test, the higher the CRP amount, the higher probability of being in RA group.
According to multivariate Logistic Regression assay, the odd ratios for ADA in synovial fluid and serum were 1.62 and 2.20, respectively (Table 2). This showed that for every elevation in ADA amount in synovial fluid, there was 60% raise in the probability of being in RA group and for every elevation in ADA amount in serum this chance raised more than double.
Table 2.
multi variant analysis between synovial fluid ADA and RA with Logistic Regression assay
| Samples | Odds Ratio | P-value | SD | 95% confidence Interval |
|---|---|---|---|---|
| Synovial fluid ADA (IU/L) | 1.62 | 0.002 | 1.19 | 2.19 |
| Serum ADA (IU/L) | 2.20 | 0.011 | 1.19 | 4.05 |
The important point was that the relation between the amount of ADA in synovial fluid and presence of RA was stronger than this relation for serum ADA (using Wald test).
Despite the interesting statistical model that is shown in Table 2, the important issue is having a cut-off point to relieve the patients’ complaints, affected by either RA or OA (Table 2).
For determining cut-off point of ADA in synovial fluid, at first a separation model was prepared for ADA level in synovial fluid and ROC curve was sketched and analyzed. According to this analysis, synovial fluid ADA cut-off point, with the highest specificity and sensitivity, was about 20. According to this cut-off point, the sensitivity and specificity were 86.7% and 80%, respectively. If the patient was classified in OA or RA group, based on the mentioned standards, underneath area of ROC curve was estimated to be 90.3% (confidence interval: 82.4% - 98.3 %) (Figure 1).
Figure 1.
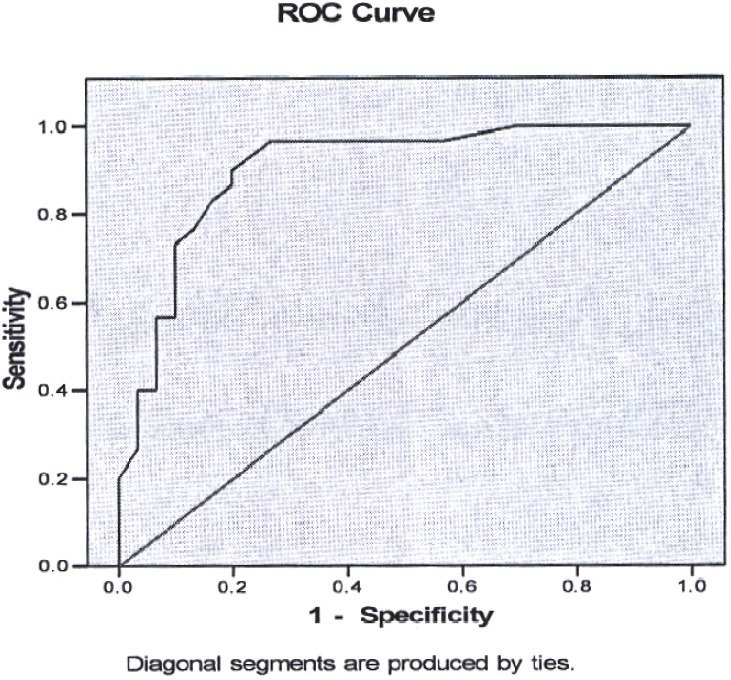
ROC curve for determination cut-off point of synovial fluid ADA in RA group.
With the same method, the cut-off point was estimated to be 15 based on 93.3% sensitivity and 53.3% specificity. Underneath area of ROC curve for mentioned quantities will be 77.1% with confidence limit of 65.2% to 89.0%. Although it is statistically significant, it may not be appropriate due to low specificity for diagnosis or even screening approaches.
If we make a common model with Logistic model based on above cut-off point for serum and synovial ADA, the following results will be achieved (Table 3, Figure 2).
Table 3.
Logistic Regression statistical assay model based on cut-off point for serum (15IU/L) and synovial ADA (20IU/L).
| Sample | Odds Ratio | P-value | 95% confidence interval |
|---|---|---|---|
| Mean serum ADA | 28.5 | 0.002 | 3.4-235.56 |
| Mean synovial fluid ADA | 40.5 | < 0.001 | 6.8-241.1 |
Figure 2.
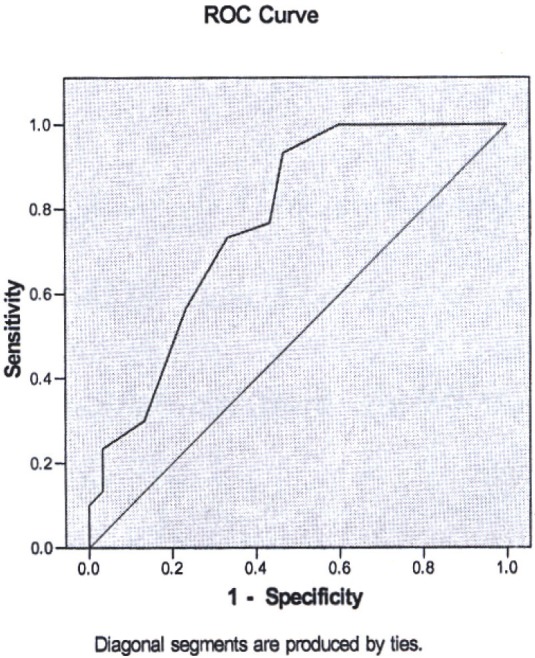
ROC curve for determination cut-off point of serum ADA in RA group.
According to this model, the expected cut-off point for synovial fluid ADA was 20 and for serum was15. According to reported OR, if the ADA level of synovial fluid raised over 20 units, individual chance for being in RA group would raise more than 40 folds and if ADA amount in serum increased more than 15 units, the chance of being in RA group would raise more than 28 folds.
If we want to enter CRP variant to these models due to co-linearity between CRP and serum ADA level, the effect of serum ADA level will be weakened in statistical model and will be deleted. Therefore, CRP should enter to model with synovial fluid ADA detached from serum ADA. This subject repeats during ESR entering. In fact, ESR severely had co-linearity with CRP, which was not unexpected, because both of them are acute phase reactants. (One of the most reasons for co-linearity is solidarity of variants), but both variants could be entered easily with synovial fluid's ADA in one table (Table 4, Figure 3).
Table 4.
Co-linearity between CRP, ESR and serum ADA level on cut-off point for serum (15IU/L ) and synovial ADA(20IU/L) using Logistic Regression statistical assay.
| Group | Odds Ratio | P-value | 95% confidence Intervall |
|---|---|---|---|
| Mean synovial fluid ADA | 1.35 | 0.016 | 1.73 |
| CRP | 81.32 | 0.022 | 3520.27 |
| ESR | 1.13 | 0.04 | 1.29 |
Figure 3.
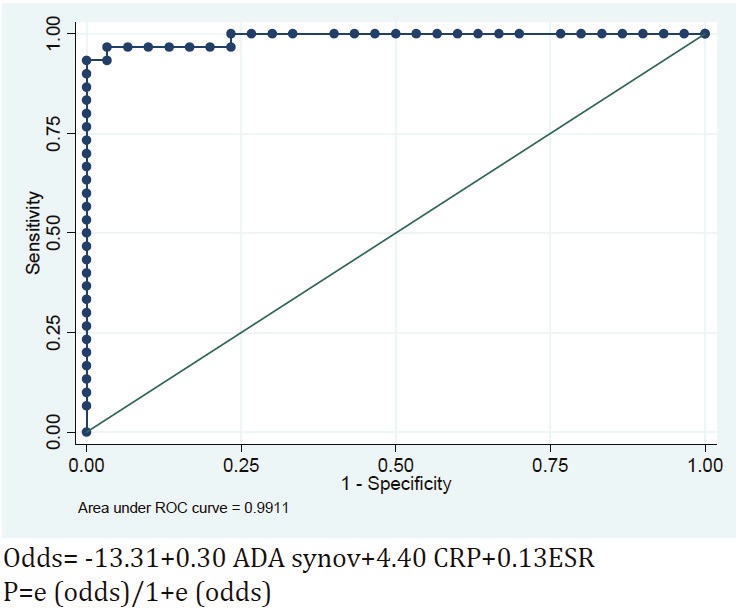
ROC .
This model classifies participants carefully, so that the underneath area of ROC curve will approximate almost 100 % (99.11%). In other words, based on ESR, CRP and synovial fluid's ADA quantities, we could differentiate between RA and OA.
If we want to determine patients condition based on model n.2 of Logistic Regression, we must measure every patient’s ESR and CRP quantities, in addition to synovial fluid's ADA level.
As it is seen, CRP is an appropriate prognostic factor, even if used alone and in most RA patients the amount is above 1.
According to ROC curve analysis of this model on all the 60 participants, the cut-off point of Regression equation was estimated to be 0.7 (with 93% sensitivity 93% and 100% specificity). If we assume the cut-off point to be 0.06, the sensitivity and specificity for the participated groups will be equal and both will be 96.7%.
Discussion
Considering the chronic course of RA and not completely known pathology of the disease and the destructive debilitating nature of RA, it is important to determine the level of inflammation and response to therapy. Therefore, due to non-specific clinical features and insufficient diagnostic tests, competency of synovial fluid ADA for diagnosing RA was evaluated in this study.
According to previous studies, serum ADA level and average of synovial fluid ADA are different among Asian countries. But none of studies had determined cut-off point to compare results. On the other hand, all had shown a significant relation between the average level of ADA in synovial fluid and/or serum and activation of RA. So, due to these evidences, it seems essential to do a research to verify competency of ADA to diagnose join effusion in RA and also to determine a cut-off point.
Meanwhile, ADA is a marker for cellular immune activity and knowing the pathophysiology of disease can reveal the inflammation trigger factors and finally disclose new therapeutic approaches [10].
Our results showed that the average level of serum and synovial fluid ADA have statistically significant difference between RA and OA. Furthermore, synovial fluid ADA can be used as an appropriate screening test for diagnosing Rheumatoid joint effusions and underneath of ROC curve is about 100% (99.11%), based on sketch model (Figure 1). According to synovial fluid ADA and patient's CRP and ESR levels, we can distinguish between RA and OA. In other words, the two previous tests fortified synovial fluid ADA specificity.
But the important point that must be considered is that the control group was chosen from noninflammatory patients. Therefore, we suggest further studies on other diseases like Lupus, reactive arthritis and even crystal arthritis.
In 2001, Hitogloue et al. evaluated activity of total ADA in juvenile rheumatoid arthritis and systemic lupus erythematous patients in different stages of the diseases. They showed that higher levels of serum ADA had close association with activity and relapse of the diseases [12].
In another study performed by Sari et al. in 2003, the correlation of serum activity level of ADA and its isoenzymes with disease activity of RA was assessed. Results showed that activity of total ADA and ADA2 had correlation with RA activity. They concluded that this non invasive test in concordance with CRP and ESR could be used as a biomarker to diagnose inflammation [13].
CRP is released by liver cells and by cytokines secreted from Macrophages. Recent studies have shown that CRP is a pre-inflammatory factor that causes Monocytes stimulation to secret IL6 and TNFα, which all directly activate macrophages [14]. According to our study, CRP level of RA patients was significantly higher than that of the control group. In 2003, Kilmanuiuk et al found that high levels of CRP related with active inflammation of RA [15].
IN 2004, Yildirim et al. studied the relationship between acute phase reactants and RA activity level. They found that CRP had the most diagnostic value among other acute phase reactants [16].
To take advantage of our study, we must review the pathophysiology of adenosine secretion. Endogenous adenosine has anti-inflammatory function, but some local mechanisms decrease its concentration in emission area. First, sympathic enervation causes adenosine release suppression, second, elevation of ADA activity which causes adenosine disturbance and finally decreases enzyme activity which disintegrates ATP to adenosine [4]. So it seems the aim of RA treatment is to save adenosine to prevent inflammatory cycle. Therefore, new drug should be aimed to elevate local adenosine release and to decrease its disturbance. One of the present drugs that have been successful in treatment of RA is methotrexate which prevents disease progression via inhibitory mechanism on adenosine distribution, but it has multiple complications. So we could try to produce a drug with similar molecular structure.
References
- 1.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 2.Rothschild BM, Turner KR, DeLuca MA. Symmetrical erosive peripheral polyarthritis in the Late Archaic Period of Alabama. Science. 1988;241:1498–1501. doi: 10.1126/science.3047874. [DOI] [PubMed] [Google Scholar]
- 3.Iwaki-Egawa S, Watanabe Y, Matsuno H. Correlations between matrix metalloproteinase-9 and adenosine deaminase isozymes in synovial fluid from patients with rheumatoid arthritis. J Rheumatol. 2001;28:485–489. [PubMed] [Google Scholar]
- 4.Chan ES, Fernandez P, Cronstein BN. Adenosine in inflammatory joint diseases. Purinergic Signal. 2007;3:145–152. doi: 10.1007/s11302-006-9046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wortmann RL, Veum JA, Rachow JW. Purine catabolic enzymes in human synovial fluids. Adv Exp Med Biol. 1989;253A:393–398. doi: 10.1007/978-1-4684-5673-8_64. [DOI] [PubMed] [Google Scholar]
- 6.Iwaki-Egawa S, Yamamoto T, Watanabe Y. Human plasma adenosine deaminase 2 is secreted by activated monocytes. Biol Chem. 2006;387:319–321. doi: 10.1515/BC.2006.042. [DOI] [PubMed] [Google Scholar]
- 7.Davis MA, Ettinger WH, Neuhaus JM, Hauck WW. Sex differences in osteoarthritis of the knee. The role of obesity. Am J Epidemiol. 1988;127:1019–1030. doi: 10.1093/oxfordjournals.aje.a114878. [DOI] [PubMed] [Google Scholar]
- 8.Brandt K, editor. Textbook of Rheumatology. Fifth Edition. Philadelphia: W.B. Saunders; 1997. Osteoarthritis: Clinical patterns and pathology. [Google Scholar]
- 9.Jordan JM, Luta G, Renner JB, Linder GF, Dragomir A, Hochberg MC, Fryer JG. Self-reported functional status in osteoarthritis of the knee in a rural southern community: the role of sociodemographic factors, obesity, and knee pain. Arthritis Care Res. 1996;9:273–278. doi: 10.1002/1529-0131(199608)9:4<273::aid-anr1790090412>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Yagawa K, Okamura J. Role of adenosine deaminase in activation of macrophages. Infect Immun. 1981;32:394–397. doi: 10.1128/iai.32.1.394-397.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pettersson T, Klockars M, Weber TH, von Essen R. Adenosine deaminase activity in joint effusions. Scand J Rheumatol. 1988;17:365–369. doi: 10.3109/03009748809105272. [DOI] [PubMed] [Google Scholar]
- 12.Hitoglou S, Hatzistilianou M, Gougoustamou D, Athanassiadou F, Kotsis A, Catriu D. Adenosine deaminase activity and its isoenzyme pattern in patients with juvenile rheumatoid arthritis and systemic lupus erythematosus. Clin Rheumatol. 2001;20:411–416. doi: 10.1007/s100670170005. [DOI] [PubMed] [Google Scholar]
- 13.Sari RA, Taysi S, Yilmaz O, Bakan N. Correlation of serum levels of adenosine deaminase activity and its isoenzymes with disease activity in rheumatoid arthritis. Clin Exp Rheumatol. 2003;21:87–90. [PubMed] [Google Scholar]
- 14.Ballou SP, Lozanski G. Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine. 1992;4:361–368. doi: 10.1016/1043-4666(92)90079-7. [DOI] [PubMed] [Google Scholar]
- 15.Klimiuk PA, Sierakowski S, Chwiecko J. [Serum interleukin 6 (il-6A) concentration correlates with matrix metalloproteinases and their tissue inhibitors in rheumatoid arthritis] . Pol Arch Med Wewn. 2003;109:119–123. [PubMed] [Google Scholar]
- 16.Yildirim K, Karatay S, Melikoglu MA, Gureser G, Ugur M, Senel K. Associations between acute phase reactant levels and disease activity score (DAS28) in patients with rheumatoid arthritis. Ann Clin Lab Sci. 2004;34:423–426. [PubMed] [Google Scholar]


