Abstract
Findings from previous research assessing sleep quality in caregivers are inconsistent due to differences in sleep assessment methods. This study evaluated sleep in dementia caregivers using a comprehensive sleep assessment utilizing an ambulatory polysomnography (PSG) device. Twenty caregivers and twenty non-caregivers rated their perceived sleep quality, stress, and depressive symptoms; provided samples of cortisol and inflammatory biomarkers; and completed an objective sleep assessment using a portable PSG device. Caregivers reported greater perceived stress than non-caregivers. Next, the groups had different sleep architecture: caregivers spent less proportion of their sleep in restorative sleep stages compared to non-caregivers. Further, levels of C-reactive protein and awakening salivary cortisol were greater in caregivers than in non-caregivers, and these measures were related to sleep quality. Our findings indicate that sleep disruption is a significant concomitant of caregiving and may affect caregiver’s health. Sleep quality of caregivers might be a useful target for a clinical intervention.
Keywords: caregiver, chronic stress, sleep quality, sleep architecture, ambulatory PSG
Introduction
Poor sleep is a common complaint in a growing population of caregivers of relatives with dementia, and sleep quality has been linked to pro-inflammatory and pro-coagulant activity associated with health problems such as cardiovascular disease.1, 2 However, despite common reports of decreased subjective sleep quality in caregivers,3–5 to date findings from studies assessing objective sleep quality in this population have been inconsistent. On one hand, investigators have reported poorer sleep in caregivers suggesting a detrimental effect of caregiving on sleep quality.5–9 On the other hand, some researchers have found no differences in objectively measured sleep between caregivers and non-caregivers even though caregivers reported their sleep to be worse.3, 10 Based on the latter findings it has been suggested that caregivers’ interpretation of the situation might affect their judgment and lead to misperception of their sleep quality.3, 10
One of the reasons for such inconsistencies in findings from previous studies might be the way sleep quality has been measured by different investigators. Assessing sleep is challenging in humans, and ways of evaluating sleep include self-report measures (questionnaires and sleep diaries), overnight actigraphy, and polysomnography (PSG).11 Though easy to obtain, self-reports of sleep disturbances tend to vary in accuracy, not always correlating with objective sleep assessments.8, 12 Actigraphy has been gaining popularity as an objective and a non-obtrusive way to assess sleep,2, 8, 12, 13 but it does not allow for examining sleep architecture.14 The gold standard of sleep assessment, PSG, is typically performed in a sleep laboratory. This presents a logistical problem for evaluating caregivers who provide in-home care for relatives with illness. Additionally, being in a strange environment of a sleep lab might interfere with a person’s normal sleep. Ambulatory PSG might be necessary for sleep research in caregivers who are often unable to spend a night in a sleep laboratory because of caregiving responsibilities at home. Additionally, previous research indicated that because sleep is evaluated in participants’ natural environment, ambulatory PSG is not associated with significant first night effects evident when PSG is performed in the laboratory setting,15 which means fewer recording nights might be sufficient for a reliable sleep assessment.
To date, very few studies attempted to assess caregivers’ sleep quality using some form of PSG that can be performed at home.1, 7, 10 Further, although sleep quality was a primary outcome in these studies, participants were not consistently screened for sleep disorders1, 7. The presence of pre-existing sleep disorders in study samples might have made the results of some investigations less interpretable. Additionally, age and gender emerged as important factors when assessing sleep.1, 7 Thus, when evaluating sleep quality in caregivers it is important to recruit caregivers who are generally healthy and free of diagnosed sleep problems. Next, controlling for the effects of age and gender might be necessary for clear interpretation of the findings.
To address the above-mentioned issues highlighted in previous research, we conducted a study that compared sleep quality between two groups of generally healthy older adults screened for significant medical problems including diagnosed sleep disorders: dementia caregivers (caregivers) and age-and –gender-matched non-caregivers (non-caregivers). Both groups completed sleep assessment that was conducted using an ambulatory PSG device capable of collecting detailed information about sleep quality including sleep architecture in participants’ home environment.16 Utilizing this device and focusing on sleep architecture in our participants allowed us to extend previous findings related to objective sleep quality in caregivers, which was the main goal of the study.
Based on previous findings indicating that caregivers and non-caregivers spend similar amount of time sleeping although caregivers report poorer sleep,10 we hypothesized that caregivers might spend their sleep time in lighter and less restorative sleep stages, which can be evaluated comparing sleep architecture between the two groups. Our main hypothesis was that compared to non-caregivers, dementia caregivers would demonstrate lower sleep quality when measured objectively by ambulatory PSG. Specifically, compared to non-caregivers, caregivers will spend a greater proportion of time in lighter sleep stages.
Additionally, to assess relationships between sleep architecture and pro-inflammatory and pro-coagulant activity reportedly increased in caregivers and linked to sleep,1, 2, 17 we collected several biomarkers of inflammation and stress from our participants. We hypothesized that caregivers will have increased inflammatory activity compared to non-caregivers, and we specifically predicted that measures of inflammation will be related to sleep architecture variables.
Methods
Participants
Twenty caregivers and twenty non-caregivers were recruited from the Portland, OR, metropolitan area. All potential subjects signed the consent form and completed screening prior to participating in any study procedures. General inclusion criteria for both groups were ages 50 to 85 and being in good physical and cognitive health (as evidenced by scoring 25 or more on the Modified Telephone Interview for Cognitive Status, TICS-m).18 Potential subjects were excluded if they reported a serious current medical condition such as cancer or current mental health problem such as untreated major depression that might significantly affect physiologic or cognitive functioning. Further, people reporting an excessive alcohol intake or illicit drug use were not eligible to participate. Potential participants were also excluded if they reported diagnosed sleep disorders, indicated self-reported difficulties with falling asleep or staying asleep, or stated they used medications to help with sleep on a regular basis. Those on medications known to affect CNS function or impact sleep (e.g., steroids or neuroleptics) or on a new medication for less than 2 months were also excluded. The exclusion criteria were assessed by a self-report during a screening interview. The main rationale for the exclusion criteria was to exclude people with underlying conditions that might affect sleep or other study assessments, thus limiting interpretability of the results.
To be eligible for the caregiver group, subjects had to be primary caregivers for a relative with dementia. The caregivers were part of a mind-body intervention study to reduce stress.19 Caregivers were recruited by posting study flyers at the university neurology clinic and in the community, as well as by advertising in the local media and local caregiver support groups. The caregiver assessments for the current cross-sectional study occurred at the baseline visit prior to randomization for the interventional trial.
To be eligible to participate in the non-caregiver group, the subjects had to be free of any caregiving duties and not reporting any major stress (e.g. death of a loved one) at the time of the study. Non-caregivers were recruited from the community by posting study flyers at adult community centers and other public locations. To control for seasonal effects subjects for both groups were recruited in several interleaved cohorts. Caregiver cohorts were recruited first followed by recruitment of non-caregivers who would match the most recent caregivers’ cohort. The criteria for matching of non-caregivers were being the same gender and being within ± 5 age range of the index caregiver. The study protocol was approved by the Oregon Health & Science University Institutional Review Board.
Study Procedures
Eligible participants were invited for a study visit conducted at the laboratory. To minimize the effects of circadian variation on blood biomarkers of inflammation, laboratory visits were scheduled at similar times during the day. During the visit participants completed self-report measures and gave blood samples for inflammatory biomarkers. At the end of the laboratory visit, research personnel set up and turned on the 24-hour ambulatory PSG device for sleep, as well as awake, recording. At that time the participants were instructed to follow their normal routine during the rest of the day and night while the device was recording. The following day a member of the research team went to the participant’s home and disconnected the recording device. At that time participants were given a kit for collecting 3 salivary cortisol samples at home. The samples were returned to the laboratory by mail.
Measurements
Self-report Measures of Sleep, Stress, and General Functioning
Pittsburgh Sleep Quality Index (PSQI), an instrument that measures sleep quality in the previous month and discriminates between good and poor sleepers, was used to evaluate perceived sleep quality.20
Epworth Sleepiness Scale (ESS) was used to assess participants’ daytime sleepiness. ESS requires participants rate the likelihood of dozing off in a number of common situations.21
Perceived Stress Scale (PSS), a 10-item measure assessing frequency of occurrence of stressful experiences in the previous week, was used to evaluate perceived stress.22
Center for Epidemiologic Studies Depression Scale 10 - item version (CESD-10) assessing prevalence of depressive symptoms in the previous week was used to evaluate depressive symptoms.23
Question 9 of the SF-36 scale (Energy and Fatigue subscore) assessing participants’ fatigue and general physiological functioning in the previous month was used to evaluate participants’ perceived fatigue level.24
Additionally, the Revised Memory and Behavior Problem Checklist (RMBPC) was administered to caregivers for description of caregiver burden. It is a measure of behaviors of the care recipient and the caregiver’s own reactions to the reported behaviors.25
Biomarkers of Stress and Inflammation
Salivary cortisol level was measured as a biomarker of stress. The samples were collected using Sarstedt Salivettes ® at home on three occasions: at bedtime, upon awakening, and 30 minutes after awakening. Subjects were asked to refrain from food, alcohol, tobacco, or citrus one hour prior to sampling. Samples were stored in a refrigerator and returned to the OHSU at participant’s earliest convenience by mail. The samples were spun and stored at −80F, then assessed using an enzyme-linked immunoassays (ELISA) kit (Active Cortisol EIA, Diagnostic Systems Laboratory, Webster, Texas) as previously described.26
Several markers of increased inflammation related to chronic stress were obtained through blood assays. Specifically, the analyses were completed for the levels of cytokines interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), and C-reactive protein (CRP). The cytokines were measured in duplicate using ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. CRP was quantified in duplicate by a chemiluminescent assay system (Siemens Medical Solutions Diagnostics, Los Angeles, CA).
Objective Sleep Assessment
Objective measures of overnight sleep quality were obtained using a portable PSG data collection system, a battery-powered device capable of recording signals from multiple domains including electroencephalogram (EEG), electro-oculogram (EOG), electrocardiogram (ECG), respiration, and movement for 24 hours or longer.16 The system recorded physiological data that were later processed offline using Brain Vision Analyzer (Brain Products GmbH, Inc., Gilching, Germany). During the laboratory assessment prior to overnight recording EEG electrodes were applied in standard International System 10–20 locations27 with the EEG-scalp impedances equal to or below 5 kohm. The gold plated electrodes were affixed with small gauzes soaked in collodion to keep them in place for overnight recording. The recording included 5 channels of bipolar EEG (F3-C3, P3-O1, F4-C4, P4-O2, and either Fz-Cz or Cz- to linked mastoid reference) recorded with high- pass filtering at 0.5Hz and low-pass filtering at 64 Hz and at a rate of 512 per second. Two additional electrodes recording EOG signal were placed diagonally, one lateral to the right superior and the other lateral to the left inferior orbit. ECG electrodes were attached just below clavicles to collect information about heart rate. Two accelerometers (one taped on the top of the participant’s head and another on the upper arm) were used to record muscle activity and movement. Respiration rate was evaluated utilizing an elastic respiration belt (Ambu Sleepmate, Glen Burnie, MD) placed around participant’s abdomen. All electrodes, accelerometers, and respiration belt were connected to the ambulatory device contained in a carrying bag. Importantly, participants were not constrained in their movements during the recording, and the bag with the device could be worn over the shoulder, as a fanny pack, or placed next to the participant during sleep. The only activities participants were not able to do during the recording were bathing or showering to avoid submerging the device under water. The recording was ended and electrodes removed the following morning at the participant’s residence. At the same time research personnel collected a brief sleep diary completed by the participant during the recording period with the information about bedtimes and wake times and any interruptions in sleep during the recording. This information was used to assist with data processing and sleep scoring. To minimize the burden on study participants PSG recording were conducted in participants’ homes only for one night.
Data Processing
Processing of the overnight data for all subjects was performed using Brain Vision Analyzer software. The montage for data analyses consisted of two frontal channels (F3-C3, F4-C4), two posterior channels (P3-O1, P4-O2), the EOG channel, and 2 accelerometer channels. The data were segmented into consecutive 30-second epochs. Sleep stages were scored manually by an assessor blinded to the participant’s group as Stage W for awake epochs; Stage N1and Stage N2 for non-Rapid Eye Movements (NREM) sleep epochs; Stage N3 for NREM slow-wave sleep epochs; and Stage R for REM sleep epochs. The scoring was done according to the most recent sleep scoring criteria28 with modifications to incorporate the use of accelerometers in place of EMG for stage R scoring and alternative placement of EEG electrodes. The following variables were obtained for the sleep analyses: total time in bed (TIB), total sleep time (TST), sleep onset latency (SOL) determined as subject-reported lights out time to the first epoch of any sleep in minutes, wake after sleep onset (WASO) determined as the minutes awake (in Stage W) after sleep onset, number of arousals determined as sum of all arousals during the TST, percent sleep efficiency determined as a ratio between TST and TIB, as well as percent of TST spent in each sleep stage (N1, N2, N3, and R).
All collected data were screened prior to conducting any analyses for quality assessment. Transformations (natural logarithm, square root, or rank order) were used for variables that had skewed distributions. For the variables not meeting normality assumption even after attempting transformations we used non-parametric tests.
Statistical Analyses
All data analyses were performed using PASW Statistic 18.0 (SPSS Inc., Chicago, IL). The alpha level was set at .05.
Primary Analyses
Group Differences in Self-reports
A single overall scale score was used as an outcome variable for each of the self-report measures participants completed (PSS, CESD-10, SF-36 question 9, PSQI, and ESS). To test the group differences we performed analyses of covariance (ANCOVAs) with a score for each scale, group (caregivers vs. non-caregivers) as a between-subjects factor, and age as a covariate.
Group Differences in Stress and Inflammation Biomarkers
To evaluate group differences between the levels of inflammatory markers and salivary cortisol at different time points we used ANCOVAs with group as a between-subjects factor and age as a covariate.
Group Differences in Objective Sleep Measures
To assess group differences in objective sleep quality parameters we used multivariate analyses of covariance (MANCOVAs) with group as a between-subjects factor, and age as a covariate. The primary MANCOVA was conducted with selected sleep architecture parameters (percentages of sleep time spent in each stage) and SOL as dependent variables.
Secondary Analyses
Group Differences in Objective Sleep Measures
To assess group differences in basic sleep quality parameters a secondary MANCOVA was performed with basic sleep parameters including TIB, TST, WASO, arousals during sleep, and sleep efficiency as dependent variables. The variables used for secondary analyses were previously shown to be similar between the groups.29
Relationships Among Variables
Pearson bivariate correlations were used to evaluate relationships between pairs of stress-related variables (PSS and cortisol) and sleep quality measures (both subjective and objective) regardless of group membership.
Predicting Sleep Quality from Group and Depressive Symptoms
Since previous findings suggested influences of depressive symptoms on sleep quality, 6, 10, 30 we created hierarchical linear regression models for each objective sleep quality outcome that differed significantly between the groups to determine the proportion of variance accounted for by depressive symptoms beyond the variance accounted for by age and group membership. Age was entered in the first step, group membership was entered in the second step, and depressive symptoms score from CESD-10 was entered in the last step.
Results
The presented data were obtained from twenty caregivers and twenty non-caregivers (sample mean age, 65.7 years; 97.5 % white). For the caregiver group, 64 potential subjects contacted the researchers and were screened for eligibility, 31 signed the consent form and participated in the study, with data from 20 participants available for statistical analyses. For the non-caregiver group, 60 potential subjects contacted the researchers and were screened for eligibility, 25 signed the consent form and participated in the study, with data from 20 available for the analyses. The reasons for data loss included PSG device battery failure during the overnight recording and storing to memory errors. The equipment-related errors were random and not systematic.
Group Characteristics
Table 1 contains information about the study groups. The resulting samples of caregivers and non-caregivers were matched on gender, p = 1.00; age, p = .31; and education level, p = .38. Because being a primary caregiver was the eligibility criteria for the study, only one participant from caregiver group was employed part-time, while in non-caregiver group 40 % were employed, p < .01. The participants in both groups were similar in health status: hypertension controlled with medication was the most commonly reported problem in both groups. Other conditions reported by study participants included mild asthma, non-insulin dependent diabetes, thyroid deficiency, and acid reflux, all controlled with medication. Few participants in both groups reported a history of depression which was controlled with medication.
Table 1.
Group demographics, medical diagnoses, and self-report measures
| Caregivers (n = 20) | Non-caregivers (n = 20) | Sig. (p) | |
|---|---|---|---|
| Demographics | |||
| Gender (Number females/males) | 18/2 | 18/2 | 1.00 |
| Age (years), Mean (range, SD) | 64.50 (50–76, 7.13) | 66.95 (55–76, 7.89) | .309 |
| Education level (years), Mean (SD) | 15.75 (2.07) | 16.40 (2.52) | .379 |
| Employment status (% employed)** | 5 | 40 | .008 |
| Medical diagnoses | |||
| Hypertension (%) | 50 | 30 | .197 |
| History of depression (%) | 10 | 5 | .548 |
| Other medical diagnoses (%) | 45 | 50 | .752 |
| Self-reports | |||
| PSS, Mean (SD)** | 19.15 (5.72) | 12.50 (7.22) | .004 |
| CESD-10, Mean (SD)*** | 10.80 (4.56) | 4.95 (4.15) | .000 |
| SF-36 (Q. 9), Mean (SD)** | 25.80 (6.30) | 32.80 (7.73) | .004 |
| PSQI, Mean (SD)*** | 8.31 (3.38) | 4.35 (2.30) | .000 |
| ESS, Mean (SD) | 6.63 (4.43) | 5.05 (3.22) | .315 |
p < .001,
p < .01
Abbreviations: PSS= Perceived Stress Scale, CESD-10 = Center for Epidemiologic Studies Depression Scale (10-item version), PSQI = Pittsburgh Sleep Quality Index, ESS = Epworth Sleepiness Scale.
The caregivers were either spouses (70%) or children (30%) of the person with dementia, and with a single exception care recipients were present at home during the overnight recording. The RMBPC scores (mean +/− SD) indicating frequency of occurrence of particular behaviors suggested moderate severity of problems in care recipients for depression subscale (9.53 +/− 6.05), disruptive behavior subscale (9.05 +/− 6.21), memory problems subscale (18.89 +/− 8.87), and overall (37.37+/− 17.75). The RMBPC scores, indicating caregiver reaction to care recipient’s behaviors, were on average in the low to medium range for depression subscale (9.79 +/− 8.62), disruptive behavior subscale (10.47 +/− 6.76), memory problems subscale (8.16 +/− 5.31), and overall (28.42 +/− 17.86).
Group Differences in Self-report Measures
The results of ANCOVAs comparing groups on self-report measures demonstrated that compared to non-caregivers, caregivers reported significantly greater perceived stress, F (1, 37) = 9.67, p < .01, depressive symptoms, F (1, 37) = 18.94, p < .001, and fatigue, F (1, 37) = 9.30, p < .01.
For the subjective sleep and daytime sleepiness measures ANCOVAs revealed no differences between the groups on ESS, F (1, 35) = 1.04, p = .32, but significantly more problems with sleep on PSQI in the caregiver group compared to the non-caregiver group, F (1, 35) = 16.40, p < .001. Moreover, the average PSQI score for the caregiver group was 8.31 (SD = 3.38), which is above the score of 5 indicating poor sleep quality on this instrument.18 Group means and standard deviations (SD) for each self-report measure are presented in Table 1.
Group Differences in Salivary Cortisol and Inflammatory Biomarkers
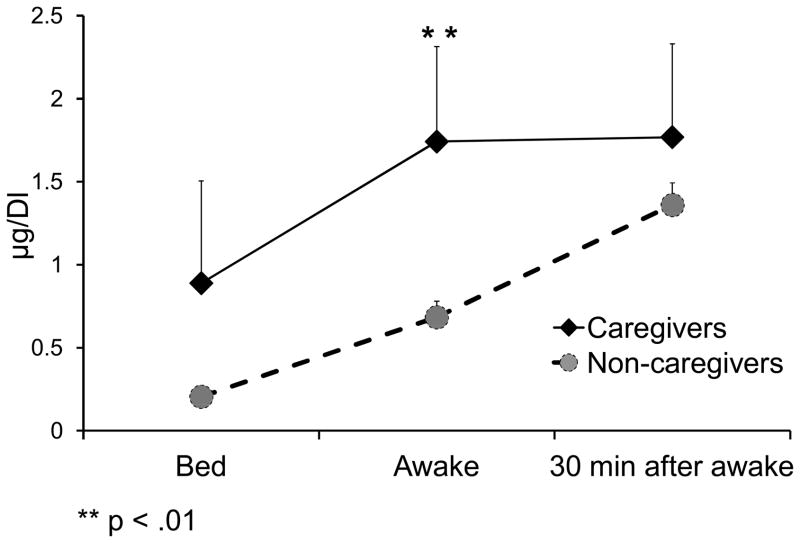
Caregivers had significantly higher mean salivary cortisol levels (in μg/dL) on awakening (1.7 ± 2.29) compared to non-caregivers, (0.68 ± 0.44), F (1, 34) = 11.26, p = .002. No other differences in salivary cortisol were observed (Figure 1). Further, levels of IL-6 (in pg/mL) were similar between caregivers (2.38 ±1.72) and non-caregivers (2.73 ± 2.61), F (1, 29) = 1.15, p = .29. There was also no difference between caregivers (1.09 ± 0.65) or non-caregivers, (2.39 ± 4.65) in TNF-α levels (in pg/mL), F (1, 29) = .07, p = .80. However, caregivers demonstrated significantly greater CRP levels (in mg/L) (3.96 ± 4.41) than did non-caregivers, (2.19 ± 3.56), F (1, 29) = 9.778, p = .004.
Figure 1.
Salivary cortisol levels
Group Differences in Objective Sleep Measures
Table 2 presents the between-group differences in objectively measured sleep quality. The primary analysis of objectively measured sleep outcomes demonstrated significant differences between the groups: The overall MANCOVA for the main effect of a group was statistically significant, F (1, 37) = 2.83, p = .03. Tests of between-subjects effects indicated no significant differences between the groups for the percent sleep time in Stage N2, F (1, 37) = 2.15, p = .15 or Stage N3, F (1, 37) = 2.80, p = .10. However, compared to non-caregivers, caregivers on average spent greater proportions of their sleep in Stage N1, F (1, 37) = 4.56, p = .04 but smaller proportions of their sleep in Stage R, F (1, 37) = 5.16, p = .03. Moreover, caregivers took significantly longer to fall asleep than did non-caregivers, F (1, 37) = 4.56, p = .04.
Table 2.
Group differences in objective sleep quality measures
| Caregivers, Mean (SD) n = 20 |
Non-caregivers, Mean (SD) n = 20 |
Sig. (p) | Partial η 2 | |
|---|---|---|---|---|
| primary analysis | ||||
| Sleep onset latency (min)* | 17.32 (21.18) | 8.67 (13.15) | .039 | .110 |
| TST in Stage N1 (%)* | 22.79 (10.64) | 17.80 (6.63) | .039 | .110 |
| TST in Stage N2 (%) | 48.00 (8.32) | 43.87 (8.97) | .151 | .055 |
| TST in Stage N3 (%) | 11.42 (8.68) | 15.47 (7.27) | .103 | .070 |
| TST in Stage R (%)* | 17.93 (8.54) | 22.94 (7.04) | .029 | .122 |
| Secondary analysis | ||||
| Time in bed (min) | 480.69 (101.82) | 473.16 (97.67) | .711 | .004 |
| Total sleep time (min) | 409.52 (98.24) | 428.71 (77.99) | .460 | .015 |
| Wake after sleep onset (min) | 53.85 (64.97) | 35.93 (28.09) | .459 | .015 |
| Arousals (#) | 51.30 (24.68) | 41.00 (13.17) | .221 | .040 |
| Sleep efficiency (%): NP | 85.27 (12.49) | 91.03 (4.01) | .330 | - |
p < .05
Abbreviations: NP = non-parametric test used due to violation of normality assumption, TST = total sleep time.
Secondary MANCOVA assessing basic sleep parameters showed that caregivers and non-caregivers had similar TIB, TST, WASO, arousals during sleep, and sleep efficiency.
Relationships Involving Sleep Quality Measures
Table 3 contains details of simple bivariate correlations among selected variables assessing stress, depressive symptoms, and sleep quality.
Table 3.
Simple correlations (r) among variables of stress, depression, and sleep quality
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. PSS score | – | .565*** | .821*** | .171 | .431** | .132 | .255 | .369* | −.329* | −.352* |
| 2. Salivary cortisol (wake) | – | – | .545*** | .317 T | .407* | .101 | .338* | .248 | −.358* | −.326 T |
| 3. CESD-10 score | – | – | – | .157 | .571*** | .150 | .269 T | .224 | −.187 | −.381* |
| 4. CRP | – | – | – | – | .220 | .278 | .312 T | .380* | −.439* | −.324 T |
| 5. PSQI overall score | – | – | – | – | – | .301 T | .256 | .021 | .026 | −.333* |
| 6. SOL | – | – | – | – | – | – | −.049 | .043 | −.081 | .096 |
| 7. % TST in N1 | – | – | – | – | – | – | – | −.029 | −.502*** | −.588*** |
| 8. % TST in N2 | – | – | – | – | – | – | – | – | −.569*** | −.478** |
| 9. % TST in N3 | – | – | – | – | – | – | – | – | – | .176 |
| 10. % TST in R | – | – | – | – | – | – | – | – | – |
.10 >p>.05,
p < .05,
p < .01,
p <= .001
Abbreviations: PSS = Perceived Stress Scale, CESD-10 = Center for Epidemiologic Studies Depression Scale (10-item version), CRP = C-reactive protein, PSQI = Pittsburgh Sleep Quality Index, SOL = sleep onset latency, TST = total sleep time.
As expected, subjective and objective sleep quality measures were related. In particular, subjective sleep quality was correlated with percent of TST in Stage R (r = −.33, p < .05).
Sleep measures were also correlated with perceived stress and cortisol levels. For example, perceived stress was related to percent TST in Stage N2 (r = .37, p = .02), Stage N3 (r = −.33, p = .04), and Stage R (r = −.35, p = .03). Similarly, awakening cortisol level was related to percent TST in Stage N1 (r = .34, p = .04), Stage N3 (r = −.36, p = .03), and Stage R (r = −.33, p = .05).
Next, inflammatory biomarkers such as CRP were related to sleep architecture variables. Specifically, CRP levels were associated with percent TST in Stage 2 (r = .38, p = .03) and Stage N3 (r = −.44, p = .01).
Besides correlations involving stress-related variables and biomarkers of stress and inflammation, both subjective and objective sleep quality measures were associated with depressive symptoms. In particular, depressive symptoms were positively related to overall PSQI scores (r = .57, p < .001) and negatively related to percent TST in Stage R (r = −.38, p = .02).
Models Predicting Sleep Quality
We conducted three hierarchical multiple regression analyses to predict each of the three objective sleep quality measures that were significantly different between caregivers and non-caregivers: SOL, percent TST in Stage N1, and percent TST in Stage R. The results of the analyses are presented in Table 4.
Table 4.
Results of hierarchical multiple regression analyses for primary outcome sleep variables different between groups
| Outcome | Predictor | rs | β | Adj R2 | ΔR2 | p value for ΔR2 change |
|---|---|---|---|---|---|---|
| SOL | Age | .152 | .154 | −.016 | .010 | .535 |
| Overall model | Group membership | .285 | .356 | .071 | .109 | .039 |
| p = .199 | CESD score | −.030 | −.037 | .046 | .001 | .851 |
| Percent TST in Stage N1 | Age | .303 | .308 | .033 | .058 | .134 |
| Overall model | Group membership | .153 | .191 | .116 | .103 | .039 |
| p = .047 | CESD score | .186 | .231 | .129 | .034 | .222 |
| Percent TST in Stage R | Age | −.242 | −.246 | .003 | .029 | .296 |
| Overall model | Group membership | −.129 | −.161 | .102 | .119 | .029 |
| p = .031 | CESD score | −.261 | −.324 | .150 | .068 | .086 |
Abbreviations: rs = semi-partial correlation; Adj R2 = adjusted R2; ΔR2 = R2 change
Significant results for overall model and ΔR2 are in bold font.
The overall regression for predicting SOL was not statistically significant, R = .35, R2 = .12, adjusted R2 = .05, F (3, 36) = 1.63, p = .20, indicating that none of the predictors accounted for the significant proportion of variance in the outcome variable.
For the model predicting percent of TST in Stage N1, the overall regression including all predictors (age, group membership, and depressive symptoms) was significant, R = .44, R2 = .20, adjusted R2 = .13, F (3, 36) = 2.92, p < .05. The t ratios for individual regression slopes were examined for the variables in the step where they first entered the analysis. In step 1 age did not reach significance, t (38) = 1.53, p = .13; R2 increment for the step was .06. Being in a caregiver group in the next step significantly increased the R2, t (37) = 2.14, p = .04, R2 inc = .10. Finally, CESD-10 score entered in step 3 did not significantly increase the R2, t (36) = 1.24, p = .22, R2 inc = .03 suggesting that depressive symptoms were not significantly contributing to the overall variance predicted for TST in Stage N1 beyond the variance predicted by age and group membership.
For the model predicting percent TST in Stage R, the overall regression including all predictors was significant, R = .46, R2 = .22, adjusted R2 = .15, F (3, 36) = 3.30, p = .03. The t ratios for individual regression slopes were examined for the variables in the step where they first entered the analysis. In step 1 age did not reach significance, t (38) = −1.06, p = .30; R2 increment for the step was .03. Being in a caregiver group in the next step significantly increased the R2, t (37) = −2.27, p = .03, R2 inc = .12. Finally, CESD-10 score entered in step 3 indicated a non-significant trend to increase the R2, t (36) = −1.77, p = .09, R2 inc = .07.
The models predicting percent of TST in Stage N1 and percent of TST in Stage R suggested that depressive symptoms did not significantly contribute to the overall variance beyond the variance predicted by age and group membership. However, it is important to note that there was a high degree of shared variance between the variables used in steps 2 and 3 (group membership and depressive symptoms, respectively), and this presence of high covariance might complicate interpretation of the results.
Discussion
This study indicated that commonly reported subjective complaints of poor sleep quality in caregivers might be explained by objectively measured sleep architecture changes in this population. Specifically, using an ambulatory home PSG recording and comparing sleep architecture between caregivers and non-caregivers we demonstrated that caregivers spent less proportion of their sleep in restorative sleep stages compared to non-caregivers, an important finding that hasn’t been previously reported.
Low sleep quality in caregivers is a real problem, not a misperception
Our results added to the evidence indicating lower sleep quality in caregivers,1, 5, 7, 9, 30 with levels of sleep disturbance severity that are comparable to those typically endorsed by insomnia sufferers.5 To date lower sleep quality in caregivers has been confirmed not only by subjective but also by objective sleep assessments. An actigraphic study reported that older dementia caregivers demonstrated worse sleep, characterized by fewer minutes asleep and longer SOLs than non-caregivers.8 Studies using PSG indicated shorter sleep duration along with greater sleep disturbances (more WASO and poorer sleep efficiency) in caregivers compared to non-caregivers.1 In agreement with some of the previous findings, our results indicated that caregivers take longer to fall asleep than non-caregivers. However, though caregivers in our study also had less sleep time, greater WASO, and lower sleep efficiency than non-caregivers, the group differences on these measures were not statistically significant. Small sample size used in our study might be one potential explanation for this lack of statistical significance.
The results of investigations demonstrating lower sleep quality in caregivers have been challenged by researchers observing no differences in sleep quality between caregivers and non-caregivers.3, 10 Because caregivers in these studies did not differ from non-caregivers on objective sleep quality measures but subjectively rated their sleep quality lower than non-caregivers did, it was proposed that caregivers might be misperceiving their sleep quality and exaggerating their sleep problems. Our findings argue against this proposed “misperception” of sleep quality by caregivers. Our group of caregivers also did not significantly differ from non-caregivers on TST, WASO, or sleep efficiency; however, caregivers in our study spent proportionally more sleep in the less restful stage N1 and less sleep in Stage R. Further, in our sample perceived sleep quality was associated with objectively measured sleep variables, such as percent TST in Stages N2 and R. Therefore, caregivers’ subjective reports of poor sleep quality might in fact reflect an adequate perception of poor sleep resulting from less sleep time in restorative sleep stages. Because the finding of differences in sleep architecture between caregivers and non-caregivers has not been consistently reported in previous research, more studies assessing caregivers’ sleep with objective measures including ambulatory PSG are needed to confirm this proposition.
Methodological issues in sleep assessment
Methodological issues are important to consider in interpreting research results on the topic published to date. Study samples, inclusion criteria, sleep assessment methods, number of nights assessed, and other variables might affect findings significantly. As a rule, the studies suggesting poorer caregiver sleep quality tend to have larger sample sizes and, therefore, greater power to detect group differences. In contrast, researchers failing to report any differences in sleep quality between caregivers and controls used small samples.10 Interestingly, however, though the sample was relatively small, this above mentioned study10 utilized three nights of ambulatory PSG recording, but the majority of the studies using PSG1, 7 including ours relied on a single recording night. While previous research suggested that first night effect is minimal when using ambulatory PSG, and no significant differences over three nights on any PSG sleep parameters were observed when ambulatory PSG was used in subjects’ own homes,15 it is possible that several recording nights might be beneficial when assessing sleep with PSG.
To the best of our knowledge differences in sleep architecture between caregivers and non-caregivers have not been previously reported; however, sleep architecture has been compared between caregivers and non-caregivers in previous studies.1, 7, 10 What are the possible reasons for the differences in findings related to sleep architecture between these studies and our study? First, age and gender emerged as important factors when assessing sleep,1 with increasing age and male gender being associated with poorer sleep quality. Therefore it might be critical to match caregiver and non-caregiver groups on these variables. Further, although the sleep quality was a primary outcome for the mentioned-above studies, participants were not always consistently screened for sleep disorders1, 7, which might have made the results of some investigations less interpretable.
In conducting our study we took into consideration the lessons learned from previous research. We recruited generally healthy participants who were screened by self-report for sleep impairments, excluding those with diagnosed sleep disorders, or with serious sleep complaints from participation. Additionally, we recruited participants who were matched on gender and age.
Importantly, the ambulatory PSG system used in our study allowed assessment of sleep including sleep architecture in caregivers’ own homes, a habitual environment, possibly resulting in a more valid sleep quality assessment. Of note, to measure activity during sleep we used two accelerometers placed on a participant’s head and arm rather than chin electromyography to produce less discomfort during the ambulatory and sleep recording. Additionally, while other researchers1, 7, 10 prepared the sleep recording equipment in the evening at participants’ homes, we set up all the recording equipment in the laboratory during the afternoon visit, which allowed our participants to acclimate to the device before sleep recording. Though wearing the device and having electrodes on one’s head and upper body might have interfered with some aspects of participants’ routine (e.g. ability to shower), our participants reported no significant interference of the device with any other activities including their sleep.
Sleep quality and general health
Understanding sleep issues in caregivers deserves close attention. Recently sleep has been called a “new vital sign“3, 31 with poor sleep linked to endocrine changes and immune dysfunction,32, 33 cognitive deficits,34–36 cardiovascular risk,1 and other health issues.31 Adding to this evidence, we demonstrated that caregivers showing lower sleep quality had increased salivary cortisol on awakening. Next, compared to non-caregivers, caregivers in our study had greater CRP levels, and the CRP levels were positively related to the percentage of TST spent in Stage N2 and negatively related to percentage of TST spent in Stage N3. Thus the evidence linking caregiving to decreased sleep quality associated with indicators of general health and physiologic functioning is accumulating.
Current study limitations
Several limitations of our study must be taken into consideration when interpreting our results. First, the study sample size was small, which could have led to insufficient power to detect significant group differences in some variables of interest. Further, we did not make any adjustments for multiple comparisons when analyzing study data, which could have resulted in inflated Type I error. However, some or our primary results would have been significant even with a conservative Boneferroni adjustment. Next, our caregivers were recruited as part of a clinical trial for stress reduction, and all of them reported being stressed. Though it has been documented that caregivers of relatives with dementia commonly experience stress37, 38 our caregiver sample might not be entirely representative of general caregiver population. Additionally, we did not obtain the data about caregivers’ sleeping arrangements that might have impacted the sleep quality in this group. However, understanding the causes of lower sleep quality in caregivers was not a primary focus of the study.
Further, our results might have been influenced by the increased presence of depressive symptoms that are common in caregivers and may affect sleep quality.6, 10, 30 Even though people with current untreated depression were excluded from the study, those with a history of depression or stable on SSRIs could participate. Notably, caregivers in our sample endorsed significantly more depressive symptoms than non-caregivers, similar to previous research,10, 30, 39 and depressive symptoms were strongly correlated with sleep measures (Tables 3 and 4). Hierarchical linear regression analyses indicated that depressive symptoms did not account for a significant proportion of variance beyond the variance accounted for by age and group membership for any of the sleep quality measures. However, high covariance between group membership and depressive symptoms makes the interpretation of the results difficult, and the role depressive symptoms might play in understanding sleep quality in caregivers should be investigated further. Future studies assessing sleep in caregivers and non-caregivers might benefit from including a group of depressed non-caregivers for elucidating effects of depressive symptoms on caregiver sleep quality.
Next, the PSG device used in our study did not include a commonly used EMG channel and used accelerometers instead. In particular, this modification might have influenced the accuracy of Stage R scoring in our study. While our device’s montage might be slightly different from the full clinical PSG montage prohibiting us from diagnosing specific sleep problems, it allowed us to obtain the general information about the sleep architecture that was the primary focus of the study. It is important to note, however, that currently there are no data available to draw direct comparisons between our PSG device and a clinical PSG system.
Lastly, the results for the inflammatory biomarkers for our study were based on a single collection which is not ideal for the measures displaying diurnal variability, and the limited reliability of a single collection might be viewed as a limitation in our study. However, our samples have been collected within a reasonably small window and recent analysis of reproducibility of some measures used in our study indicated an acceptable within-individual reproducibility of these measures.40
In conclusion, our study added to the evidence indicating lower sleep quality in caregivers compared to non-caregivers, and to the best of our knowledge our findings are the first to indicate that caregivers differ from non-caregivers on sleep architecture. Future studies assessing sleep architecture in a larger sample of caregivers are needed to confirm our results. Understanding specific sleep problems in caregivers might be an important step to creating interventions to promote health and well being in this population.
Acknowledgments
This research was conducted at the Oregon Health & Science University and supported by NIH grants U19AT002656, T32AT002688, K24AT005121, P30AG008017 and UL1RR024140.
This research was supported by National Institutes of Health (NIH) grants U19AT002656, T32AT002688, K24AT005121, P30AG008017, and UL1RR024140. We thank Helane’ Wahbeh for help conducting the study and Andy Fish for administrative support.
Footnotes
Some of the data from the manuscript were presented in a poster at the American Psychosomatic Society 68th Annual Scientific Meeting, March 10 - 14, 2010, in Portland, OR.
Contributor Information
Irina Fonareva, Department of Behavioral Neuroscience, Oregon Health & Science University, Portland, OR 97239, USA
Alexandra M. Amen, Department of Neurology, Oregon Health & Science University, Portland, OR 97239, USA
Daniel P. Zajdel, Department of Neurology, Oregon Health & Science University, Portland, OR 97239, USA
Roger M. Ellingson, Department of Neurology, Oregon Health & Science University, Portland, OR 97239, USA
Barry S. Oken, Departments of Neurology and Behavioral Neuroscience, Oregon Health & Science University, Portland, OR 97239, USA
References
- 1.Mills PJ, Ancoli-Israel S, von Kanel R, et al. Effects of gender and dementia severity on Alzheimer’s disease caregivers’ sleep and biomarkers of coagulation and inflammation. Brain Behav Immun. 2009;23(5):605–610. doi: 10.1016/j.bbi.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Kanel R, Ancoli-Israel S, Dimsdale JE, et al. Sleep and biomarkers of atherosclerosis in elderly Alzheimer caregivers and controls. Gerontology. 2010;56(1):41–50. doi: 10.1159/000264654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCurry SM, Gibbons LE, Logsdon RG, et al. Insomnia In Caregivers Of Persons With Dementia: Who Is At Risk And What Can Be Done About It? Sleep Med Clin. 2009;4(4):519–526. doi: 10.1016/j.jsmc.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: contributing factors and treatment implications. Sleep Med Rev. 2007;11(2):143–153. doi: 10.1016/j.smrv.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilcox S, King AC. Sleep complaints in older women who are family caregivers. J Gerontol B Psychol Sci Soc Sci. 1999;54(3):P189–198. doi: 10.1093/geronb/54b.3.p189. [DOI] [PubMed] [Google Scholar]
- 6.Beaudreau SA, Spira AP, Gray HL, et al. The relationship between objectively measured sleep disturbance and dementia family caregiver distress and burden. J Geriatr Psychiatry Neurol. 2008;21(3):159–165. doi: 10.1177/0891988708316857. [DOI] [PubMed] [Google Scholar]
- 7.McKibbin CL, Ancoli-Israel S, Dimsdale J, et al. Sleep in spousal caregivers of people with Alzheimer’s disease. Sleep. 2005;28(10):1245–1250. doi: 10.1093/sleep/28.10.1245. [DOI] [PubMed] [Google Scholar]
- 8.Rowe MA, McCrae CS, Campbell JM, et al. Sleep pattern differences between older adult dementia caregivers and older adult noncaregivers using objective and subjective measures. J Clin Sleep Med. 2008;4(4):362–369. [PMC free article] [PubMed] [Google Scholar]
- 9.Teel CS, Press AN. Fatigue among elders in caregiving and noncaregiving roles. West J Nurs Res. 1999;21(4):498–514. doi: 10.1177/01939459922044009. discussion 514–420. [DOI] [PubMed] [Google Scholar]
- 10.Castro CM, Lee KA, Bliwise DL, et al. Sleep patterns and sleep-related factors between caregiving and non-caregiving women. Behav Sleep Med. 2009;7(3):164–179. doi: 10.1080/15402000902976713. [DOI] [PubMed] [Google Scholar]
- 11.Morgenthaler TI, Lee-Chiong T, Alessi C, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30(11):1445–1459. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoekert M, der Lek RF, Swaab DF, et al. Comparison between informant-observed and actigraphic assessments of sleep-wake rhythm disturbances in demented residents of homes for the elderly. Am J Geriatr Psychiatry. 2006;14(2):104–111. doi: 10.1097/01.JGP.0000192481.27931.c5. [DOI] [PubMed] [Google Scholar]
- 13.Sivertsen B, Omvik S, Havik OE, et al. A comparison of actigraphy and polysomnography in older adults treated for chronic primary insomnia. Sleep. 2006;29(10):1353–1358. doi: 10.1093/sleep/29.10.1353. [DOI] [PubMed] [Google Scholar]
- 14.Conradt R, Brandenburg U, Ploch T, Peter JH. Actigraphy: methodological limits for evaluation of sleep stages and sleep structure of healthy probands. Pneumologie. 1997;51 (Suppl 3):721–724. [PubMed] [Google Scholar]
- 15.Sharpley AL, Solomon RA, Cowen PJ. Evaluation of first night effect using ambulatory monitoring and automatic sleep stage analysis. Sleep. 1988;11(3):273–276. doi: 10.1093/sleep/11.3.273. [DOI] [PubMed] [Google Scholar]
- 16.Ellingson RM, Eriksen K, Schaller JJ, et al. Second generation complementary and alternative medicine physiologic data collection and monitoring research platform. Conf Proc IEEE Eng Med Biol Soc. 2008:1270–1273. doi: 10.1109/IEMBS.2008.4649395. [DOI] [PubMed] [Google Scholar]
- 17.von Kanel R, Dimsdale JE, Mills PJ, et al. Effect of Alzheimer caregiving stress and age on frailty markers interleukin-6, C-reactive protein, and D-dimer. J Gerontol A Biol Sci Med Sci. 2006;61(9):963–969. doi: 10.1093/gerona/61.9.963. [DOI] [PubMed] [Google Scholar]
- 18.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry, Neurophysiology, and Behavioral Neurology. 1993;6:103–110. [Google Scholar]
- 19.Oken BS, Fonareva I, Haas M, et al. Pilot controlled trial of mindfulness meditation and education for dementia caregivers. J Altern Complement Med. 2010;16(10):1031–1038. doi: 10.1089/acm.2009.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 22.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 23.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 24.Ware JE, Snow KK, Kosinski M, Gandek B, THI . SF-36R Health Survey Manual and Interpretation Guide. New England Medical Center; Boston, MA: 1993. [Google Scholar]
- 25.Teri L, Truax P, Logsdon R, et al. Assessment of behavioral problems in dementia: the revised memory and behavior problems checklist. Psychol Aging. 1992;7(4):622–631. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- 26.Wahbeh H, Kishiyama SS, Zajdel D, Oken BS. Salivary cortisol awakening response in mild Alzheimer disease, caregivers, and noncaregivers. Alzheimer Dis Assoc Disord. 2008;22(2):181–183. doi: 10.1097/WAD.0b013e31815a9dff. [DOI] [PubMed] [Google Scholar]
- 27.American EEG Society guidelines in EEG and evoked potentials. Guideline I: minimum technical requirements for performing clinical electroencephalography. J Clin Neurophysiol. 1994 [Google Scholar]
- 28.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, Ill: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 29.Fonareva IG, Amen AM, Zajdel DP, et al. Cognitive Processes in Older Adults: Potential Impact of Stress and Physiological Functioning. Poster presentation at the Cognitive Neuroscience Society Annual meeting; San Francisco, CA. 2009. [Google Scholar]
- 30.Kochar J, Fredman L, Stone KL, Cauley JA. Sleep problems in elderly women caregivers depend on the level of depressive symptoms: results of the Caregiver--Study of Osteoporotic Fractures. J Am Geriatr Soc. 2007;55(12):2003–2009. doi: 10.1111/j.1532-5415.2007.01434.x. [DOI] [PubMed] [Google Scholar]
- 31.Wilson JF. Is sleep the new vital sign? Ann Intern Med. 2005;142(10):877–880. doi: 10.7326/0003-4819-142-10-200505170-00026. [DOI] [PubMed] [Google Scholar]
- 32.Bryant PA, Trinder J, Curtis N. Sick and tired: Does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4(6):457–467. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- 33.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 34.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49(9):1185–1189. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 35.Jelicic M, Bosma H, Ponds RW, et al. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS) Int J Geriatr Psychiatry. 2002;17(1):73–77. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- 36.Lee S, Kawachi I, Grodstein F. Does caregiving stress affect cognitive function in older women? J Nerv Ment Dis. 2004;192(1):51–57. doi: 10.1097/01.nmd.0000106000.02232.30. [DOI] [PubMed] [Google Scholar]
- 37.Aguglia E, Onor ML, Trevisiol M, et al. Stress in the caregivers of Alzheimer’s patients: an experimental investigation in Italy. Am J Alzheimers Dis Other Demen. 2004;19(4):248–252. doi: 10.1177/153331750401900403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoskins S, Coleman M, McNeely D. Stress in carers of individuals with dementia and Community Mental Health Teams: an uncontrolled evaluation study. J Adv Nurs. 2005;50(3):325–333. doi: 10.1111/j.1365-2648.2005.03396.x. [DOI] [PubMed] [Google Scholar]
- 39.Pinquart M, Sorensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging. 2003;18(2):250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- 40.Karakas M, Baumert J, Greven S, et al. Reproducibility in serial C-reactive protein and interleukin-6 measurements in post-myocardial infarction patients: results from the AIRGENE study. Clin Chem. 2010;56(5):861–864. doi: 10.1373/clinchem.2010.143719. [DOI] [PubMed] [Google Scholar]