Abstract
Objectives
The purpose of this study was to assess the effect of scar tissue composition on engraftment of progenitor cells into infarcted myocardium.
Background
Scar tissue formation after myocardial infarction creates a barrier that severely compromises tissue regeneration, limiting potential functional recovery.
Methods
In vitro: A tricell patch (Tri-P) was created from peritoneum seeded and cultured with induced pluripotent stem cell–derived cardiomyocytes, endothelial cells, and mouse embryonic fibroblasts. The expression of fibrosis-related molecules from mouse embryonic fibroblasts and infarcted heart was measured by Western blot and quantitative reverse transcriptase polymerase chain reaction. In vivo: A Tri-P was affixed over the entire infarcted area 7 days after myocardial infarction in mice overexpressing adenylyl cyclase 6 (AC6). Engraftment efficiency of progenitor cells in hearts of AC6 mice was compared with that of control wild-type (WT) mice using a combination of in vivo bioluminescence imaging, post-mortem ex vivo tissue analysis, and the number of green fluorescent protein–positive cells. Echocardiography of left ventricular (LV) function was performed weekly. Hearts were harvested for analysis 4 weeks after Tri-P application. Mouse embryonic fibroblasts were stimulated with forskolin before an anoxia/reoxygenation protocol. Fibrosis-related molecules were analyzed.
Results
In AC6 mice, infarcted hearts treated with Tri-P showed significantly higher bioluminescence imaging intensity and numbers of green fluorescent protein–positive cells than in WT mice. LV function improved progressively in AC6 mice from weeks 2 to 4 and was associated with reduced LV fibrosis.
Conclusions
Application of a Tri-P in AC6 mice resulted in significantly higher induced pluripotent stem cell engraftment accompanied by angiomyogenesis in the infarcted area and improvement in LV function.
Keywords: adenylyl cyclases, angiomyogenesis, induced pluripotent stem cell engraftment, myocardial infarction, tricell patch
Myocardial fibrosis, a key contributor to cardiac dysfunction after myocardial infarction (MI), presents as a secondary response to the pathophysiologic remodeling of longstanding coronary artery disease including ischemia, obstruction, and microvascular abnormalities (1). Cardiac fibroblasts and myofibroblasts are responsible for post-MI remodeling, which occurs via regulation of extracellular matrix (ECM) (2), as evidenced by increased collagen types I and III in the interstitial and perivascular spaces (3).
Decreased apoptosis, hypertrophy, and fibrosis in the infarcted heart have been demonstrated with stem cell–based therapy (4,5). The pluripotency of stem cells has made transplantation of stem/progenitor cells a primary research focus of myocardial tissue regeneration. However, the accumulation of ECM and myofibroblasts in areas of tissue injury after ischemia-induced MI presents a potential barrier that impairs penetration of reparative stem/progenitor cells mobilized from peripheral reservoirs including cell patches. Therefore, the role played by cardiac fibroblasts in the production and degradation of ECM may be critical in regulating cardiac remodeling and in permitting stem/progenitor cell mobilization into infarcted myocardium.
In addition to mechanical stimuli and cytokines, myofibroblast formation is regulated most notably by transforming growth factor beta (3). Cyclic adenosine monophosphate (cAMP), a ubiquitous second messenger, is regulated by adenylyl cyclases, which play a critical role in cell function via the activation of cAMP-dependent protein kinase A (PKA) (6). cAMP-elevating agents appear to be negative regulators of fibroblast function that can inhibit the profibrotic effects of transforming growth factor beta in cardiac fibroblasts via inhibition of extracellular signal–regulated kinase (ERK) 1/2 Smad signaling pathway (7). AC6, a dominant adenylyl cyclase isoform in mammalian cardiac myocytes, appears to exert favorable effects on heart function (8). Activation of AC6 has been reported to correlate with decreased collagen production in the infarcted myocardium (3). Although the specific role of collagen deposition in fibrotic myocardium on stem/progenitor cell engraftment and MI repair is currently unknown, overexpression of cardiac-specific AC6 reduces collagen density in scar tissue and decreases this barrier to progenitor cell migration. This could potentially increase induced pluripotent stem cell (iPSC) engraftment, promote penetration from the cell patch into the infarcted area, and enhance functional differentiation within the infarcted area after implantation of the cell patch, such as enhanced angiomyogenesis, allowing for improved cardiac function.
Methods
A detailed methods section is available in the Online Appendix.
Results
In vitro study
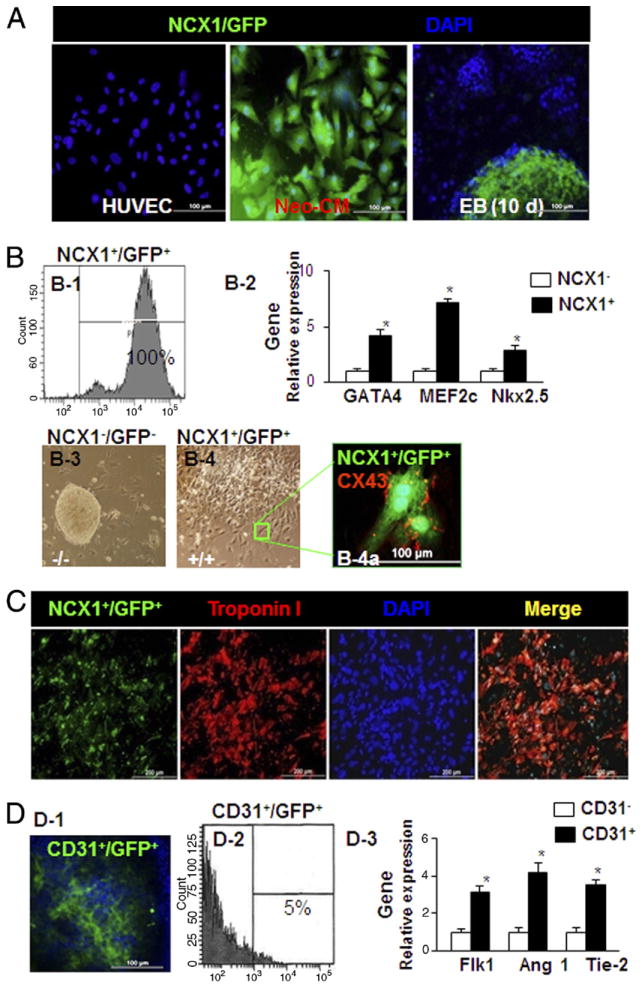
Characteristics of cardiac-specific NCX1-promoter represent cardiogenic differentiation in iPSCs and isolated endothelial cells from iPSCs. Human umbilical vein endothelial cells, neonatal rat cardiomyocytes (Neo-CMs), and embryoid bodies (EBs) derived from iPSCs were transduced with pLVX-NCX1-Fluc-Puro-IRES-ZsGreen1 lentivirus. Human umbilical vein endothelial cells served as the negative cardiomyocyte (CM) control, rat Neo-CMs were used as the positive CM control and ZsGreen1 (green fluorescent protein [GFP]) was detected in Neo-CMs and at 10 days in EBs (Fig. 1A). After 2 passages treated with puromycin (1.5 μg/ml), the brightest GFP-positive (GFP+) iPSCs were further selected to generate GFP+ subclones. Fluorescence-activated cell sorting (FACS) for the selected clone of NCX1+/GFP+ cells derived from iPSCs, in which EBs differentiated for 14 days show 100% GFP+ (Fig. 1B-1). Quantitative reverse transcriptase polymerase chain reaction showed that FACS sorting of positive cells (NCX1+/GFP+) resulted in significantly greater expression of CM markers (GATA4, MEF2c, and Nkx2.5) than in NCX1− control cells (Fig. 1B-2). EBs from iPSCs followed by an additional 4-day adherent phase were differentiated in a monolayer and developed prominent beating areas with sustained contractile activity. In contrast to NCX1−/GFP− cells (Fig. 1B-3), NCX1+/GFP+ cells were very active with more robust differentiation morphologically into CMs (Fig. 1B-4). All NCX1+ cells were derived from the EBs-cardiac progenitor cells (CPCs). More than 65% of the cells expressing GFP displayed spontaneous contraction. Immunofluorescence of Cx43 showed that Cx43+ spots were principally localized to cell-cell contacts between CMs, suggesting that gap-junctional intercellular communication plays a major role in the synchronous beating of CMs (Fig. 1B-4a). Cells from the same clone showed uniformly high GFP expression, and differentiation into CMs was confirmed by the CM-specific marker troponin I staining (Fig. 1C, red). Current experimental protocol yields about 5% CD31+/GFP+ cells detected at 14 days in EBs (Figs. 1D-1 and 1D-2). Interestingly, quantitative reverse transcriptase polymerase chain reaction showed that CD31+/GFP+ cells exhibited significantly greater expression of vascular markers (Flk1, angiopoietin-1, and Tie-2) than in CD31− control cells (Fig. 1D-3). A representative cardiomyocyte mechanical response to electrical stimulation at frequencies from 0.0 to 5.0 Hz was measured by using video edge detection (Fig. 1E).
Figure 1. Construction and Validation of iPSC-Derived Cardiac Progenitor Cells Labeled With NCX1+/GFP+ (NCX1/ZsGreen1).
(A) Fluorescence images of 3 cell lines after transfection with lentivirus-based NCX1 promoter. Neonatal rat cardiomyocytes (Neo-CM) served as the positive cardiomyocyte (CM) control and human umbilical vein endothelial cells (HUVEC) served as the negative control. Embryoid bodies (EB) from induced pluripotent stem cells (iPSC) on day 10 were used to determine the efficiency of lentivirus-based NCX1/ZsGreen1 vector (NCX1/GFP) transduction for iPSC differentiation to cardiac progenitor cells (CPC). (B) High-quality CPC overexpressing NCX1+/GFP+ were obtained after treatment with puromycin (1.5 μg/ml) and sorted by fluorescence-activated cell sorting (FACS) (B-1). Amplified gene expression was confirmed in NCX1+ and NCX1− by quantitative reverse transcriptase polymerase chain reaction (qPCR) (B-2). In contrast to NCX1−/GFP− cells (B-3), NCX1+/GFP+ cells, sorted by FACS, were differentiated (B-4) and developed prominent beating areas with sustained contractile activity for an additional 4 days. Positive expression of gap junction was identified by CX43 (B-4a). (C) Cells from the same clone show uniformly high GFP (NCX1/ZsGreen1) expression and differentiation into CM as confirmed by the CM-specific marker troponin I staining (red). (D) CD31+ cells identified by immunostaining (D-1), percentage of CD31+/GFP+ cells from 14-day-old EB sorted by FACS (D-2), and vascular gene expression obtained by qPCR (D-3). *p < 0.05 versus CD31+ cells. All values expressed as mean ± SEM; 6 for each group.
(E) Patches actively contract and relax in response to electrical stimulation. A representative CM patch derived from NCX1+/GFP+ iPSC was electrically stimulated at frequencies from 0.0 to 5.0 Hz. (F) Characteristics of iPSC-derived tricell patch (Tri-P). Tri-P containing all 3 cell types (CM + endothelial cells [EC] + mouse embryonic fibroblast [MEF]) promoted vessel formation by significant expression of CD31 (green) in comparison with patches containing only CM or CM and EC. Troponin I was used to identify CM (red), 4,6-diamino-2-phenylindole (DAPI) was used to identify all nuclei (blue). (G) Quantitative data for vessel number in 3 types of cell cultures. *p < 0.05 versus CM or CM + EC. All values expressed as mean ± SEM; n = 6 in each group. (H) Quantitative data showed no significant difference in vessel number in Tri-P despite increasing ratios of MEF to EC and CM. Differentiated CM from NCX1+/GFP+ iPSC. †p < 0.05 versus EC. All values expressed as mean ± SEM; n = 6 for each group. Ang1 = angiopoietin-1.
Characteristics of iPSC-derived tricell patch
Patches derived using only CMs and endothelial cells (ECs) demonstrated clumps of CD31+ cells, but did not organize into blood vessels. Immunofluorescent stained cell patches containing CMs alone (Fig. 1F, top), CMs and ECs (Fig. 1F, middle), and CMs + ECs + MEFs (tricell patch [Tri-P]) (Fig. 1F, bottom) were used to determine optimal cell ratios. Tri-P had a significant increase in the number of vessels compared with CMs or CMs + ECs. Quantitative analysis demonstrated that the addition of MEFs to the constructs containing CMs and ECs produced structurally organized vascularized cardiac muscle (Fig. 1G) (n = 4). Comparison of the Tri-P containing ECs with MEF ratios of 0.5:1, 1:1, and 2:1 revealed no significant difference in the number of vessels despite increasing MEF concentration (Fig. 1H). Addition of MEFs to the constructs containing CMs and ECs resulted in uniformly higher EC density in Tri-P regardless of concentration of MEFs.
The role of AC6 overexpression in collagen synthesis
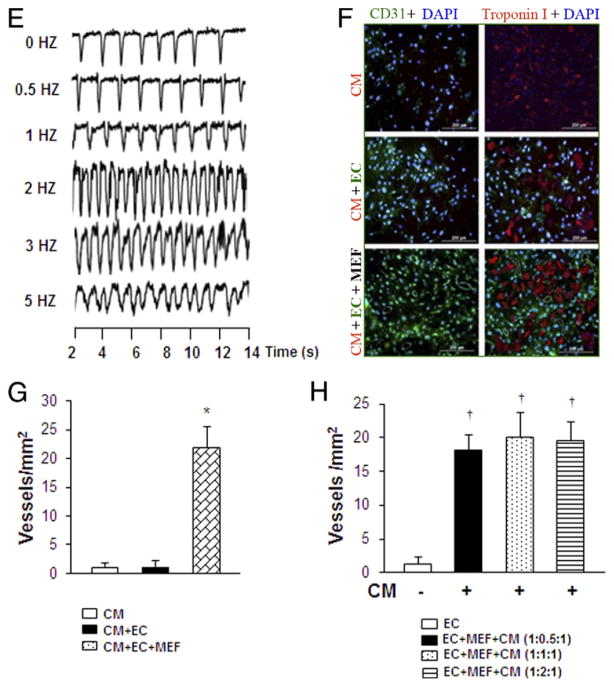
Forskolin, which is able to directly increase the levels of cAMP (7), was used to mimic the effect of AC6 overexpression on inhibition of collagen synthesis in vitro. The phosphorylation of the cAMP response element-binding factor (CREB), a cellular transcription factor, was significantly increased in MEFs exposed to anoxia/reoxygenation after pretreatment with forskolin (10 μM for 30 min) (Figs. 2A and 2B-1), whereas the levels of phosphorylated ERK (Fig. 2B-2), pSmad-3 (Fig. 2B-3), collagen I (Fig. 2C-1), and collagen type III expression (Fig. 2C-2) were significantly decreased. Levels of pSmad-2 (Fig. 2B-4) showed no difference compared with vehicle control (Figs. 2A, 2B, and 2C). Interestingly, the forskolin effects on p-CREB, p-ERK, pSmad-3, collagen I, and collagen III were abolished in MEFs pretreated with H89, a selective inhibitor of cAMP-dependent PKA (Figs. 2A to 2C).
Figure 2. Effect of AC6 Overexpression in Cardiac Fibrosis.
In vitro study. (A to C) Effect of forskolin (FOK), an agent that elevates cyclic adenosine monophosphate (cAMP), mimicked adenylyl cyclase 6 (AC6) overexpression in mouse embryonic fibroblasts (MEF). Various signaling pathways known to be involved in myofibroblast formation were assessed by Western blotting (A and B) and quantitative reverse transcriptase polymerase chain reaction (qPCR) (C) under anoxia/reoxygenation conditions. *p < 0.05 versus vehicle control. All values expressed as mean ± SEM; n = 6 for each group. (D) The expression of AC6 in the heart tissue in both AC6 transgenic mice and WT mice by Western blot. (E) Expression of collagen types I (E-1) and III (E-2) by qPCR in the heart tissue of AC6 and WT mice 24 h, 1 week, and 4 weeks after myocardial infarction. Collagen I (E-3) and III (E-4) expression in the heart tissue of AC6 and WT mice after treatment with either tricell patch (Tri-P) or MEF patch (MEF-P) alone. WT mice. *p < 0.05 versus WT mice; †p < 0.05 versus WT mice at 24 h; ‡p < 0.05 versus MEF-P. All values expressed as mean ± SEM; n = 6 for each group. CON = vehicle control; H89 = N-[2-(p-bromocin-namylamino) ethyl]-5-isoquinolinesulfonamide; pCREB = phosphorylated cAMP response element-binding protein; pERK = phosphorylated extracellular signal-regulated kinase; pSmad2 = phosphorylated Smad2; pSmad3 = phosphorylated Smad3; WT = wild-type (control).
AC6 transgenic mice were used to further explore these findings in vivo. Compared with WT mice (control group), significantly greater AC6 expression was confirmed in the heart tissue of AC6 transgenic mice than in WT mice (Fig. 2D). No significant difference was detected in collagen types I (Fig. 2E-1) and III (Fig. 2E-2) at 24 h post-MI. However, collagen type I in AC6 heart tissue was significantly reduced 1 week after MI, and both collagen types I and III were significantly reduced in AC6 transgenic mice 4 weeks after MI compared with WT mice (Figs. 2E-1 and 2E-2). Furthermore, collagen type I in WT heart tissue was significantly increased 1 week after MI, and both collagen types I and III were significantly increased in WT mice 4 weeks after MI compared with 24 h after MI (Figs. 2E-1 and 2E-2). In addition, expression of collagen types I and III was significantly reduced 4 weeks after either MEF or Tri-P treatment in AC6 mice compared with WT mice, and these changes were further decreased in the Tri-P treatment group compared with the MEF treatment group in both WT and AC6 transgenic mice (Figs. 2E-3 and 2E-4).
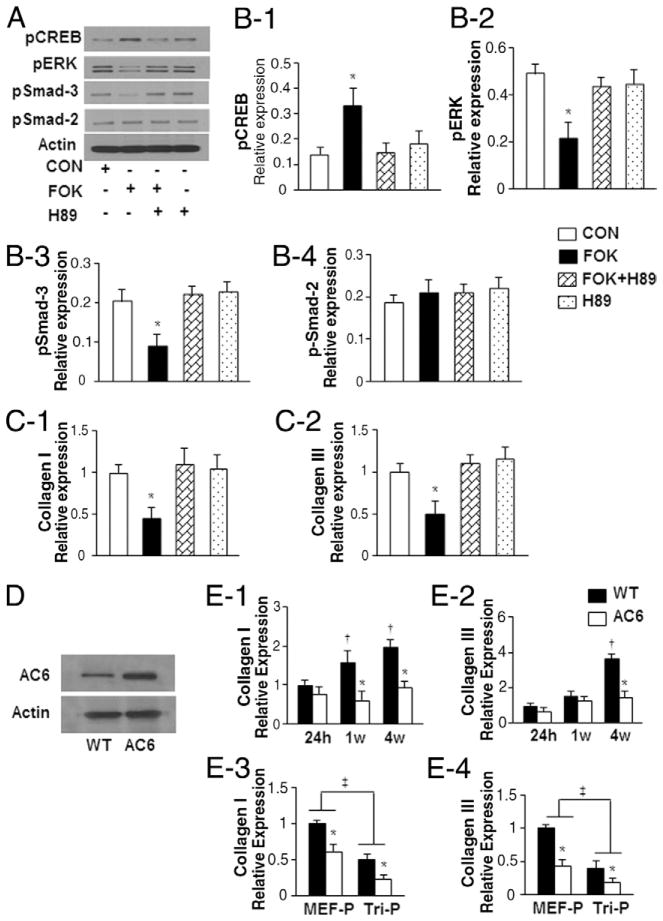
Tri-P on AC6 transgenic mice attenuated heart remodeling and fibrosis
Left ventricular (LV) fibrosis, assessed by Masson trichrome staining 4 weeks after MEF patch or Tri-P implantation in WT mice and AC6 transgenic mice was not different after MEF-only patch implantation (Fig. 3A). However, fibrosis was decreased in both WT and AC6 Tri-P groups compared with the MEF patch group (p < 0.05) (Fig. 3B). Within the Tri-P groups, fibrosis was decreased significantly in AC6 mice compared with WT counterparts (p < 0.05) (Fig. 3B).
Figure 3. Assessment of LV Fibrosis In Vivo.
(A) Heart slices at 4 weeks after cell patch transplantation stained with Masson trichrome. (B) Quantification of the percentage of left ventricular (LV) fibrotic area. WT + MEF-P = wild-type mouse treated with mouse embryonic fibroblast patch after MI; AC6 + MEF-P = AC6 gene transgenic mouse treated with mouse embryonic fibroblast patch after myocardial infarction; Tri-P = tricell patch. *p < 0.05 versus WT + MEF-P; †p < 0.05 versus WT + Tri-P. All values expressed as mean ± SEM; n = 6 for each group.
Tri-P on AC6 mice facilitated iPSC-derived CPC engraftment and angiomyogenesis
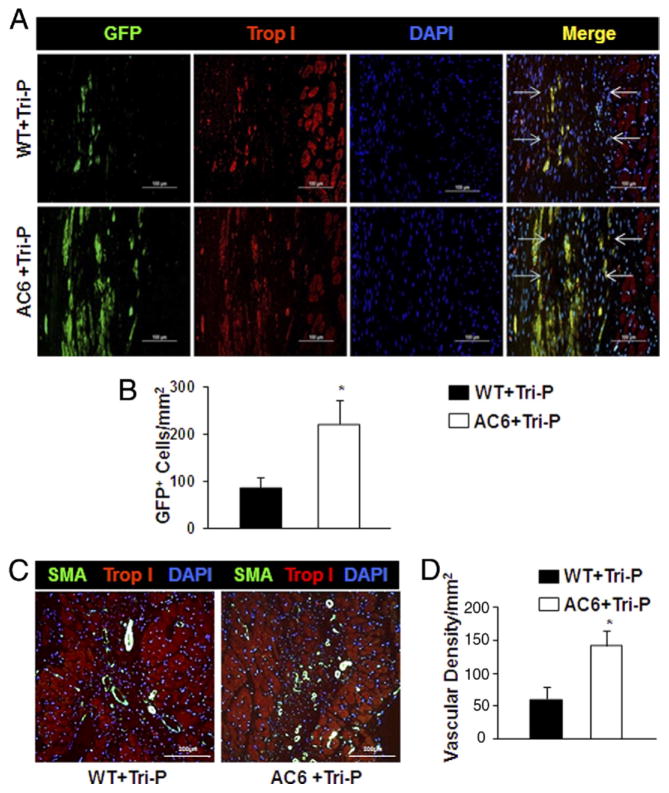
GFP lentiviral construct transduced cells retained uniformly high GFP expression and CM differentiation confirmed by troponin I+ immunostaining, a CM marker, at 4 weeks after Tri-P. These GFP+ cells had migrated from the Tri-P and penetrated the LV infarcted area of both WT and AC6 mice. However, significantly more GFP+ cells were found in AC6 mice compared with WT mice (Fig. 4A), suggesting that reduced collagen synthesis in AC6 mice enhanced CPC engraftment (Fig. 4B). No differences were detected between WT + MEF patch and AC6 + MEF patch groups (data not shown).
Figure 4. Effect of Cardiac AC6 Overexpression on iPSC-Derived CPC Migration and Angiomyogenesis 4 Weeks After Tri-P Implantation.
(A) Immunofluorescence staining showed a cluster of green fluorescent protein positive (GFP+) cells originating from the tricell patch (Tri-P) penetrated into the infarcted area (green fluorescence) and differentiated into cardiomyocytes, which were identified by the cardiac-specific marker troponin I (red). The infarct margins are identified by arrows. (B) The number of GFP+ cells was significantly greater in adenylyl cyclase 6 (AC6) + Tri-P group than WT + Tri-P group. *p < 0.05 versus WT + Tri-P. All values expressed as mean ± SEM; n = 6 for each group. (C) Blood vessel formation was confirmed by α-smooth muscle actin (α-SMA) (green) in AC6 + Tri-P group and WT + Tri-P group. (D) Vascular density (α-SMA staining) in WT and AC6 mice 4 weeks after Tri-P. All values expressed as mean ± SEM. *p < 0.05 versus WT + Tri-P; n = 6 for each group.
An increased number of newly formed blood vessels, identified by α-SMA antibody staining, were observed in AC6 mice compared with WT mice 4 weeks after Tri-P (Figs. 4C and 4D). No differences were observed between WT + MEF patch and AC6 + MEF patch groups (data not shown).
Echocardiographic findings
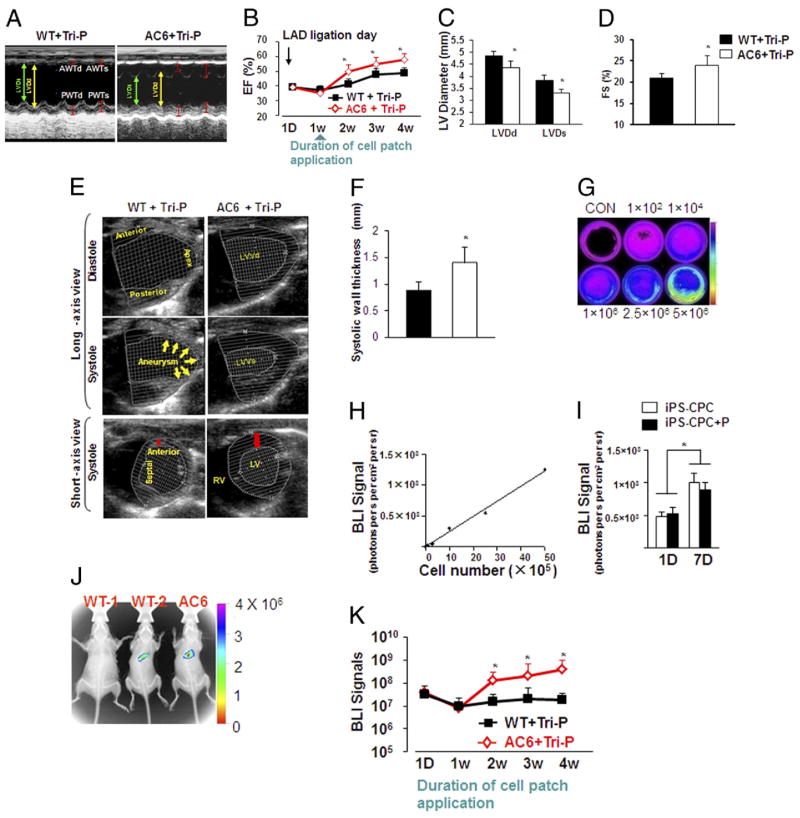
The LV cavity was dilated and the anterior wall thickness was reduced in WT mice hearts at 4 weeks after Tri-P. LV dilation was less in the AC6 mice hearts than in WT mice hearts (Fig. 5A). The ejection fraction (EF) index was similar at day 1 and 1 week after Tri-P, but showed significant improvement only in AC6 mice hearts starting at 2 weeks after Tri-P (Fig. 5B). LV end-diastolic diameter, LV end-systolic diameter, and fractional shortening (FS) improved in AC6 mice compared with WT mice (Figs. 5C and 5D). Anterior wall thinning and apical aneurysmal behavior was documented in WT mice with Tri-P. In contrast, the LV anterior wall thickness and LV performance were significantly improved in AC6 mice 4 weeks after Tri-P (Figs. 5E to 5F). Echocardiographic analysis of all treatment groups was performed to determine whether AC6 affected LV remodeling and function restoration (Online Table 1). No significant differences were detected between WT mice and AC6 mice pre-MI in LV end-diastolic diameter, LV end-systolic diameter, EF, and FS. At 4 weeks post-MI, AC6 mice showed significant increases in EF and FS for MI, MI + MEF patch, and MI + Tri-P but were not significantly different in LV end-diastolic diameter and LV end-systolic diameter (Table 1). in LV end-diastolic diameter, LV end-systolic diameter, EF, and FS were further improved in post-MI AC6 mice after Tri-P compared with no patch or only MEF patch treatment in both AC6 and WT mice 4 weeks after MI. These data support the conclusion that Tri-P treatment in AC6 mice plays an important role in attenuating LV remodeling after MI.
Figure 5. Cardiac Function Assessed by Echocardiography 4 Weeks After Tri-P Implantation.
(A) M-mode echocardiograms in 2 groups: WT + Tri-P and AC6 + Tri-P (see Online Table 1 for all groups). (B) Quantitative data for ejection fraction index (EF) up to 4 weeks in WT and AC6 Tri-P mice (see Online Table 1 for all groups). (C and D) Quantitative data LVDd, LVDs, and FS shown 4 weeks after Tri-P implantation. (E and F) Heart function analysis by long-axis and short-axis views. Short-axis views confirmed the thickness in the anterior wall (red bar) between WT + Tri-P and AC6 + Tri-P. WT + Tri-P and AC6 + Tri-P, WT mouse, and AC6 mouse treated with Tri-P, respectively. White dotted lines indicate endocardium and epicardium. LVDd, left ventricular end-diastolic diameters; All values expressed as mean ± SEM. *p < 0.05 versus WT + Tri-P; n = 6 for each group. (G) Bioluminescent imaging (BLI) in various iPSC-CPC plates. (H) Linear correlation of cell numbers and BLI signals (photons/s/cm2 per steridian). (I) BLI intensity to identify iPSC-CPC proliferation in both cell culture dishes (iPSC-CPC) and seeded isolated peritoneum (iPSC-CPC + P) in vitro at days 1 (1D) and 7 (7D). All values expressed as mean ± SEM. *p < 0.05 versus 1D, 6 for each group. (J) In vivo tracking of differentiated cardiomyocytes derived from iPSC-CPC with BLI and x-ray overlay at 4 weeks after cell patch. treatment. A stronger BLI signal was observed in AC6 mice compared with WT mice. WT-1 = animal heart cell patch without gene transfer as background control; WT-2 and AC6 = WT mouse and AC6 mouse treated with Tri-P, respectively. (K) Serial quantitative analysis of BLI signals up to 4 weeks after Tri-P application. WT + Tri-P and AC6 + Tri-P = WT mouse and AC6 mouse treated with Tri-P, respectively. All values expressed as mean ± SEM. *p < 0.05 versus WT + Tri-P; n = 6 for each group. AC6 = AC6 mouse treated with Tri-P; FS = fractional shortening; LAD = left anterior descending artery; LVDs = left ventricular end-systolic diameter; WT-1 = animal heart cell patch without gene transfer as background control; WT-2 = wild type mouse treated with Tri-P; other abbreviations as in Figures 1 and 2.
Bioluminescent imaging for tracking iPSC-CPC survival and proliferation after Tri-P transplantation
We confirmed the successful construction of a reporter gene (GFP/Fluc) vector in iPSC-CPC overexpressing NCX1/Fluc. Bioluminescent imaging (BLI) signal intensity was measured at day 1 and day 7 to characterize iPSC-CPC proliferation in cell culture dishes and in the cell-seeded isolated peritoneum in vitro. The BLI signal intensity correlated with cell numbers (Fig. 5H). There were no significant differences in cell proliferation between cells in culture dishes and cells seeded in the peritoneum. However, the BLI signal intensity was significantly higher on day 7 than on day 1 in both conditions (Fig. 5I). Noninvasive BLI and X-ray image overlays obtained at day 1 and at 1, 2, 3, and 4 weeks after Tri-P were used to track the transplanting iPSC-CPC in vivo. Mice with nontransduced Tri-P served as negative controls for the background BLI signal (WT-1) (Fig. 5J). BLI signal was detected in both WT and AC6 mice after cell patch implantation (Figs. 5J and 5K). No differences in BLI signal intensity were detected between AC6 and WT mice 1 week after Tri-P. The BLI signal intensity increased progressively from weeks 2 to 4 after Tri-P in AC6 mice but not in WT mice (Figs. 5J and 5K). BLI intensity was low and not different in WT + MEF patch and AC6 + MEF patch (data not shown). These notable in vivo BLI signal data obtained in AC6 overexpressing mice with the Tri-P were confirmed with histologic analysis and supported by echocardiography.
Discussion
Cardiac tissue patch engineering offers an attractive approach to the replacement of dead or damaged myocardium after MI. In a recent study, we reported the use of a cardiac tissue patch to repair MI in rats (9). The patch consisted of genetically modified progenitor cells that had been differentiated in culture on a peritoneal membrane. For a cardiac tissue patch prepared in culture to be a viable therapeutic option for treating MI in humans, it will likely require CMs to restore heart contractility, ECs, and smooth muscle cells for new blood vessels to supply oxygen and nutrients, and stromal cells (e.g., fibroblasts) for ECM support. In addition, it will be necessary to avoid host rejection of the engrafted tissue patch. Recently, iPSCs generated from skin fibroblasts have led to the prospect of using self-specific (autologous) stem cell lines. We selected a natural biomaterial (peritoneum) as the substrate for culture of CPCs derived from homologous iPSCs and fibroblasts as a repair strategy for reverse remodeling of ischemic tissue. To obtain sufficient numbers of CMs and their precursor cells with activated fetal gene programs, iPSCs were enriched using viral vectors encoding markers under the control of the CM-specific promoter NCX1. FACS was used to identify and sort these cell lineages.
Purification of specific cell lineages derived from iPSCs is vitally important for the prevention of teratoma formation. To avoid or minimize teratoma formation, we used lentiviral vectors containing specific cardiac promoters for both GFP and puromycin resistance (Puro). GFP was used for further purification of CMs and precursors by FACS. Continuous CM selection in puromycin (1.5 μg/ml)-enriched media removed non-CMs. Consequently, no teratoma formation was observed in any iPSC-derived progenitor cell Tri-P hearts.
iPSC-based therapy appears to be a promising modality for the repair of ischemic myocardium (10). Current methods of cell delivery (intravascular, direct tissue injection) offer inadequate guidance to the target tissues. To overcome this obstacle, the gene-manipulated Tri-P provides a supportive microenvironment for appositional cell engraftment, migration, and survival.
The major findings of this study are: 1) genetically manipulated iPSCs with cardiac-specific NCX1 promoter significantly enhanced CM differentiation; 2) forskolin increased phosphorylated CREB levels and inhibited both ERK-mediated myofibroblast formation and collagen synthesis; 3) overexpression of AC6 in infarcted myocardium reduced the collagen barrier and enhanced migration and engraftment of Tri-P progenitor cells, which was associated with LV function restoration; 4) MEF in the Tri-P was associated with enhanced new vessel formation, crucial for angiogenesis in ischemic heart disease (our results are consistent with those of Souders et al. [11]); and 5) Tri-P applied in AC6 transgenic mice after MI appears to attenuate LV remodeling as measured by increased LV anterior wall thickness and angiomyogenesis, with decreased LV collagen deposition and tissue fibrosis.
Rapid myofibroblast proliferation and enhanced ECM synthesis after MI are associated with heart failure and arrhythmias (12). In addition, scar formation impairs penetration of reparative stem/progenitor cells into infarcted areas. It is well established that activation of cardiac AC6 expression decreases collagen density and increases function of the failing ischemic heart (13). Particularly, the increase in cAMP production by AC6 overexpression appears to be a negative regulator of fibroblast activation (6). To determine the effect of enhanced cAMP levels on fibroblast activation and collagen synthesis, we treated MEF cells in vitro with forskolin, which is known to increase cAMP mimicking AC6 overexpression. We observed that forskolin increased the phosphorylation of the cellular transcription factor CREB significantly, while decreasing the phosphorylation of ERK, Smad-3, and expression of collagen types I and III. As reported previously, CREB phosphorylation is regulated by cAMP, which catalyzes PKA activation (14). The most commonly used PKA inhibitor, H89, attenuates the favorable effect on fibrosis. Interestingly, H89 alone had no effect on basal activity of CREB or ERK. These results are consistent with the recent report that cAMP-elevating agents inhibit cardiac fibroblasts largely through inhibiting ERK1/2 phosphorylation and the Smad signaling pathway (7). The findings that we report here confirm a significant down-regulation of collagen types I and III in infarcted hearts of mice overexpressing AC6. Taken together, these results implicate an important role for the AC6-cAMP-CREB signaling pathway in collagen synthesis.
To test our hypothesis that collagen density determines iPSC-derived progenitor cell engraftment, BLI was performed together with immunohistochemical analysis. After lentiviral vector transfection, the reporter gene was integrated into the cell’s chromosome and was subsequently found in daughter cells. This enabled us to use the BLI signal as an indicator of cell engraftment or of cell death (15). BLI and x-ray imaging were performed as an overlay 4 weeks after Tri-P transplantation to track the fate of transplanted iPSCs-CPCs. A BLI signal was detected in both AC6 mice and WT mice with MI as early as 24 h after Tri-P implantation. Compared with Tri-P–treated WT mice, iPSCs-CPCs produced stronger BLI signals between weeks 2 and 4 post-Tri-P implantation in AC6 mice. This result is confirmed with immunohistochemical staining. A significantly higher number of GFP+ cells were found in the infarcted area of AC6 mice compared with WT mice. Similarly, the differences in BLI signals and number of GFP+ cells were consistent with improvements in LV function, suggesting that the number of iPSC-derived progenitor cells in the infarcted area may play an important role in the restoration of heart function.
In developing this new approach to myocardial tissue repair, we addressed earlier limitations by selecting an endogenous biocompatible tissue substrate that: 1) serves as an effective reservoir for cell delivery; 2) provides nutrition from outside the adjacent/adherent epicardium (newly formed blood vessel precursors in the cell patch) and angiogenesis within the infarcted areas; 3) dampens systolic aneurysmal bulging over the ischemic area and prevents aneurysmal stretching of peri-ischemic myocardium; 4) strengthens the myocardial wall and reduces wall stress; 5) reduces LV collagen deposition and prevents or reverses further LV remodeling; 6) manipulates genes so that progenitor cells release cyto-protective factors that exert beneficial effects on angiogenesis, progenitor cell survival, and cell migration into the scarred myocardium; and 7) allows progenitor cells to become embedded in the matrix of the underlying infarcted myocardium resulting in a stronger, more adherent bond with the homologous peritoneum patch.
Our approach is based in part on successful tissue engineering techniques used to restore LV function in mouse, rat, rabbit, canine, and pig models (16). Human clinical trials with similar strategies are now beginning to appear (17). When considering clinical application, a suitably sized greater omentum or peritoneal sample can be easily harvested in humans. We acknowledge that other laboratories have reported that a cell patch is not only suitable for treatment of acute MI but is also effective for heart tissue repair or regeneration after long-term LV remodeling (18). Previous studies have reported that mice with cardiac AC6 expression have better post-MI cardiac function than transgenic-negative siblings with identical changes in LV dimension (13). Activation of cardiac AC6 expression increases function of the failing ischemic heart by reducing the rate of apoptosis by about 50% in the border zone and in remote areas of the heart (13). In addition, cardiac AC6 expression also increases Akt activity and phospholamban phosphorylation in cardiac myocytes (19), which potentially plays an important role in cell survival after Tri-P implantation and also in restoration of heart function.
The role of cardiac AC6 expression in heart function was further confirmed by using AC6 deletion mice where deletion of AC6 was associated with reduced LV contractile function due to impaired cardiac cAMP generation and calcium handling (20). We used echocardiography to detect and differentiate the effects of the Tri-P application on LV function in mice overexpressing AC6 on LV function. We observed that LV remodeling was significantly improved after Tri-P treatment of AC6 mice as indicated by a reduction of LV chamber volume, an increase in LV FS, and calculated EF (Fig. 5, Online Table 1). The salutary outcomes include increased LV wall thickness at the infarct region, attenuated LV dilation, and improved LV function indices. We acknowledge that the current surgical procedure for cell patch transplantation is invasive and requires thoracotomy. This issue may reduce enthusiasm for and potential significance of this approach for some prospective users. However, a novel endoscopic device for minimally invasive transplantation of cell patches using video-assisted thoracoscopic surgery is now available and offers a minimally invasive approach as an alternative method to applying cell patches to regions of acute or chronic MI (17).
Conclusions
CPCs derived from iPSCs show significantly increased engraftment associated with angiomyogenesis and improved LV function in AC6 mice that express less collagen in the infarcted myocardium. These results suggest that the density of collagen influences the penetration and engraftment of iPSCs in infarcted myocardium.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health grants HL089824, HL110740, and HL081859 (to Dr. Wang); HL-080686 and R37HL-074272 (to Dr. Ashraf).
The authors thank Christian Paul for technical assistance.
Abbreviations and Acronyms
- AC6
adenylyl cyclase 6
- BLI
bioluminescent imaging
- cAMP
cyclic adenosine monophosphate
- CERB
cyclic adenosine monophosphate response element-binding factor
- CM
cardiomyocyte
- CPC
cardiac progenitor cell
- EB
embryoid body
- EC
endothelial cell
- ECM
extracellular matrix
- ERK
extracellular signal–regulated kinase
- EF
ejection fraction
- FACS
fluorescence-activated cell sorting
- FS
fractional shortening
- GFP
green fluorescent protein
- iPSC
induced pluripotent stem cell
- LV
left ventricular
- MEF
mouse embryonic fibroblast
- MI
myocardial infarction
- Neo-CM
neonatal rat cardiomyocyte
- PKA
protein kinase A
- Tri-P
tricell patch
APPENDIX
For a detailed Methods section and supplemental table, please see the online version of this article.
Footnotes
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Ho CY, Lopez B, Coelho-Filho OR, et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363:552–63. doi: 10.1056/NEJMoa1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diez J, Lopez B, Gonzalez A, Querejeta R. Clinical aspects of hypertensive myocardial fibrosis. Curr Opin Cardiol. 2001;16:328–35. doi: 10.1097/00001573-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Swaney JS, Patel HH, Yokoyama U, et al. Adenylyl cyclase activity and function are decreased in rat cardiac fibroblasts after myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H3216–20. doi: 10.1152/ajpheart.00739.2007. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava D, Ivey KN. Potential of stem-cell-based therapies for heart disease. Nature. 2006;441:1097–9. doi: 10.1038/nature04961. [DOI] [PubMed] [Google Scholar]
- 5.Singla DK. Stem cells in the infarcted heart. J Cardiovasc Transl Res. 2010;3:73–8. doi: 10.1007/s12265-009-9151-4. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Ostrom RS, Insel PA. cAMP-elevating agents and adenylyl cyclase overexpression promote an antifibrotic phenotype in pulmonary fibroblasts. Am J Physiol Cell Physiol. 2004;286:C1089–99. doi: 10.1152/ajpcell.00461.2003. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Sun SQ, Hassid A, Ostrom RS. cAMP inhibits transforming growth factor-beta-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and Smad signaling in cardiac fibroblasts. Mol Pharmacol. 2006;70:1992–2003. doi: 10.1124/mol.106.028951. [DOI] [PubMed] [Google Scholar]
- 8.Tang T, Gao MH, Lai NC, et al. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation. 2008;117:61–9. doi: 10.1161/CIRCULATIONAHA.107.730069. [DOI] [PubMed] [Google Scholar]
- 9.Huang W, Zhang D, Millard RW, et al. Gene manipulated peritoneal cell patch repairs infarcted myocardium. J Mol Cell Cardiol. 2010;48:702–12. doi: 10.1016/j.yjmcc.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–16. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–76. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 13.Lai NC, Tang T, Gao MH, et al. Activation of cardiac adenylyl cyclase expression increases function of the failing ischemic heart in mice. J Am Coll Cardiol. 2008;51:1490–7. doi: 10.1016/j.jacc.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funaki C, Hodges RR, Dartt DA. Identification of the Raf-1 signaling pathway used by cAMP to inhibit p42/p44 MAPK in rat lacrimal gland acini: role in potentiation of protein secretion. Invest Ophthalmol Vis Sci. 2010;51:6321–8. doi: 10.1167/iovs.10-5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun N, Lee A, Wu JC. Long term non-invasive imaging of embryonic stem cells using reporter genes. Nat Protoc. 2009;4:1192–201. doi: 10.1038/nprot.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao SY, Siu CW, Liu Y, et al. Attenuation of left ventricular adverse remodeling with epicardial patching after myocardial infarction. J Card Fail. 2010;16:590–8. doi: 10.1016/j.cardfail.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Maeda M, Yamato M, Kanzaki M, Iseki H, Okano T. Thoracoscopic cell sheet transplantation with a novel device. J Tissue Eng Regen Med. 2009;3:255–9. doi: 10.1002/term.161. [DOI] [PubMed] [Google Scholar]
- 18.Chen CH, Wei HJ, Lin WW, et al. Porous tissue grafts sandwiched with multilayered mesenchymal stromal cell sheets induce tissue regeneration for cardiac repair. Cardiovasc Res. 2008;80:88–95. doi: 10.1093/cvr/cvn149. [DOI] [PubMed] [Google Scholar]
- 19.Gao MH, Tang T, Guo T, et al. Adenylyl cyclase type VI increases Akt activity and phospholamban phosphorylation in cardiac myocytes. J Biol Chem. 2008;283:33527–35. doi: 10.1074/jbc.M805825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang T, Lai NC, Hammond HK, et al. Adenylyl cyclase 6 deletion reduces left ventricular hypertrophy, dilation, dysfunction, and fibrosis in pressure-overloaded female mice. J Am Coll Cardiol. 2010;55:1476–86. doi: 10.1016/j.jacc.2009.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.