Abstract
Background
The auditory brainstem response (ABR) test is frequently employed to estimate hearing sensitivity and assess the integrity of the ascending auditory system. In persons who cannot participate in conventional tests of hearing, a short-acting general anesthetic is used, recordings are obtained, and the results are compared with normative data. However, several factors (e.g., anesthesia, temperature changes) can contribute to delayed absolute and interpeak latencies, making it difficult to evaluate the integrity of the person’s auditory brainstem function.
Purpose
In this study, we investigated the latencies of ABR responses in children who received general anesthesia.
Research Design
Between subject.
Study Sample
Twelve children between the ages of 29 and 52 mo, most of whom exhibited a developmental delay but normal peripheral auditory function, comprised the anesthesia group. Twelve participants between the ages of 13 and 26 yr with normal hearing thresholds comprised the control group.
Data Collection and Analysis
ABRs from a single ear from children, recorded under general anesthesia, were retrospectively analyzed and compared to those obtained from a control group with no anesthesia. ABRs were generated using 80 dB nHL rarefaction click stimuli. T-tests, corrected for alpha slippage, were employed to examine latency differences between groups.
Results
There were significant delays in latencies for children evaluated under general anesthesia compared to the control group. Delays were observed for wave V and the interpeak intervals I–III, III–V, and I–V.
Conclusions
Our data suggest that caution is needed in interpreting neural function from ABR data recorded while a child is under general anesthesia.
Keywords: ABR, children, general anesthesia
The auditory brainstem response (ABR) is detected by placing electrodes on the scalp and separating the response from background EEG (electroencephalography) measures using averaging and amplification techniques. It consists of a series of peaks that occur within 10 msec after an abrupt stimulus such as a click. Waves I and II originate from the VIII nerve while waves III–V depend on neural functioning of the cochlear nucleus through the lateral lemniscus (Møller and Jannetta, 1982; Møller et al, 1981). The ABR test serves two main purposes in clinical audiology. It can be used as a measure of hearing sensitivity and to examine functional integrity of the VIIIth nerve and auditory brainstem neurons.
As a measure of hearing sensitivity, the ABR test is usually performed when more traditional behavioral methods (e.g., hand-raising techniques, conditioned play audiometry, visual reinforcement audiometry) yield inconclusive results or cannot be performed because of the child’s age or developmental status. At a high stimulus level, waves I through V can be observed in normal hearing individuals. As the stimulus level is reduced, early ABR waves disappear (Selters and Brackmann, 1977). However, wave V, which is typically the most robust wave of the ABR, can be detected for stimuli that are close to behavioral thresholds.
The ABR can be used to evaluate neurologic function of the auditory pathways. In this procedure, a high level click stimulus (e.g., 70 or 80 dB nHL) is presented to each ear, and the absolute and interpeak latencies are measured. Although the absolute latencies can be influenced by peripheral hearing loss, the interpeak latencies (e.g., I–III, III–V, I–V) are generally accepted as measures of central neural conduction time (Eggermont and Don, 1986) and are influenced by myelination of the nerve fibers. The ABR interpeak latencies are not adultlike until approximately 2 yr of age (Hecox and Galambos, 1974; Salamy, 1984); therefore, age-specific normative data are employed in evaluation of ABR latencies (Gorga et al, 1989; Issa and Ross, 1995). Alternatively the latencies may be compared to one’s own clinical norms. However, these normative databases are typically obtained by recording ABRs under natural sleep and/or oral sedation. In our clinical experience the latencies obtained when ABRs are recorded in a child under general anesthesia are prolonged and often outside of clinical norms.
Several factors such as anesthesia (e.g., sevoflurane, isoflurane, enflurane) and temperature changes associated with undergoing anesthesia are known to increase neural conduction time (Dubois et al, 1982; Thornton et al, 1983; Manninen et al, 1985; Markand et al, 1987; Schwender et al, 1995). With the exception of wave I, which is generated by the distal portion of the VIII nerve (Møller and Jannetta, 1982), an increase in central conduction time would result in delayed ABR absolute and interpeak latencies (e.g., III, V, I–III, III–V, I–V). This paper is a retrospective analysis of the latency measures of ABRs recorded to high level click stimuli from children while under general anesthesia. ABRs from a control group, not under general anesthesia, were examined for comparison purposes. Several factors that can potentially influence latency characteristics are reviewed.
METHOD
Subjects
This study was approved by the University of Arizona Human Subjects and Tucson Medical Center (TMC) Human Research Committees. For the anesthesia group, medical records and data files, for children between the ages of 29 and 52 mo who had previously undergone ABR testing under general anesthesia at TMC were reviewed. Data files obtained between August 1, 2008 and February 28, 2010 were analyzed. Children and adults with normal hearing were recruited for a control group.
Inclusion Criteria
Only ABR test results obtained from children and adults having normal peripheral auditory function were used in this study. Children who had a diagnosed condition known to influence neural function (e.g., bacterial meningitis, hydrocephalus, periventricular leukomalacia) were excluded from data analyses. The following criteria were used as evidence for normal peripheral auditory function.
Normal otoscopy (i.e., clear ear canal, absence of visible pathology or pressure equalization tube).
Transient evoked otoacoustic emissions (OAEs), elicited using an 80 dB SPL peak equivalent (pe) level click, present in at least three of four frequency bands including one band between 3000 and 4000 Hz with a signal-to-noise ratio of at least 6 dB and a reproducibility score of at least 70% in each of the three bands using the Bio-logic Scout OAE System (ver. 3.45.00) or the Otodynamics EZ-Screen 2 (ver. 6).
Because the children undergoing ABR testing under general anesthesia were not testable using behavioral audiometric techniques, inclusion criteria included a wave V present at a click level of 20 dB nHL and a wave V present to a 500 and 4000 Hz toneburst presented at or lower than 30 and 25 dB nHL, respectively. For the control group, pure tone audiometric thresholds of 20 dB HL or better for the frequencies 500–4000 Hz were required for inclusion.
Anesthesia Group
Twelve children met inclusion criteria for at least one ear. If only one ear of a participant met inclusion criteria, it was selected for analysis. If both ears met inclusion criteria, the ear included in analyses was randomly selected. The mean age of the children in the anesthesia group was 38.3 mo (SD 5 8.4 mo). Most of the children in this data set had a developmental delay. For seven children the primary concern was a speech delay. Three children had a diagnosis of autism, and one was diagnosed with Down syndrome. The final child had no reported developmental issues but a unilateral atresia. Diagnosis was based on chart review and/or parental or caregiver case history. Note that the children in the speech delay group may not have had a final diagnosis at time of testing. That is, the hearing evaluation is typically one of the first referrals when a child has a delay. Further developmental evaluations may ultimately result in other diagnoses. There was a higher prevalence of males (n = 11) compared to females (n = 1) in this data set. This is expected given that developmental delays are much more common for male than female children (Simpson et al, 2003).
Control Group
Because of the difficulties in obtaining quiet EEG samples in children ages 2–6 yr without the use of sedation, the control group’s mean age (19.7 yr, SD 5 3.8 yr) was higher than the anesthesia group and ranged from 13 to 26 yr. However, the interpeak intervals of the ABR are known to be “adultlike” by at least 2 yr of age (Hecox and Galambos, 1974; Salamy, 1984). The control group had the same gender distribution as the experimental group (i.e., 11 males/1 female). The ear chosen for analyses was selected prior to data analyses to match the distribution in the anesthesia group.
Procedure
For all participants, ABR testing was performed using the Bio-logic Auditory Evoked Potential System (ver. 6.2.0). ABR tests for the control group were conducted in a quiet room at TMC. The participant was allowed to recline in a comfortable chair for the duration of testing and was asked to relax with eyes closed.
ABR tests under general anesthesia were performed at TMC in the Ambulatory Surgery Department or in the recovery areas of MRI/CT (magnetic resonance imaging/computerized tomography scan). The purpose of testing in all cases was to estimate hearing sensitivity in a child who was difficult to test using behavioral audiometric techniques.
The procedures for general anesthesia consisted of the following: All participants had a preoperative evaluation performed by an anesthesiologist before the study. A few children received Midazolam (Versed®) before induction of anesthesia. After the preoperative evaluation, participants were taken to the test study location and anesthetized using the technique of mask induction with the anesthetic sevoflurane with or without nitrous oxide. Simultaneous to the mask induction, standard anesthetic monitoring devices were applied to each child (pulse oximetry, electrocardiogram, noninvasive arterial blood pressure). Intravenous therapy (IV) access was established, and a laryngeal mask was inserted after the participant reached the appropriate depth of anesthesia. After insertion of the laryngeal mask, spontaneous breathing was maintained. In addition to the above vitals, end-tidal carbon dioxide was also continuously monitored. The ABR test involves minimal stimulation and requires only that the participant not move during the exam. Therefore, the anesthetic was conducted in such a manner as to provide the least amount of gas to maintain each child motionless during the test. Once anesthetized and stable, otoscopy was performed followed by otoacoustic emissions and ABR testing. At the conclusion of the test, the anesthetic was discontinued, and the child was allowed to spontaneously emerge from the anesthetic. The laryngeal mask was removed, and the child was taken to the Post Anesthesia Care Unit to complete the recovery process.
ABR Protocol
For all participants, a single channel recording was used with high forehead as the noninverting electrode. The earlobe or mastoid ipsilateral to the stimulus was used as the inverting electrode site while the earlobe or mastoid contralateral to the stimulus was used as the ground electrode. All impedances were less than 5 kohms. A high level ABR was obtained by presenting 80 dB nHL, 100 microsec rarefaction clicks through an insert earphone using a rate of 21.1 clicks per second. Two recordings were obtained to ensure repeatability. The recording parameters consisted of a 16 msec time window. The bioelectrical activity was amplified 100,000 times and filtered 100–3000 Hz. Artifact reject was set to 10 mv for children under general anesthesia. Approximately 500 averages were obtained for each recording. Because of the high click level and the low noise condition (child under general anesthesia), the recordings were clear and highly reproducible with approximately 500 averages. For the control group, an artifact reject of 10 or 20 mv was used depending on the amplitude of the ongoing EEG. Approximately 1500 averages were obtained for each recording.
Data Analysis
The analysis of the ABR waveforms consisted of peak latency for waves I, III, and V. The two waveforms were overlapped on the computer screen, and a cursor was used to obtain measurements. Peak latencies for waves I and III were measured at the highest peak. If the highest peak occurred at different points for the two recordings, an average of the two was taken as latency. If the wave was a plateau or two equal peaks separated by one or more lower points, the median was taken as latency. Peak latency for wave V was measured at the highest peak if there was a clearly identifiable wave IV. If waves IV and V were a complex, wave V was measured at the farthest excursion before the trough of the complex.
Interrater Measurements
Latency measures from all ears were randomly selected and reevaluated by two “blinded” undergraduate students who were trained using the peak-picking rules and then jointly interpreted the ABR waveforms. Interrater measurements consisted of comparing the measures obtained by the first author with those obtained from the undergraduate student measurements. The percent of repeated measures falling within one bin (plus or minus 0.07 msec) of the first measurement was 96%. When discrepant measurements were identified between raters (i.e., greater than 0.07 msec), the measurements were reevaluated by all three raters and resolved by reviewing the peak-picking rules and discussion.
RESULTS
Figure 1 displays the average ABRs recorded from the control group (top panel) and the children under general anesthesia (bottom panel). While wave I and III latencies are very similar for participants in both groups, wave V latency is prolonged for the anesthesia group compared to the control group.1 To examine group differences, the absolute and interpeak latencies from ABRs obtained from children under general anesthesia were compared to the latencies obtained from the control group. Bonferroni adjusted t-tests (p < 0.008) were performed on absolute latencies and interpeak intervals. A two-tailed t-test was used for the wave I absolute latency comparison and one-tailed t-tests for all other comparisons since the prediction was for longer absolute wave III and V latencies and interpeak latencies for the anesthesia compared to control group. Table 1 displays the means and standard deviations as well as significant differences obtained between groups. The children under general anesthesia exhibited a longer wave V latency as well as longer I–III, III–V, and I–V interpeak intervals compared to the control group.
Figure 1.
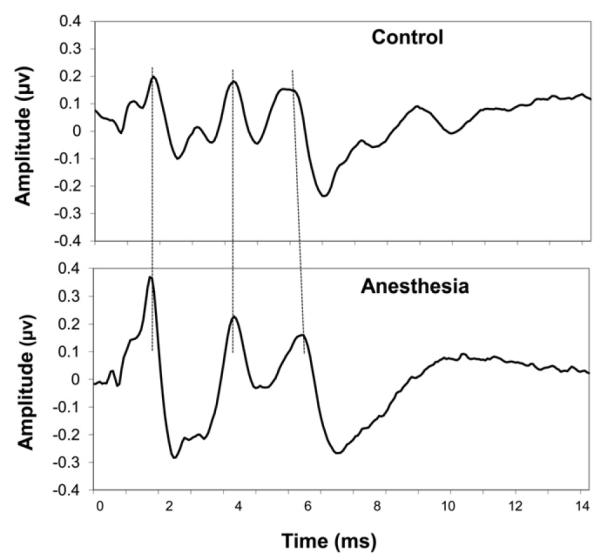
Mean ABR waveforms for the control and anesthesia group. Dotted lines were added to facilitate comparisons of the major waves between groups.
Table 1.
Mean Wave Latencies (SD in parentheses) for Anesthesia and Control Groups
| I | III | V | I–III | III–V | I–V | |
|---|---|---|---|---|---|---|
| Anesthesia | 1.46 (0.09) | 3.90 (0.13) | 5.95 (0.18) | 2.44 (0.13) | 2.05 (0.15) | 4.49 (0.19) |
| Control | 1.56 (0.12) | 3.81 (0.14) | 5.65 (0.12) | 2.25 (0.14) | 1.84 (0.17) | 4.09 (0.16) |
| P value | 0.05 | 0.06 | 0.00005* | 0.0008* | 0.002* | 0.000006* |
Significant.
DISCUSSION
In our sample of young children who under went general anesthesia for the purpose of audiological assessment, the ABR wave V and interpeak intervals for I–III, III–V, and I–V were prolonged compared to a control group. One possible but unlikely explanation for these differences is that participants in the control group were significantly older than the children under general anesthesia. However, as previously noted, the ABR latencies are thought to be adultlike by age 2 yr. In addition, our control group interpeak latencies and standard deviations are very similar to those obtained by Gorga et al (1989) for a group of 30- to 33-mo-old children under natural sleep or sedation such as choral hydrate (Table 2).2 Therefore, factors other than age, such as the effects of anesthesia, core body temperature, or intrinsic neurological dysfunction, should be explored as the source of prolonged latencies for ABRs obtained under general anesthesia.
Table 2.
ABR Mean Interpeak Latencies in msec, SD in Parentheses, for Control Group Compared to Results Obtained by Gorga et al (1989)
| I–III | III–V | I–V | |
|---|---|---|---|
| Control Group | 2.25 (0.14) | 1.84 (0.17) | 4.09 (0.16) |
| Gorga et al (30–33 mo) | 2.21 (0.16) | 1.90 (0.18) | 4.12 (0.23) |
Note: The ABRs in the Gorga et al study were elicited from 30- to 33-mo-old infants using 13/sec rarefaction clicks presented at 80 dB nHL via circumaural earphones. ABRs were recorded in infants under natural sleep or oral sedation.
Core Body Temperature
Anesthesia can cause vasodilation and decreased core body temperature which in turn can increase the ABR interpeak intervals (Markand et al, 1987). Although major surgical procedures such as abdominal or thoracic surgery and long surgical procedures can result in severe hypothermia (Sessler, 1997; Kasai et al, 2002), minor surgeries or those procedures requiring no surgical incision are expected to result in smaller changes in body temperature. For example, Ikeda et al (1999) found a mean drop of 0.8°C (SD of 0.2) in adults undergoing minor oral surgery after 1 hr of induction of anesthesia using sevoflurane. Stockard et al (1978) suggest that a 0.5° C change in core body temperature would produce changes in interpeak latency of 0.075, 0.04, and 0.04 msec for the I–V, I–III, and III–V interpeak latencies, respectively. However, one may not be able to presume similar temperature changes in infants and children. The pediatric population in general is susceptible to heat loss due to a large surface area to body mass ratio and is not efficient in preserving and generating body heat (Sessler, 2000). Therefore, core body temperature decreases might contribute to delayed latencies under general anesthesia.
Anesthesia
According to a review by Banoub et al (2003) the volatile anesthetics are associated with small increases in ABR latency because they depress brainstem neuronal activity. Banoub et al point out that the volatile anesthetics slow down neural transmission with effects on synaptic transmission being greater than effects on axonal conduction. According to these authors, polysynaptic pathways will be more influenced than oligosynapic pathways, with the brainstem auditory evoked potentials less affected by volatile anesthetics compared to later evoked potentials that arise from thalamic and cortical neural generators.
Several studies have found increased ABR latencies when recordings are obtained from patients under general anesthesia compared to pre-anesthesia (Dubois et al, 1982; Thornton et al, 1983; Manninen et al, 1985; Schwender et al, 1995). Thornton et al (1983) found that enflurane was associated with increased ABR wave latencies (particularly wave III and V) in six adults. In a study of 10 adults, in which body temperature was carefully controlled, Manninen et al (1985) found that isoflurane and isoflurane-nitrous oxide significantly increased ABR latencies for waves III, IV, and V compared to pre-anesthesia recordings.
Developmental Delays
Most of the children in the current study were diagnosed with some type of developmental delay. To date, ABRs obtained in developmentally delayed children recorded using slow rate click stimuli, similar to the rate used in our study, do not provide clear evidence of auditory brainstem dysfunction in developmentally delayed children. For example, Olsén et al (2002) examined click evoked ABRs in 42 children (mean age of 8 yr) who were born premature. Thirty-one percent had minor developmental dysfunction, and 32% had periventricular leukomalacia. They found no significant differences in ABR absolute or interpeak latencies for these children compared to a group of age-matched normally developing children who were not born premature. Tharpe et al (2006) found no significant delays in absolute or interpeak latencies for 12 normal hearing autistic children, ages 3:2–10:30 yr, compared to age and gender matched children. King et al (2002) found no delays for click evoked ABRs for a group of children with language based learning impairments, and Filippini and Schochat (2009) found no differences in a click evoked ABR for individuals with an auditory processing disorder compared to a control group.
Although other studies have described click evoked ABR abnormalities in children with autism (Student and Sohmer, 1978; Tanguay et al, 1982; Taylor et al, 1982), they have been criticized because of methodological concerns (see Klin, 1993).
The click evoked ABR, when obtained using a slow presentation rate, may not be sensitive to subtle neural dysfunction at the brainstem level. However, more recent studies have found that ABRs recorded using speech stimuli (Cunningham et al, 2001; King et al, 2002; Filippini and Schochat, 2009) or clicks presented at a fast rate (Basu et al, 2010) may indicate neural dysfunction at the brainstem level for some language-impaired children.
CONCLUSION
Our analysis of ABR latencies demonstrates that young children who are difficult to test behaviorally may have delayed ABR latencies when recorded under general anesthesia using click stimuli presented at a slow rate. We reviewed several variables, in isolation, that can contribute to delays in central conduction time (i.e., decreased body temperature, anesthesia, developmental delay). However, a combination of these factors may be responsible for delayed ABR latencies under general anesthesia. Synergistic effects between core body temperature and level of anesthetic also exist (see Vitez et al, 1974). In addition, there may be some children or groups of children who show a greater change in their ABR latencies, compared to other children, when under anesthesia or with core body temperature changes. Careful monitoring of core body temperature and level of anesthetic is critical in ABR interpretation of neural function when performed under general anesthesia. Without careful monitoring, it is difficult to determine the reasons for prolonged latencies. If possible, ABR evaluations for the purpose of evaluating neural function should be conducted under natural sleep to avoid the undesired factors that can alter ABR latency when anesthetized.
Acknowledgments
The authors thank Theodore Glattke for his comments on an earlier version of this manuscript. We would also like to thank Zina Bernal and Berit Bilquist for assisting with data analyses.
Abbreviations
- ABR
auditory brainstem response
- EEG
electroencephalography
- TMC
Tucson Medical Center
Footnotes
While amplitude differences in the ABRs obtained for our two groups could not be compared, our anesthesia group appeared to have enlarged wave I amplitudes. Future studies are needed to confirm and further explore this observation.
A study by Pillion et al (2010) found no differences in ABR interpeak latencies when obtained in the same subjects with and without the use of chloral hydrate, which does not produce complete anesthesia. Further explorations of the effects of chloral hydrate on ABR central conduction time in young children may be warranted.
Portions of this study were presented at the annual meeting of the American Auditory Society, March 2010, Scottsdale, AZ.
REFERENCES
- Banoub M, Tetzlaff JE, Schubert A. Pharmacologic and physiologic influences affecting sensory evoked potentials. Anesthesiology. 2003;99:716–737. doi: 10.1097/00000542-200309000-00029. [DOI] [PubMed] [Google Scholar]
- Basu M, Krishnan A, Weber-Fox C. Brainstem correlates of temporal auditory processing in children with specific language impairment. Dev Sci. 2010;13:77–91. doi: 10.1111/j.1467-7687.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Nicol T, Zecker SG, Bradlow A, Kraus N. Neurobiologic responses to speech in noise in children with learning problems: deficits and strategies for improvement. Clin Neurophysiol. 2001;112:758–767. doi: 10.1016/s1388-2457(01)00465-5. [DOI] [PubMed] [Google Scholar]
- Dubois MY, Sato S, Chassy J, Macnamara TE. Effects of enflurane on brainstem auditory evoked responses in humans. Anesth Analg. 1982;61:898–902. [PubMed] [Google Scholar]
- Eggermont JJ, Don M. Mechanism of central conduction time prolongation in brainstem auditory evoked potentials. Arch Neurol. 1986;43:116–120. doi: 10.1001/archneur.1986.00520020010007. [DOI] [PubMed] [Google Scholar]
- Filippini R, Schochat E. Brainstem evoked auditory potentials with speech stimulus in the auditory processing disorder. Braz J Otorhinolaryngol. 2009;75:449–455. doi: 10.1016/S1808-8694(15)30665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga MP, Kaminski JR, Beauchaine KL, Jesteadt W, Neely ST. Auditory brainstem responses from children three months to three years of age: normal patterns of response II. J Speech Hear Res. 1989;32:281–288. doi: 10.1044/jshr.3202.281. [DOI] [PubMed] [Google Scholar]
- Hecox K, Galambos R. Brain stem auditory evoked responses in human infants and adults. Arch Otolaryngol. 1974;99:30–33. doi: 10.1001/archotol.1974.00780030034006. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Sessler DI, Kikura M, Kazama T, Ikeda K, Sato S. Less core hypothermia when anesthesia is induced with inhaled sevoflurane than with intravenous propofol. Anesth Analg. 1999;88:921–924. doi: 10.1097/00000539-199904000-00044. [DOI] [PubMed] [Google Scholar]
- Issa A, Ross HF. An improved procedure for assessing ABR latency in young subjects based on a new normative data set. Int J Pediatr Otorhinolaryngol. 1995;32:35–47. doi: 10.1016/0165-5876(94)01110-j. [DOI] [PubMed] [Google Scholar]
- Kasai T, Hirose M, Yaegashi K, Matsukawa T, Takamata A, Tanaka Y. Preoperative risk factors of intraoperative hypothermia in major surgery under general anesthesia. Anesth Analg. 2002;95:1381–1383. doi: 10.1097/00000539-200211000-00051. [DOI] [PubMed] [Google Scholar]
- King C, Warrier CM, Hayes E, Kraus N. Deficits in auditory brainstem pathway encoding of speech sounds in children with learning problems. Neurosci Lett. 2002;319:111–115. doi: 10.1016/s0304-3940(01)02556-3. [DOI] [PubMed] [Google Scholar]
- Klin A. Auditory brainstem responses in autism: brainstem dysfunction or peripheral hearing loss? J Autism Dev Disord. 1993;23:15–35. doi: 10.1007/BF01066416. [DOI] [PubMed] [Google Scholar]
- Manninen PH, Lam AM, Nicholas JF. The effects of isoflurane and isoflurane-nitrous oxide anesthesia on brainstem auditory evoked potentials in humans. Anesth Analg. 1985;64:43–47. [PubMed] [Google Scholar]
- Markand ON, Lee BI, Warren C, et al. Effects of hypothermia on brainstem auditory evoked potentials in humans. Ann Neurol. 1987;22:507–513. doi: 10.1002/ana.410220410. [DOI] [PubMed] [Google Scholar]
- Møller A, Jannetta P. Evoked potentials from the inferior colliculus in man. Electroencephalogr Clin Neurophysiol. 1982;53:612–620. doi: 10.1016/0013-4694(82)90137-7. [DOI] [PubMed] [Google Scholar]
- Møller A, Jannetta P, Bennett M, Møller M. Intracranially recorded responses from the human auditory nerve: new insights into the origin of brain stem evoked potentials (BSEPs) Electroencephalogr Clin Neurophysiol. 1981;52:18–27. doi: 10.1016/0013-4694(81)90184-x. [DOI] [PubMed] [Google Scholar]
- Olsén P, Yliherva A, Pääkkö E, Järvelin MR, Tolonen U. Brainstem auditory-evoked potentials of 8-year-old preterm children in relation to their psycholinguistic abilities and MRI findings. Early Hum Dev. 2002;70:25–34. doi: 10.1016/s0378-3782(02)00066-x. [DOI] [PubMed] [Google Scholar]
- Pillion JP, Bibat G, Naidu S. Effects of sedation on auditory brainstem response in Rett syndrome. Pediatr Neurol. 2010;42:331–334. doi: 10.1016/j.pediatrneurol.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamy A. Maturation of the auditory brainstem response from birth through early childhood. J Clin Neurophysiol. 1984;1:293–329. doi: 10.1097/00004691-198407000-00003. [DOI] [PubMed] [Google Scholar]
- Schwender D, Conzen P, Klasing S, Finsterer U, Poppel E, Peter K. The effects of anesthesia with increasing end-expiratory concentrations of sevoflurane on midlatency auditory evoked potentials. Anesth Analg. 1995;81:817–822. doi: 10.1097/00000539-199510000-00027. [DOI] [PubMed] [Google Scholar]
- Selters WA, Brackmann DE. Acoustic tumor detection with brain stem electric response audiometry. Arch Otolaryngol. 1977;103:181–187. doi: 10.1001/archotol.1977.00780210037001. [DOI] [PubMed] [Google Scholar]
- Sessler DI. Perioperative thermoregulation and heat balance. Ann N Y Acad Sci. 1997;813:757–777. doi: 10.1111/j.1749-6632.1997.tb51779.x. [DOI] [PubMed] [Google Scholar]
- Sessler DI. Perioperative heat balance. Anesthesiology. 2000;92:578–596. doi: 10.1097/00000542-200002000-00042. [DOI] [PubMed] [Google Scholar]
- Simpson GA, Colpe L, Greenspan S. Measuring functional developmental delay in infants and young children: prevalence rates from the NHIS-D. Paediatr Perinat Epidemiol. 2003;17:68–80. doi: 10.1046/j.1365-3016.2003.00459.x. [DOI] [PubMed] [Google Scholar]
- Stockard JJ, Sharbrough FW, Tinker JA. Effects of hypothermia on the human brainstem auditory response. Ann Neurol. 1978;3:368–370. doi: 10.1002/ana.410030416. [DOI] [PubMed] [Google Scholar]
- Student M, Sohmer H. Evidence from auditory nerve and brainstem evoked responses for an organic brain lesion in children with autistic traits. J Autism Child Schizophr. 1978;8:13–20. doi: 10.1007/BF01550274. [DOI] [PubMed] [Google Scholar]
- Tanguay PE, Edwards RM, Buchwald J, Schwafel J, Allen V. Auditory brainstem evoked responses in autistic children. Arch Gen Psychiatry. 1982;39:174–180. doi: 10.1001/archpsyc.1982.04290020040008. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Rosenblatt B, Linschoten L. Auditory brainstem response abnormalities in autistic children. Can J Neurol Sci. 1982;9:429–433. doi: 10.1017/s0317167100044346. [DOI] [PubMed] [Google Scholar]
- Tharpe AM, Bess FH, Sladen DP, Schissel H, Couch S, Schery T. Auditory characteristics of children with autism. Ear Hear. 2006;27:430–441. doi: 10.1097/01.aud.0000224981.60575.d8. [DOI] [PubMed] [Google Scholar]
- Thornton C, Catley DM, Jordan C, Lehane JR, Royston D, Jones JG. Enflurane anaesthesia causes graded changes in the brainstem and early cortical auditory evoked response in man. Br J Anaesth. 1983;55:479–486. doi: 10.1093/bja/55.6.479. [DOI] [PubMed] [Google Scholar]
- Vitez TS, White PF, Eger EI. Effects of hypothermia on halothane MAC and isoflurane MAC in the rat. Anethesiology. 1974;41:80–81. doi: 10.1097/00000542-197407000-00020. [DOI] [PubMed] [Google Scholar]


