Abstract
The PDGF family members are potent mitogens for cells of mesenchymal origin and serve as important regulators of cell migration, survival, apoptosis, and transformation. Tumor-derived PDGF ligands are thought to function in both autocrine and paracrine manners, activating receptors on tumor and surrounding stromal cells. PDGF-C and -D are secreted as latent dimers, unlike PDGF-A and -B. Cleavage of the CUB domain from the PDGF-C and -D dimers is required for their biological activity. At present, little is known about the proteolytic processing of PDGF-C, the rate-limiting step in the regulation of PDGF-C activity. Here we show that the breast carcinoma cell line, MCF7, engineered to overexpress PDGF-C, produces proteases capable of cleaving PDGF-C to its active form. Increased PDGF-C expression enhances cell proliferation, anchorage independent cell growth, and tumor cell motility by autocrine signaling. In addition, MCF7-produced PDGF-C induces fibroblast cell migration in a paracrine manner. Interestingly, PDGF-C enhances tumor cell invasion in the presence of fibroblast, suggesting a role of tumor-derived PDGF-C in tumor-stromal interactions. In the present study, we identify tissue plasminogen activator (tPA) and matriptase as major proteases for processing of PDGF-C in MCF7 cells. In in vitro studies, we also show that urokinase plasminogen activator (uPA) is able to process PDGF-C. Furthermore, by site-directed mutagenesis, we identify the cleavage site for these proteases in PDGF-C. Lastly, we provide evidence suggesting a 2-step proteolytic processing of PDGF-C involving creation of a hemidimer, followed by growth factor domain dimer (GFD-D) generation.
Keywords: PDGF-C, Breast Cancer, Serine Proteases, proteolytic processing, hemidimer
INTRODUCTION
Platelet-derived growth factor (PDGF) regulates a diverse array of cellular processes such as cell proliferation and motility under normal physiological conditions as well as during the pathogenesis of a number of human diseases [1–5]. Immunohistochemical analysis of human breast cancer tissues showed localized, membranous PDGF receptor expression/activation in periepithelial stromal cell populations, suggesting a paracrine stimulation of adjacent stromal tissue by breast tumor cells [6]. Moreover, a recent study of invasive ductal breast carcinomas demonstrated an association between PDGF receptor alpha (α-PDGFR) staining and lymph node metastasis [7]. In experimental models of breast cancer, PDGF initiates a human breast carcinoma desmoplastic response via paracrine signaling [8, 9]. PDGF autocrine signaling was shown to be essential for tumor formation and metastasis in Ras-mediated mammary tumors as well as in MMTV-Neu/TGF-β transgenic mice [10]. Importantly, our recent tissue microarray analysis of 216 patients with invasive breast cancer showed that increased PDGF-C expression correlates with lymph node metastases, increased Ki-67 proliferation staining, and lower rates of 7-yr disease-free survival (Meng et al, manuscript under review). Furthermore, a role for PDGF-C in malignancy was suggested by the observation that Ewing sarcoma cell lines showed increased expression and secretion of PDGF-C [11, 12]. When a dominant negative PDGF-C construct was expressed in these cell lines, they showed a reduction of anchorage independent growth, but not a full reversion of the phenotype [11, 12]. PDGF-C autocrine signaling has also been suggested for the initiation and progression of brain tumors such as glioblastoma and medulloblastoma [13, 14]. However, little is known about the role of PDGF-C in breast cancer at the present.
While the classical PDGF ligands A and B are secreted as active heterodimer or homodimers, newly identified PDGF ligands C and D are secreted as latent homodimers containing an N-terminal CUB domain and a C-terminal growth factor domain (GFD) [15, 16]. Previous reports demonstrated that plasmin and a component of fetal bovine serum were capable of processing latent PDGF-C into the growth factor domain, and identified the PDGF-C cleavage site to be between K225/A226 by N-terminal sequencing of GFD of PDGF-C isolated from BHK-570 cells [15, 17]. A later study reported that PDGF-C is a substrate of tissue plasminogen activator (tPA) in in vitro biochemical assays, and R231 in the hinge region of PDGF-C is essential for its cleavage by tPA [18]. Proteolytically activated PDGF-C stimulates its cognate receptor, α-PDGFR, but can also activate β-PDGFR via αβ-PDGFR heterodimerization [15, 17].
The goals of the present study are to identify breast carcinoma-produced proteases responsible for extracellular proteolytic cleavage of PDGF-C, a key step to initiate PDGF-C/PDGFR signaling, and to investigate the potential oncogenic activities of PDGF-C in breast cancer. Here we identified tissue plasminogen activator (tPA), urokinase plasminogen activator (uPA), and matriptase as potential activators of PDGF-C in breast cancer. We show that the full-length PDGF-C (FL-PDGF-C) dimer undergoes proteolytic cleavage in a two-step process, creating a hemidimer containing one chain of FL-PDGF-C monomer and one chain of GFD-PDGF-C monomer followed by GFD dimer (GFD-D). Interestingly, while K225 is the putative proteolytic cleavage site, LLGK (aa 222–225) motif appears to be critical for the first cleavage for the generation of hemidimer; both the LLGK and RKSR (aa 231–234) motifs in the hinge region between the CUB and GFD domains of PDGF-C are essential for the second cleavage for the generation of the GFD dimer. Importantly, increased PDGF-C expression in MCF7 cells increased cell proliferation, anchorage independent cell growth, and tumor cell motility, demonstrating a potential oncogenic activity of PDGF-C in breast cancer. Importantly, we also provide evidence that PDGF-C potentiates tumor cell invasion through paracrine signaling in fibroblasts.
MATERIALS AND METHODS
Cell Culture and Reagents
Cell lines used in this manuscript were purchased from American Type Culture Collection (ATCC) and maintained as recommended. MCF7 human breast carcinoma cells, the resultant MCF7 transfectant cell lines, and murine NIH3T3 fibroblasts were cultured in a humidified 5% CO2 incubator with DMEM/F12 medium supplemented with 10% bovine serum. BSC-1 and CV-1 green monkey kidney cells were cultured in DMEM supplemented with 10% fetal bovine serum. All cell lines were supplemented with 100units/ml penicillin, 100units/ml streptomycin, 2mM glutamine, and fungizone. The proteases tPA and uPA and the protease inhibitors PAI-1, TAPI, and PDX-Portland were purchased from EMD Biosciences (San Diego, CA). HAI-1 was obtained from R&D Systems (Minneapolis, MN). Aprotinin and Leupeptin were purchased from Sigma-Aldrich (St. Louis, MO).
Construction of Viral PDGF-C Expression Vectors
A reverse transcription-PCR approach was taken to clone PDGF-C into a vaccinia expression vector. Total RNA was isolated from the prostate cancer cell lines DU145 and PC3 using Trizol reagent and used in cDNA synthesis reactions using SuperScript RT-III (Invitrogen, Carlsbad, CA). The resultant cDNAs were then used in PCR reactions that yielded a 1071-bp product containing the 1035-bp ORF of PDGF-C encoding FL-PDGF-C. Additionally, this product contained the restriction sites for NcoI and BamHI at the 5’ and 3’ ends, respectively, of the PDGF-C ORF. Further, to the carboxyl-terminus of the PDGF-C product we added a 6X HIS epitope tag (forward, 5’-catgccatggggagcctcttcgggcttctc-3’; reverse 5’-cgggatccctaatggtgatggtgatgatgtcctcctgtgctccctct-3’; the 6X HIS tag is underlined). This product was then digested with NcoI and BamHI and inserted into the NcoI/BamHI site of the vaccinia virus expression vector pTF7-ECM1 (a kind gift from Dr. R. Fridman, Wayne State University). Fidelity of the in-frame sequence encoding the PDGF-C FL: HIS fusion protein was confirmed by DNA sequencing (Elim Biopharmaceuticals, Inc., Hayward, CA). This plasmid is referred to as pTF7-PDGF-C FL: HIS. The pTF7-PDGF-C FL: HIS construct was then used as a template in site-directed mutagenesis to create point mutants. Mutations were made at lysine 225 to alanine (K225A), arginines 231 and 234 to alanine (R231/R234A), and lysine 225, arginines 231 and 234 to alanine (K225A/R231A/R234A). Following the manufacturer’s protocol for the Quikchange Site-directed Mutagenesis Kit (Stratagene, Valencia, CA), primers were designed to create a single mutant at K225A (forward 5’-ccaacttggcaacttcttggcgcggcttttgtttttgg-3’; reverse 5’-ccaaaaacaaaagccgcgccaagaagttgccaagttgg-3’) and a double mutant at R231A/234A (forward 5’-ggcttttgtttttggagcaaaatccgcagtggtggatctg-3’; reverse 5’-cagatccaccactgcggattttgctccaaaaacaaaagcc-3’). After confirmation of sequencing, the pTF7-PDGF-C FL: HIS R231A/234A construct was used as a template for creation of a triple mutant at K225A/R231A/R234A using the same primers that generated the K225A mutant. The sequencing of this construct was then confirmed.
Production of Recombinant PDGF-C (rPDGF-C) Protein
Established vaccinia virus protocols [19] were followed to generate rPDGF-C ligand. Briefly, CV-1 cells were first infected with the recombinant vaccinia virus vTF7-3, which expresses the T7 RNA polymerase. After 30 min. of infection, the cells were washed with PBS and transfected with the plasmid pTF7-PDGF-C FL: HIS, using Effectene reagent (Qiagen, Valencia, CA). Expression of the FL-PDGF-C protein inserted into the pTF7 plasmid is reliant on infection of the cells by vTF7-3. Cell-host machinery then transcribes the gene of interest. Forty-eight hours after co-infection/transfection with vaccinia virus and pTF-7 PDGF-C FL: HIS, the serum-free conditioned media (CM) was collected and cleared of cellular debris by a 5 minute centrifugation at 2000 × g. The resultant CM containing FL-PDGF-C protein was used in subsequent PDGF-C processing experiments.
Construction of a Mammalian PDGF-C Expression Vector
To insert FL-PDGF-C into the mammalian expression vector pcDNA3.1 (+) (Invitrogen), a double restriction enzyme digest was performed. The wild-type PDGF-C FL: HIS sequence was first cut from pIND-PDGF-C FL: HIS with the enzymes AflII and BamHI. The resulting product was then gel purified and ligated into the pcDNA3.1 (+) plasmid. This plasmid is referred to as pcDNA3.1-PDGF-C FL: HIS.
Establishment of a FL-PDGF-C-Overexpressing MCF7 Breast Carcinoma Cell Line
Human breast carcinoma MCF7 cells were transfected with pcDNA3.1-PDGF-C FL: HIS and pcDNA3.1 (+) empty vector using Effectene reagent and then selected using 400µg/ml Geneticin (G418) in complete culture medium for 12 days. The resulting resistant cells were pooled together and are referred to as MCF7-PDGF-C and MCF7-Neo, respectively. Overexpression of FL-PDGF-C was confirmed by reverse transcription-PCR as follows: total RNA was extracted from cells using Trizol Reagent (Invitrogen). Five µg of total RNA from each cell line was used to synthesize cDNA using SuperScript RT-III (Invitrogen). Resultant cDNA was then used as a template in a PCR reaction, using Taq Polymerase (Promega, Valencia, CA) and the following primers: forward, 5’-tccagcaacaaggaacagaa-3’ and reverse, 5’-gggtcttcaagcccaaatct-3’. These primers amplify a 200-bp product that represents part of the CUB domain in the FL-PDGF-C protein. This primer pair does not allow for discrimination of endogenously and exogenously expressed FL-PDGF-C. Glyceradehyde-3-phosphate dehydrogenase (GAPDH) expression was used as a positive control with the following primers: forward, 5’-atcaccatcttccaggagcga-3’ and reverse, 5’-gccagtgagcttcccgttca-3’.
Custom Antibody Raised Against the PDGF-C Growth Factor Domain
An antibody was raised against the PDGF-C protein using synthetic peptide (N’-CGRKSRVVDLNLLTEEVRLYSC-C’) representing a portion of the PDGF-C Growth Factor Domain (GFD) (amino acids 230–250). The resultant antibody was affinity purified (Zymed Biomedical, So. San Francisco, CA) and is referred to as anti-PDGF-C GFD Ab.
PDGF-C-mediated Paracrine Activation of α-PDGFR
MCF7-PDGF-C and MCF7-Neo cells were cultured in serum-free media for 48h. The resultant CM was collected and centrifuged for 5 min. at 2000 rpm to remove cellular debris. Serum-starved NIH3T3 cells were treated with this CM from the MCF7 transfectants for 10 min. Lysates were collected using RIPA lysis buffer [0.5% sodium deoxycholate, 1% NP-40, 50mM Tris (pH=7.6), 2mM EGTA, 2mM EDTA, 150mM NaCl, 2mM sodium orthovanadate, 1mM phenylmethylsulfonyl fluoride, 1mM sodium fluoride, and protein inhibitor cocktail tablet (Roche)]. The lysates were centrifuged for 20 min. at 4°C, 12000 × g to remove debris, and the supernatant was collected. Total protein concentrations were determined using BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL). The lysates (500µg) were then used for immunoprecipitation with an α-PDGFR antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and protein G-agarose beads (Pierce Biotechnology). Immunoprecipitates were washed three times with RIPA lysis buffer and resolved by reducing SDS-PAGE. Tyrosine-phosphorylated α-PDGFR was detected by immunoblot using a phosphotyrosine antibody (Upstate Biotechnology, Lake Placid, NY). Total levels of α-PDGFR were detected using the same antibody used for immunoprecipitation. Activation assays were repeated three independent times.
Inhibition Experiments with MCF7-PDGF-C Cells
MCF7-PDGF-C cells were seeded in 6-well plates, washed three times with PBS, and incubated in the presence of serum-free medium with the protease inhibitors Leupeptin, Aprotinin, E64, E64c, TAPI, PAI-1, HAI-1, a tPA blocking antibody, or a uPA blocking antibody for 48h. After incubation, the resultant CM was collected and analyzed by SDS-PAGE under reducing conditions, immunoblotted, and probed with anti-PDGF-C GFD Ab. Inhibitory analysis was observed in three separate experimental runs.
Ni-NTA Concentration of rPDGF-C
rPDGF-C expressed by vaccinia virus contains a 6X-HIS tag at the C-terminal end of FL-PDGF-C. Therefore, a Ni-affinity protocol was used to immunoprecipitate the FL-PDGF-C and/or GFD forms. Briefly, 10X binding buffer (500mM sodium phosphate, 10mM imidazole, and 0.5% Tween-20) was added to CM collected from vaccinia virus co-infected/transfected FL-PDGF-C expressing cells in the presence of a 50% Ni-nitrilotriacetic acid (NTA)-agarose bead slurry (Qiagen). The media and beads were then rocked overnight at 4°C, after which time the beads are spun down by centrifugation and washed twice in wash buffer (1X binding buffer, 300mM NaCl). The rPDGF-C was then eluted from the beads by incubating them overnight at 4°C in the presence of 10mM EDTA. This PDGF-C eluate was then aliquoted and stored at −80°C until needed for subsequent experiments.
In vitro Cleavage of rPDGF-C by tPA, uPA, or Matriptase
Latent rPDGF-C was incubated with various concentrations of human tPA (50mM Tris-HCl pH=7.5, 50mM NaCl) or human uPA (50mM Tris-HCl pH=8.8, 50mM NaCl, 0.1% PEG-4000) for 16–18h at 37°C. For matriptase experiments, latent rPDGF-C was incubated for 2h with varying concentrations of the matriptase catalytic domain (kind gift of Dr. C-Y Lin, University of Maryland) at 37°C (50mM Tris-HCl pH 7.5, 100mM NaCl). After incubation, the products were analyzed by SDS-PAGE under reducing or non-reducing conditions and immunoblotted with anti-PDGF-C GFD Ab. PDGF-C protease cleavage experiments were verified in three independent experiments.
Plasminogen-casein or Casein Zymography
MCF7-PDGF-C and -Neo CM were resolved on an 11% plasminogen-casein SDS-PAGE for 1h at 35mA on ice. After electrophoresis, the gel was washed with 2.5% Triton-X100 twice, before incubation overnight at 37°C in 0.1M glycine pH 8.0. The following day the gel was stained for 2 hours at room temperature with gentle agitation in 0.1% amido black. After the incubation, the gel was destained in 30% MeOH, 10% acetic acid. Finally, the gel was placed in a softening solution of 5% glycerol/5% acetic acid for 30 minutes before drying overnight. The gel was subsequently imaged on a Microtek Scanmaker i900 using Photoshop Elements software. Zymographic analysis was repeated three different times.
Scratch Migration Assay
NIH3T3 fibroblasts were seeded in a 6-well plate and allowed to attach overnight at 37°C. The next day, these cells were washed once with PBS and incubated for 30 minutes at 37°C with 1.0ml SF DMEM/F12 media + 25µg/ml mitomycin C. After incubation, the media was aspirated, and an injury line was scraped with a 1–200µl yellow pipette tip. The cells were carefully washed once with PBS. Next, 2.0ml of either MCF7-PDGF-C or –Neo CM was added to each of 3 wells. The cells were then placed in the incubator at 37°C for 24 hours. Pictures were taken of the cells every 8 hours to monitor closure of the gap. Using NIH ImageJ, closure was assessed as a percentage of cleared area remaining at time 0, 8, and 16 hrs. Migration assays were repeated three independent times.
Soft Agar Analysis
MCF7-PDGF-C and -Neo cells were embedded in a 0.35% Bacto agarose (BD, Franklin Lakes, NJ) gel at 5,000 cells/mL and laid a 0.6% agarose bottom layer. Experiments were performed in triplicates. Cells were allowed to grow for 2 weeks, then stained with 4% Geimsa (Riedel-de Haën, Germany). Colonies ranging from 200–500 µm in size were counted using the Optronix GelCount (Oxford, England). Soft agar analysis was observed in three separate experimental runs.
WST-1 Proliferation Assay
MCF7-PDGF-C and -Neo cells were plated in a 96-well plate at 2,000 cells per well. Experiments were carried out in sets of 6 wells per cell line. Cells were then treated with the WST-1 (Roche, Indianapolis, IN) reagent per the manufacturer’s recommendation for 3 hours then read on a Benchmark microplate reader (Bio-Rad, Hercules, CA ) at 450nm. PDGF-C mediated cell proliferation was verified in three independent experiments.
Matrigel Cell Invasion
MCF7-PDGF-C and -Neo cells were plated in serum-free media at a density of 3.75×105 cells/mL into Matrigel coated transwells (BD, Franklin Lakes, NJ). Transwells were then placed in 24-well containing either serum free media or a confluent layer of serum-starved NIH3T3 cells (co-culture). Invasion was permitted to occur over 48 hours, then transwells were cleaned and stained with 0.9% Crystal Violet. The number of invading cells was counted in 5 different high-powered fields using an inverted Nikon TMS microscope (Melville, NY). Cell invasion analysis was repeated three different times.
RESULTS
MCF7 cells process PDGF-C into its active growth factor domain
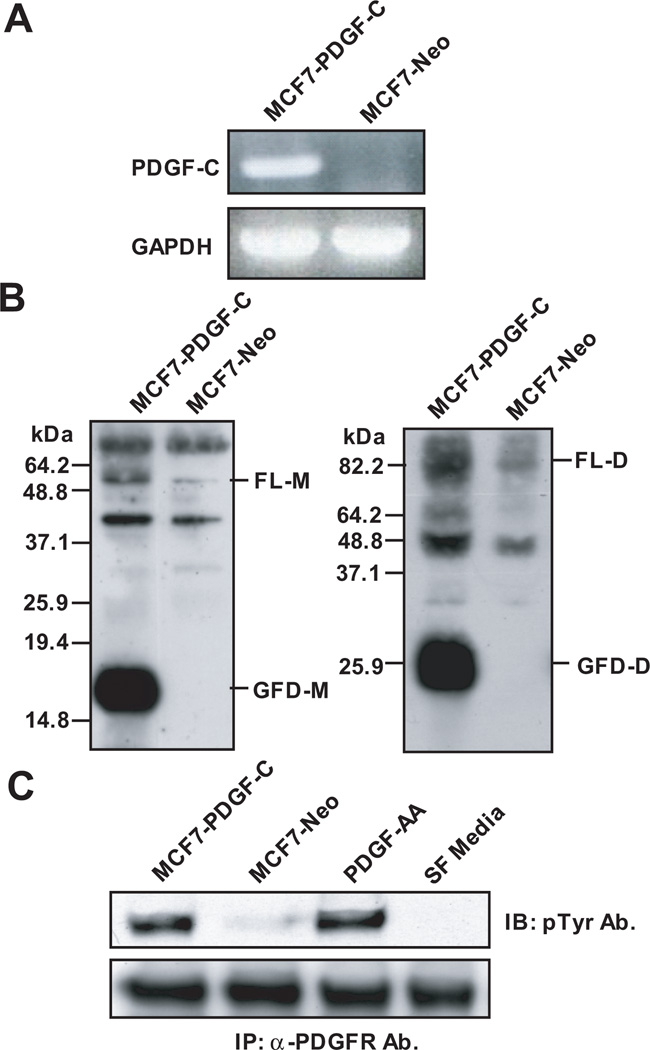
To examine whether breast cancer cells contain the protease(s) responsible for PDGF-C activation and to examine the role of PDGF-C in breast cancer progression, we established MCF7 cells engineered to express PDGF-C (MCF7-PDGF-C) or a control vector (MCF7-Neo) as described in “Materials and Methods.” PDGF-C overexpression in MCF7-PDGF-C cells was confirmed by RT-PCR analysis (Fig. 1A). Importantly, immunoblot analysis in a reducing condition using conditioned media (CM) collected from these cells in serum-free media detected both the full length monomer (FL-M) of PDGF-C with a molecular weight (Mr) of ~48kDa as well as the processed growth factor domain monomer (GFD-M) of PDGF-C with Mr ~17kDa (Fig. 1B, left panel). Consistently, immunoblot analysis in a non-reducing condition confirmed FL dimer (FL-D) of PDGF-C (~85 kDa) and GFD dimer (GFD-D) of PDGF-C (~26 kDa) (Fig. 1B, right panel). These results demonstrate that breast cancer cells express proteases capable of processing PDGF-C independent of a component in serum, which was previously reported to be a major enzyme to process PDGF-C to its active form [17]. Importantly, we found that breast carcinoma-processed GFD-PDGF-C dimer was biologically active for induction of α-PDGFR phosphorylation, as demonstrated by a receptor activation assay in NIH3T3 fibroblasts (Fig. 1C).
Figure 1. Expression and processing of platelet-derived growth factor-C (PDGF-C) in MCF7.
A. PDGF-C levels in MCF7-PDGF-C (Lane 1) and -Neo (Lane 2) were examined by RT-PCR. B. Serum-free (SF) conditioned media (CM) collected from MCF7-PDGF C or -Neo cells were analyzed by immunoblot analysis using anti-PDGF-C growth factor domain Ab in reducing (left panel) and non-reducing (right panel) conditions. FL-M, full-length monomer; GFD-M, growth factor domain monomer; FL-D, full-length dimer; GFD-D, growth factor domain dimer. C. Serum-starved NIH3T3 fibroblasts were stimulated for 10 min with CM collected from MCF7-PDGF-C (Lane 1) or MCF7-Neo (Lane 2) cells, SF media with 20ng/ml PDGF-AA (Lane 3, positive control), or SF media (Lane 4, negative control). Cell lysates were immunoprecipitated using anti-PDGF-C Ab and activated α-PDGFR was detected by immunoblot using a pTyr Ab.
Tumor-derived PDGF-C mediates both paracrine and autocrine signaling
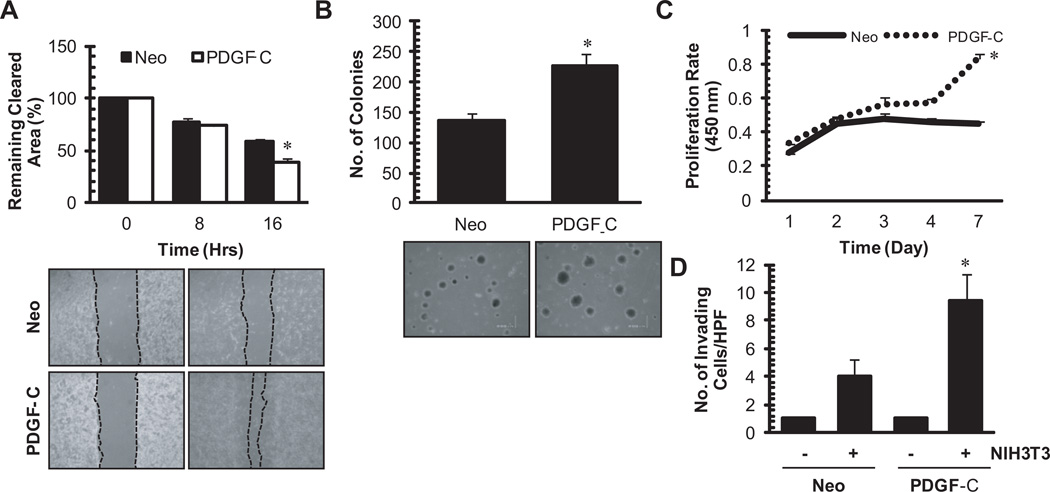
Since PDGF was shown to induce a desmoplastic response in breast cancer [9], we sought to determine whether tumor-derived PDGF-C induces fibroblast cell migration. To this end, a scratch migration assay was performed, whereby NIH3T3 fibroblasts treated with CM collected from MCF7-PDGF-C cells demonstrated an increased ability to migrate compared to Neo-treated control (Figure 2A). Next we examined the autocrine effects of tumor-derived PDGF-C on induction of transformed phenotype and tumor cell growth. As shown in Figure 2B and C, increased PDGF-C expression resulted in an increase in anchorage-independent cell growth and an increased cell proliferation as assessed by a soft agar assay and a WST-1 proliferation assay, respectively. Lastly, to assess the effect of PDGF-C on tumor cell invasion, matrigel invasion assays were performed. MCF7 cells were barely invasive in a matrigel invasion assay regardless of PDGF-C expression (Figure 2D). However, when MCF7-PDGF-C and MCF7-Neo cells were seeded on top of transwell filters coated with growth factor reduced-matrigel in the presence of NIH3T3 fibroblasts in the lower chamber, MCF7-PDGF-C cells were more invasive than the Neo control. This suggests that fibroblasts respond to tumor-derived PDGF-C, resulting in secretion of factors that potentiate the invasive phenotype in tumor cells. Taken together, these results suggest the potential oncogenic activities of PDGF-C in breast tumor growth, its mediation of tumor-stromal interactions, and tumor cell invasion via both autocrine and paracrine manners.
Figure 2. In vitro transformative properties of PDGF-C in MCF7 cells.
A (paracrine signaling). A scratch migration assay of NIH3T3 was performed in the presence of conditioned media collected from MCF7-neo or –PDGF-C cells. Using NIH ImageJ, closure of the gap was quantified as a percentage of cleared area remaining at time 0, 8, and 16 from three independent experiments (top panel). Representative 40X images of time 0 and 16 hours are displayed (bottom panel). B and C (autocrine signaling). Anchorage-independent growth and proliferation of MCF7-neo or –PDGF-C cells were assessed by a soft agar colony formation assay (B) and WST-1 cell proliferation assay (C), respectively. Positive colonies were quantified from three separate experiments using Optronix GelCount (Oxford, England) in panel B and cell proliferation was quantified from three independent WST-1 assays in panel C. D (autocrine and double paracrine signaling). Matrigel invasion assay of MCF7-neo or –PDGF-C cells through a modified Boyden chamber was performed in the absence or presence of NIH3T3 fibroblasts in the bottom chamber. Quantitation is averaged results of three separate experiments. *p<0.05.
A serine protease processes FL-PDGF-C in MCF7 cells
The above results indicate that the MCF7 cell line provided a good model to elucidate which protease(s) were capable of activating this growth factor in the context of breast carcinoma cells. To identify the class of protease responsible for the proteolytic processing of FL-PDGF-C, MCF7 cells were cultured in serum-free conditions in the presence of several class-specific protease inhibitors. As shown in Figure 3A, aprotinin, a serine protease inhibitor, effectively inhibited FL-PDGF-C processing in these cells. Unlike aprotinin, the cysteine protease inhibitor leupeptin, the cathepsin inhibitors E64 and E64c, the matrix metalloproteinase (MMP) inhibitor TAPI, and the furin inhibitor PDX-Portland showed no significant inhibition of FL-PDGF-C processing, indicating a specific role of serine proteases in the activation of FL-PDGF-C in breast carcinoma. This finding is consistent with a previous report that the serine protease, tPA, is responsible for the processing of PDGF-C in human fibroblasts [20].
Figure 3. PDGF-C is processed by serine proteases, specifically tPA and uPA.
A. MCF7-PDGF-C cells were incubated in SF media with the various class specific inhibitors for 48h; the collected CM was resolved under reducing SDS-PAGE and immunoblotted with an anti-PDGF-C GFD Ab. B. Left panel, MCF7-PDGF-C cells were incubated with SF media without or with PAI-1. Right panel, MCF7-PDGF-C (Lanes 1 and 3) or MCF7-neo (Lanes 2 and 4) cells were incubated with SF media containing tPA or uPA specific inhibitors for 48h; the collected CM was resolved under reducing SDS-PAGE and immunoblotted with an anti-PDGF-C GFD Ab. C. MCF7-PDGF-C (Lane 3) and MCF7-Neo (Lane 4) CM was run in a plasminogen-dependent zymogram with r-tPA (Lane 1) and r-uPA (Lane 2) serving as positive activity controls. D. rPDGF-C was generated by co-infecting/transfecting CV-1 cells with vaccinia virus and the pTF7-PDGF-C: HIS construct. After 48h of serum-starvation the CM was collected. This CM was concentrated using Ni-NTA agarose beads overnight. After washing of the beads, rPDGF-C was eluted with 10mM EDTA then rPDGF-C was incubated with tPA or uPA overnight. Finally, the resultant products were resolved by SDS-PAGE and immunoblotted with an anti-PDGF-C GFD Ab. E. rPDGF-C was incubated with tPA, uPA, streptokinase, and streptokinase+glu-plasminogen overnight, and the resultant products were resolved by SDS-PAGE and immunoblotted with an anti-PDGF-C GFD Ab.
To determine whether the plasminogen activator system of proteases plays a role in FL-PDGF-C processing in MCF7 cells, conditioned media was collected from MCF7-PDGF-C cells in the presence of the tPA/uPA specific inhibitor, PAI-1, or in the presence of blocking antibodies against tPA and uPA. As shown in Figure 3B, PAI-1 effectively, although not completely, inhibited proteolytic cleavage of FL-PDGF-C into GFD-PDGF-C. Similarly, tPA blocking antibodies and to a lesser degree uPA blocking antibodies were able to inhibit the processing of PDGF-C. The tPA or uPA activities in CM from MCF7-PDGF-C and MCF7-Neo were confirmed by plasminogen-casein zymography, as shown in Figure 3C. These findings suggest that MCF7-produced tPA and, to a lesser extent, uPA activate PDGF-C.
Both tPA and uPA are able to process PDGF-C in vitro
To determine if recombinant tPA and/or uPA can directly process PDGF-C, we first generated recombinant PDGF-C proteins (rPDGF-C) using the vaccinia virus system, followed by purification and concentration using Ni-NTA agarose beads. When rPDGF-C was incubated with increasing concentrations of tPA or uPA overnight at 37°C, both tPA and uPA were able to process rPDGF-C into the GFD-PDGF-C (Fig. 3D), suggesting PDGF-C is a substrate for tPA and uPA. However, if plasminogen contamination during purification of rPDGF-C exists, uPA/tPA-mediated rPDGF-C processing may be indirect, as tPA and uPA are potent activators of plasminogen to plasmin, an enzyme known to cleave PDGF-C [15, 21]. To address this, rPDGF-C was incubated with 100nM streptokinase, a bacterial plasminogen activator. Streptokinase activates plasminogen in a non-proteolytic manner and streptokinase itself has no proteolytic activity of its own [22]. As shown in Figure 2E, in the presence of streptokinase, there was no processing of PDGF-C to its growth domain form, indicating no significant amount of plasminogen to be activated for the processing of PDGF-C in our experimental condition. As a positive control to ensure that streptokinase is functional, rPDGF-C was incubated with streptokinase and 0.3nM plasminogen. As shown in Figure 3E (lane 5), streptokinase-activated plasminogen completely processed rPDGF-C into a smaller size of GFD-PDGF-C. These results demonstrate that both tPA and uPA are able to directly process PDGF-C to its GFD form in vitro, independent of plasmin.
Identification of tPA/uPA cleavage sites within the hinge domain of PDGF-C
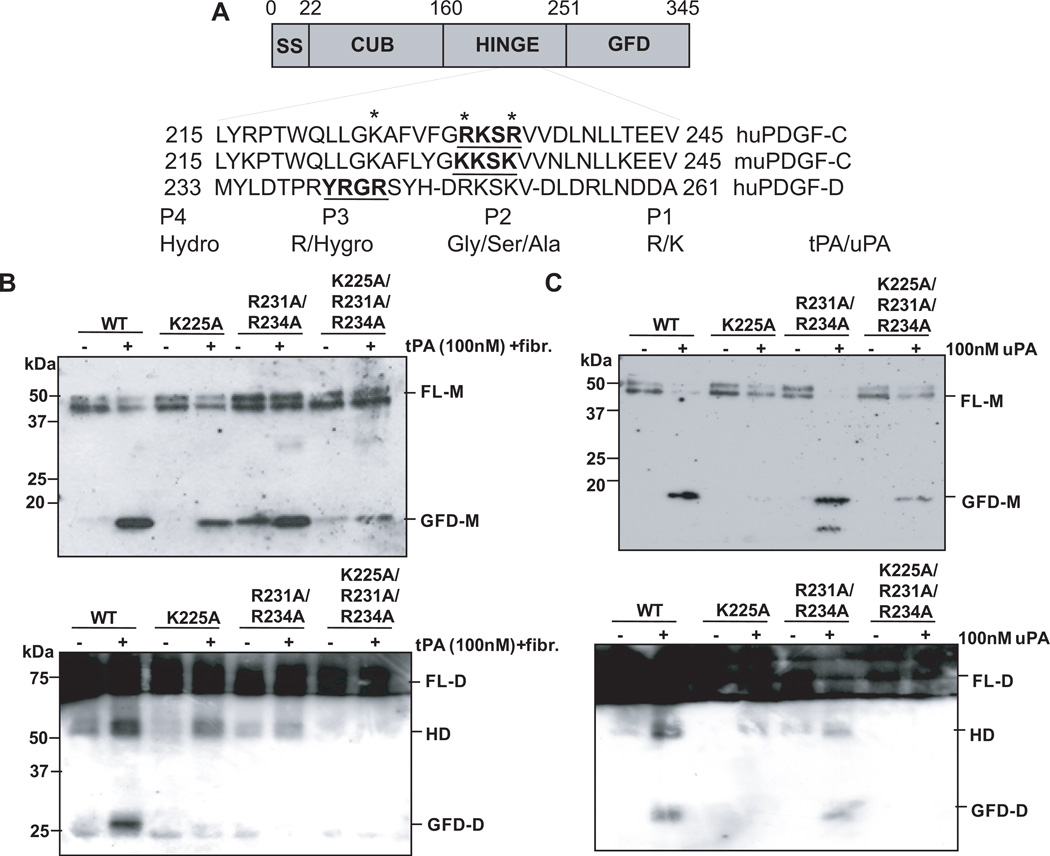
Gilbertson, et.al. predicted the serum-sensitive cleavage site of PDGF-C to be RKSR (amino acid residues 231–234), but identified the PDGF-C cleavage site to be between K225/A226 by N-terminal sequencing of the GFD of PDGF-C isolated from BHK-570 cells [17]. A later study reported through mutagenic analysis that R231 in the hinge region of PDGF-C is essential for cleavage by tPA [18]. Taking these results together with our amino acid sequence analysis, we focused on two putative sites, LLGK (aa 222–225) and RKSR (aa 231–234), for uPA and/or tPA-mediated proteolytic cleavage of PDGF-C, as depicted in Figure 4A. By site-directed mutagenesis and the vaccinia virus expression system, rPDGF-C mutants with lysine 225 mutated to alanine (K225A), arginines 231 and 234 to alanines (R231A/R234A), and lysine 225 and arginines 231 and 234 to alanines (K225A/R231A/R234A) were generated. It should be noted that since our study regarding tPA processing of PDGF-C parallels two studies that have been performed by Fredriksson et al. [18, 20], we followed the protocols contained within these studies for comparison of tPA processing of PDGF-C. The greatest inhibition of tPA-mediated proteolytic cleavage was observed in the K225A/R231A/R234A mutant, while the R231A/R234A mutant showed the least inhibition of processing by tPA when the cleavage products were analyzed in a reducing condition (Fig. 4B, top panel). Interestingly, when these samples were analyzed under the non-reducing condition, GFD dimer was generated from WT rPDGF-C proteins only (Fig. 4B bottom panel, lane 2). With the K225A or R231A/R234A mutants, generation of GFD dimer was almost completely inhibited with a noticeable accumulation of a product around Mr~50kD (Fig. 4B bottom panel, lanes 4 and 6). This product has been termed a hemidimer, containing one FL-PDGF-C monomer and one GFD-PDGF-C monomer [20]. When the uPA cleavage site was examined in PDGF-C, K225 in the LLGK motif appears to be more critical than the arginine residues (R231 and R234) in the RKSR motif (Fig. 4C, top panel). Similarly to the tPA-mediated PDGF-C processing, GFD dimer formation was drastically inhibited in these mutants (Fig.4C, bottom panel). These results suggest that K225 in the LLGK motif within the hinge region between the CUB and GFD is critical for tPA- and uPA-mediated first cleavage of PDGF-C for the generation of the hemidimer. However, both LLGK and RKSR motifs are important for the second cleavage for the generation of the GFD-PDGF-C dimer.
Figure 4. Mutational analysis identifies tPA and uPA cleavage sites.
A. Comparison of sequence alignments between human PDGF-C, murine PDGF-C, and human PDGF-D. Mutagenesis sites are marked with asterisks and the tPA/uPA substrate specificity in the P1–P4 residues are shown. B. Western blot analysis of PDGF-C wild-type (WT), PDGF-C K225A, PDGF-C R231A/234A, and PDGF-C K225A/R231A/R234A mutants under reducing (left panel) and non-reducing (right panel) conditions when incubated with 100nM of tPA 4h at 37°C in the presence of fibrinogen fragments. C. Western blot analysis of PDGF-C WT, PDGF-C K225A, PDGF-C R231A/R234A, and PDGF-C K225A/R231A/R234A under reducing (left panel) and non-reducing (right panel) conditions when incubated with 100nM of uPA overnight at 37°C.
Matriptase is able to process PDGF-C in vitro
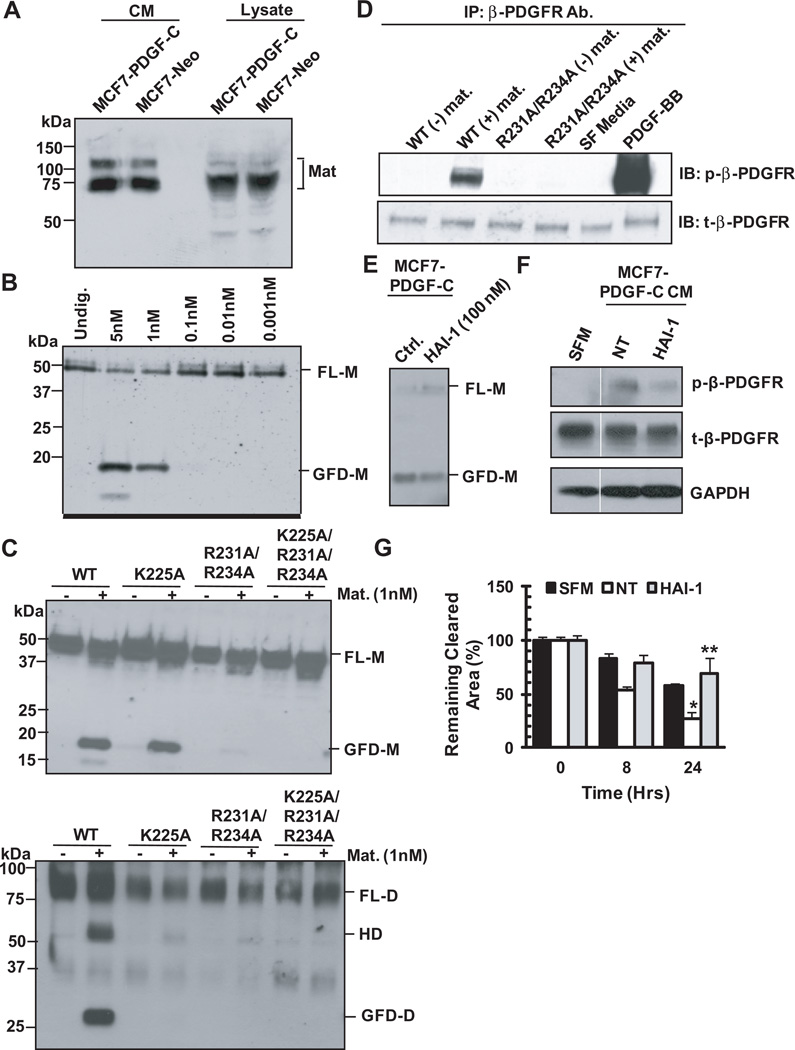
Incomplete inhibition of PDGF-C processing in the presence of the tPA/uPA inhibitor, PAI, in MCF7 cells (Fig. 3B) suggested the presence of additional serine proteases with the capacity to process PDGF-C. Interestingly, the serine protease matriptase was shown to have substrate specificity for the amino acid motif RXSR by phage display [23]. Since this putative matriptase cleavage site was found in the RKSR motif in the hinge region of PDGF-C, we sought to investigate if matriptase can process PDGF-C. First, we confirmed matriptase expression in MCF7 cells by immunoblot analysis, as shown in Figure 5A. When rPDGF-C was incubated with varying concentrations of the purified catalytic domain of matriptase for 2 hours, FL-PDGF-C was processed into GFD-PDGF-C by 1-5 nM matriptase, as shown in Figure 5B. To assess if the cleavage site for matriptase was the same as for tPA and uPA, rPDGF-C R231A/R234, K225A, and K225/R231A/R234A mutants were also incubated with 1nM matriptase for 2 hours at 37°C. Matriptase-treated rPDGF-C samples were then analyzed by immunoblot analysis under reducing and non-reducing conditions. Similarly to uPA and tPA, K225 was critical for matriptase cleavage of FL-PDGF-C into GFD-PDGF-C as detected under the reducing condition (Fig. 5C, top panel). However, none of these PDGF-C mutant dimers underwent matriptase-mediated proteolytic cleavage in both chains for the generation of the GFD dimer as analyzed under the non-reducing condition (Fig. 5C, bottom panel), suggesting that both LLGK and GRSR motifs are required for matriptase generation of GFD-PDGF-C dimer.
Figure 5. Matriptase is capable of processing PDGF-C.
A. Western blot analysis of MCF7-PDGF-C and -Neo CM and lysates for matriptase expression. Blot was probed with anti-matriptase Ab (kind gift of Dr. C-Y Lin, University of Maryland) under the non-reducing condition. B. rPDGF-C WT incubated with varying concentrations of matriptase 2h at 37°C and then resolved on SDS-PAGE under reducing conditions C. rPDGF-C WT and mutants were incubated with 1nM matriptase for 2h at 37°C and then resolved on SDS-PAGE under reducing conditions (top panel) or non-reducing conditions (bottom panel). D. rPDGF-C WT or R231A/R234A were incubated 2h at 37°C with 1nM matriptase before being suspended in SF DMEM/F12 media and then added to NIH3T3 cells for 10 min. Subsequently, the lysates from these cells were analyzed by western blot for the presence of phospho-β-PDGFR. Total β-PDGFR was used as a loading control. MCF7-PDGF-C cells were treated with the matriptase inhibitor, HAI-1, and PDGF-C processing (E) and biological activity (F) were monitored. In the same gel, an unnecessary lane separating SFM and NT treatment was removed in panel E. A white line is drawn to demarcate this change. G. Conditioned media from (E) were utilized to assess NIH3T3 cell migration. * and ** p<0.05.
To determine whether the GFD dimer and/or hemidimer generated by matriptase have biological activity, we performed the following experiment. WT rPDGF-C and R231A/R234A mutant were incubated with 1nM matriptase for 2 hours at 37°C. The processed PDGF-C was then suspended in serum-free media and utilized for the treatment of serum-starved NIH3T3 cells for 10 minutes, followed by immunoblot analysis using anti-phospho-β-PDGFR Ab. β-PDGFR activation in NIH3T3 cells was chosen as readout for PDGF-C activity for the following reasons. NIH3T3 cells express a high level of β-PDGFR and PDGF-C was shown to activate β-PDGFR via αβ-PDGFR heterodimerization [17]. As shown in Figure 5D, WT rPDGF-C processed by matriptase was biologically active and induced phosphorylation of PDGFR, whereas the hemidimer or GFD monomer generated by matriptase cleavage of R231A/R234A PDGF-C mutant exhibited no biological activity.
To determine whether MCF7-produced matriptase is critical for PDGF-C processing and signaling, MCF7-PDGF-C cells were treated with HAI-1, an endogenous inhibitor of matriptase. Generation of the GFD of PDGF-C was reduced, accompanied with accumulation of FL-PDGF-C (Fig. 5E), supporting a role for matriptase in the proteolytic processing of PDGF-C in breast carcinoma. Next, we examined the biological consequence of reduced PDGF-C processing on its ability to activate PDGFR and induce cell motility in a paracrine manner. To this end, serum-starved fibroblasts were treated with conditioned media (CM) collected from MCF7-PDGF-C cells in the absence or presence of HAI-I and analyzed for the status of β–PDGFR activation and for their motility. As shown in Figure 5F, β–PDGFR activation was less apparent by treatment with CM from HAI-1 treated cells compared to the non-treated cells. Accordingly, NIH3T3 cell migration was greatly inhibited following treatment with CM from HAI-1 treated MCF7-PDGF-C cells compared to the control (Fig. 5G). Taken together, these results demonstrated a critical role for matriptase in the activation of PDGF-C signaling.
DISCUSSION
The present study demonstrated that both tPA and uPA can process latent FL-PDGF-C into the active form, whereas our previous study found that uPA, but not tPA, is capable of processing latent PDGF-D into the active form [24]. tPA and uPA belong to the chymotrypsin family of serine proteases, and share a high degree of structural similarity and primary physiological substrates such as plasminogen [25–27]. Unlike plasmin, they display highly restricted substrate specificity and play unique roles under the physiological and pathological conditions. It is thought that tPA is involved in vascular fibrinolysis, while uPA has been implicated in the immune response, tissue remodeling, angiogenesis, cancer growth, and metastasis [25, 28]. Since tPA and uPA have differing physiologic roles yet are similar structurally and exhibit a high degree of substrate specificity, several studies have been performed to understand their basis for recognizing substrate sequences [23, 29–33]. These studies utilized substrate phage display, phage substrate subtraction library, and positional scanning-synthetic combinatorial library (PS-SCL) techniques to determine specificity. Briefly, substrate phage display identifies the most labile substrates, and substrate subtraction identifies the most selective substrates for a given enzyme, while PS-SCL allows a rapid and minimally difficult technique for the determination of proteolytic substrate specificity. Both tPA and uPA prefer arginine over lysine in the P1 position; however, uPA has less preference for arginine than for lysine, indicating that uPA is a less specific protease than tPA [32]. Both enzymes show similar specificity for Ser/Gly/Ala at the P2 position [23, 33]. Further, the P3 and P4 residues have been shown to be the primary determinants in substrate recognition between tPA and uPA with a P3 arginine and P4 large hydrophobic residue being the most selective for tPA over uPA [30]. In our mutagenesis assays, PDGF-C processing still occurs with R231A mutation. This may result from an introduction of a new cleavage site as a result of mutation. Indeed, the R231A mutation introduces a potential cleavage site at K232 with Ala in the P2 site (R231A) [33]. However, although these studies provide insights into the ability of tPA and uPA to cleave certain peptide sequences, it was suggested that the P1–P4 peptide sequences alone are not enough for recognition and cleavage in vivo; there is also a requirement for protease-protein interactions at nearby or distant sites [29]. Consistent with the notion that uPA is a less specific protease than tPA, our previous [24] and present studies found that tPA can cleave PDGF-C only, while uPA can process both PDGF-C and -D.
A previous report by Fredriksson, et al. has demonstrated that tPA processing of PDGF-C requires R231 in the RKSR (aa 231–234) motif in the hinge region to mediate proteolytic activation of PDGF-C [18]. This conclusion was reached by site-directed mutagenesis of PDGF-C followed by in vitro cleavage assay with tPA and a PDGF receptor activation assay. While this conclusion is valid, our study provided an alternative K225 processing site and further insight into PDGF-C processing by tPA as well uPA and matriptase. Our results indicate that K225 in the LLGK (aa 222–225) motif is more critical for the first cleavage generation of the PDGF-C hemidimer. In line with this finding, the K225 site was the cleavage site of PDGF-C sequenced by Gilbertson et al. in BHK-570 cells [17]. More importantly, our study showed that both the LLGK and RKSR motifs are essential for generation of the biologically active GFD-PDGF-C dimer. These results suggest differences in the structure of the hinge regions of the two PDGF-C chains for the recognition/access of serine proteases to latent PDGF-C for proteolytic activation. Our results also provide important information that the presence of GFD-PDGF-C detected under reducing conditions may not be a good predictor for the biological activity of PDGF-C.
The present study also identified matriptase as a potential activator of PDGF-C in breast cancer. Matriptase was first identified as the major gelatinase in hormone-dependent human breast cancer cells [34], and since its initial discovery, matriptase has been cloned by several laboratories and has shown a restricted epithelial expression profile in both human and murine tissues [35–40]. At present, only a handful of substrates have been identified, including proteinase-activated receptor-2 (PAR-2), single chain uPA, the pro-form of MT-SP1, and hepatocyte growth factor (HGF) [23, 41]. Importantly, increasing evidence suggested a critical role for matriptase in human cancers, as it is highly expressed in carcinomas of the head and neck, mesothelium, breast, ovary, cervix, prostate, lung, and gastrointestinal cancers [42–45]. Dysregulated matriptase activity is thought to directly affect the extracellular microenvironment during cancer progression through altering processing of matrix components, could act through downstream effector molecules such as growth factors and receptors, chemokines, or protease zymogens, leading to their activation or deactivation. Our finding suggests the possibility of matriptase’s oncogenic activity involving activation of PDGF-C signaling.
The relative biological significance of tPA, uPA, and matriptase for the regulation of PDGF-C activity in vivo is likely to depend on the bioavailability of these enzymes in a given microenvironment. The expression and role of the plasminogen system has been extensively studied during tumor progression and showed increased expression/activity of uPA and tPA in several tumor types including breast cancer, gliomas, non-small cell lung cancer, pancreatic cancer, prostate cancer [46–50]. In a recent study of breast cancer patient tissue samples, uPA and uPAR expression was correlated with increasing tumor stage and in breast cancer bone metastases by in situ hybridization analyses [51]. However, a role for tPA in breast cancer is unclear. One study showed that tPA expression in breast cancer is not associated with the malignant or benign state [52]. Another report states that low intratumoral tPA expression levels correlate with aggressiveness and poor prognosis for breast cancer patients [53]. Irrespective of these reports, increased PDGF-C expression and serine protease-mediated proteolytic activation of PDGF-C in human breast carcinoma MCF7 cells resulted in fibroblast migration in a paracrine manner as well as increased MCF7 cell proliferation and anchorage independent growth in an autocrine manner. In addition, serine protease-activated PDGF-C signaling induced an invasive phenotype in MCF7 cells in the presence of fibroblasts. These results suggest the potential for regulatory crosstalk between extracellular serine proteases and PDGF signaling as being critical for carcinoma growth as well as for tumor-stromal interactions leading to tumor invasion.
Furthermore, we utilized the Oncomine database (Compendium Biosciences), which is a compendium of published cDNA arrays from over 100 institutions that allows for gene comparison across multiple array studies, in an effort to obtain information as to which serine protease(s) is (are) likely to be available for PDGF-C activation during breast cancer progression. Using this tool, we found that among the PDGF family members, PDGF-C and its cognate receptor α-PDGFR were more likely to be upregulated during breast cancer progression. Interestingly, while only one out of 19 studies reported an increase in tPA expression in breast carcinoma, almost all studies found increases in matriptase and uPA in breast cancer tissues (Supplementary Table 1). Lastly, our laboratory (Meng et al., manuscript under review) demonstrated in a tissue microarray analysis of 216 patients with invasive breast cancer that increased PDGF-C expression correlates with lymph node metastasis, increased Ki-67 proliferation staining, and lower rates of 7-yr disease free survival, providing compelling evidence for PDGF-C in human breast cancer progression. Taken together, matriptase and uPA are more likely than tPA to be the relevant enzymes for PDGF-C activation, contributing to breast cancer progression.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH/National Cancer Institute RO1 Grants CA064139 and CA123362 (to H-R. C. K.), as well as the Ruth L. Kirschstein National Research Service Award T32-CA009531 (to N.H). We thank Dr. Shijie Sheng for thoughtful discussions and Dr. M. Katie Conley-LaComb with her assistance in the preparation of this manuscript and the Translational Research Core Facility of the Karmanos Cancer Institute for their assistance with analysis of soft agar colony assay.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
Conceived and designed the experiments: NJH, AJN, CVU HRCK. Performed the experiments: NJH, AJN, LSM. Analyzed the data: NJH, AJN, LSM. Wrote the paper: NJH, AJN, CVU, HRCK.
REFERENCES
- 1.Deuel TF. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3:443–492. doi: 10.1146/annurev.cb.03.110187.002303. [DOI] [PubMed] [Google Scholar]
- 2.Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46:155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- 3.Rosenkranz S, Kazlauskas A. Evidence for distinct signaling properties and biological responses induced by the PDGF receptor alpha and beta subtypes. Growth Factors. 1999;16:201–216. doi: 10.3109/08977199909002130. [DOI] [PubMed] [Google Scholar]
- 4.Anderberg C, Li H, Fredriksson L, Andrae J, Betsholtz C, Li X, Eriksson U, Pietras K. Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts. Cancer Res. 2009;69:369–378. doi: 10.1158/0008-5472.CAN-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, Kaminker J, Ferrara N. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Coltrera MD, Wang J, Porter PL, Gown AM. Expression of platelet-derived growth factor B-chain and the platelet-derived growth factor receptor beta subunit in human breast tissue and breast carcinoma. Cancer Res. 1995;55:2703–2708. [PubMed] [Google Scholar]
- 7.Carvalho I, Milanezi F, Martins A, Reis RM, Schmitt F. Overexpression of platelet-derived growth factor receptor alpha in breast cancer is associated with tumour progression. Breast Cancer Res. 2005;7:R788–R795. doi: 10.1186/bcr1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker RA. The complexities of breast cancer desmoplasia. Breast Cancer Res. 2001;3:143–145. doi: 10.1186/bcr287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao ZM, Nguyen M, Barsky SH. Human breast carcinoma desmoplasia is PDGF initiated. Oncogene. 2000;19:4337–4345. doi: 10.1038/sj.onc.1203785. [DOI] [PubMed] [Google Scholar]
- 10.Jechlinger M, Sommer A, Moriggl R, Seither P, Kraut N, Capodiecci P, Donovan M, Cordon-Cardo C, Beug H, Grunert S. Autocrine PDGFR signaling promotes mammary cancer metastasis. J Clin Invest. 2006;116:1561–1570. doi: 10.1172/JCI24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zwerner JP, May WA. PDGF-C is an EWS/FLI induced transforming growth factor in Ewing family tumors. Oncogene. 2001;20:626–633. doi: 10.1038/sj.onc.1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zwerner JP, May WA. Dominant negative PDGF-C inhibits growth of Ewing family tumor cell lines. Oncogene. 2002;21:3847–3854. doi: 10.1038/sj.onc.1205486. [DOI] [PubMed] [Google Scholar]
- 13.Lokker NA, Sullivan CM, Hollenbach SJ, Israel MA, Giese NA. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res. 2002;62:3729–3735. [PubMed] [Google Scholar]
- 14.Andrae J, Molander C, Smits A, Funa K, Nister M. Platelet-derived growth factor-B and -C and active alpha-receptors in medulloblastoma cells. Biochem Biophys Res Commun. 2002;296:604–611. doi: 10.1016/s0006-291x(02)00917-8. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellstrom M, Bostrom H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A, Eriksson U. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2:302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- 16.Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, Alitalo K, Eriksson U. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol. 2001;3:512–516. doi: 10.1038/35074588. [DOI] [PubMed] [Google Scholar]
- 17.Gilbertson DG, Duff ME, West JW, Kelly JD, Sheppard PO, Hofstrand PD, Gao Z, Shoemaker K, Bukowski TR, Moore M, Feldhaus AL, Humes JM, Palmer TE, Hart CE. Platelet-derived growth factor C (PDGF-C), a novel growth factor that binds to PDGF alpha and beta receptor. J Biol Chem. 2001;276:27406–27414. doi: 10.1074/jbc.M101056200. [DOI] [PubMed] [Google Scholar]
- 18.Fredriksson L, Ehnman M, Fieber C, Eriksson U. Structural requirements for activation of latent platelet-derived growth factor CC by tissue plasminogen activator. J Biol Chem. 2005;280:26856–26862. doi: 10.1074/jbc.M503388200. [DOI] [PubMed] [Google Scholar]
- 19.Fuerst TR, Earl PL, Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987;7:2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredriksson L, Li H, Fieber C, Li X, Eriksson U. Tissue plasminogen activator is a potent activator of PDGF-CC. Embo J. 2004;23:3793–3802. doi: 10.1038/sj.emboj.7600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei H, Velez G, Hovland P, Hirose T, Kazlauskas A. Plasmin is the major protease responsible for processing PDGF-C in the vitreous of patients with proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2008;49:42–48. doi: 10.1167/iovs.07-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunamneni A, Abdelghani TT, Ellaiah P. Streptokinase--the drug of choice for thrombolytic therapy. J Thromb Thrombolysis. 2007;23:9–23. doi: 10.1007/s11239-006-9011-x. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem. 2000;275:26333–26342. doi: 10.1074/jbc.M002941200. [DOI] [PubMed] [Google Scholar]
- 24.Ustach CV, Kim HR. Platelet-derived growth factor D is activated by urokinase plasminogen activator in prostate carcinoma cells. Mol Cell Biol. 2005;25:6279–6288. doi: 10.1128/MCB.25.14.6279-6288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collen D, Lijnen HR. Basic and clinical aspects of fibrinolysis and thrombolysis. Blood. 1991;78:3114–3124. [PubMed] [Google Scholar]
- 26.Lamba D, Bauer M, Huber R, Fischer S, Rudolph R, Kohnert U, Bode W. The 2.3 A crystal structure of the catalytic domain of recombinant two-chain human tissue-type plasminogen activator. J Mol Biol. 1996;258:117–135. doi: 10.1006/jmbi.1996.0238. [DOI] [PubMed] [Google Scholar]
- 27.Spraggon G, Phillips C, Nowak UK, Ponting CP, Saunders D, Dobson CM, Stuart DI, Jones EY. The crystal structure of the catalytic domain of human urokinase-type plasminogen activator. Structure. 1995;3:681–691. doi: 10.1016/s0969-2126(01)00203-9. [DOI] [PubMed] [Google Scholar]
- 28.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 29.Coombs GS, Dang AT, Madison EL, Corey DR. Distinct mechanisms contribute to stringent substrate specificity of tissue-type plasminogen activator. J Biol Chem. 1996;271:4461–4467. doi: 10.1074/jbc.271.8.4461. [DOI] [PubMed] [Google Scholar]
- 30.Ke SH, Coombs GS, Tachias K, Navre M, Corey DR, Madison EL. Distinguishing the specificities of closely related proteases. Role of P3 in substrate and inhibitor discrimination between tissue-type plasminogen activator and urokinase. J Biol Chem. 1997;272:16603–16609. doi: 10.1074/jbc.272.26.16603. [DOI] [PubMed] [Google Scholar]
- 31.Ke SH, Coombs GS, Tachias K, Corey DR, Madison EL. Optimal subsite occupancy and design of a selective inhibitor of urokinase. J Biol Chem. 1997;272:20456–20462. doi: 10.1074/jbc.272.33.20456. [DOI] [PubMed] [Google Scholar]
- 32.Rijken DC, Groeneveld E. Substrate specificity of tissue-type and urokinase-type plasminogen activators. Biochem Biophys Res Commun. 1991;174:432–438. doi: 10.1016/0006-291x(91)91434-e. [DOI] [PubMed] [Google Scholar]
- 33.Harris JL, Backes BJ, Leonetti F, Mahrus S, Ellman JA, Craik CS. Rapid and general profiling of protease specificity by using combinatorial fluorogenic substrate libraries. Proc Natl Acad Sci U S A. 2000;97:7754–7759. doi: 10.1073/pnas.140132697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi YE, Torri J, Yieh L, Wellstein A, Lippman ME, Dickson RB. Identification and characterization of a novel matrix-degrading protease from hormone-dependent human breast cancer cells. Cancer Res. 1993;53:1409–1415. [PubMed] [Google Scholar]
- 35.Lin CY, Anders J, Johnson M, Sang QA, Dickson RB. Molecular cloning of cDNA for matriptase, a matrix-degrading serine protease with trypsin-like activity. J Biol Chem. 1999;274:18231–18236. doi: 10.1074/jbc.274.26.18231. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi T, Shuman MA, Craik CS. Reverse biochemistry: use of macromolecular protease inhibitors to dissect complex biological processes and identify a membrane-type serine protease in epithelial cancer and normal tissue. Proc Natl Acad Sci U S A. 1999;96:11054–11061. doi: 10.1073/pnas.96.20.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim MG, Chen C, Lyu MS, Cho EG, Park D, Kozak C, Schwartz RH. Cloning and chromosomal mapping of a gene isolated from thymic stromal cells encoding a new mouse type II membrane serine protease, epithin, containing four LDL receptor modules and two CUB domains. Immunogenetics. 1999;49:420–428. doi: 10.1007/s002510050515. [DOI] [PubMed] [Google Scholar]
- 38.List K, Szabo R, Molinolo A, Nielsen BS, Bugge TH. Delineation of matriptase protein expression by enzymatic gene trapping suggests diverging roles in barrier function, hair formation, and squamous cell carcinogenesis. Am J Pathol. 2006;168:1513–1525. doi: 10.2353/ajpath.2006.051071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oberst MD, Singh B, Ozdemirli M, Dickson RB, Johnson MD, Lin CY. Characterization of matriptase expression in normal human tissues. J Histochem Cytochem. 2003;51:1017–1025. doi: 10.1177/002215540305100805. [DOI] [PubMed] [Google Scholar]
- 40.Tanimoto H, Underwood LJ, Wang Y, Shigemasa K, Parmley TH, O'Brien TJ. Ovarian tumor cells express a transmembrane serine protease: a potential candidate for early diagnosis and therapeutic intervention. Tumour Biol. 2001;22:104–114. doi: 10.1159/000050604. [DOI] [PubMed] [Google Scholar]
- 41.Lee SL, Dickson RB, Lin CY. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem. 2000;275:36720–36725. doi: 10.1074/jbc.M007802200. [DOI] [PubMed] [Google Scholar]
- 42.Oberst M, Anders J, Xie B, Singh B, Ossandon M, Johnson M, Dickson RB, Lin CY. Matriptase and HAI-1 are expressed by normal and malignant epithelial cells in vitro and in vivo. Am J Pathol. 2001;158:1301–1311. doi: 10.1016/S0002-9440(10)64081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riddick AC, Shukla CJ, Pennington CJ, Bass R, Nuttall RK, Hogan A, Sethia KK, Ellis V, Collins AT, Maitland NJ, Ball RY, Edwards DR. Identification of degradome components associated with prostate cancer progression by expression analysis of human prostatic tissues. Br J Cancer. 2005;92:2171–2180. doi: 10.1038/sj.bjc.6602630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng L, Cao J, Zhang X. Expression of serine protease SNC19/matriptase and its inhibitor hepatocyte growth factor activator inhibitor type 1 in normal and malignant tissues of gastrointestinal tract. World J Gastroenterol. 2005;11:6202–6207. doi: 10.3748/wjg.v11.i39.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanimoto H, Shigemasa K, Tian X, Gu L, Beard JB, Sawasaki T, O'Brien TJ. Transmembrane serine protease TADG-15 (ST14/Matriptase/MT-SP1): expression and prognostic value in ovarian cancer. Br J Cancer. 2005;92:278–283. doi: 10.1038/sj.bjc.6602320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chernicky CL, Yi L, Tan H, Ilan J. Tissue-type plasminogen activator is upregulated in metastatic breast cancer cells exposed to insulin-like growth factor-I. Clin Breast Cancer. 2005;6:340–348. doi: 10.3816/CBC.2005.n.038. [DOI] [PubMed] [Google Scholar]
- 47.Goh KY, Poon WS, Chan DT, Ip CP. Tissue plasminogen activator expression in meningiomas and glioblastomas. Clin Neurol Neurosurg. 2005;107:296–300. doi: 10.1016/j.clineuro.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Laurell H, Bouisson M, Berthelemy P, Rochaix P, Dejean S, Besse P, Susini C, Pradayrol L, Vaysse N, Buscail L. Identification of biomarkers of human pancreatic adenocarcinomas by expression profiling and validation with gene expression analysis in endoscopic ultrasound-guided fine needle aspiration samples. World J Gastroenterol. 2006;12:3344–3351. doi: 10.3748/wjg.v12.i21.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Offersen BV, Pfeiffer P, Andreasen P, Overgaard J. Urokinase plasminogen activator and plasminogen activator inhibitor type-1 in nonsmall-cell lung cancer: relation to prognosis and angiogenesis. Lung Cancer. 2007;56:43–50. doi: 10.1016/j.lungcan.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 50.Pulukuri SM, Estes N, Patel J, Rao JS. Demethylation-linked activation of urokinase plasminogen activator is involved in progression of prostate cancer. Cancer Res. 2007;67:930–939. doi: 10.1158/0008-5472.CAN-06-2892. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Fisher JL, Field CL, Zhou H, Harris TL, Henderson MA, Choong PF. Urokinase plasminogen activator system gene expression is increased in human breast carcinoma and its bone metastases--a comparison of normal breast tissue, non-invasive and invasive carcinoma and osseous metastases. Breast Cancer Res Treat. 2000;61:1–12. doi: 10.1007/s10549-004-6659-9. [DOI] [PubMed] [Google Scholar]
- 52.Jankun J, Merrick HW, Goldblatt PJ. Expression and localization of elements of the plasminogen activation system in benign breast disease and breast cancers. J Cell Biochem. 1993;53:135–144. doi: 10.1002/jcb.240530206. [DOI] [PubMed] [Google Scholar]
- 53.Corte MD, Verez P, Rodriguez JC, Roibas A, Dominguez ML, Lamelas ML, Vazquez J, Garcia Muniz JL, Allende MT, Gonzalez LO, Fueyo A, Vizoso F. Tissue-type plasminogen activator (tPA) in breast cancer: relationship with clinicopathological parameters and prognostic significance. Breast Cancer Res Treat. 2005;90:33–40. doi: 10.1007/s10549-004-2624-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.