Abstract
Although radon gas is a known cause of lung cancer, the association between residential radon and mortality from non-malignant respiratory disease has not been well characterised.
The Cancer Prevention Study-II is a large prospective cohort study of nearly 1.2 million Americans recruited in 1982. Mean county-level residential radon concentrations were linked to study participants' residential address based on their ZIP code at enrolment (mean±sd 53.5±38.0 Bq·m−3). Cox proportional hazards regression models were used to estimate adjusted hazard ratios (HR) and 95% confidence intervals (CI) for non-malignant respiratory disease mortality associated with radon concentrations. After necessary exclusions, a total of 811,961 participants in 2,754 counties were included in the analysis.
Throughout 2006, there were a total of 28,300 non-malignant respiratory disease deaths. Radon was significantly associated with chronic obstructive pulmonary disease (COPD) mortality (HR per 100 Bq·m−3 1.13, 95% CI 1.05–1.21). There was a significant positive linear trend in COPD mortality with increasing categories of radon concentrations (p<0.05).
Findings suggest residential radon may increase COPD mortality. Further research is needed to confirm this finding and to better understand possible complex inter-relationships between radon, COPD and lung cancer.
Keywords: Chronic obstructive pulmonary disease, cohort study, radon, USA
In 1988, the International Agency for Research on Cancer designated radon as a known cause of lung cancer, based on studies of underground miners exposed to high levels of the gas prior to the adequate ventilation of mines [1]. Radon further decays into a series of radon daughters, some of which emit α-particles capable of damaging cellular DNA [2]. Radon, formed during the radioactive decay of uranium-238, is present in air, soil and water. Upon release from the Earth's crust, radon enters homes through cracks in the foundations and accumulates primarily in the basement and lower living areas [3]. Combined analyses of case–control studies conducted in North America and Europe have also implicated residential exposure to radon as a risk factor for lung cancer [4–7].
Although radon may affect other non-malignant respiratory diseases, epidemiological evidence is sparse [2, 8]. Archer et al. [9] reported a positive association between radon and non-malignant respiratory disease mortality in an early study of uranium miners in the Colorado Plateau, USA. Mapel et al. [10] reported an inverse association between underground mining duration and lung function in a cross-sectional study of uranium miners in New Mexico, USA. There are also reports of uranium miners with chronic diffuse interstitial fibrosis, although a causal link with radon could not be established [8, 11, 12].
This article examines the association between residential radon and non-malignant respiratory disease mortality in the American Cancer Society Cancer Prevention Study (CPS)-II. CPS-II is a large, well-established general population cohort, with detailed, individual-level risk factor data collected at enrolment. We recently observed a positive association between mean county-level residential radon concentrations and lung cancer mortality in the CPS-II [13]. A 15% (95% CI 1–31%) increase in lung cancer mortality was observed for each 100-Bq·m−3 increase in radon concentration. CPS-II provides an excellent data resource to evaluate whether exposure to residential radon is associated with non-lung cancer mortality, including non-malignant respiratory disease mortality. Some of the results presented in this article have been included in a previous abstract [14].
METHODS AND MATERIALS
Study population
CPS-II is a prospective study comprising of nearly 1.2 million subjects enrolled by >77,000 volunteers in 1982. Ethics approval for the CPS-II was obtained from the Emory University School of Medicine Human Investigations Committee (Atlanta, GA, USA). Participants were recruited from all 50 US states, as well as from the District of Columbia and Puerto Rico. The participants were mainly friends and family members of the volunteers, were ≥30 yrs of age, and had at least one family member aged ≥45 yrs. A four-page self-administered questionnaire completed at enrolment captured data on a range of demographic, lifestyle and medical factors, including ZIP code of residence.
Follow-up of CPS-II participants for vital status has been conducted every 2 yrs. In 1984, 1986 and 1988, vital status was obtained from the study volunteers, and confirmed by obtaining the corresponding death certificate. Since 1989, follow-up has been conducted through computerised links to the National Death Index [15]. A total of 2,840 (0.2%) participants had their follow-up terminated in September 1988 due to insufficient information to link to the National Death Index. Over 99% of all known deaths have been assigned a cause. Deaths were classified by the underlying cause of death according to the International Classification of Disease 9th and 10th editions [16, 17].
Of the 1,184,881 CPS-II participants, subjects were excluded due to: missing vital status (n=419); prevalent cancer at enrolment (except non-melanoma skin cancer) (n=82,329); missing ZIP code (n=99,479) or county data (n=22,872); missing data on radon (see below; n=5,836); or other individual level covariates of interest including cigarette smoking (n=161,985). A total of 811,961 participants in 2,754 counties were retained in the final analytic cohort. Throughout 2006, there were a total of 28,300 non-malignant respiratory deaths observed in 16,554,617 person-yrs of follow-up.
Ecological measures of residential radon
Study participants were assigned to a primary county of residence using five-digit ZIP code information provided at enrolment, according to the ZIP code boundaries of the 1980 US Census [18]. Ecological indicators of residential radon concentrations were obtained from the Lawrence Berkeley National Laboratory (LBL; Berkeley, CA, USA) and the University of Pittsburgh (Pittsburgh, PA, USA). A detailed description is provided elsewhere [13]. In brief, at LBL, both short- and long-term indoor radon monitoring data were used, along with a variety of geological, soil, meteorological and housing data, including location of screening measurements within the home, to predict annual average radon concentrations in the main living areas of homes in 3,079 US counties [19, 20].
Cohen [21, 22] compiled a database of mean county-level residential radon concentrations based on a series of screening measurements made in a non-random sample of homes in 1,601 US counties by researchers at the University of Pittsburgh, the US Environmental Protection Agency and other state-level sources from the mid-1980s to the early 1990s. Data were excluded from counties where there were <10 radon measurements or from states with high rates of migration (Florida, California and Arizona) [22] and were normalised to the data of the US National Residential Radon Survey (NRRS), a long-term national residential radon survey conducted in 125 US counties [3].
Mean county-level residential radon concentrations were linked to study participants as indicators of historical residential radon exposure. Mean LBL county-level residential radon concentrations ranged from 6.3 to 265.7 Bq·m−3 (1 pCi·L−1 is equivalent 37 Bq·m−3), with a mean±sd of 53.5±38.0 Bq·m−3.
Social and demographic ecological covariates
Data on a range of social and demographic ecological covariates were compiled for 18,484 participant ZIP codes from the 1990 US Census including: median household income and per cent black, Hispanic, post-secondary education, unemployment and poverty [23].
Statistical analysis
Cox proportional hazards regression models were used to examine the independent effects of mean county-level residential radon concentrations on non-malignant respiratory disease mortality [24]. The baseline hazard in the proportional hazards model was stratified by 1-yr age categories, sex, race (white, black or other) and state of residence at enrolment [13]. Follow-up time since enrolment (1982) was used as the time axis. The survival times of those still alive at the end of follow-up were censored.
Estimated hazard ratios (HRs) and 95% confidence intervals (CIs) were adjusted for a range of individual level risk factors including: education; marital status; body mass index (BMI); BMI squared; cigarette smoking status; cigarettes per day; cigarettes per day squared; years smoked; years smoked squared; age started smoking <18 yrs for both current and former smokers; passive smoking (hours exposed at home, work or other); quintiles of vegetable/fruit/fibre and fat intake; occupational exposures (asbestos, chemicals/acids/solvents, coal or stone dusts, coal tar/pitch/asphalt, formaldehyde and diesel engine exhaust); as well as an “occupational dirtiness index” specifically designed for CPS-II [13, 25].
Potential effect modification was examined on the additive and multiplicative scales. On the additive scale, estimates of the relative excess risk due to interaction, attributable proportion and synergy index (and associated 95% CIs) were calculated according to the method of Zou [26] for the analysis of four by two tables. On the multiplicative scale, interaction terms between radon and selected risk factors were entered into proportional hazards models. Two-sided p-values were calculated to assess the significance of the interaction term using the likelihood ratio statistic. In order to assess the impact of attained age, time-dependent variables were constructed by allowing subjects to be included in the risk set at each death time if they met the age criteria for the model (<70, 70–79 or ≥80 yrs). The functional form of the relationship between residential radon and mortality was assessed using the Supremum test [27]. The proportional hazard assumption was tested by assessing the significance of an interaction term between radon and follow-up time.
Analyses were also undertaken using a random-effects Cox model, originally developed to take into account complex spatial patterns in the data in air pollution research in the CPS-II [28, 29]. Analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) and our random-effects Cox regression programme [28, 29]. Ethical approval was obtained from the Ottawa Hospital (Ottawa, ON, Canada).
RESULTS
The distribution of selected CPS-II participant characteristics is presented in table 1. The majority of participants were aged 40–69 yrs at enrolment, had more than a high school education and were never-smokers. Mean county-level residential radon concentrations varied according to several participant characteristics: there was a tendency for higher radon concentrations to be observed among subjects who were white, had a lower level of education, a higher BMI or who were never-smokers. Radon concentrations tended to be higher in the Northeast and Midwest and lowest in the South.
Table 1– Distribution of selected participant characteristics at enrolment in 1982, American Cancer Society Cancer Prevention Study-II.
| Characteristics | Subjects | Radon Bq·m−3 |
| Overall | 811961 (100) | 53.5±38.0 |
| Age at enrolment yrs | ||
| <40 | 37262 (4.6) | 50.1±35.4 |
| 40–49 | 173768 (21.4) | 54.0±37.9 |
| 50–59 | 297108 (36.6) | 54.2±38.5 |
| 60–69 | 213231 (26.3) | 53.1±38.0 |
| 70–79 | 76633 (9.4) | 52.4±37.5 |
| ≥80 | 13959 (1.7) | 51.9±36.9 |
| Race | ||
| White | 770352 (94.9) | 54.2±38.2 |
| Black | 29832 (3.7) | 40.2±28.3 |
| Other | 11777 (1.5) | 39.3±32.1 |
| Sex | ||
| Male | 362600 (44.7) | 53.8±38.2 |
| Female | 449361 (55.3) | 53.2±37.8 |
| Education | ||
| Less than HS | 106668 (13.1) | 55.2±38.9 |
| HS | 262853 (32.4) | 56.8±39.5 |
| More than HS | 442440 (54.5) | 51.1±36.6 |
| BMI kg·m−2 | ||
| <18.5 | 13685 (1.7) | 50.3±36.1 |
| 18.5–24.9 | 402003 (49.5) | 52.2±37.2 |
| 25.0–29.9 | 299755 (36.9) | 54.6±38.6 |
| ≥30.0 | 96518 (11.9) | 55.6±39.1 |
| Marital Status | ||
| Single | 25564 (3.2) | 51.7±36.7 |
| Married | 691267 (85.1) | 54.1±38.2 |
| Other | 95130 (11.7) | 49.7±36.0 |
| Cigarette smoking status | ||
| Never | 375087 (46.2) | 55.5±39.0 |
| Current | 152033 (18.7) | 51.5±36.4 |
| Former | 203253 (25.0) | 51.2±36.9 |
| Pipe/cigar only | 81588 (10.1) | 53.4±37.9 |
| Region | ||
| Northeast | 170281 (21.0) | 58.3±42.3 |
| South | 257243 (31.7) | 35.6±21.7 |
| Midwest | 234952 (28.9) | 73.7±36.6 |
| West | 149485 (18.4) | 46.9±40.3 |
Data are presented as n (%) or mean±sd. HS: high school; BMI: body mass index. Radon data was obtained from the Lawrence Berkeley National Laboratory (Berkeley, CA, USA).
Table 2 presents adjusted HRs (95% CIs) for non-malignant respiratory disease mortality in relation to a 100-Bq·m−3 increase in mean county-level residential radon concentrations. In the fully adjusted model, a significant positive association was observed between radon and non-malignant respiratory disease mortality overall (HR 1.08, 95% CI 1.03–1.13) and chronic obstructive pulmonary disease (COPD) specifically (HR 1.13, 95% CI 1.05–1.21) using the LBL data. There was a significant positive linear trend of increasing categories of radon concentrations with both COPD and non-malignant respiratory mortality (p<0.05). However, the association with non-malignant respiratory death was not apparent upon exclusion of COPD (HR 1.03, 95% CI 0.96–1.09). Figure 1 shows adjusted HRs (95% CIs) for COPD mortality according to continuous and categorical indicators of radon concentrations. There was no significant departure from a linear exposure-response relationship (p>0.05). Similar results were obtained using the data of Cohen [21, 22].
Table 2– Non-malignant respiratory disease mortality per 100 Bq·m−3 mean county-level residential radon concentrations at enrolment in 1982 follow-up 1982–2006, American Cancer Society Cancer Prevention Study-II.
| Cause of death | ICD-9 | ICD-10 | Cohen data | LBL data | ||||
| Deaths n | Minimally adjusted# | Fully adjusted¶ | Deaths n | Minimally adjusted# | Fully adjusted¶ | |||
| Diseases of the respiratory system | 460–519 | J00-J98 | 20406 | 1.04 (0.98–1.09) | 1.11 (1.05–1.17) | 28300 | 1.01 (0.96–1.06) | 1.08 (1.03–1.13) |
| Pneumonia and influenza | 480–487 | J10-J18 | 6440 | 1.06 (0.96–1.17) | 1.08 (0.98–1.19) | 9058 | 0.99 (0.91–1.07) | 1.01 (0.92–1.10) |
| COPD and allied conditions | 490–496 | J19-J46 | 9664 | 1.02 (0.94–1.11) | 1.14 (1.05–1.23) | 13541 | 1.02 (0.95–1.09) | 1.13 (1.05–1.21) |
| All other respiratory diseases | All not specified | All not specified | 4302 | 1.03 (0.92–1.16) | 1.07 (0.95–1.20) | 5701 | 1.02 (0.92–1.13) | 1.05 (0.95–1.16) |
Data are presented as hazard ratio (95% confidence interval), unless otherwise stated. ICD: International Classification of Diseases; LBL: Lawrence Berkeley National Laboratory (Berkeley, CA, USA); COPD: chronic obstructive pulmonary disease. #: age, race, sex and state stratified. ¶: age, race, sex and state stratified and adjusted for education, marital status, body mass index, body mass index squared, cigarette smoking status, cigarettes per day, cigarettes per day squared, duration of smoking, duration of smoking squared, age started smoking, passive smoking, vegetable/fruit/fibre consumption, fat consumption, industrial exposures, and occupation dirtiness index. Data of Cohen from [21, 22].
Figure 1–
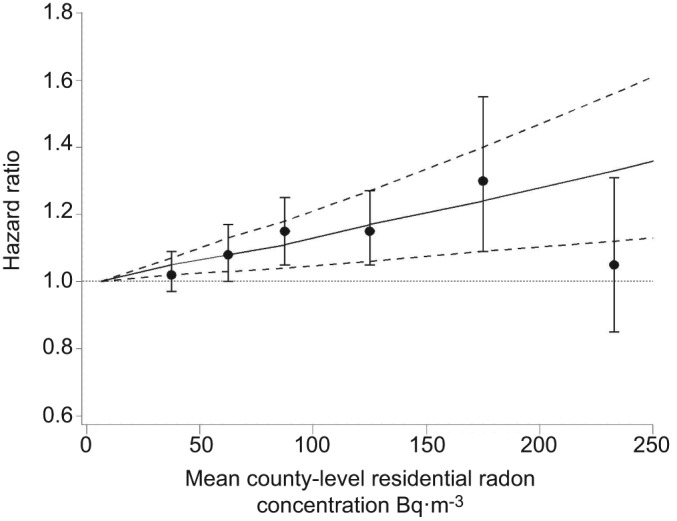
Adjusted hazard ratios with 95% confidence intervals (- - - -) for chronic obstructive pulmonary disease mortality in relation to continuous (–––) and categorical indicators of mean Lawrence Berkeley National Laboratory (Berkeley, CA, USA) county-level residential radon concentrations at enrolment in 1982 and follow-up from 1982–2006, American Cancer Society Cancer Prevention Study-II. Reference category: <25 Bq·m−3.
Results for COPD mortality were similar when excluding individuals who reported a history of any previous lung disease (asthma, emphysema or chronic bronchitis) at enrolment (HR 1.12, 95% CI 1.03–1.22). Results were robust to the adjustment of six socio-demographic ecological covariates (n=811,373; HR 1.11, 95% CI 1.04–1.19) and ambient ozone concentrations (average of 1977–2000 annual spring and summer daily maximum [30], correlation with radon -0.09) (n=404,519; HR 1.16, 95% CI 1.03–1.31) in the model. Results were also insensitive to allowance for spatial clustering at either the ZIP, county or state level in analysis using the random-effects Cox model. Similar results were observed upon exclusion of participants (n=104,821) who lived in their current neighbourhood at enrolment for <5 yrs (HR 1.13, 95% CI 1.05–1.22). There was no evidence that the proportional hazards assumption was violated (p>0.05).
There was no significant effect modification of the radon–COPD association by cigarette smoking, passive smoking or ambient ozone concentrations on either the additive (table 3) or multiplicative scales (table 4). However, results varied by age at enrolment and region (p<0.05) with a somewhat stronger association observed in participants aged ≥65 yrs at enrolment compared to participants aged <64 yrs, and among participants in the Northeast and West; no association was observed in the South (table 4).
Table 3– Measures of additive interaction between mean county-level residential radon concentration, cigarette smoking and other inhalable agents for chronic obstructive pulmonary disease mortality, follow-up 1982–2006, American Cancer Society Cancer Prevention Study-II#.
| RERI | Attributable proportion | Synergy index | |
| Cigarette smoking | 0.61 (-0.38–1.73) | 0.08 (-0.06–0.20) | 1.11 (0.94–1.31) |
| Industrial exposures | -0.06 (-0.34–0.25) | -0.05 (-0.38–0.14) | 0.76 (0.19–3.08) |
| Passive smoke | -0.08 (-0.17–0.03) | -0.06 (-0.17–0.01) | 0.70 (0.39–1.26) |
| Ambient ozone | -0.04 (-0.18–0.11) | -0.04 (-0.22–0.07) | 0.29 |
Data for radon concentration was obtained from the Lawrence Berkeley National Laboratory (Berkeley, CA, USA). RERI: relative excess risk due to interaction. #: exposures categorised as: mean county-level residential radon concentrations <148 Bq·m−3 and ≥148 Bq·m−3; cigarette smoking never or ever; industrial exposures no or yes; passive smoking in home none or any; ambient ozone concentrations <57.1 ppb and ≥57.1 ppb. Cox regression models were fitted with the baseline hazard stratified by age, race, sex and state, and adjusted for education, marital status, body mass index, body mass index squared, cigarette smoking status, cigarettes per day, cigarettes per day squared, duration of smoking, duration of smoking squared, age started smoking, passive smoking, vegetable/fruit/fibre consumption, fat consumption, industrial exposures, and occupation dirtiness index where appropriate.
Table 4– Chronic obstructive pulmonary disease mortality per 100 Bq·m−3 mean county-level residential radon concentrations at enrolment in 1982 interacted with selected risk factors on the multiplicative scale, American Cancer Society Cancer Prevention Study-II.
| Characteristic | Deaths n | Fully adjusted HR# (95% CI) | p-value |
| Age at enrolment yrs | |||
| <65 | 7800 | 1.10 (1.01–1.19) | |
| ≥65 | 5741 | 1.20 (1.08–1.33) | <0.01 |
| Attained age yrs¶ | |||
| <70 | 2376 | 1.06 (0.91–1.24) | |
| 70–79 | 5500 | 1.18 (1.07–1.31) | |
| ≥80 | 5665 | 1.11 (1.00–1.23) | 0.12 |
| Sex | |||
| Male | 7414 | 1.07 (0.98–1.17) | |
| Female | 6127 | 1.20 (1.09–1.33) | 0.27 |
| Education | |||
| Less than HS | 2923 | 1.14 (0.99–1.33) | |
| HS | 4589 | 1.18 (1.05–1.32) | |
| More than HS | 6029 | 1.06 (0.95–1.19) | 0.38 |
| Cigarette smoking | |||
| Never | 1797 | 1.03 (0.86–1.25) | |
| Current | 6585 | 1.13 (1.02–1.24) | |
| Former | 3912 | 1.08 (0.94–1.24) | 0.24 |
| Industrial exposures | |||
| No | 10268 | 1.16 (1.07–1.26) | |
| Yes | 3273 | 1.03 (0.89–1.18) | 0.21 |
| Passive smoking at home+ | |||
| None | 1570 | 1.04 (0.85–1.27) | |
| Any | 227 | 1.03 (0.56–1.90) | 0.89 |
| Region§ | |||
| Northeast | 2646 | 1.20 (1.10–1.31) | |
| South | 4359 | 0.94 (0.81–1.08) | |
| Midwest | 3695 | 1.12 (1.02–1.22) | |
| West | 2841 | 1.21 (1.10–1.32) | 0.02 |
| Ambient ozone concentrationsƒ | |||
| <57.1 ppb | 3232 | 1.21 (0.97–1.53) | |
| ≥57.1 ppb | 3311 | 1.17 (1.01–1.36) | 0.32 |
Data for radon concentration was obtained from the Lawrence Berkeley National Laboratory (Berkeley, CA, USA). HR: hazard ratio; HS: high school. #: age, race, sex and state stratified and adjusted for education, marital status, body mass index, body mass index squared, cigarette smoking status, cigarettes per day, cigarettes per day squared, duration of smoking, duration of smoking squared, age started smoking, passive smoking, vegetable/fruit/fibre consumption, fat consumption, industrial exposures, and occupation dirtiness index where appropriate; ¶: race, sex and state stratified and adjusted for cigarette smoking status, cigarettes per day, cigarettes per day squared, duration of smoking, duration of smoking squared, age started smoking; +: never-smokers; §: HR (95% confidence interval) and p-value were calculated without stratification by state; ƒ: participants with missing ozone data excluded cut-off points based on median participant ozone value.
DISCUSSION
Findings of this large prospective study showed a significant positive association between residential radon and COPD mortality (HR per 100 Bq·m−3 1.13, 95% CI 1.05–1.21). Findings were robust to the control of a variety of socio-demographic ecological variables, ambient ozone concentrations and potential spatial clustering in the data. Similar results were obtained upon exclusion of individuals who reported a history of any previous lung disease at enrolment.
COPD includes chronic bronchitis and emphysema. It leads to a progressive loss of airflow and can ultimately be fatal [31]. Although cigarette smoking is a major known risk factor, other risk factors include occupational dusts and fumes, air pollution and genetic susceptibility [31, 32]. COPD is also associated with multiple comorbidities, such as other respiratory, cardiovascular and malignant diseases, including lung cancer, possibly due to chronic pulmonary and systemic inflammation [31, 33–35]. Jerrett et al. [30] recently reported a positive association between long-term ambient ozone concentrations and non-malignant respiratory disease mortality in the CPS-II.
Early research findings provide limited evidence of an association between radon and non-malignant respiratory disease [2, 8]. Results from animal studies have shown pulmonary fibrosis and emphysema with exposure to either radon progeny alone or in combination with uranium ore dust [8, 12]. In recent epidemiological studies, a significant excess in mortality from non-malignant respiratory disease was observed in a cohort of French uranium miners [36]. However, there was no trend in mortality with cumulative radon exposure and, when excluding silicosis, the excess in mortality disappeared. Excesses in mortality due to silicosis, other and unspecified pneumoconiosis, and pulmonary fibrosis were observed among uranium miners in the Colorado Plateau [37]. There was also a significant increasing trend in pulmonary fibrosis with increasing categories of cumulative radon exposure. However, occupational dust exposure could confound these associations. We found a significant positive association between radon and COPD mortality in a large general population study. There were also no clear associations observed between radon and mortality from any other malignant or non-malignant disease beyond lung cancer [13] and COPD (reported herein) in the CPS-II [14].
Radiation-induced lung damage, including radiation pneumonitis and lung fibrosis, is also observed following radiation therapy for lung or other thoracic tumours [38]. α-radiation exposure was associated with respiratory dysfunction in Mayak nuclear workers (Ozyorsk, Russia) [39]. The Japanese Life Span Study of atomic bomb survivors also yielded significant increases in respiratory mortality in relation to acute, whole body, γ- and neutron radiation exposure [40]. In contrast, there was no association between external radiation and respiratory mortality in a 15-country collaborative study of nuclear workers [41].
The present study is based on ecological indicators of residential radon. Previous studies have examined associations between county-level residential radon concentrations and county-level mortality rates [2, 22]. However, such studies are limited by cross-level bias and confounding due to cigarette smoking and other individual-level risk factors that may vary across counties [8, 42, 43]. Here, mean county-level residential radon concentrations were assigned to individual CPS-II participants and mortality health effects estimated with detailed adjustment for individual level cigarette smoking status (including both linear and square terms for amount and duration of smoking) and other potential confounders.
Ecological radon data were either estimated using a statistical model based on available short- and long-term monitoring, geological, meteorological and housing data (LBL), or were based on a non-random series of short-term screening measurements normalised to the data of the US NRRS [21, 22]. These data are subject to radon measurement error, seasonal and yearly variability, and within-county variability, probably resulting in some degree of downwards bias and reduced precision of relative risk estimates [13, 44–46]. However, our previous estimates of increased lung cancer mortality associated with ecological indicators of residential radon were compatible with estimates obtained from combined analyses of residential case–control studies [13]. There were also no data on time–activity patterns or residential mobility from enrolment. Results from a study in Iowa, USA revealed that attempts to minimise misclassification through restricting participant selection to long-term household residents and compiling detailed retrospective mobility assessments with measures of radon in multiple locations both within and outside of the home resulted in an improved ability to detect associations with lung cancer [47]. In the combined analysis of seven North American case–control studies of residential radon and lung cancer, restriction of the analysis to subjects with limited residential mobility (reporting at most two addresses in the previous 5–30 yrs) also tended to strengthen the association between residential radon exposure and lung cancer risk [4, 5]. Although participants here reported living in their current neighbourhood at enrolment for a mean±sd of 19.4±14.1 yrs, results were virtually unchanged upon restriction of the analysis to participants who lived in the same neighbourhood at enrolment for ≥5 yrs.
There was no updated information on cigarette smoking available beyond enrolment in the full CPS-II. Previously, results for radon-associated lung cancer mortality were restricted a priori to the first 6 yrs of follow-up (1982–1988) in order to most accurately control for smoking status [13]. However, here, an extended follow-up period (1982–2006) was used in an attempt to maximise statistical power to detect possible associations with causes of death other than lung cancer by maximising the number of observed deaths. With a mean age at enrolment of 57 yrs, it is unlikely that participants would begin smoking during follow-up. Results for COPD mortality in the first 6 yrs of follow-up (1982–1988) were also similar to, although less precise than (HR 1.18, 95% CI 0.96–1.44), those obtained in the full follow-up time-period, and there was no evidence that the proportional hazard assumption was violated. Residential radon concentrations and cigarette smoking were also inversely correlated [6, 7, 13, 43] and negative confounding of both radon-associated lung cancer [13] and COPD mortality by cigarette smoking was observed. Although findings were somewhat stronger in current, as opposed to never- or former smokers, there was no significant effect modification by cigarette smoking status observed on either the additive or multiplicative scales.
Another limitation of the present study is the mortality-based design. Inferences for less fatal diseases may be less reliable to those that are more highly fatal. COPD on death certificates is also probably underreported [34, 48], which may result in non-differential misclassification that is more pronounced in younger subjects. Although respiratory failure typically accounts for the majority of deaths in patients with severe COPD, lung cancer and cardiovascular disease are reported in patients with mild disease [34].
This large prospective study suggests that residential exposure to radon may increase COPD mortality. Although it is unclear whether radon may lead to the induction or exacerbation of COPD (or both), radon may lead to pulmonary inflammation and damage associated with both COPD and lung cancer. The present findings require replication in other settings. If confirmed, airway dysfunction may represent an early indicator of the radon effect. Further research is needed to confirm this finding, and to better understand possible complex inter-relationships between radon, COPD and lung cancer.
Acknowledgments
The authors would like to thank J. Calle (American Cancer Society, Atlanta, GA, USA) for valuable contributions in the development of the study, and G. Thurston and K. Ito (both New York University, Tuxedo, NY, USA) for providing the ozone data used in this manuscript.
Footnotes
Support Statement
M.C Turner was supported by a Canada Graduate Scholarship from the Canadian Institutes of Health Research. D. Krewski is the Natural Sciences and Engineering Research Council Chair in Risk Science at the University of Ottawa (Ottawa, ON, Canada).
Statement of Interest
None declared.
REFERENCES
- 1.International Agency for Research on Cancer Monographs on the Evaluation of Carcinogenic Risk to Humans: Manmade Fibres and Radon. Lyon, International Agency for Research on Cancer, 1988 [Google Scholar]
- 2.National Research Council Health Risks of Radon and Other Internally Deposited Alpha-Emitters. Bier IV. Washington, National Academy Press, 1988 [PubMed] [Google Scholar]
- 3.Marcinowski F, Lucas RM, Yeager WM. National and regional distributions of airborne radon concentrations in U.S. homes. Health Phys 1994; 66: 699–706 [DOI] [PubMed] [Google Scholar]
- 4.Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology 2005; 16: 137–145 [DOI] [PubMed] [Google Scholar]
- 5.Krewski D, Lubin JH, Zielinski JM, et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A 2006; 69: 533–597 [DOI] [PubMed] [Google Scholar]
- 6.Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case–control studies. BMJ 2005; 330: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darby S, Hill D, Deo H, et al. Residential radon and lung cancer – detailed results of a collaborative analysis of individual data on 7,148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand J Work Environ Health 2006; 32: 1–84 [PubMed] [Google Scholar]
- 8.National Research Council Health Effects of Exposure to Radon. Bier VI. Washington, National Academy Press, 1999 [Google Scholar]
- 9.Archer VE, Gillam D, Wagoner JK. Respiratory disease mortality among uranium miners. Ann NY Acad Sci 1976; 271: 280–293 [DOI] [PubMed] [Google Scholar]
- 10.Mapel DW, Coultas DB, James DS, et al. Ethnic differences in the prevalence of nonmalignant respiratory disease among uranium miners. Am J Public Health 1997; 87: 833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archer V. Radon, silicosis, and lung cancer. Health Phys 1996; 70: 268–269 [PubMed] [Google Scholar]
- 12.Archer V, Renzetti A, Doggett R, et al. Chronic diffuse interstitial fibrosis of the lung in uranium miners. J Occup Environ Med 1998; 40: 460–474 [DOI] [PubMed] [Google Scholar]
- 13.Turner MC, Krewski D, Chen Y, et al. Radon and lung cancer in the American Cancer Society Cohort. Cancer Epidemiol Biomarkers Prev 2011; 20: 438–448 [DOI] [PubMed] [Google Scholar]
- 14.Turner MC, Krewski D, Chen Y, et al. Residential radon and non-lung cancer mortality in the American Cancer Society Cohort. Am J Epidemiol 2011; 173: Suppl. 1, S98 [Google Scholar]
- 15.Calle EE, Terrell DD. Utility of the National Death Index for ascertainment of mortality among Cancer Prevention Study II participants. Am J Epidemiol 1993; 137: 235–241 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization International Classification of Diseases: Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death. Geneva, World Health Organization, 1977 [Google Scholar]
- 17.World Health Organization ICD-10: International Statistical Classification of Diseases and Related Health Problems. Geneva, Switzerland, World Health Organization, 1992 [Google Scholar]
- 18.U. S. Department of Commerce, Bureau of the Census Census of Population and Housing 1980 (United States): Summary Tape File 3B. ICPSR version. Washington, U.S. Dept. of Commerce, Bureau of the Census, 1980 [Google Scholar]
- 19.Price PN, Nero A, Revzan K, et al. Predicted GM and other parameters by county in the U. S. http://eande.lbl.gov/IEP/high-radon/files.html Date last accessed: May 20, 2010.
- 20.Price PN, Nero AV. Joint analysis of long- and short-term radon monitoring data from the Northern U.S. Environ Int 1996; 22: Suppl. 1, S699–S714 [Google Scholar]
- 21.Cohen BL. Compilation and integration of studies of radon levels in U.S. homes by states and counties. Crit Rev Environ Cont 1992; 22: 243–364 [Google Scholar]
- 22.Cohen BL. Test of the linear – no threshold theory of radiation carcinogenesis for inhaled radon decay products. Health Phys 1995; 68: 157–174 [DOI] [PubMed] [Google Scholar]
- 23.U. S. Department of Commerce, Bureau of the Census Census of Population and Housing, 1990 (United States): Summary Tape File 3B. ICPSR version. Washington, U.S: Dept. of Commerce, Bureau of the Census, 1990 [Google Scholar]
- 24.Cox D. Regression models and life tables. J R Stat Soc B 1972; 34: 187–220 [Google Scholar]
- 25.Siemiatycki J, Krewski D, Shi Y, et al. Controlling for potential confounding by occupational exposures. J Toxicol Env Health A 2003; 66: 1591–1603 [DOI] [PubMed] [Google Scholar]
- 26.Zou GY. On the estimation of additive interaction by use of the four-by-two table and beyond. Am J Epidemiol 2008; 168: 212–224 [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Wei L, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika 1993; 80: 557–572 [Google Scholar]
- 28.Krewski D, Jerrett M, Burnett RT, et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res Rep Health Eff Inst 2009; 140: 5–114 [PubMed] [Google Scholar]
- 29.Ma R, Krewski D, Burnett RT. Random effects Cox models: a Poisson modeling approach. Biometrika 2003; 90: 157–169 [Google Scholar]
- 30.Jerrett M, Burnett RT, Pope CA, III, et al. Long-term ozone exposure and mortality. N Engl J Med 2009; 360: 1085–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viegi G, Pistelli F, Sherrill DL, et al. Definition, epidemiology and natural history of COPD. Eur Respir J 2007; 30: 993–1013 [DOI] [PubMed] [Google Scholar]
- 32.Salvi S, Barnes P. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009; 374: 733–743 [DOI] [PubMed] [Google Scholar]
- 33.Punturieri A, Szabo E, Croxton TL, et al. Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J Natl Cancer Inst 2009; 101: 554–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sin DD, Anthonisen NR, Soriano JB, et al. Mortality in COPD: role of comorbidities. Eur Respir J 2006; 28: 1245–1257 [DOI] [PubMed] [Google Scholar]
- 35.Turner MC, Chen Y, Krewski D, et al. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med 2007; 176: 285–290 [DOI] [PubMed] [Google Scholar]
- 36.Vacquier B, Caer S, Rogel A, et al. Mortality risk in the French cohort of uranium miners: extended follow-up 1946–1999. Occup Environ Med 2008; 65: 597–604 [DOI] [PubMed] [Google Scholar]
- 37.Schubauer-Berigan M, Daniels R, Pinkerton L. Radon exposure and mortality among white and American Indian uranium miners: an update of the Colorado Plateau cohort. Am J Epidemiol 2009; 169: 718–730 [DOI] [PubMed] [Google Scholar]
- 38.Bentzen SM, Skoczylas JZ, Bernier J. Quantitative clinical radiobiology of early and late lung reactions. Int J Radiat Biol 2000; 76: 453–462 [DOI] [PubMed] [Google Scholar]
- 39.Belyaeva ZD, Osovets SV, Scott BR, et al. Modeling of respiratory system dysfunction among nuclear workers: a preliminary study. Dose Response 2008; 6: 319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preston DL, Shimizu Y, Pierce DA, et al. Studies of mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950–1997. Radiat Res 2003; 160: 381–407 [DOI] [PubMed] [Google Scholar]
- 41.Cardis E, Vrijheid M, Blettner M, et al. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: estimates of radiation-related cancer risks. Radiat Res 2007; 167: 396–416 [DOI] [PubMed] [Google Scholar]
- 42.Stidley CA, Samet JM. A review of ecologic studies of lung cancer and indoor radon. Health Phys 1993; 65: 234–251 [DOI] [PubMed] [Google Scholar]
- 43.Puskin JS. Smoking as a confounder in ecologic correlations of cancer mortality rates with average county radon levels. Health Phys 2003; 84: 526–532 [DOI] [PubMed] [Google Scholar]
- 44.Darby S, Deo H, Doll R, et al. A parallel analysis of individual and ecologic data on residential radon and lung cancer in south-west England. J R Stat Soc A 2001; 164: 193–203 [Google Scholar]
- 45.Lagarde F, Pershagen G. Parallel analyses of individual and ecologic data on residential radon, cofactors, and lung cancer in Sweden. Am J Epidemiol 1999; 149: 268–274 [DOI] [PubMed] [Google Scholar]
- 46.Armstrong BG. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med 1998; 55: 651–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Field R, Smith B, Steck D, et al. Residential radon exposure and lung cancer: variation in risk estimates using alternative exposure scenarios. J Expo Anal Environ Epidemiol 2002; 12: 197–203 [DOI] [PubMed] [Google Scholar]
- 48.Jensen HH, Godtfredsen NS, Lange P, et al. Potential misclassification of causes of death from COPD. Eur Respir J 2006; 28: 781–785 [DOI] [PubMed] [Google Scholar]


