Abstract
We previously reported that osteoprotegerin (OPG) is regulated by pathways associated with pulmonary arterial hypertension (PAH), and is present at elevated levels within pulmonary vascular lesions and sera from patients with idiopathic PAH (IPAH). Since OPG is a naturally secreted protein, we investigated the relationship between serum OPG and disease severity and outcome in patients with IPAH and animal models. OPG mRNA expression was measured in pulmonary artery smooth muscle cells (PASMC) from pulmonary arteries of patients with and without IPAH. Serum concentrations of OPG were measured in a retrospective and prospective group of patients. OPG levels were compared with phenotypic data and other putative PAH biomarkers. Prognostic significance was assessed and levels compared with healthy controls. Correlation of OPG and pulmonary vascular remodeling was also performed in rodent models of PAH. OPG mRNA was significantly increased 2-fold in PASMC isolated from explanted PAH lungs compared with control. Serum OPG concentrations were markedly elevated in IPAH compared with controls. In Cohort 1 OPG levels significantly correlated with mean right atrial pressure and cardiac index, while in Cohort 2 significant correlations existed between age-adjusted OPG levels and gas transfer. In both cohorts an OPG concentration above a ROC-derived threshold of 4728 pg/ml predicted poorer survival. In two rodent models, OPG correlated with the degree of pulmonary vascular remodeling. OPG levels are significantly elevated in patients with idiopathic PAH and are of prognostic significance. The role of OPG as a potential biomarker and therapeutic target merits further investigation.
Keywords: biomarker, osteoprotegerin, pulmonary arterial hypertension
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a rare, progressive condition characterized by abnormal intimal and medial proliferation within the pulmonary arterial bed[1] resulting in elevation of pulmonary vascular resistance and subsequent right heart failure.[2] Current biomarkers such as brain natriuretic peptide (BNP or NT-proBNP) reflect right ventricular load rather than pulmonary arterial remodeling. Subsequently, there has been increased interest in identifying biomarkers for PAH that reflects this vasculopathy.
Osteoprotegerin (OPG) is a soluble member of the tumor necrosis factor (TNF) receptor family which acts as a decoy receptor for receptor activator of nuclear factor-kB ligand (RANKL) in the regulation of osteoclast differentiation.[3] OPG can also block the binding of TNF-related apoptosis-inducing ligand (TRAIL) with its membrane-associated death receptors thus preventing apoptosis.[4] We have previously demonstrated that several molecular pathways previously associated with PAH including reduced bone morphogenetic protein receptor type II (BMPR-2) expression stimulate OPG expression and secretion from pulmonary artery smooth muscle cells (PASMC).[5] Furthermore, we have shown increased protein expression of OPG within remodeled pulmonary vascular lesions and serum from patients with idiopathic PAH (IPAH) compared to controls. Interestingly, elevated levels of OPG have also been observed in PAH associated with congenital systemic to pulmonary shunts.[6] Altered levels and prognostic importance of OPG have been described in systemic cardiovascular disease,[7–10] diabetes[11–13] and connective tissue disease.[14,15] We therefore hypothesized that serum concentrations of OPG would provide meaningful prognostic information in patients with IPAH.
We now report our findings utilizing both an initial retrospective and subsequent prospective collection of patients with IPAH from two separate UK centers. Critically, we demonstrated for the first time that OPG mRNA is upregulated in PASMCs isolated from patients with IPAH, suggesting that OPG is produced locally within remodeled vessels, and likely contributes significantly to serum levels. We also report significant relationships between serum OPG, pulmonary hemodynamics, and survival.
MATERIALS AND METHODS
OPG gene expression in PASMC
RNA was isolated from PASMC grown from explanted pulmonary arteries of three patients with IPAH, and three controls (two emphysema, one lung cancer) as previously described.[16] The RNA was reverse transcribed using Superscript III (Invitrogen, Paisley, UK) and OPG gene expression measured using TaqMan quantitative PCR (Assay ID: Hs00171068_m1, Applied Biosystems, Warrington, UK). The relative quantity of OPG was assessed against 18S ribosomal RNA using the delta-delta comparative CT method.
Clinical subjects
Serum samples were obtained from two cohorts of patients with IPAH attending two large designated UK pulmonary vascular disease units. Cohort 1 consisted of 35 patients from Papworth Hospital, Cambridge (28 of whom were prevalent cases on targeted therapy), who were sampled between 2001 and 2006. Cohort 2 (validation cohort) consisted of 23 treatment-naïve incident cases at the Royal Hallamshire Hospital, Sheffield, who were sampled during 2009 and 2011. A control group of 35 volunteers without PH or significant cardiorespiratory disease were age and gender matched for Cohort 2. PAH was defined as mean pulmonary arterial pressure (mPAP) ≥25 mmHg in association with a normal pulmonary capillary wedge pressure of ≤15 mmHg. Exclusion criteria included associated forms of PAH, FEV1or FVC <60% predicted, or significant parenchymal lung disease on CT imaging. Serum was obtained peripherally in patients in Cohort 1 and at diagnostic right heart catheterization (RHC) in Cohort 2 unless the patient had undergone an isotope perfusion scan on the same day, in which case a peripheral sample was obtained the following day. All samples were obtained, stored, and analyzed in accordance with prior ethical approval from North Sheffield Research Ethics Committee via the Sheffield NIHR Cardiovascular Biomedical Research Unit Biobank, and Papworth Research Ethics Committee. OPG levels for patients from Cohort 1 have previously been reported but not interrogated for prognostic significance.[5]
Animals
PAH was induced in male Sprague Dawley rats (Charles River, UK) by subcutaneous injection of moncrotaline (Sigma, Poole, UK). Lung harvest was performed at days 2, 7, 14, 21, and 28 postinjection. Male ApoE-/- and ApoE-/-/IL-R1-/- mice 10-12 weeks of age (7 per group) were fed Paigen diet for 8 weeks as previously described.[17] Where stated disease progression was modified by administration of human IL-1Ra or placebo control (Amgen Inc., Thousand Oaks, Calif, USA. MTA 200517250-001) as previously described.[17] Lungs were harvested at 8 weeks and the pulmonary vascular remodeling was quantified as previously described.[17] All animal experiments were approved by the University of Sheffield Project Review Committee and conformed to UK Home Office ethical guidelines.
OPG measurement
OPG concentration in patient[18] and rodent[17] serum was measured using an enzyme-linked immunosorbent assay (ELISA) as previously described.
Statistical analysis
Baseline data was described using mean (standard deviation) or median (interquartile range). Comparison between groups was performed using the independent t-test and one-way ANOVA with Bonferonni post hoc analysis for parametric data and the Mann–Whitney or Kruskal–Wallis with Dunn's post hoc analysis tests for nonparametric data. Categorical data were compared with the χ2 test. Correlations were assessed using Pearson's test and multivariate linear regression. Optimal thresholds for survival analysis were identified using Receiver-Operated Characteristics (ROC) analysis. Event-free survival (death or lung transplant) was assessed using the Kaplan-Meier method with a census date of July 1, 2009 in Cohort 1 and July 18, 2011 in Cohort 2. A P value of <0.05 was taken as significant throughout. Statistical analysis was performed using SPSS 19 (SPSS; Chicago, IL, USA) and GraphPad Prism 5.0d (San Diego, CA, USA) software.
RESULTS
OPG mRNA expression is increased in human PASMC
We previously showed that OPG protein expression is increased within both concentric and plexiform lesions from patients with IPAH compared to controls.[5] To determine whether de novo synthesis by resident PASMC could be the source of this OPG protein, we firstly examined OPG expression in human pulmonary artery smooth muscle cells isolated from patients with IPAH. We found a significant 2-fold (P<0.05, Fig. 1) increase in OPG mRNA in un-stimulated PA-SMC from patients with IPAH compared with control PA-SMC isolated from non-PAH explanted lungs. These data suggest that OPG within remodeled lesions can be produced locally, and likely contributes significantly to circulating levels. Serum levels of OPG may therefore reflect the degree or activity of the underlying pulmonary vascular remodeling.
Figure 1.
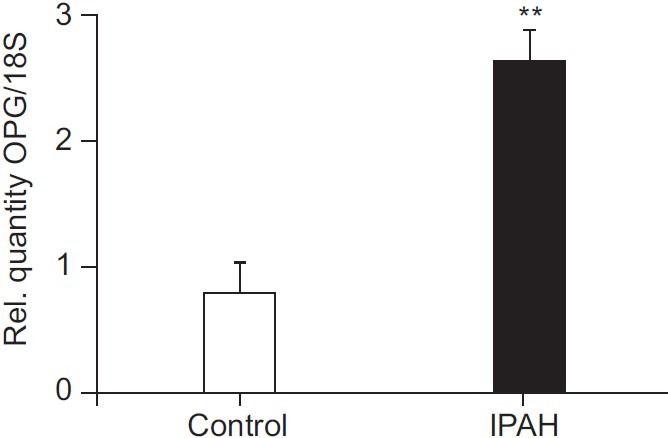
OPG mRNA is elevated in PASMC from patients with IPAH. Bar graph shows TaqMan derived mRNA expression of OPG in explanted PASMC from patients with idiopathic pulmonary arterial hypertension (IPAH) and controls, normalised using ΔΔCT with 18S rRNA as the endogenous control gene. Bars represent mean±SEM, n=3. **P<0.01 compared to control cells.
Patient characteristics, OPG concentrations and prognostic strength in a retrospective cohort
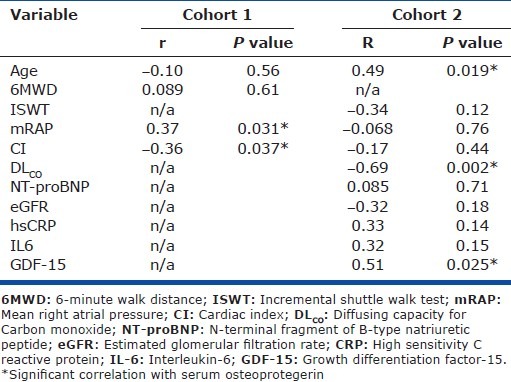
Baseline demographics and serum OPG concentration for both cohorts and the control are shown in Table 1. The majority (79%) of patients in Cohort 1 were receiving targeted therapy: (24%) bosentan; 6 (17%) epoprostenol; 2 (6%) intravenous iloprost; 2 (6%) nebulized iloprost; 1 (3%) sildenafil; and 8 (23%) combination therapy at the time of sampling (Table 1). Median serum OPG concentrations in patients with IPAH from Cohort 1 were significantly higher than in control (4807 vs. 1352 pg/ml, P<0.001; Fig. 2A). Median time between RHC and serum sampling in Cohort 1 was 2.2 (0.72, 10.5) months. OPG levels correlated positively with mRAP (r=0.37, P=0.03) and inversely with CI (r=-0.36, P=0.04; Table 2). No correlations were observed with age, exercise capacity or WHO functional class nearest to date of sampling. Sixteen patients in Cohort 1 had RHC performed within 90 days of sampling; in these patients OPG concentration correlated positively with mean right atrial pressure (mRAP; r=0.57, P=0.03).
Table 1.
Patient demographics and serum concentrations
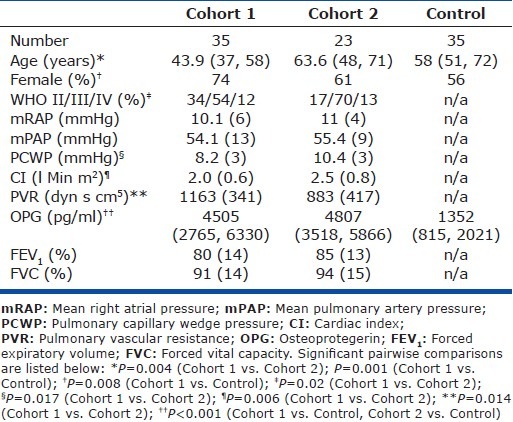
Figure 2.
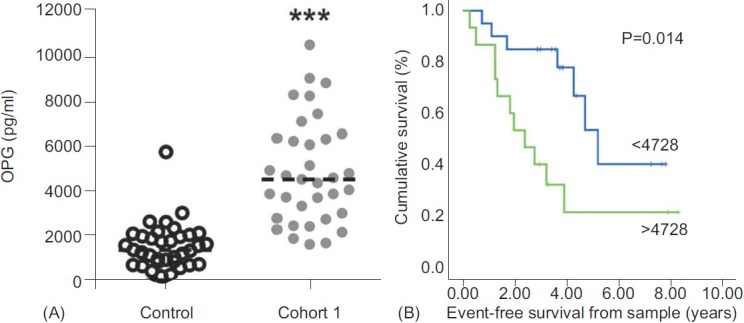
Retrospective analysis of serum OPG levels in patients with IPAH. (A) Scatter plot shows serum level of OPG in control and IPAH serum (Cohort 1) samples from retrospective analysis of prevalent cases. Dotted line represents the median, n=35. ***P<0.001 compared to control samples. (B) Assessment of serum OPG concentration against event-free survival. There was a significantly higher event-free survival in patients with serum OPG concentration below 4728 pg/ml (light gray line) having a 3-year survival of 84% compared with vs. 40% in patients with concentrations above 4728 pg/ ml (dark gray line).
Table 2.
Correlations between OPG, exercise capacity, TLCO and selected biomarkers
Median follow-up from date of sampling to census in Cohort 1 was 3.27 (1.81, 4.26) years. Fourteen patients died during follow-up while four patients underwent lung transplantation. The area under the curve in ROC analysis for the prediction of 4-year event-free survival by serum OPG concentration was 0.72 with an optimal threshold of 4728 pg/ml (true positive 73%, false positive 22%). A significant difference in event-free survival was demonstrated using this threshold (3-year survival 85% vs. 40%, P=0.014; Fig. 2B).
Patient characteristics, OPG concentrations and prognostic strength in a prospective, treatment-naïve cohort
Baseline demographics and serum OPG concentration for Cohort 2 and controls are shown in Table 1. Patients in Cohort 2 were older, had a higher mean cardiac index (CI) and lower pulmonary vascular resistance than in Cohort 1. All patients were treatment-naïve (Table 1) and all sampling was performed at or within 24 hours of RHC. Median OPG concentrations (4505 pg/ml) were significantly higher than in controls (1352 pg/ ml, P<0.001; Fig. 3A) while there was no significant difference compared with Cohort 1 (4807 pg/ml, P=ns). In Cohort 2, OPG concentrations correlated positively with age (r=0.49, P=0.019) and inversely with DLCO (r=-0.69, P=0.02; Table 2). When adjusted for age the correlation between OPG and DLCO persisted. No correlations between OPG and pulmonary hemodynamics, exercise capacity or WHO functional class were observed.
Figure 3.
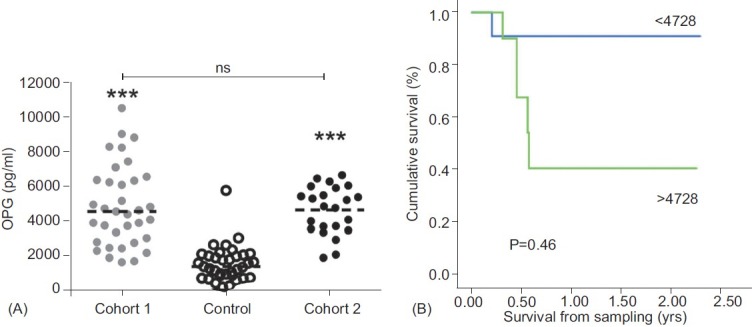
Prospective analysis of serum OPG levels in patients with IPAH. (A) Scatter plot shows serum level of OPG in control and retrospective prevalent cases of IPAH (Cohort 1) and prospectively collected samples of treatment naïve incident cases of IPAH (Cohort 2). Dotted line represents the median, n=35 of control and Cohort 1, and 23 for Cohort 2. ***P<0.001 compared to control samples. (B) Assessment of serum OPG concentration against event-free survival. There was a significantly higher event-free survival in patients with serum OPG concentration below 4728 pg/ml (light gray line) having a 1-year survival of 91% compared with vs. 41% in patients with concentrations above 4728 pg/ml (dark gray line).
Median follow-up from date of sampling to census in Cohort 2 was 0.62 (0.38, 1.5) years. Patients were treated with targeted therapies in keeping with current guidelines.[19] Six patients died during follow-up. The previously identified threshold of 4728 pg/ml predicted mortality in Cohort 2 (1 year survival 91% vs. 41%, P=0.046: Fig. 3B). Neither OPG nor traditional important prognostic factors such as pulmonary hemodynamics predicted survival in either Cohort 1 or 2 by univariate Cox regression analysis.
Comparison of OPG with previously recognized putative biomarkers
Correlations of several previously described putative biomarkers were explored in the treatment-naïve Cohort 2. NT-Pro BNP correlated inversely with CI (r=-0.45, P=0.037) and incremental shuttle walking distance (r=-0.52, P=0.016) and positively with mRAP (r=0.53, P=0.011) but not with age, WHO functional class or OPG level. In this cohort no threshold of NT-Pro BNP could be identified which significantly predicted outcome. No correlations between creatinine, red cell distribution width, GDF-15, IL-1β, IL-6, IL-8, IL-10, and IL-12p70 and pulmonary hemodynamics were observed. OPG correlated positively with GDF-15 (r=0.53, P=0.025; Table 2).
Serum OPG correlates with disease progression and severity in pre-clinical models
Utilizing serum and histomorphological data collected from our preclinical animal models, we examined whether there was any correlation between serum OPG and pulmonary vascular remodeling during disease progression and regression. In the monocrotaline rat model there was a significant correlation between pulmonary vascular remodeling as assessed by media/cross sectional area (media/CSA) of small pulmonary arterioles (<50 μm) and serum OPG (R2=0.63, P<0.0001; Fig. 4A) over 28 days. We have recently described the beneficial effects of treatment with interleukin-1 receptor antagonist (IL-1Ra) in a mouse model of severe pulmonary hypertension that is associated with obliterative pulmonary vascular lesions.[17] To further examine whether OPG would also track with treatment of established disease, we subsequently examined the relationship between serum OPG and media/CSA in these mice and again found a significant correlation (R2=0.37, P<0.05: Fig. 4B).
Figure 4.
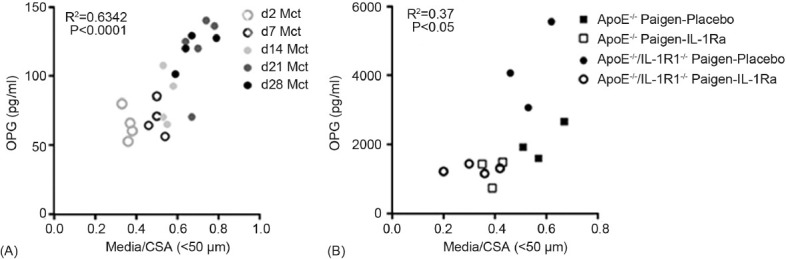
Serum OPG levels correlate with pulmonary vascular remodeling in rodent models of PAH. Graphs show a significant correlation of serum OPG with media/cross-sectional area (Media/CSA) in (A) a time course of disease development in the monocrotaline rat model, and (B) the fat-fed ApoE−/− mouse model of PAH with disease regression following treatment with interleukin-1 receptor antagonist (IL-1Ra).
DISCUSSION
We previously reported that levels of OPG protein are increased in pulmonary artery lesions and sera from patients with IPAH.[5] Critically, this is the first study to provide evidence that this increased protein expression is driven by resident PASMCs. We subsequently examined the prognostic utility of OPG as a biomarker in both a restrospective and subsequent prospective clinical study. We demonstrated in these two separate cohorts that IPAH is characterized by significantly higher serum OPG levels than control patients. We also showed that serum OPG correlates significantly with hemodynamic markers of severity such as mRAP and CI in a predominantly prevalent population (Cohort 1), while in an older incident population (Cohort 2) there was a significant correlation with DLCO even when adjusted for age. Most importantly, we observed that OPG levels >4728 pg/ml predicted poorer survival in both cohorts. In addition we provide further evidence of the utility of OPG as a biomarker for pulmonary vascular remodeling by showing a significant correlation between serum levels and vascular remodeling in two animal models of pulmonary hypertension during disease progression but encouragingly, also during regression.
These findings are in keeping with a previous study in which OPG was found to be elevated in patients with PAH associated with congenital systemic to pulmonary shunts[6] and also with several studies which have found OPG to be an important prognostic factor in left ventricular disease.[7–9] Interestingly, in a cohort of patients with acute coronary syndrome, OPG was shown to be of strong prognostic value for mortality and heart failure hospitalization especially in patients with OPG >4540 pg/ml, a very similar level to the prognostic threshold observed in the present study.[20]
There is a major unmet need for a marker of pulmonary vascular remodeling. While we cannot rule out a contribution of other sources of OPG in serum, the evidence that PA-SMC express higher levels of OPG mRNA certainly suggests that the remodeling of the distal pulmonary arterial bed during disease progression contributes significantly to OPG concentrations in the bloodstream. Furthermore, we found significant correlations between serum OPG levels and pulmonary artery remodeling in 2 rodent models. The origin of OPG being in the pulmonary arteries is further supported by the lack of correlation of OPG levels with markers of right ventricular function such as mRAP, CI and NT pro-BNP in Cohort 2. Interestingly we also observed a significant correlation between DLCO and OPG. Pulmonary diffusing capacity of carbon monoxide is dependent on the alveolar membrane diffusing capacity and pulmonary capillary blood volume. Significantly abnormal lung function or a significant degree of parenchymal lung disease were exclusion criteria in the present study and it could therefore be hypothesized that this correlation was observed due to high levels of OPG reflecting a higher degree of pulmonary arterial vasculopathy and hence a lower pulmonary capillary blood volume.
As has been noted above, however, that elevated OPG levels have been previously observed in left ventricular failure[9] and elsewhere this has been found to occur in both ischemic and non-ischemic forms of cardiomyopathy.[21] It is therefore also possible that a proportion of the serum OPG observed in the present may study arise from the right ventricle. This would be supported by the significant correlations observed with both mRAP and CI in Cohort 1.
It is interesting to note that there was a difference in observed correlations between OPG and other parameters in the two cohorts. There are several possible explanations for this difference. Firstly, patients in the more recent group (Cohort 2) were significantly older than in the earlier group, consistent with the increasing age of patients reported elsewhere.[22] Secondly, the majority of patients in Cohort 1 were already receiving disease-modifying therapies which may have affected OPG levels. A reduction in levels of another chemokine, monocyte chemoattractant protein-1, after treatment with prostacyclin, has previously been observed in patients with IPAH.[23] The effect of disease-modifying therapy on OPG and other chemokines will be assessed in a larger cohort with a longer follow-up. Thirdly, median time between OPG sampling and RHC was significant in Cohort 1 while all patients in Cohort 2 had sampling performed either at or within 24 hours of RHC. In the present study we also did find correlations between previously identified novel biomarkers and pulmonary hemodynamics but it is acknowledged that Cohort 2 is relatively small compared with previous studies.[24]
In conclusion, OPG mRNA expression is increased in PA-SMC from patients with IPAH while serum levels are also significantly elevated when compared to controls. Levels of OPG >4728 pg/ml are associated with an increased chance of death or transplantation. These findings certainly warrant further investigation of OPG as both a biomarker and a potential therapeutic target.
ACKNOWLEDGMENTS
The authors would like to acknowledge funding from the Medical Research Council UK, the British Heart Foundation, the National Institute for Health Research Sheffield Cardiovascular Biomedical Research Unit, and Cambridge University Hospitals Biomedical Research Center. We are also grateful to all the patients, nurses and support staff who contributed to this study.
Footnotes
Source of Support: Medical Research Council Career Development Award (G0800318, AL); British Heart Foundation Clinical Research Training Fellowship (FS/08/061/25740, AGH), the National Institute for Health Research Sheffield Cardiovascular Biomedical Research Unit, and Cambridge University Hospitals Biomedical Research Center
Conflict of Interest: None declared.
REFERENCES
- 1.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43:25S–32S. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 2.Rubin L. Primary pulmonary hypertension. New Engl J Med. 1997;336:111–7. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 3.Simonet W, Lacey D, Dunstan C, Kelley M, Chang M, Luthy R, et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 4.Emery J, McDonnell P, Burke M, Deen K, Lyn S, Silverman C, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–7. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 5.Lawrie A, Waterman E, Southwood M, Evans D, Suntharalingam J, Francis S, et al. Evidence of a role for osteoprotegerin in the pathogenesis of pulmonary arterial hypertension. Am J Pathol. 2008;172:256–64. doi: 10.2353/ajpath.2008.070395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brun H, Holmstrøm H, Thaulow E, Damås JK, Yndestad A, Aukrust P, et al. Patients with pulmonary hypertension related to congenital systemic-to-pulmonary shunts are characterized by inflammation involving endothelial cell activation and platelet-mediated inflammation. Congenit Heart Dis. 2009;4:153–9. doi: 10.1111/j.1747-0803.2009.00297.x. [DOI] [PubMed] [Google Scholar]
- 7.Andersen GØ, Knudsen EC, Aukrust P, Yndestad A, Øie E, Müller C, et al. Elevated serum osteoprotegerin levels measured early after acute ST-elevation myocardial infarction predict final infarct size. Heart. 2011;97:460–5. doi: 10.1136/hrt.2010.206714. [DOI] [PubMed] [Google Scholar]
- 8.Lieb W, Gona P, Larson M, Massaro J, Lipinska I, Keaney JFs, Jr, et al. Biomarkers of the Osteoprotegerin Pathway: Clinical Correlates, Subclinical Disease, Incident Cardiovascular Disease, and Mortality. Arterioscler Thromb Vasc Biol. 2010;30:1849–54. doi: 10.1161/ATVBAHA.109.199661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Røysland R, Masson S, Omland T, Milani V, Bjerre M, Flyvbjerg A, et al. Prognostic value of osteoprotegerin in chronic heart failure: The GISSI-HF trial. Am Heart J. 2010;160:286–93. doi: 10.1016/j.ahj.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Stępień E, Wypasek E, Stopyra K, Konieczyńska M, Przybyło M, Pasowicz M. Increased levels of bone remodeling biomarkers (osteoprotegerin and osteopontin) in hypertensive individuals. Clin Biochem. 2011;44:826–31. doi: 10.1016/j.clinbiochem.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Chang YH, Lin KD, He SR, Hsieh MC, Hsiao JY, Shin SJ. Serum osteoprotegerin and tumor necrosis factor related apoptosis inducing-ligand (TRAIL) are elevated in type 2 diabetic patients with albuminuria and serum osteoprotegerin is independently associated with the severity of diabetic nephropathy. Metabolism. 2011;60:1064–9. doi: 10.1016/j.metabol.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen L, Tarnow L, Hansen T, Parving H, Flyvbjerg A. Plasma osteoprotegerin levels are associated with glycaemic status, systolic blood pressure, kidney function and cardiovascular morbidity in type 1 diabetic patients. Eur J Endocrinol. 2006;154:75–81. doi: 10.1530/eje.1.02049. [DOI] [PubMed] [Google Scholar]
- 13.Waluź-Miarka M, Katra B, Fedak D, Czarnecka D, Miarka P, Woźniakiewicz E, et al. Osteoprotegerin is associated with markers of atherosclerosis and body fat mass in type 2 diabetes patients. Int J Cardiol. 2011;147:335–6. doi: 10.1016/j.ijcard.2010.12.094. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH, Chen HA, Liao HT, Liu CH, Tsai CY, Chou CT. Soluble receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoprotegerin in ankylosing spondylitis: OPG is associated with poor physical mobility and reflects systemic inflammation. Clin Rheumatol. 2010;29:1155–61. doi: 10.1007/s10067-010-1543-y. [DOI] [PubMed] [Google Scholar]
- 15.Castellino G, Corallini F, Bortoluzzi A, Corte RL, Monaco AL, Secchiero P, et al. The tumour necrosis factor-related apoptosis-inducing ligand-osteoprotegerin system in limited systemic sclerosis: A new disease marker? Rheumatology (Oxford) 2010;49:1173–6. doi: 10.1093/rheumatology/keq064. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Long L, Southwood M, Rudarakanchana N, Upton P, Jeffery T, et al. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res. 2005;96:1053–63. doi: 10.1161/01.RES.0000166926.54293.68. [DOI] [PubMed] [Google Scholar]
- 17.Lawrie A, Hameed AG, Chamberlain J, Arnold N, Kennerley A, Hopkinson K, et al. Paigen Diet-Fed Apolipoprotein E Knockout Mice Develop Severe Pulmonary Hypertension in an Interleukin-1-Dependent Manner. Am J Pathol. 2011;179:1693–705. doi: 10.1016/j.ajpath.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holen I, Croucher P, Hamdy F, Eaton C. Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells. Cancer Res. 2002;62:1619–23. [PubMed] [Google Scholar]
- 19.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 20.Omland T, Ueland T, Jansson AM, Persson A, Karlsson T, Smith C, et al. Circulating osteoprotegerin levels and long-term prognosis in patients with acute coronary syndromes. J Am Coll Cardiol. 2008;51:627–33. doi: 10.1016/j.jacc.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 21.Ueland T, Yndestad A, Øie E, Florholmen G, Halvorsen B, Frøland SS, et al. Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation. 2005;111:2461–8. doi: 10.1161/01.CIR.0000165119.62099.14. [DOI] [PubMed] [Google Scholar]
- 22.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, et al. Pulmonary arterial hypertension: Baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–87. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 23.Katsushi H, Kazufumi N, Hideki F, Katsumasa M, Hiroshi M, Kengo K, et al. Epoprostenol therapy decreases elevated circulating levels of monocyte chemoattractant protein-1 in patients with primary pulmonary hypertension. Circ J. 2004;68:227–31. doi: 10.1253/circj.68.227. [DOI] [PubMed] [Google Scholar]
- 24.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920–7. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]


