Abstract
This paper reviews how biomedical engineers, in collaboration with physicians, biologists, chemists, physicists and mathematicians, have developed models to explain how the impact of vascular interventions on blood flow predicts subsequent vascular repair. These models have become increasingly sophisticated and precise - propelling us towards optimization of cardiovascular therapeutics in general and personalizing treatments for patients with cardiovascular disease.
Keywords: atherosclerosis, endothelium, blood flow
Atherosclerosis, the most common cause of disease and death in the developed world, affects all arterial beds. The clinical treatment for atherosclerosis has evolved tremendously over many decades, with percutaneous interventions such as stents and/or grafts becoming almost a commodity in patient care. Despite technological improvements, restenosis, and stent and graft thrombosis continue to hamper the success of the implants. It has been suggested that only modifications of interventions personalized to individual state and vascular geometries can prevent flow- and drug-related post-implantation complications. Several in vitro and ex vivo models have been developed to explain partially the molecular mechanisms of atherogenesis, thrombosis or restenosis. It is now possible to tailor these models to include patient-specific characteristics in the search for personalized solutions.
In vitro flow chambers
The endothelium is a unique organ. As a monolayer of cells exposed to blood flow, the endothelium expresses an ordered array of biochemical regulators that ensure blood fluidity along and through the vessels, nutrient transport and an appropriate control of coagulation if the lining integrity is compromised. Endothelial cells are constantly in direct contact with blood flow and are flow-sensitive. Hence, the expression of proteins is triggered by and depends upon local hemodynamics. As the vascular system is a closed universe, the interaction between flow and the endothelium can be studied in a specific fashion and controlled environments using in vitro and ex vivo model systems––flow chambers.
In 2002, Blackman, García-Cardeña and Gimbrone introduced the Dynamic Flow System (DFS)1 as a culmination of decades of research on the effects of flow on endothelial cell biology.2, 3 The DFS consists of tissue culture wells seeded with functional endothelial cells, under controlled humidity, temperature and CO2 levels and a medium feeding system. A conical device coupled to a motor rotates and generates a controlled flow over cultured endothelial cells. Endothelial cells in static conditions act differently than those subject to flow. Arterial flow reshapes the cytoskeleton, and reduces eNOS mRNA expression. Later, Dr. Gimbrone’s group used the DFS to define athero-prone and athero-protective waveforms4 and showed how endothelial cells exposed to athero-prone waveforms expressed a pro-inflammatory phenotype, including increase of NF-κB, VCAM and e-Selectin, among others. Our group reported similar findings in 2005 using a different flow chamber, proving that the activity of eNOS and production of prostaglandins was dependent on flow frequency5. Here endothelial cells were seeded in silicone tubular structures and connected to a perfusion loop. Media circulated through the system using a programmable pump that delivered well-defined and physiologically-matched velocities and shear stresses.
Ex vivo flow chambers
Much earlier, Badimon et al. designed the first ex vivo chamber,6 using de-endothelialized swine arteries, to prove the relevance of shear rates on platelet deposition. Replacing the de-endothelialized artery by a collagen strip proved the key role of collagen in post-interventional thrombogenicity. This 1986 ex vivo model is still a valid tool to evaluate the effects of anticoagulant drugs7 or other thrombogenicity relevant questions. This model, however, does not recapitulate the interaction between smooth muscle cells and the remaining endothelial cells nor the full architecture of the vessel. Another interesting approach was introduced in 2002, when Kolandaivelu and Edelman designed an ex vivo system to study stent thrombogenicity.8, 9 A peristaltic pump is substituted by a rotor and the inertial flow imposed is customizable so the waveforms are totally controlled. This model has been used to study and optimize stent designs and enlighten different aspects of stent thrombosis.10
Cellular co-culture
Endothelial cells are not only in direct contact with blood flow but interact with their underlying cellular neighbors. Many of the questions one may ask cannot be answered if considering one cell type alone. Balcells et al. proved that the mTOR pathway in endothelial cells is promoted by alterations in flow, but to a far lesser extent when intact vascular smooth muscle cells were present in the system. These findings confirm the importance of the big picture in understanding endothelial function. At a microscopic scale, several groups have applied surface patterning and engineered test chambers to study cell-cell crosstalk. A number of strategies have been developed; Chen’s bow-ties or Bhatia’s micropatterned cocultures are good examples. Chen and colleagues showed how VE-cadherin is responsible for contact growth inhibition and the role of this molecule in regulating how cells adhere to the extracellular matrix.11 Bhatia and colleagues engineered several microscale devices to study cell-cell interaction in liver cells. Liver sinusoidal endothelial cells (LSEC) have a very particular phenotype that is hard to maintain in vitro. To maintain phenotype, Bhatia’s group co-cultured LSECs with hepatocytes and fibroblasts on collagen.12 These findings suggest that the optimal strategy to create a physiologically relevant in vitro model depends on the cell type and the in vivo micro- and macro-environment simulated. There is no unique solution to how many variables should be included when engineering biological models.
Computational models
Computational models have gained increasing acceptance as valuable tools to predict physical, chemical and biological phenomena in arteries. Computational fluid dynamics (CFD) simulations can predict with micrometric precision several physical and biological variables as velocity, shear stress and drug distribution profiles. This is particularly important in stent design, where biological analytical methods cannot provide the required precision to define micrometer based events. There is an increasing interest in understanding how micro-alterations and separation in flow caused by stent struts may affect local drug delivery13, 14 and stent thrombogenicity10 Recent studies15 couple the computational results with in vivo experience, but this is only possible when classic mass transport equations and molecule-specific uptake kinetics are considered together, and with accurate input parameters derived and validated from in vivo conditions15, 16 CFD simulations are limited by their input validity, but when validated, they offer a platform for repeatable, quantifiable assay of effects across a spectrum of dependent parameters, device and formulation modifications, and environmental conditions––a feature unachievable with biological and animal testing. Computational models have become so helpful that FDA is considering their use when approving new medical devices.17
Materials science and vascular grafts
Ever since Voorhees et al. replaced diseased blood vessels with tubes of synthetic fabric18 in 1952, there have been numerous attempts to design a vascular graft that not only matches the mechanical properties of the native vasculature, but also promotes cell growth, facilitates extracellular matrix production and inhibits thrombogenicity. Approximately 1.4 million US patients per year require arterial prostheses19. Synthetic polymer materials, especially expanded polytetrafluoroethylene (ePTFE) and polyethylene terephtalate (Dacron®), are the dominant materials used for applications requiring large-diameter (>6 mm) vessels.20 In smaller diameter grafts thrombogenicity and compliance mismatch re-occlude the bypassed vessel,21, 22 limiting the use of synthetic grafts in applications such as coronary artery bypass surgeries (one third of the arterial prosthesis procedures performed each year). In these cases, autologous vasculature is used unless precluded by disease, trauma or anatomic abnormalities.19 The high occlusion rates of synthetic polymer materials has motivated further strategies to functionalize the luminal surface of graft materials such as coatings, chemical and protein modifications and endothelial cell seeding. Although there have been some promising results, synthetic grafts still induce chronic inflammation and thrombogenicity.23 More recently, tissue-engineered vascular grafts (TEVG) have emerged to overcome the limitations of synthetic grafts. In TEVG, a synthetic or natural biodegradable material acts as a scaffold where vascular cells are seeded. The extracellular matrix produced by the cells gradually replaces the degrading scaffold. The matrix created––decellularized or not––is then implanted to the patient.20 This technology provides more favorable hemodynamics, mechanical properties and suture force retention than previous synthetic grafts.24 Polyglycolic acid (PGA) is the preferred scaffold material as it is biocompatible and safely degrades through hydrolysis. Biomaterials engineers may adapt the hydrolysis kinetics to the specific patient needs by copolymerization with other polymers. Although tissue-engineered vascular grafts have yielded promising results; the long culture periods required, the low proliferative capacity of cells isolated from elderly patients and the mechanical immaturity of the vessels at the time of in vivo implantation remain major drawbacks.
Future approaches to engineer arterial models
We presented a novel engineered arterial model that provides a personalized platform for cardiovascular architecture and lesion morphology. Three-dimensional images of patient arterial structures are reproduced using CATIA®, a computer aided design commercial software, and saved as IGS, CAM or STL files (Fig. 1), providing the precise coordinates of a given arterial bifurcation. CAM image files are used to create Teflon® molds to manufacture PDMS scaffolds in forms and shapes that mimic the specific vascular bed (Fig. 2). The PDMS scaffolds are coated with fibronectin as extracellular matrix protein and subsequently, layer-by-layer assembled with all three cellular components of the arterial wall: fibroblasts, smooth muscle cells and endothelial cells. Using the STL files, a support is 3D printed so the PDMS scaffolds may be fit in their tridimensional physiological position and the full architecture of the vessel recapitulated. Using the IGS files, we perform CFD simulations to predict the map of velocities and shear stresses along the bifurcations.
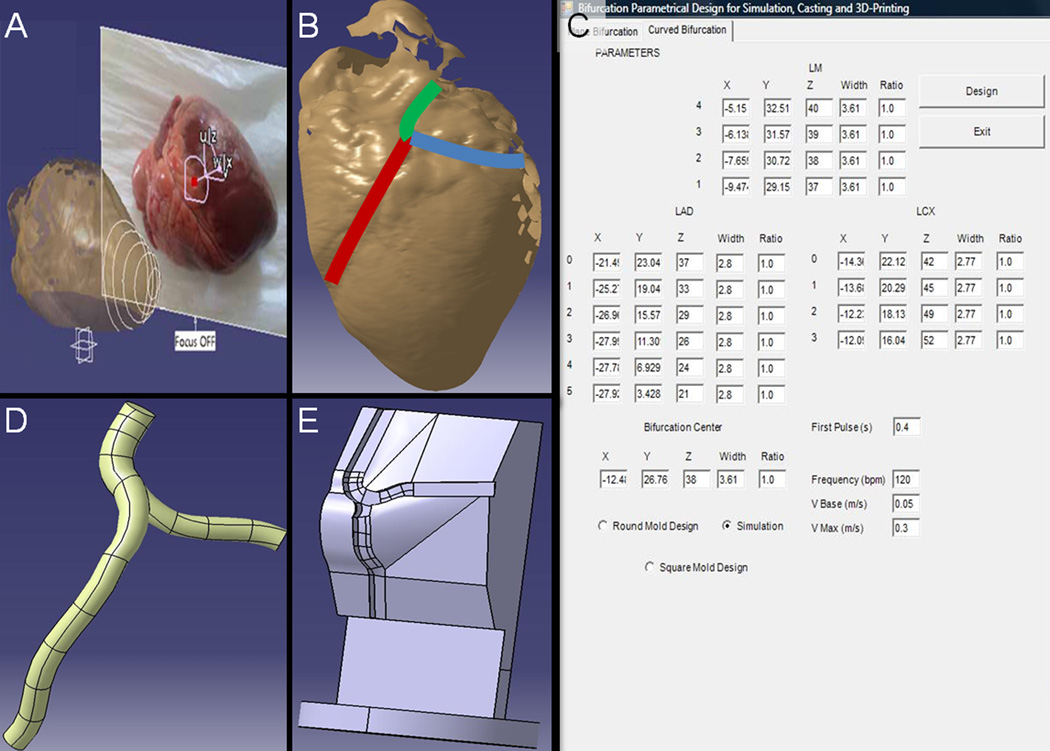
Figure 1.
A computational platform for design of scaffold-supports and flexible polymer constructs that reproduces complex individual arterial geometries and their underlying myocardium. (A) Swine hearts scanned using a Roland Active Piezo Sensor 3D laser scanner. A physician or pathologist defines where along the length of the artery of the scanned image (B) dimensions will be obtained and borders defined. The density of the determinations defines an effective mesh size for the reconstruction and allows for increased precision at specific areas of interest, e.g. at bifurcations. The coordinates that are determined represent arterial dimensions and are introduced in a custom designed Visual Basic® interface (C), which encodes up to four different macro files for CATIA®, ending up in IGS, STL and CAM files. Each file is used for a different aspect of reconstruction. The IGS file (D) is used for computational fluid dynamics simulation in TDyn®, the CAM file is used to drive formulation of Teflon® molds that will be used to mold PDMS scaffolds. STL files allow for direct 3D printing of the selected arterial architecture as a flexible polymer structure and their curved acrylonitrile-butadiene-styrene (ABS) support that mimics the contour established by the underlying myocardium (E).
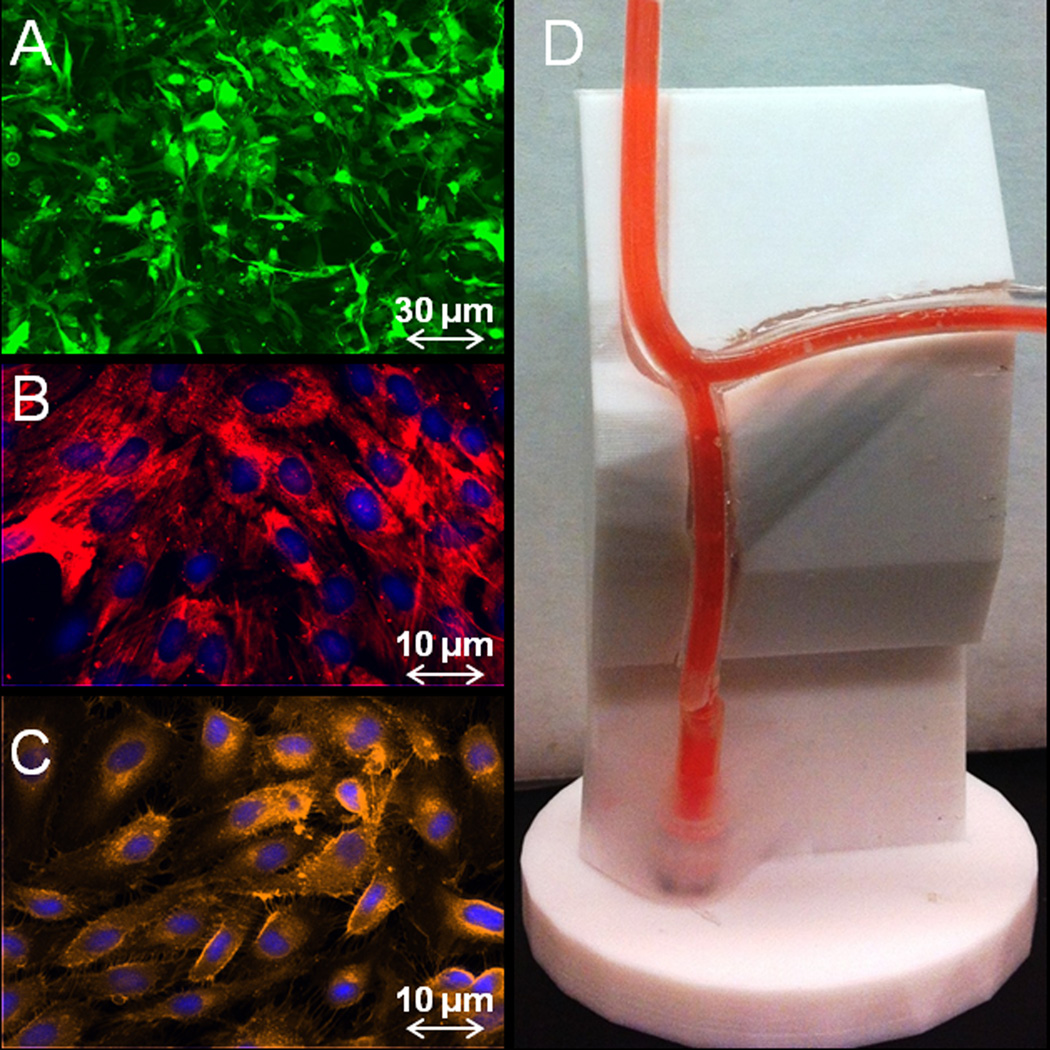
Figure 2.
A trilaminate cell-lined tubular structure is created from three human cell types, aortic adventitial fibroblasts (A; GFP retrovirus, green), aortic smooth muscle cells (B; smooth muscle cell α-actin, red) and coronary artery endothelial cells (C; PECAM-1, orange). Cell layers are created sequentially by seeding in PDMS scaffolds (D) that are upheld on an ABS support that recapitulates the exact architecture of the scanned heart.
Our engineered arterial model serves as a platform for experiments that can help answer an array of biological and clinically relevant questions. The ability to control flow enables examination and definition of the complete shear stress map imposed by the architecture of the vessel and, simultaneously, description of the molecular signaling interaction between cells upstream and downstream of a vascular region with flow disruptions. The cell-seeded scaffolds have also been used to estimate monocyte attachment and thrombogenicity ex vivo. For in vitro testing of stents, the model provides flexibility to evaluate thrombogenicity and immune response as a function of their design (both from the mechanical and the chemical point of view) and their position along the bifurcation. Coupled with mechanical strain and testing, the model can help elucidate mechanisms of stent fracture. The co-culture of vascular cells gives the scaffolds a unique perspective of arterial physiology by combining the effects of flow gradients and inter-cellular signaling. The integration of CFD into these models (Fig. 3) allows our simulations to evaluate the impact of vascular architecture on flow, and hence guide predictions about where wall shear stress gradients and flow disruptions arise. The correlation of clinical events with serial simulations driven by time-dependent dimensional changed in CVD patient images will help establish a solid model that correlates fluid flow disruptions and biomarkers predicting CVD. As a scaffold, the platform has the potential of casting bio-implantable vascular grafts. Once the geometry of the patient has been studied, the casted graft would have the optimal fluid dynamic conditions to minimize the graft’s risks like thrombosis or restenosis.
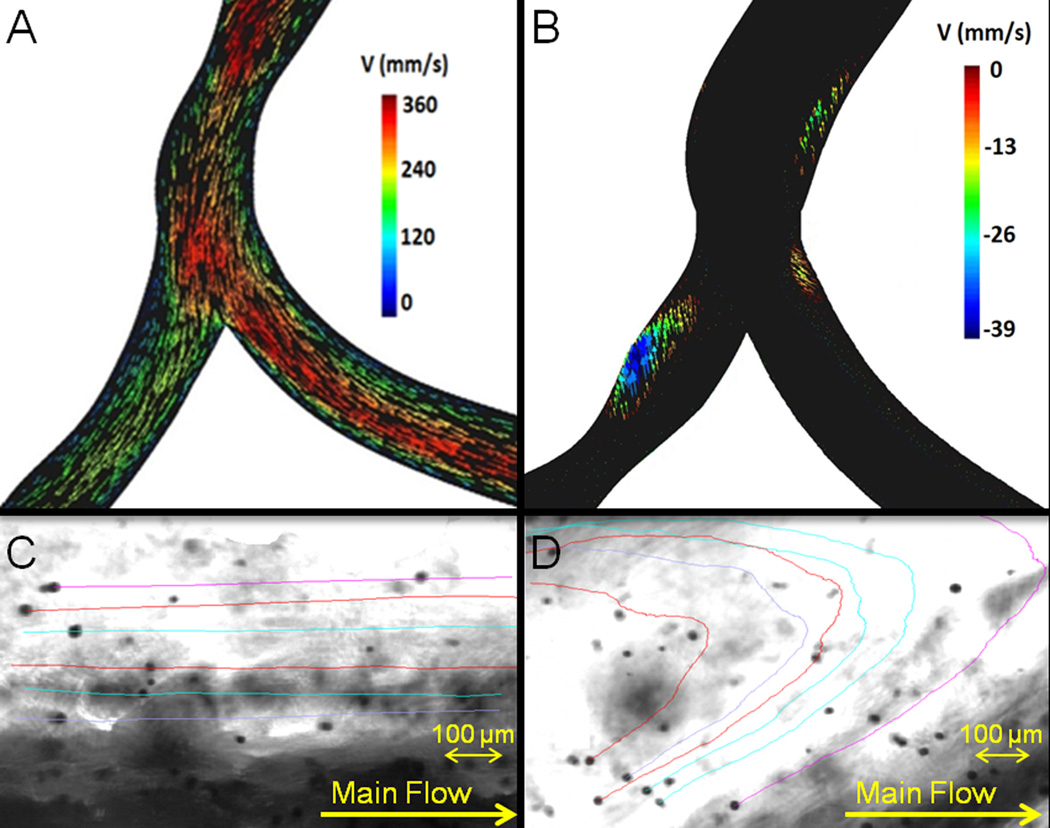
Figure 3.
Computational fluid dynamics (CFD) simulations for a scanned model of a swine left coronary artery bifurcation. Results are vector maps in mm/s at the instant of a pulse (A) and 0.005 seconds after the pulse (B). Patterns of flow stagnation and recirculation in the vicinity of the branch point with a minimal speed of −38mm/s. Results were confirmed by tracking microparticles flowing through the PDMS bifurcated scaffolds, using a 295 fps camera connected to an optical microscope. Microparticles with their associated trajectories in a high shear stress laminar steady flow region (C) and a low shear stress recirculation region (D).
Our model combines the most advanced available technologies to recapitulate and study the macro- and micro-environment of vascular cells in specialized geometries, such as bifurcations. The unification of those tools under a single platform may help predict the outcome of a given drug or intervention in a patient-specific manner, so that patients can receive fully personalized treatment. CFD can predict the wall shear stress gradients and the drug uptake kinetics, 3D printers can loyally reproduce the architecture of the vessels and novel grafts can be manufactured in biocompatible implantable materials to replace the diseased, damaged or constitutionally dangerous vasculature. Only the mixture of sciences in biomedical engineering can achieve these objectives at a minimal economical and ecological cost.
Acknowledgments
This work has been supported by Ministerio de Innovación Plan Nacional BFU2009-09804, NIH grant NIH/NIGMS RO1/GM049039, Generalitat de Catalunya FI-DGR 2011, Barcelona Chamber of Commerce, Fundació Empreses IQS, and POSIMAT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Blackman BR, Garcia-Cardena G, Gimbrone MA., Jr A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. J Biomech Eng. 2002;124:397–407. doi: 10.1115/1.1486468. [DOI] [PubMed] [Google Scholar]
- 2.DePaola N, Gimbrone MA, Jr, Davies PF, Dewey CF., Jr Vascular endothelium responds to fluid shear stress gradients. Arteriosclerosis and thrombosis : a journal of vascular biology / American Heart Association. 1992;12:1254–1257. doi: 10.1161/01.atv.12.11.1254. [DOI] [PubMed] [Google Scholar]
- 3.Davies PF, Remuzzi A, Gordon EJ, Dewey CF, Jr, Gimbrone MA., Jr Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci U S A. 1986;83:2114–2117. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and - resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balcells M, Fernandez Suarez M, Vazquez M, Edelman ER. Cells in fluidic environments are sensitive to flow frequency. J Cell Physiol. 2005;204:329–335. doi: 10.1002/jcp.20281. [DOI] [PubMed] [Google Scholar]
- 6.Badimon L, Badimon JJ, Galvez A, Chesebro JH, Fuster V. Influence of arterial damage and wall shear rate on platelet deposition. Ex vivo study in a swine model. Arteriosclerosis. 1986;6:312–320. doi: 10.1161/01.atv.6.3.312. [DOI] [PubMed] [Google Scholar]
- 7.Molins B, Pena E, Padro T, Casani L, Mendieta C, Badimon L. Glucose-regulated protein 78 and platelet deposition: Effect of rosuvastatin. Arterioscler Thromb Vasc Biol. 2010;30:1246–1252. doi: 10.1161/ATVBAHA.110.205112. [DOI] [PubMed] [Google Scholar]
- 8.Kolandaivelu K, Edelman ER. Environmental influences on endovascular stent platelet reactivity: An in vitro comparison of stainless steel and gold surfaces. J Biomed Mater Res A. 2004;70:186–193. doi: 10.1002/jbm.a.30023. [DOI] [PubMed] [Google Scholar]
- 9.Kolandaivelu K, Edelman ER. Low background, pulsatile, in vitro flow circuit for modeling coronary implant thrombosis. J Biomech Eng. 2002;124:662–668. doi: 10.1115/1.1517062. [DOI] [PubMed] [Google Scholar]
- 10.Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich KL, Giddings VL, Coleman L, Wong GK, Edelman ER. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400–1409. doi: 10.1161/CIRCULATIONAHA.110.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson CM, Chen CS. Ve-cadherin simultaneously stimulates and inhibits cell proliferation by altering cytoskeletal structure and tension. Journal of cell science. 2003;116:3571–3581. doi: 10.1242/jcs.00680. [DOI] [PubMed] [Google Scholar]
- 12.March S, Hui EE, Underhill GH, Khetani S, Bhatia SN. Microenvironmental regulation of the sinusoidal endothelial cell phenotype in vitro. Hepatology. 2009;50:920–928. doi: 10.1002/hep.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolachalama VB, Levine EG, Edelman ER. Luminal flow amplifies stent-based drug deposition in arterial bifurcations. PloS one. 2009;4:e8105. doi: 10.1371/journal.pone.0008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolachalama VB, Tzafriri AR, Arifin DY, Edelman ER. Luminal flow patterns dictate arterial drug deposition in stent-based delivery. Journal of controlled release : official journal of the Controlled Release Society. 2009;133:24–30. doi: 10.1016/j.jconrel.2008.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzafriri AR, Vukmirovic N, Kolachalama VB, Astafieva I, Edelman ER. Lesion complexity determines arterial drug distribution after local drug delivery. Journal of controlled release : official journal of the Controlled Release Society. 2010;142:332–338. doi: 10.1016/j.jconrel.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro MA, Putman CM, Cebral JR. Computational fluid dynamics modeling of intracranial aneurysms: Effects of parent artery segmentation on intra-aneurysmal hemodynamics. AJNR. American journal of neuroradiology. 2006;27:1703–1709. [PMC free article] [PubMed] [Google Scholar]
- 17.Hariharan P, Giarra M, Reddy V, Day SW, Manning KB, Deutsch S, Stewart SF, Myers MR, Berman MR, Burgreen GW, Paterson EG, Malinauskas RA. Multilaboratory particle image velocimetry analysis of the fda benchmark nozzle model to support validation of computational fluid dynamics simulations. J Biomech Eng. 2011;133:041002. doi: 10.1115/1.4003440. [DOI] [PubMed] [Google Scholar]
- 18.Voorhees AB, Jr, Jaretzki A, 3rd, Blakemore AH. The use of tubes constructed from vinyon "n" cloth in bridging arterial defects. Annals of surgery. 1952;135:332–336. doi: 10.1097/00000658-195203000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Browning MB, Dempsey D, Guiza V, Becerra S, Rivera J, Russell B, Hook M, Clubb F, Miller M, Fossum T, Dong JF, Bergeron AL, Hahn M, Cosgriff-Hernandez E. Multilayer vascular grafts based on collagen-mimetic proteins. Acta biomaterialia. 2012;8:1010–1021. doi: 10.1016/j.actbio.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, Carter R, Williams TP, Prichard HL, Dey MS, Begelman KG, Niklason LE. Readily available tissue-engineered vascular grafts. Science translational medicine. 2011;3:68ra69. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 21.Greenwald SE, Berry CL. Improving vascular grafts: The importance of mechanical and haemodynamic properties. The Journal of pathology. 2000;190:292–299. doi: 10.1002/(SICI)1096-9896(200002)190:3<292::AID-PATH528>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 22.Klinkert P, Post PN, Breslau PJ, van Bockel JH. Saphenous vein versus ptfe for above-knee femoropopliteal bypass. A review of the literature. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2004;27:357–362. doi: 10.1016/j.ejvs.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Ravi S, Chaikof EL. Biomaterials for vascular tissue engineering. Regenerative medicine. 2010;5:107–120. doi: 10.2217/rme.09.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasaki K, Kojima K, Kodama S, Paz AC, Chambers M, Umezu M, Vacanti CA. Bioengineered three-layered robust and elastic artery using hemodynamically-equivalent pulsatile bioreactor. Circulation. 2008;118:S52–S57. doi: 10.1161/CIRCULATIONAHA.107.757369. [DOI] [PubMed] [Google Scholar]