Abstract
Pulmonary hypertension (PH) may affect survival in cystic fibrosis (CF) and can be assessed on echocardiographic measurement of the pulmonary acceleration time (PAT). The study aimed at evaluating PAT as a tool to optimize timing of lung transplant in CF patients. Prospective multicenter longitudinal study of patients with forced expiratory volume in 1 second (FEV1) ≤60% predicted. Echocardiography, spirometry and nocturnal oximetry were obtained as part of the routine evaluation. We included 67 patients (mean FEV1 42±12% predicted), among whom 8 underwent lung transplantation during the mean follow-up of 19±6 months. No patients died. PAT was determined in all patients and correlated negatively with systolic pulmonary artery pressure (sPAP, r=–0.36, P=0.01). Patients in the lowest PAT tertile (<101 ms) had lower FEV1 and worse nocturnal oxygen saturation, and they were more often on the lung transplant waiting list compared to patients in the other tertiles. Kaplan–Meier curves showed a shorter time to lung transplantation in the lowest PAT tertile (P<0.001) but not in patients with sPAP>35 mmHg. By multivariate analysis, FEV1and nocturnal desaturation were the main determinants of reduced PAT. A PAT<101 ms reduction is a promising tool for timing of lung transplantation in CF.
Keywords: cystic fibrosis, echocardiography, pulmonary acceleration time, pulmonary hypertension, pulmonary transplantation
INTRODUCTION
Cystic fibrosis (CF) is among the leading causes of chronic respiratory insufficiency in adolescents and young adults. Lung transplantation is the treatment of last resort for end-stage lung disease. Given the shortage of lungs for transplantation, adequate selection of transplant recipients, and optimal timing of lung transplantation in individual patients are crucial.
Pulmonary hypertension (PH) is a major component of advanced CF lung disease.[1,2] Although PH is usually moderate in CF, its severity varies greatly across patients and its presence may negatively affect outcomes.[3]
Therefore, evaluating PH may help to guide treatment decisions in CF patients, including referral for lung transplantation. However, studies on the prognostic impact of PH in CF are scarce, and most of them are retrospective, have small sample sizes, or are confined to patients with end-stage lung disease.[1–7]
Furthermore, limited data are available on the prevalence and determinants of PH in CF.
PH is classically defined as a mean pulmonary artery pressure (PAP) greater than 25 mmHg as measured by right heart catheterization. In practice, however, noninvasive echocardiography is used to estimate systolic PAP (sPAP) from the peak tricuspid regurgitation velocity (TRV) using the modified Bernouilly equation.[3] PH is defined as an echocardiographic sPAP value greater than 35 mmHg. However, tricuspid regurgitation may be absent in patients with CF, particularly those in the pediatric age range. The pulmonary artery acceleration time (PAT) measured on the Doppler pulmonary artery flow correlates strongly with mean PAP[8] and can be measured in most children and adults.[8,9]
We hypothesized that the severity of PH affected survival and that PAT was more informative than echocardiographic sPAP in CF. We assessed these hypotheses by conducting a large, prospective multicenter study in children and adults with CF who underwent echocardiography with echocardiographic sPAP and PAT determination, spirometry, and nocturnal oximetry during their routine annual evaluation. Patients were then followed up for at least 1 year. The primary endpoint was the number of patients who died or underwent lung transplantation during follow-up. No death occurred, and the prognosis was therefore evaluated based only on time to lung transplantation.
MATERIALS AND METHODS
Patients
Patients with CF were recruited prospectively during their routine annual evaluation at two pediatric and four adults CF centers between April 2008 and April 2009. Inclusion criteria were presence of two CFTR mutations or one CFTR mutation with a sweat chloride test >60 mmol/l and a characteristic phenotype, age ≥ 7 years, ability to perform a reproducible forced expiratory maneuver with a forced expiratory volume in one second (FEV1) ≤ 60% of predicted value in a stable clinical state defined by the absence of a respiratory exacerbation since at least one month or a patient finishing an at least 10 days intravenous antibiotic treatment. Sputum bacteriology and lung function tests including arterial blood gas measurements were obtained routinely.[10–12] We determined the vital status and lung transplantation status of each patient on 1 May 2010. All the parents of children, children with sufficient understanding, and all adults, gave informed written consent for the study, which was approved by the local ethics committee.
Echocardiographic measurements
Echocardiograms were performed as recommended by the American Society of Echocardiography.[13] TRV was measured and the transtricuspid pressure gradient was calculated using the modified Bernoulli equation.[14,15] Right atrial pressure was evaluated by measuring the inferior venous cava diameter and its variations over the breathing cycle. Systolic pulmonary artery pressure (PAP) was estimated by the sum of right atria pressure and transtricuspid gradient. The right ventricular end-diastolic diameter (RVEDD) and the left end-diastolic diameter were measured in M-mode in parasternal view and indexed to body surface area (LVEDVI). PAT was defined as the time in milliseconds from the onset of right ventricular ejection to peak systolic velocity of the forward pulmonary flow.[8] In normal individuals, pulmonary acceleration time (PAT) exceeds 110 ms and progressively shortens with the increase in pulmonary hypertension (PH).
Overnight pulse oximetry and transcutaneous carbon dioxide recording
Overnight pulse oximetry (SpO2) and transcutaneous carbon dioxide (PtcCO2) recordings were performed in room air (SenTec Digital Monitor, SenTec AG, Therwil, Switzerland).[16] We recorded the mean and minimal SpO2, number of desaturations ≥ 4%/h of recording, percentage of time spent at specific SpO2values, mean and maximum PtcCO2 and percentage of time spent at specific PtcCO2 values. Six patients had received intermittent nocturnal oxygen therapy and seven patients intermittent noninvasive positive pressure ventilation (NPPV) but all lung function tests and sleep recordings were performed on room air.
Statistical analysis
The distribution of continuous variables was checked for normality using the Kolmogorov-Smirnov test. Values for normally distributed variables are given as mean±standard deviation (SD) for quantitative data and as numbers and percentages for categorical data. We separated the patients into the following subgroups: Children (age <18 years) and adults; patients on the lung transplantation waiting list and other patients; PAT tertiles (with cut-off values of 101 and 122 ms); and sPAP < or ≥ 35 mmHg. Differences between continuous data were tested using the Mann-Whitney test for two-group comparisons and the Kruskal-Wallis test for three-group comparisons. Proportions were compared using Pearson's Chi-square test. Univariate regression analysis was performed to assess correlations linking clinical, laboratory, and echocardiographic variables. To identify variables independently associated with low PAT values, we entered the clinical, lung function and sleep variables yielding P values <0.10 by univariate analysis of associations with PAT into a binomial regression analysis model comparing the lowest PAT tertile to the two other tertiles pooled. The relationship between PAT tertile and time-to-lung transplantation was evaluated using Kaplan–Meier curves and the chi-square log-rank test. P values <0.05 were considered significant. Analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, Ill., USA).
RESULTS
Characteristics of the study population
Table 1 reports the main characteristics of the 67 CF patients by age and lung transplantation status. Table 2 shows the patients according to lung transplantation waiting list status at baseline.
Table 1.
Clinical, echocardiographic, respiratory treatment, day time blood gases, spirometry, and nocturnal gas exchange characteristics of all the patients
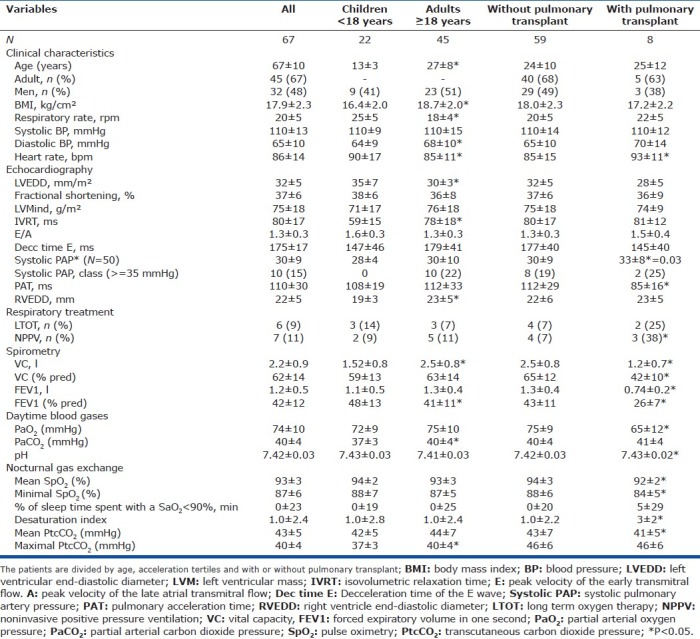
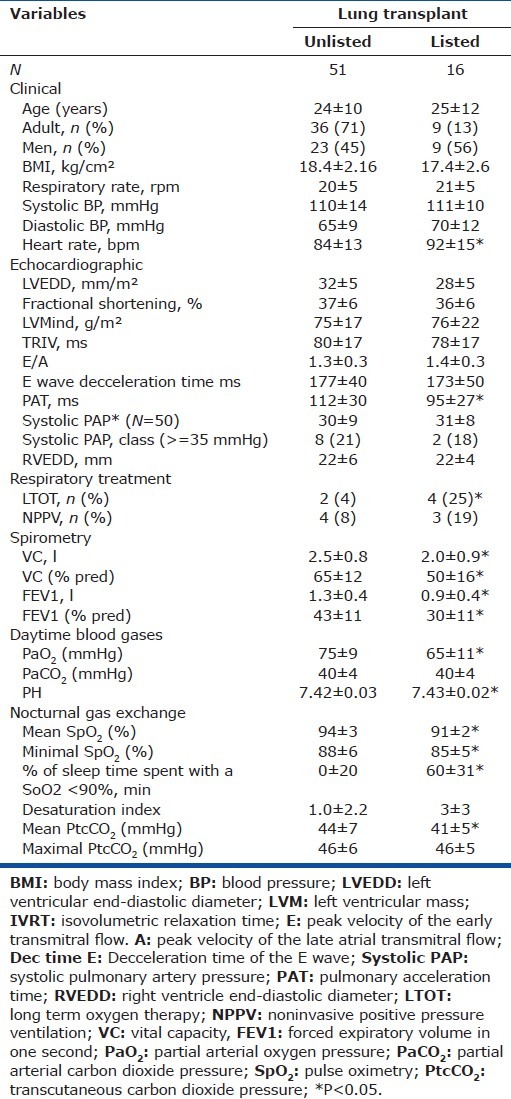
Table 2.
Characteristics of the patients divided by waiting for pulmonary transplant or not
Compared to adults, children had significantly lower values for body mass index (BMI) and diastolic blood pressure, significantly higher heart rates and FEV1 values, and significantly lower values for mean daytime and nocturnal CO2. Among the echocardiographic variables, isovolumetric relaxation time (IVRT) and right ventricular end-diastolic diameter (REVDD) were significantly lower in children than in the adults.
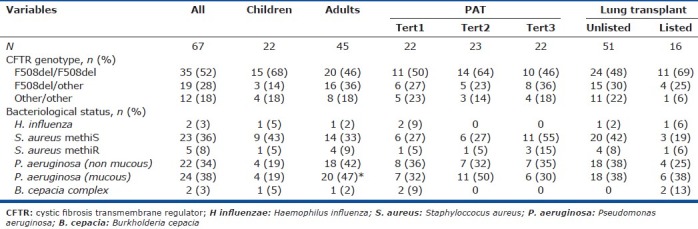
Median follow-up was 19 months. No patients died, and 8 patients underwent lung transplantation. As expected, compared to the 59 nontransplanted patients, these 8 patients had significantly higher values for heart rate and sPAP and significantly lower values for PAT, lung function parameters, nocturnal oxygenation and mean nocturnal PtcCO2, with similar maximal PtcCO2 values. Children and adults were not significantly different regarding the CFTR genotype, bacteriological status (except for a significantly higher prevalence of Pseudomonas aeruginosa pulmonary infection in adults), PAT tertile distribution or the lung transplant list status (Table 3).
Table 3.
Genetic and bacteriological status
Clinical, biological, and left ventricle echocardiographic characteristics of the population divided into PAT tertiles or systolic PAP categories
The clinical characteristics did not differ according to PAT tertiles, whereas all patients with sPAP ≥ 35 mmHg were adults and mean age in this subgroup was significantly higher than in the subgroup with sPAP<35 mmHg (Table 4). The mean deceleration time differed significantly across PAT tertiles but not between sPAP categories. The patients in the lowest PAT and those with sPAP ≥ 35 mmHg had significantly lower mean FEV1 values than the patients in the other groups. Mean vital capacity (VC) was lower in the PAT tertile than in the two other tertiles pooled, but not significantly different between the two sPAP categories. Daytime PaCO2 values were not significantly higher in the lowest PAT tertile and significantly higher in the sPAP ≥ 35 mmHg category. Finally, mean nocturnal desaturation index and maximal PtcCO2 were significantly higher in the lowest PAT tertile, and not significantly higher in the highest sPAP category.
Table 4.
Clinical, echocardiographic, respiratory treatment, day time blood gases, nocturnal gas exchange characteristics of the patients
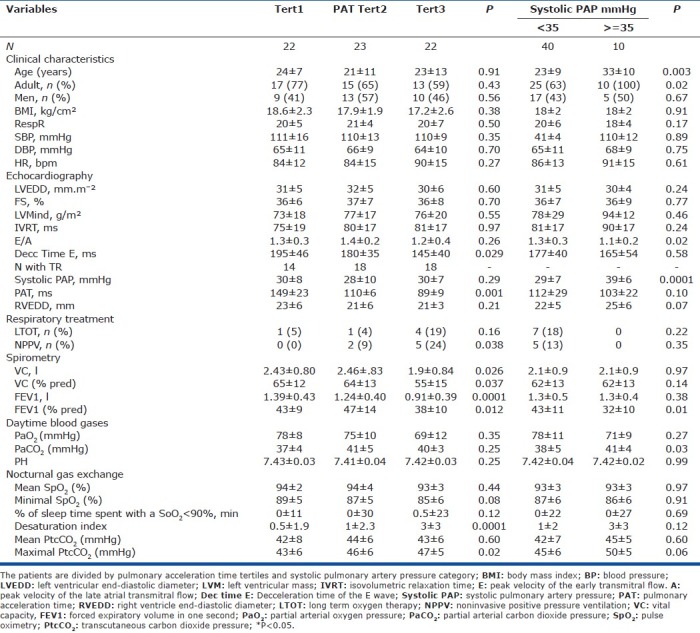
Figure 1 shows patient distribution by sPAP category and PAT tertile. The sPAP value was ≥ 35 mmHg in 10 patients, <35 mmHg in 40 patients, and not measurable in 17 patients. Mean sPAP was not significantly different between patients listed for lung transplantation and other patients. PAT was significantly lower in the listed patients (Table 2).
Figure 1.
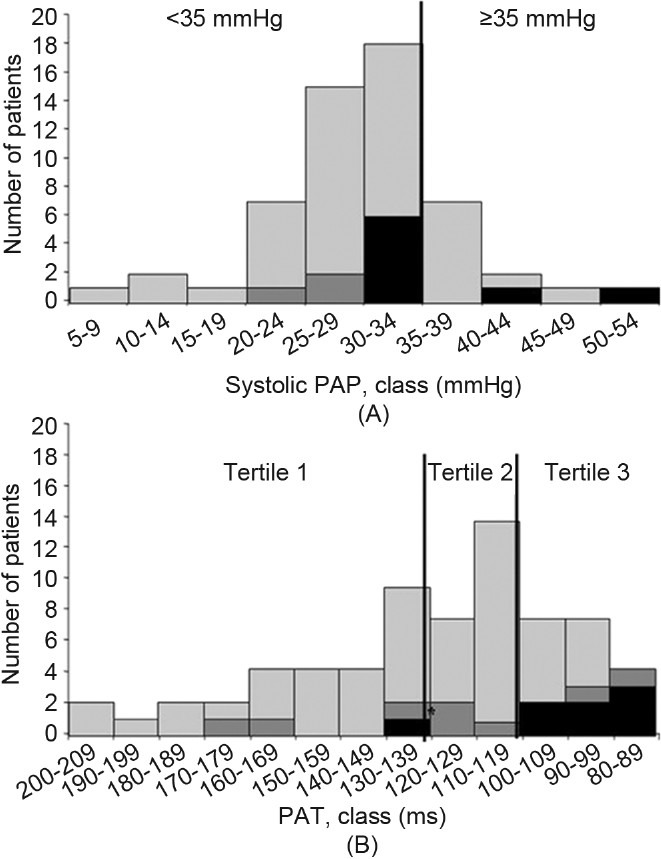
Distribution of the patients according to the systolic pulmonary arterial pressure (sPAP) (A) and pulmonary acceleration time (PAT) (B). The black bars represent the lung transplant recipients, dark gray bars the lung transplant waiting list patients, and light gray the other patients.
Correlation of echocardiographic variables with clinical, spirometric, and sleep variables
Table 5 shows that left ventricular morphology, assessed on the left end-diastolic diameter (LVEDVI), correlated negatively with age, BMI, and diastolic blood pressure, and positively with respiratory rate and heart rate. The IVRT correlated positively with age, BMI, and daytime PaCO2. The ratio of early (E) over late atrial (A) transmitral peak velocities (E/A) correlated positively with age and respiratory rate, and negatively with BMI and maximal nocturnal PtcCO2, whereas the E-wave deceleration time correlated negatively with BMI. RVEDD correlated negatively with respiratory rate.
Table 5.
Univariate correlation between echocardiographic variables and clinical and respiratory variables
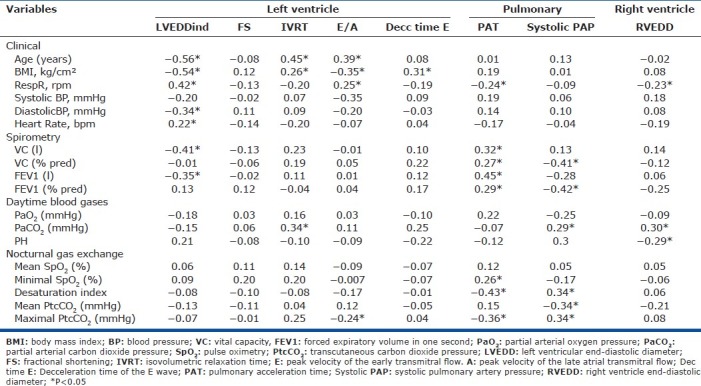
As expected, PAT correlated negatively with respiratory rate, nocturnal desaturation index and maximal PtcCO2, and positively with VC, FEV1 and minimal nocturnal SpO2. Systolic PAP correlated positively with daytime and maximal nocturnal PaCO2and the nocturnal desaturation index, and negatively with VC, FEV1 and mean nocturnal PtcCO2. Finally, PAT correlated negatively with sPAP (r=-0.36, P=0.01).
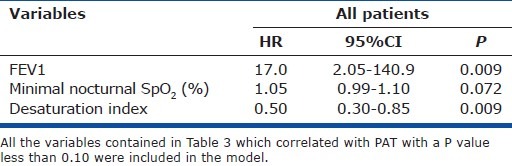
Variables associated with low PAT
Table 6 lists the determinants of PAT<101 ms (tertile 3 versus tertiles 1 and 2) identified by multivariate binomial logistic regression. Minimal nocturnal SpO2 was not significantly associated with lowest-tertile PAT. The only variables significantly associated with lowest-tertile PAT were FEV1 and nocturnal desaturation index.
Table 6.
Determinants of pulmonary acceleration time less than 101 ms (tertile 3 versus tertiles 1 and 2) by mutlivariate binomial logistic regression analysis
Prognosis value of PAT
As none of the patients died during the follow up, only time to lung transplantation was used to assess prognosis. Of the 8 patients who underwent lung transplant during the follow up, only 3 had sPAP ≥ 35 mmHg and 5 had sPAP <35 mmHg, whereas 7 had PAT values in the lowest tertile (Fig. 1). Figure 2 shows the Kaplan-Meier curves for time-to-lung transplantation according to sPAP categories and PAT tertiles. Time-to-lung transplantation did not differ significantly between the two sPAP categories. In contrast, patients in the lowest PAT tertile had significantly shorter times to lung transplantation than the patients in the two other tertiles pooled.
Figure 2.
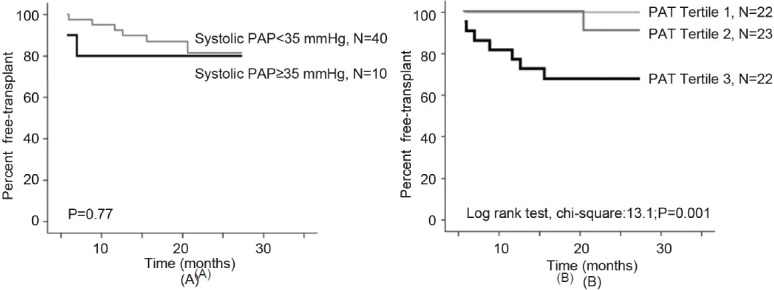
Kaplan–Meier time to lung transplantation curves according to the level of systolic pulmonary arterial pressure (sPAP) (< or ≥ 35 mmHg) (A), and according to the tertile of pulmonary acceleration time (PAT), tertile 3 representing the lowest tertile, <101 ms (B). Time to lung transplantation was significantly shorter in the lowest PAT tertile compared to the two other tertiles (P<0.001) whereas no difference was observed according to the level of sPAP.
DISCUSSION AND CONCLUSIONS
In this large, prospective multicenter study, PH, as PAT <101 ms was associated with a significantly shorter time-to-lung transplantation than higher PAT values. Thus, PAT <101 ms may hold promise as a prognostic marker in patients with CF. In addition, PAT was more informative than sPAP estimated by echocardiography; PAT but not sPAP was measurable in all patients, and the association with time-to-lung transplantation was stronger for PAT than for sPAP.
Right heart catheterization is considered the reference standard for mean PAP determination, with a value above 25 mmHg defining PH. However, this procedure is too invasive to be suitable for screening or monitoring over time.[17–19] In clinical practice, PH is often based on the sPAP value estimated from the TRV measured during echocardiography. Systolic PAP estimation by echocardiography may be difficult in CF because of lung hyperinflation, absence of tricuspid regurgitation in some patients and variations in TRV depending on respiration time and regurgitation severity.[17,20] Interestingly, a strong inverse relationship between PAT and mean PAP has been reported,[8] and we found a strong association between PAT and sPAP. A weaker association was reported previously between echocardiographic PAT and both diastolic and sPAP in children.[21] PAT is known to be inversely correlated with heart rate, which was increased in lung transplant recipients in our study. However, correcting PAT for heart rate did not improve the correlation with sPAP in other studies.[21–24]
In the present study, sPAP could not be determined in 17 (25%) patients whereas PAT could be measured in all patients. PAT correlated better with the level of lung function and nocturnal gas exchange than did sPAP. Both VC and FEV1 were significantly lower in the lowest PAT tertile compared to the two other tertiles, whereas only FEV1 % predicted was lower in the patients with sPAP ≥ 35 mmHg compared to patients with sPAP<35 mmHg. Nocturnal gas exchange did not differ significantly between the two sPAP categories, whereas the patients in the lowest PAT tertile had a significantly higher desaturation index and maximal PtcCO2 than the patients in the other two tertiles. Mean PAT was also significantly lower in a group of 103 CF patients on a lung transplant list, who had a mean FEV1 of 20±5% predicted than 17 controls (91 vs. 121 ms, P<0.01).[6]
The prevalence of PH in our group of stable patients with FEV1<60% predicted was 33% when PH was defined as PAT<101 ms and 15% when PH was defined as sPAP > 35 mmHg. These data are difficult to compare with those from other studies because of differences in patient status and selection. However, prevalence of PH in other studies was very high, ranging from 30% to 63% in patients on lung transplant lists.[3,7,25]
FEV1 was one of the two main determinants of PAT in our study, in keeping with earlier reports.[3,25] FEV1 has been shown to be the best marker of lung disease severity and a major predictor of respiratory morbidity and mortality in CF.[26] Nocturnal desaturation was the other determinant of PH in our patients. The analysis of nocturnal gas exchange is among the main, strongest points of our study, as the potential impact of nocturnal oxygen desaturation episodes on PH has not been specifically addressed in previous work.[3] Nocturnal desaturation is a well-recognized determinant of secondary PH in chronic obstructive pulmonary disease and other diseases such as heart failure.[27] One study investigated awake and sleep SpO2 values in 33 adult patients with CF.[3] By multivariate analysis, echocardiographic sPAP correlated only with awake SpO2 and not with nocturnal SpO2,[3] also in agreement with another study.[25] Of note, PAT was not investigated in these studies. A previously reported determinant of PH that was not identified in the present study is bacterial colonization. An earlier study by our group showed that PH was more frequent and more severe in patients with Burkholderia multivorans infection than in patients with infections due to other bacteria, despite a similar level of blood oxygenation, lung function and bronchiectasis.[28] The vicious circle of excessive and uncontrollable pulmonary inflammation and infection, a hallmark of CF lung disease, could play a major role in the development of PH, especially in patients who have episodes of hypoxemia. Interestingly, the two patients in the present study who harbored Burkholderia cepacia in their sputum were in the lowest PAT tertile (Table 3).
The most interesting result of our study is the correlation of PAT with time to-lung-transplantation. Of the eight patients who received lung transplants during follow up, seven had PAT values <101 ms. Numerous factors associated with mortality in CF patients have been identified. The level of lung function and exercise capacity, and above all the rate of FEV1 decline, have emerged as major risk factors.[19,26,29–31] However, survival may also be affected by other factors such as microbiology, nutritional status, age, female sex, pancreatic insufficiency, CF-related diabetes mellitus, number of respiratory exacerbations and environmental and center-related factors.[26] PH is one of the recognized risk factors for premature death.[3,19,28] In a large cohort of 149 CF patients who were listed for lung transplantation, a higher sPAP value measured during cardiac catheterization was associated with higher mortality before transplantation.[19] Interestingly, in the present study, PAT but not sPAP correlated with time-to-lung transplantation during the relatively short follow-up. This finding suggests that individual patients with PAT <101 ms may deserve consideration for inclusion on the lung transplant waiting list.
Our study has several limitations. Both practical and ethical reasons precluded routine cardiac catheterization. None of the patients died during follow-up, as all patients with very severe disease were able to undergo lung transplantation (Table 1). Therefore, survival could not be used as an endpoint, and prognosis was evaluated on time-to-lung transplantation.
PAT, which is easily and noninvasively measured during echocardiography, holds potential for diagnosis and assessing PH severity in children and adults with CF. PAT was of prognostic significance, with values lower than 101 ms being associated with a shorter time-to-lung transplantation, compared to higher values. Larger prospective studies are warranted to assess the usefulness of low PAT for determining the optimal time for listing individual patients as lung transplantation candidates.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Royce SW. Cor pulmonale in infancy and early childhood; report on 34 patients, with special reference to the occurrence of pulmonary heart disease in cystic fibrosis of the pancreas. Pediatrics. 1951;8:255–74. [PubMed] [Google Scholar]
- 2.Ryland D, Reid L. The pulmonary circulation in cystic fibrosis. Thorax. 1975;30:285–92. doi: 10.1136/thx.30.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraser KL, Tullis D, Sasson Z, Hyland RH, Thornley KS, Hanly PJ. Pulmonary hypertension and cardiac function in adult cystic fibrosis. Role of hypoxemia. Chest. 1999;115:1321–8. doi: 10.1378/chest.115.5.1321. [DOI] [PubMed] [Google Scholar]
- 4.Goldring RM, Fishman AP, Turino GM, Cohen HI, Denning CR, Anderson DH. Pulmonary hypertension and cor pulmonale in cystic fibrosis of the pancreas. J Pediatr. 1964;65:501–24. doi: 10.1016/s0022-3476(64)80286-9. [DOI] [PubMed] [Google Scholar]
- 5.Ionescu AA, Ionescu AA, Payne N, Obieta-Fresnedo I, Fraser AG, Shale DJ. Subclinical right ventricular dysfunction in cystic fibrosis. Am J Respir Crit Care Med. 2001;163:1212–8. doi: 10.1164/ajrccm.163.5.9908005. [DOI] [PubMed] [Google Scholar]
- 6.Florea VG, Florea ND, Sharma R, Coats AJ, Gibson DG, Hodson ME, et al. Right ventricular dysfunction in adult severe cystic fibrosis. Chest. 2000;118:1063–8. doi: 10.1378/chest.118.4.1063. [DOI] [PubMed] [Google Scholar]
- 7.Tonelli AR, Fernandez-Bussy S, Lodhi S, Akindipe OA, Carrie RD, Hamilton K, et al. Prevalence of pulmonary hypertension in end-stage cystic fibrosis and correlation with survival. J Heart Lung Transplant. 2010;29:865–72. doi: 10.1016/j.healun.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Kitabatake A, Inoue M, Asao M, Masuyama T, Tanouchi J, Morita T, et al. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation. 1983;68:302–9. doi: 10.1161/01.cir.68.2.302. [DOI] [PubMed] [Google Scholar]
- 9.van Dijk AP, Hopman JC, Klaessens JH, van der Werf T, Daniëls O. Is noninvasive determination of pulmonary artery pressure feasible using deceleration phase Doppler flow velocity characteristics in mechanically ventilated children with congenital heart disease? Am J Cardiol. 1996;78:1394–9. doi: 10.1016/s0002-9149(96)00643-1. [DOI] [PubMed] [Google Scholar]
- 10.Gaultier C, Boulé M, Allaire Y, Clément A, Burry A, Girard F. Determination of capillary oxygen tension in infants and children: Assessment of methodology and normal values during growth. Bull Europ Physiopath Resp. 1978;14:287–94. [PubMed] [Google Scholar]
- 11.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–34. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 12.Quanjer PH. Standardized lung function testing ;6 (Suppl. Eur Resp J. 1993;6(Suppl. 16):5–30s. [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–S66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–62. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 16.Paiva R, Krivec U, Aubertin G, Cohen E, Clément A, Fauroux B. Carbon dioxide monitoring during long-term noninvasive respiratory support in children. Intensive Care Med. 2009;35:1068–74. doi: 10.1007/s00134-009-1408-5. [DOI] [PubMed] [Google Scholar]
- 17.Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167:735–40. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 18.Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, et al. Accuracy of doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:615–21. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vizza CD, Yusen RD, Lynch JP, Fedele F, Alexander Patterson G, Trulock EP. Outcome of patients with cystic fibrosis awaiting lung transplantation. Am J Respir Crit Care Med. 2000;163:819–25. doi: 10.1164/ajrccm.162.3.9910102. [DOI] [PubMed] [Google Scholar]
- 20.Topilsky Y, Tribouilloy C, Michelena HI, Pislaru S, Mahoney DW, Enriquez-Sarano M. Pathophysiology of tricuspid regurgitation: Quantitative Doppler echocardiographic assessment of respiratory dependence. Circulation. 2010;122:1505–13. doi: 10.1161/CIRCULATIONAHA.110.941310. [DOI] [PubMed] [Google Scholar]
- 21.Serwer GA, Cougle AG, Eckerd JM, Armstrong BE. Factors affecting use of the Doppler-determined time from flow onset to maximal pulmonary artery velocity for measurement of pulmonary artery pressure in children. Am J Cardiol. 1986;58:352–6. doi: 10.1016/0002-9149(86)90076-7. [DOI] [PubMed] [Google Scholar]
- 22.Kosturakis D, Goldberg SJ, Allen HD, Loeber C. Doppler echocardiographic prediction of pulmonary arterial hypertension in congenital heart disease. Am J Cardiol. 1984;53:1110–5. doi: 10.1016/0002-9149(84)90646-5. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda M, Sekiguchi T, Sugishita Y, Kuwako K, Iida K, Ito I. Reliability of non-invasive estimates of pulmonary hypertension by pulsed Doppler echocardiography. Br Heart J. 1986;56:158–64. doi: 10.1136/hrt.56.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dabestani A, Mahan G, Gardin JM, Takenaka K, Burn C, Allfie A, et al. Evaluation of pulmonary artery pressure and resistance by pulsed Doppler echocardiography. Am J Cardiol. 1987;59:662–8. doi: 10.1016/0002-9149(87)91189-1. [DOI] [PubMed] [Google Scholar]
- 25.Rovedder PM, Ziegler B, Pinotti AF, Menna Barreto SS, Dalcin Pde T. Prevalence of pulmonary hypertension evaluated by Doppler echocardiography in a population of adolescent and adult patients with cystic fibrosis. J Bras Pneumol. 2008;34:83–90. doi: 10.1590/s1806-37132008000200004. [DOI] [PubMed] [Google Scholar]
- 26.Belkin RA, Henig NR, Singer LG, Chaparro C, Rubenstein RC, Xie SX, et al. Risk factors for death of patients with cystic fibrosis awaiting lung transplantation. Am J Respir Crit Care Med. 2006;173:659–66. doi: 10.1164/rccm.200410-1369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottlieb JD, Schwartz AR, Marshall J, Ouyang P, Kern L, Shetty V, et al. Hypoxia, not the frequency of sleep apnea, induces acute hemodynamic stress in patients with chronic heart failure. J Am Coll Cardiol. 2009;54:1706–12. doi: 10.1016/j.jacc.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fauroux B, Hart N, Belfar S, Boulé M, Tillous-Borde I, Bonnet D, et al. Burkholderia cepacia is associated with pulmonary hypertension and increased mortality among cystic fibrosis patients. J Clin Microbiol. 2004;42:5537–41. doi: 10.1128/JCM.42.12.5537-5541.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milla CE, Warwick WJ. Risk of death in cystic fibrosis patients with severely compromised lung function. Chest. 1998;113:1230–4. doi: 10.1378/chest.113.5.1230. [DOI] [PubMed] [Google Scholar]
- 30.Liou TG, Adler FR, FitzSimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153:345–52. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002;166:1550–5. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]


