Abstract
Zinc finger protein 267 (ZNF267) belongs to the family of Kruppel-like transcription factors, which regulates diverse biological processes that include development, proliferation, and differentiation. We have previously demonstrated that ZNF267 mRNA is up-regulated in liver cirrhosis, which is the main risk factor for hepatocellular carcinoma (HCC). Here, we analyzed the expression of ZNF267 in human HCC cells and tissue specimens and found a significant up-regulation compared to primary human hepatocytes and corresponding non-tumorous liver tissue. Over-expression of the transcription factor Ets-1 further enhanced ZNF267 expression, and reporter gene assays revealed that mutation of the Ets-1 binding site to the ZNF267 promotor markedly inhibited ZNF267 promotor activity. Hypoxic conditions induced Ets-1 in HCC cells via HIF1alpha activation, and hypoxia induced ZNF267 expression while HIF1alpha inhibition significantly reduced both hypoxia-induced as well as basal ZNF267 expression in HCC cells. It is known that hypoxic conditions in tumorous tissues induce the formation of reactive oxygen species (ROS), and ROS have been identified as important factor in the regulation of Ets-1 expression in tumor cells. Here, we found that ROS induction induced and ROS scavenging reduced ZNF267 expression in HCC cells, respectively. Loss and gain of function analysis applying siRNA directed against ZNF267 or transient transfection revealed that ZNF267 promotes proliferation and migration of HCC cells in vitro. These findings indicate Ets-1 and HIF1alpha as critical regulators of basal and hypoxia- or ROS-induced ZNF267 expression in HCC, and further suggest that the pro-tumorigenic effect of these factors is at least in part mediated via increased ZNF267 expression in HCC. Since ZNF267 is already elevated in cirrhosis, ZNF267 appears as promising target for both prevention as well as treatment of HCC in patients with chronic liver disease.
Keywords: Hepatocellular carcinoma, ZNF267, Kruppel-like factor
Introduction
Hepatocellular carcinoma (HCC) is currently the fifth most common cancer worldwide and the third leading cause of cancer-related deaths. The number of new cases is estimated to be more than 500,000 per year, accounting for 4% of all newly diagnosed cancers (El-Serag and Rudolph, 2007). Despite some progress in the treatment, the overall prognosis of HCC is poor, which is due to many patients at presentation already being in an advanced and un-resectable state (El-Serag et al., 2008). At least 90% of HCC cases occur in patients with chronic liver disease, and most have cirrhosis. Liver cirrhosis is characterized by an excessive deposition of extracellular matrix (ECM) proteins, and the activation of hepatic stellate cells (HSCs) is a critical step in this process as activated HSCs are the main ECM protein producing cells during hepatic fibrogenesis (Bataller and Brenner, 2005; Friedman, 2008).
The Kruppel-like factor (KLF) family of transcription factors regulates diverse biological processes that include development, differentiation, growth, and responses to external stress (McConnell and Yang, 2010). KLFs contain multiple zinc fingers, which represent one of the most common DNA binding domains. A zinc finger contains two cysteine and two histidine residues (Cys2His2 zinc finger) that coordinate a single zinc ion and fold the domain into a finger-like projection (Collins et al., 2001). Seventeen mammalian KLFs have been identified and some of them have been recognized to be involved in disorders such as inflammatory conditions, fibrosis and cancerogenesis in different organs including the liver (McConnell and Yang, 2010). KLF6 (also named human zinc finger 9 (ZF9) or COPEB) is up-regulated in liver fibrosis and is a tumor suppressor gene for HCC (Ratziu et al., 1998; Wang et al., 2007). Conversely, KLF8 is also up-regulated but promotes tumor invasion and indicates poor prognosis for HCC (Li et al., 2010).
One further member of the Kruppel-like zinc finger family is zinc finger protein 267 (ZNF267; also named human zinc finger 2 or HZF2) (Abrink et al., 1995). It contains a conserved Kruppel associated box (KRAB) domain in the amino terminal part, which is separated through a linker region from a clustered zinc finger domain (Abrink et al., 1995). The first zinc finger is followed by three degenerated fingers, and then continues with 13 zinc fingers at the carboxy terminus (Abrink et al., 1995). The function of ZNF267 is widely unknown; its mRNA is up-regulated upon nitric oxide treatment in venous endothelial cells (Schafer et al., 2000), and previously, we have demonstrated that ZNF267 mRNA is up-regulated during the activation process of human HSCs and in cirrhotic human liver tissue (Schnabl et al., 2005). Furthermore, we found that matrix metalloproteinase-10 (MMP-10) gene expression in activated HSCs is inhibited by in activated HSCs (Schnabl et al., 2005), which might result in an increased accumulation of ECM and promote liver fibrogenesis and herewith also the development of liver cirrhosis and HCC, respectively.
Consequently, in the present study we aimed to assess the expression and function of ZNF267 in HCC.
Materials and methods
Cells and cell culture
The HCC cell lines HepG2 (ATCC HB-8065), PLC (ATCC CRL-8024), Hep3B (ATCC HB-8064) and HuH-7 (JCR B0403) were cultured as described previously (Hellerbrand et al., 2008).
Primary human hepatocytes (PHHs) and hepatic stellate cells (HSCs) were isolated and cultured as described (Muhlbauer et al., 2003). In vitro activation of HSCs was achieved by cell culture on uncoated tissue culture dishes as described (Muhlbauer et al., 2006). Human liver tissue for cell isolation was obtained according to the guidelines of the charitable state-controlled foundation Human Tissue and Cell Research (HTCR) with the patient’s informed consent.
Hypoxia was induced by incubation with 2,2′-dipyridyl (DP; 100 μM; Sigma Aldrich, Deisenhofen, Germany). For pharmacological inhibition of HIF-1 activity, cells were incubated with 10 nM of echinomycin (Alexis Biochemicals, Lörrach, Germany) (Amann et al., 2009). To induce ROS formation cells were incubated with 0.75 μM arsenic trioxide (Sigma Aldrich) for the indicated periods of time.
Human tissues
Paired HCC and non-neoplastic liver tissues were obtained from 11 HCC patients undergoing surgical resection. Tissue samples were immediately snap frozen and stored at −80 °C until subsequent analysis. Informed consent was obtained from all patients and the study was approved by the local Ethics Committee.
Expression analysis
Isolation of total cellular RNA from cultured cells and tissues and reverse transcription were performed as described previously (Muhlbauer et al., 2003). Quantitative real-time PCR was performed with primers specific for ZNF267 (forward: 5′-ATG GGA GCT GTG ATC TTG AGA; reverse: 5′-GCA ATG ATG AAT GAG TAA AGA CC) and Collagen I (forward: 5′-CGG CTC CTG CTC CTC TT; reverse: 5′-GGG GCA GTT CTT GGT CTC) employing LightCycler technology (Roche, Mannheim, Germany) (Hellerbrand et al., 2005). Expression of Snail-1 and Slug mRNA was analyzed applying the QuantiTect primer assay according to the manufacturer’s instructions (Qiagen, Hilden, Germany).
Transfection experiments
Generation of the ZNF267 promoter luciferase reporter construct and a construct with a mutated Ets-1 binding site to the ZNF-promotor has been previously described (Schnabl et al., 2005). Cells (2×105 per well) were seeded into six-well plates and transfected with 0.5 mg of reporter constructs using lipofectamine plus (Invitrogen). After 24 h cells were lysed and the luciferase activity was measured. To normalize for transfection efficiency, 0.2 mg of a pRL-TK plasmid (Promega, Mannheim, Germany) was co-transfected, and renilla and luciferase activities were measured by a luminometric assay (Promega).
Furthermore, HCC cells were transfected with an Ets-1 expressing construct in pcDNA3 or the empty vector, respectively. Applying the HiPerFect method (Qiagen, Hilden, Germany), two different small interfering RNA (siRNA; Hs_ZNF267_1 and Hs_ZNF267_2; both from Qiagen) were transiently transfected into HCC cells to deplete ZNF267 expression. In parallel, HCC cells were transiently transfected with control siRNA (AllStars Negative Control siRNA; Qiagen). The transfection efficiency of siRNA in HCC cells was approximately 90%, as measured by fluorescence-activated cell sorting analysis applying Alexa Fluor 488-labeled control siRNA (Qiagen).
Proliferation and migration assays
Cell proliferation was measured using the XTT assay (Roche) (Hellerbrand et al., 2006). Migration assays were performed as previously described (Hellerbrand et al., 2008).
Statistical analysis
Statistical analyses were performed using GraphPad Prism Software (GraphPad Software, Inc., San Diego, USA). Results are expressed as mean±standard error. Comparisons between groups were made using the unpaired t-test. P-values ≤ 0.05 were considered statistically significant.
Results
ZNF267 expression in HCC
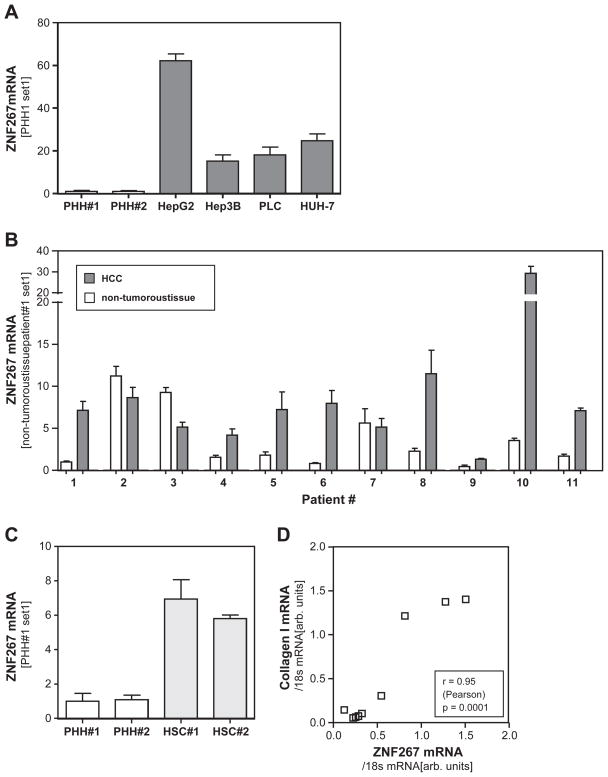
Quantitative PCR analysis revealed markedly increased ZNF267 expression in four HCC cell lines (Hepg2, Hep3B, PLC, Huh7) compared to primary human hepatocytes (PHH) (Fig. 1A).
Fig. 1.
ZNF 267 expression in HCC. (A) Expression of ZNF267 mRNA in primary human hepatocytes (PHH) and four HCC cell lines (HepG2, Hep3B, PLC, HUH-7) analyzed by quantitative real-time PCR. (B) ZNF267 mRNA expression in human HCC tissue specimens and corresponding non-tumorous tissue samples of 11 patients. (C) Expression of ZNF267 mRNA in PHH and activated human hepatic stellate cells (HSC) of 2 different donors. (D) Correlation of ZNF267 and collagen I mRNA expression in cirrhotic liver tissue of 11 patients.
Next, we assessed ZNF267 expression in HCC specimens and corresponding non-tumorous liver tissue of 11 patients. In 8 cases we observed a significant up-regulation of ZNF267 in the tumorous tissue (Fig. 1B). In only 3 HCC tissues ZNF267 expression was similar (patients #2 and #7) or slightly lower (patient #3) than in the corresponding non-tumorous tissue. It has to be noted that these 3 patients were the ones with the highest ZNF267 expression in non-tumorous tissues compared to the other 8 HCC cases, and that all 11 HCCs had developed in cirrhotic liver tissue on the ground of chronic liver injury. We have shown before that ZNF267 expression is elevated in cirrhotic livers compared to healthy control liver tissue, and that ZNF267 is up-regulated during the activation process of HSCs (Schnabl et al., 2005). The activation of HSCs is the key event of hepatic fibrosis, and activated HSC are the cellular source of collagen type I, which is the main extracellular matrix protein in cirrhotic livers (Bataller and Brenner, 2005; Friedman, 2008). Notably, activated human HSCs revealed significantly higher ZNF267 expression levels than primary human hepatocytes (Fig. 1C), and ZNF267 expression in non-tumorous but cirrhotic liver tissues revealed a striking correlation with collagen I expression (Fig. 1D). This indicates that activated HSCs are the main source of ZNF267 expression in non-tumorous (cirrhotic) liver tissue. In contrast, there was no correlation between ZNF267 and collagen I mRNA expression in HCC tissue (data not shown).
Together these findings indicate that in vitro as well as in vivo ZNF267 expression in HCC cells is significantly increased compared to (non-malignant) hepatocytes.
Regulation of ZNF267 expression in HCC cells
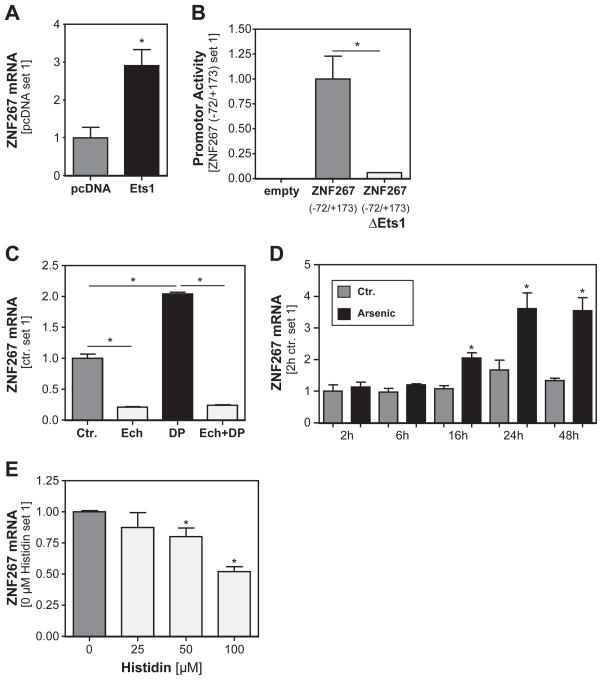
Next, we wanted to assess the molecular mechanisms responsible for the increased ZNF267 expression in HCC. Previously, we have characterized the human ZNF267 promotor and identified an Ets-1 binding site as critical for ZNF267 expression in human HSCs (Hu et al., 2005). Further, the expression of this transcription factor is increased during hepatocarcinogenesis and affects tumorigenicity of HCC cells (Jiang et al., 2001; Kanda et al., 2002). Therefore, we analyzed whether Ets-1 also affects ZNF267 expression in HCC cells and found that transient transfection with an Ets-1 expression plasmid significantly induced ZNF267 expression in Hep3B cells (Fig. 2A). Next, we transfected HCC cells with luciferase reporter plasmids containing the ZNF267 promotor or the same construct with a mutation in the Ets-1 binding site, respectively (Hu et al., 2005). Analysis of reporter gene activity revealed that mutation of the Ets1-binding site dramatically inhibited the promotor activity in Hep3B cells (Fig. 2B).
Fig. 2.
Regulation of ZNF267 expression in HCC cells. (A) Analysis of ZNF267 mRNA expression in Hep3B cells transiently transfected with an Ets-1 expression plasmid. (*: p < 0.05 compared to control plasmid pcDNA). (B) ZNF267 promoter activity in Hep3B cells transiently transfected with luciferase reporter plasmids containing the ZNF267 promoter or the same construct with a mutation in the Ets-1 binding site. (*: p < 0.05). (C) ZNF267 mRNA expression in HCC cells with or without pharmacological induction of hypoxia by DP (2,2′-dipyridyl), or HIF1alpha inhibition by echinomycin (Ech). (*: p < 0.05). (D) Expression of ZNF267 mRNA in HCC cells at different time points after incubation with the ROS inducer arsenic acid. (*: p < 0.05 compared to control). (E) Analysis of ZNF267 mRNA expression in HCC cells after treatment with the ROS scavenger histidine. (*: p < 0.05 compared to 0 μM histidine).
We have shown before that Ets-1 activity was increased in HCC cells under hypoxic conditions via HIF1alpha (Maegdefrau et al., 2009), and that pharmacologically induced hypoxia with 2,2′-dipyridyl (DP) induced HIF1alpha activity in HCC cells (Amann et al., 2009). In accordance, stimulation with DP led to a significant increase of ZNF267 expression (Fig. 2C), and conversely, preincubation with the HIF1alpha inhibitor echinomycin dramatically reduced both DP induced as well as basal ZNF267 expression in HCC cells (Fig. 2C).
Hypoxic conditions in tumorous tissues induce the formation of reactive oxygen species (ROS), and ROS have been identified as important factor in the regulation of Ets-1 expression in tumor cells (Wilson et al., 2005). In line with this, ROS induction by arsenic acid (Chou et al., 2004) led to a time-dependent induction of ZNF267 expression in HCC cells, starting after 16 h and reaching a plateau after 24 h, respectively (Fig. 2D). Conversely, incubation with the ROS scavenger histidine dose-dependently reduced ZNF267 expression in HCC cells (Fig. 2E).
In summary, these data revealed hypoxia and ROS formation as critical regulators of ZNF267 expression in HCC cells via the transcription factor Ets-1.
Functional role of ZNF267 in HCC cells
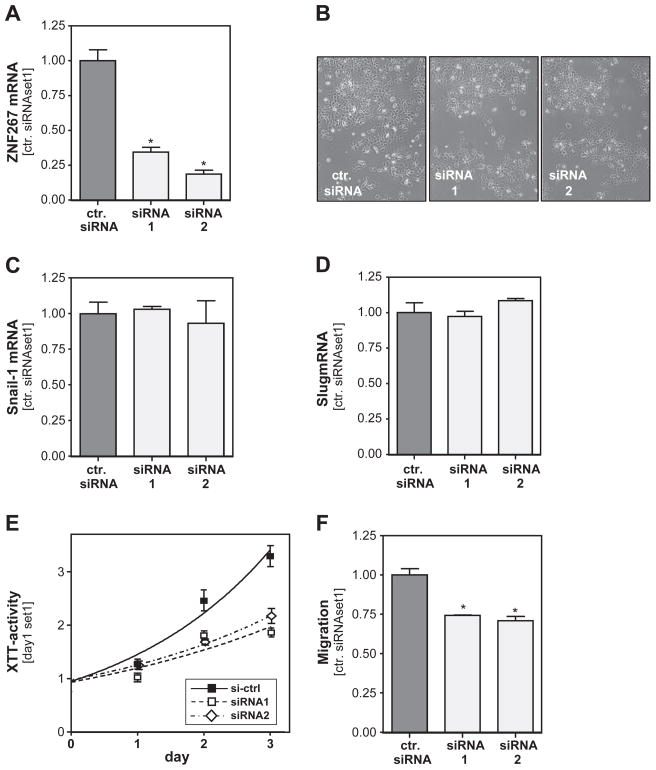
To get an insight into the functional role of increased ZNF267 expression in HCC, we transfected HCC cells with siRNA directed against ZNF267, which lead to a marked down-regulation of ZNF267 mRNA as compared to cells transfected with control siRNA (Fig. 3A). Microscopical analysis did not show morphological changes in Hep3B cells after ZNF267 suppression (Fig. 3B), and also expression of the epithelial–mesenchymal transition (EMT) markers slug and snail-1 was similar in HCC cells with and without ZNF267 suppression (Fig. 3C, D). However, functional analysis revealed that ZNF267 suppression significantly inhibited proliferation of Hep3B cells (Fig. 3E). Furthermore, in Boyden chamber assays migration of HCC cells with suppressed ZNF267 expression was significantly inhibited compared to mock transfected control cells (Fig. 3F). According results as observed in Hep3B cells were found in PLC and HepG2 cells after suppression of ZNF267 expression with siRNA (data not shown).
Fig. 3.
Functional effects of ZNF267 suppression in HCC cells. (A) ZNF267 mRNA expression in HCC cells transiently transfected with two different ZNF267 siRNAs (siRNA1 and siRNA2) and cells transfected with control siRNA. (B) Microscopical analysis of Hep3B cells after ZNF267 suppression. Analysis of snail-1 (C) and slug (D) mRNA expression in HCC cells transiently transfected with ZNF267 siRNA. (E) Proliferation of HCC cells after ZNF267 suppression determined by analysis of XTT activity. (F) Migratory activity of siRNA-treated HCC cells assessed with the Boyden chamber assays. (*: p < 0.05 compared to control).
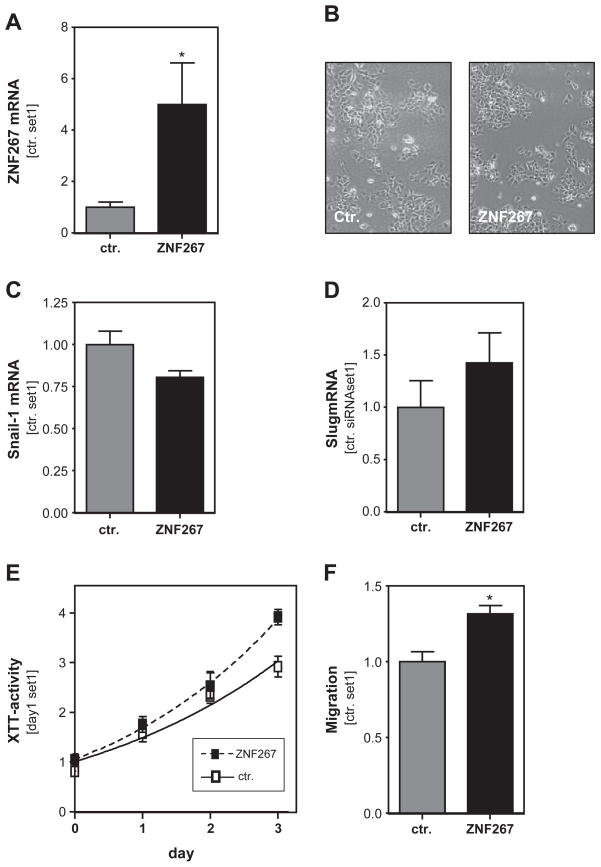
In a complementary approach, we wanted to test the effect of ZNF267 overexpression in HCC cells. Transient transfection of Hep3B cells with a ZNF267 expression plasmid (Schnabl et al., 2005) induced a significant up-regulation of ZNF267 mRNA compared to cells transfected with the empty vector (Fig. 4A). Similarly, ectopic expression of ZNF267 did not lead to morphological changes (Fig. 4B) or altered slug or snail1 expression (Fig. 4C, D). However, Hep3B cells over-expressing ZNF267 revealed significantly higher proliferation (Fig. 4E) and migratory activity (Fig. 4F) compared to control cells transfected with the empty expression vector.
Fig. 4.
Effect of ZNF267 overexpression in HCC cells. (A) ZNF267 mRNA expression in Hep3B cells transiently transfected with a ZNF expression plasmid and cells transfected with the empty vector. (B) Microscopical analysis of Hep3B cells after overexpression of ZNF267. Analysis of snail-1 (C) and slug (D) expression in HCC cells transiently transfected with ZNF267 expression plasmid. (E) Proliferation of Hep3B cells overexpressing ZNF267 determined by analysis of XTT activity. (F) Migratory activity of Hep3B cells after ectopic expression of ZNF267 assessed with the Boyden chamber assays. (* p < 0.05 compared to control).
Discussion
The aim of this study was to investigate the expression and function of ZNF267 in HCC. Assessment of human HCC tissues revealed a significantly increased ZNF267 expression compared to non-tumorous liver tissue, and the up-regulation of ZNF267 expression in HCC cell lines compared to primary human hepatocytes was even more striking. However and as already pointed out in the results section, it has to be noted that ZNF267 expression is increased in liver cirrhosis compared to healthy livers (Schnabl et al., 2005), and that the HCCs assessed in the present study arose in cirrhotic liver tissue. Although analysis of hepatic ZNF267 expression is hampered by the lack of specific antibodies, our data strongly indicate activated HSCs and not hepatocytes as cellular source of increased ZNF267 expression in non-tumorous but cirrhotic liver tissue. Thus, it appears that also in vivo liver cancer cells reveal a marked up-regulation of ZNF267 expression compared to normal hepatocytes that matches the one observed in vitro in HCC cells compared to primary hepatocytes.
We identified different mechanisms for the increased ZNF267 mRNA levels in HCC cells with the transcription factor Ets-1 as key regulator of basal as well as induced ZNF267 expression. Most impressively, mutation of the Ets-1 binding site to the ZNF267 promotor leads to an almost complete loss of reporter gene activity, while over-expression of Ets-1 approximately doubled ZNF267 expression in cancerous cells. We have shown before that HIF1alpha is a strong inducer of the transcriptional Ets-1 activity in HCC (Maegdefrau et al., 2009), and in line with this, HIF1alpha inhibition almost completely abrogated ZNF267 expression in HCC cells. Further, we have previously shown that hypoxia further enhances the already basally high HIF1alpha activity in HCC cells (Amann et al., 2009; Maegdefrau et al., 2009), and accordingly, chemically induced hypoxia led to an approximately 2-fold induction of ZNF267 expression. Hypoxia and other conditions frequently present in the tumor milieu as chronic inflammation induce the release of free radicals as reactive oxygen species (ROS), which have been shown to induce the transcription of Ets-1 in tumor cells (Wilson et al., 2005). In line with the hypothesis that Ets-1 is critical for the transcriptional regulation of ZNF267 in HCC cells, arsenic-induced ROS formation lead to marked induction ZNF267 mRNA levels, which exceeded the increase induced by chemically induced hypoxia. However and different than the effects observed in response to hypoxia, the ROS induced ZNF267 induction required a latency period of more than 12 h, which fits to the hypothesis that ROS induced Ets-1 transcription and subsequently enhanced Ets-1 levels, respectively, lead to a delayed induction of ZNF267 expression in HCC cells. Conversely, scavenging free radicals exhibited only a moderate effect on ZNF267 expression in HCC cells as compared to HIF1alpha inhibition. In summary, these findings suggest that (endogenously) high HIF1alpha activity in HCC cells is responsible for the high ZNF267 expression in HCC cells, which can be further induced via the formation of ROS under hypoxic or other conditions frequently observed in tumorous milieu. Thus, rapid expansion and inadequacy of the local vasculature resulting in hypoxia is a common feature of HCC and is associated with poor tumor outcome (Farazi and DePinho, 2006). Also the transcription factor Ets-1 is strongly linked to HCC progression, including tumor proliferation, invasion and metastasis (Jiang et al., 2001; Kanda et al., 2002; Ozaki et al., 2003). Importantly, loss and gain of function studies clearly showed that ZNF267 promotes the proliferation as well as the migratory potential of HCC cells.
In conclusion, these findings suggest that enhanced ZNF267 expression is at least one loop by which constitutively active as well as ROS or hypoxia induced Ets-1 and HIF1alpha, respectively, promote HCC progression. Furthermore, it has to be considered that we have previously shown that ZNF267 is already up-regulated in human cirrhotic liver tissue and might promote liver fibrosis through alteration of matrix degradation (Schnabl et al., 2005). Since cirrhosis is the main risk factor for HCC development, ZNF267 appears as promising target for both prevention as well as treatment of HCC in patients with chronic liver disease.
Acknowledgments
This work was supported by grants from the German Research Association (DFG) to B.S. and C.H., and the Medical Faculty of the University of Regensburg (ReForM) to C.H.
We acknowledge the Human Tissue and Cell Research (HTCR) Foundation for supporting our research by making human liver tissue available.
Abbreviations
- HCC
hepatocellular carcinoma
- HSC
hepatic stellate cell
- KLF
kruppel-like factor
- PHH
primary human hepatocytes
- ZNF
zinc finger protein
Footnotes
We are indebted to Marina Fink and Birgitta Ott-Rötzer for excellent technical assistance.
References
- Abrink M, Aveskogh M, Hellman L. Isolation of cDNA clones for 42 different Kruppel-related zinc finger proteins expressed in the human monoblast cell line U-937. DNA Cell Biol. 1995;14:125–136. doi: 10.1089/dna.1995.14.125. [DOI] [PubMed] [Google Scholar]
- Amann T, Maegdefrau U, Hartmann A, Agaimy A, Marienhagen J, Weiss TS, Stoeltzing O, Warnecke C, Scholmerich J, Oefner PJ, Kreutz M, Bosserhoff AK, Hellerbrand C. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol. 2009;174:1544–1552. doi: 10.2353/ajpath.2009.080596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Investig. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WC, Jie C, Kenedy AA, Jones RJ, Trush MA, Dang CV. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc Natl Acad Sci USA. 2004;101:4578–4583. doi: 10.1073/pnas.0306687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Stone JR, Williams AJ. All in the family: the BTB/POZ, KRAB, and SCAN domains. Mol Cell Biol. 2001;21:3609–3615. doi: 10.1128/MCB.21.11.3609-3615.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerbrand C, Bataille F, Schlegel J, Hartmann A, Muhlbauer M, Scholmerich J, Buttner R, Hofstadter F, Bosserhoff AK. In situ expression patterns of melanoma inhibitory activity 2 in healthy and diseased livers. Liver Int. 2005;25:357–366. doi: 10.1111/j.1478-3231.2005.01099.x. [DOI] [PubMed] [Google Scholar]
- Hellerbrand C, Muhlbauer M, Wallner S, Schuierer M, Behrmann I, Bataille F, Weiss T, Scholmerich J, Bosserhoff AK. Promoter-hypermethylation is causing functional relevant downregulation of methylthioadenosine phosphorylase (MTAP) expression in hepatocellular carcinoma. Carcinogenesis. 2006;27:64–72. doi: 10.1093/carcin/bgi201. [DOI] [PubMed] [Google Scholar]
- Hellerbrand C, Amann T, Schlegel J, Wild P, Bataille F, Spruss T, Hartmann A, Bosserhoff AK. The novel gene MIA2 acts as a tumour suppressor in hepatocellular carcinoma. Gut. 2008;57:243–251. doi: 10.1136/gut.2007.129544. [DOI] [PubMed] [Google Scholar]
- Hu K, Fink M, Froh M, Gabele E, Hellerbrand C, Muhlbauer M, Wiest R, Scholmerich J, Schnabl B. Characterization of the human zinc finger protein 267 promoter: essential role of nuclear factor Y. Biochim Biophys Acta. 2005;1729:14–23. doi: 10.1016/j.bbaexp.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Xu W, Lu J, He F, Yang X. Invasiveness of hepatocellular carcinoma cell lines: contribution of hepatocyte growth factor, c-met, and transcription factor Ets-1. Biochem Biophys Res Commun. 2001;286:1123–1130. doi: 10.1006/bbrc.2001.5521. [DOI] [PubMed] [Google Scholar]
- Kanda K, Nakayama T, Onizuka S, Tomioka T, Kanematsu T. Expression of the Ets-1 proto-oncogene is linked to cell differentiation of human hepatocellular carcinoma. Hepatogastroenterology. 2002;49:747–751. [PubMed] [Google Scholar]
- Li JC, Yang XR, Sun HX, Xu Y, Zhou J, Qiu SJ, Ke AW, Cui YH, Wang ZJ, Wang WM, Liu KD, Fan J. Up-regulation of Kruppel-like factor 8 promotes tumor invasion and indicates poor prognosis for hepatocellular carcinoma. Gastroenter-ology. 2010;139:2146–2157. doi: 10.1053/j.gastro.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Maegdefrau U, Amann T, Winklmeier A, Braig S, Schubert T, Weiss TS, Schardt K, Warnecke C, Hellerbrand C, Bosserhoff AK. Bone morphogenetic protein 4 is induced in hepatocellular carcinoma by hypoxia and promotes tumour progression. J Pathol. 2009;218:520–529. doi: 10.1002/path.2563. [DOI] [PubMed] [Google Scholar]
- McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlbauer M, Bosserhoff AK, Hartmann A, Thasler WE, Weiss TS, Herfarth H, Lock G, Scholmerich J, Hellerbrand C. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology. 2003;125:1085–1093. doi: 10.1016/s0016-5085(03)01213-7. [DOI] [PubMed] [Google Scholar]
- Muhlbauer M, Fleck M, Schutz C, Weiss T, Froh M, Blank C, Scholmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45:520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Ozaki I, Mizuta T, Zhao G, Zhang H, Yoshimura T, Kawazoe S, Eguchi Y, Yasutake T, Hisatomi A, Sakai T, Yamamoto K. Induction of multiple matrix metalloproteinase genes in human hepatocellular carcinoma by hepatocyte growth factor via a transcription factor Ets-1. Hepatol Res. 2003;27:289–301. doi: 10.1016/s1386-6346(03)00268-7. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Lalazar A, Wong L, Dang Q, Collins C, Shaulian E, Jensen S, Friedman SL. Zf9, a Kruppel-like transcription factor up-regulated in vivo during early hepatic fibrosis. Proc Natl Acad Sci USA. 1998;95:9500–9505. doi: 10.1073/pnas.95.16.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer U, Schneider A, Neugebauer E. Identification of a nitric oxide-regulated zinc finger containing transcription factor using motif-directed differential display. Biochim Biophys Acta. 2000;1494:269–276. doi: 10.1016/s0167-4781(00)00249-9. [DOI] [PubMed] [Google Scholar]
- Schnabl B, Hu K, Muhlbauer M, Hellerbrand C, Stefanovic B, Brenner DA, Scholmerich J. Zinc finger protein 267 is up-regulated during the activation process of human hepatic stellate cells and functions as a negative transcriptional regulator of MMP-10. Biochem Biophys Res Commun. 2005;335:87–96. doi: 10.1016/j.bbrc.2005.07.043. [DOI] [PubMed] [Google Scholar]
- Wang SP, Zhou HJ, Chen XP, Ren GY, Ruan XX, Zhang Y, Zhang RL, Chen J. Loss of expression of Kruppel-like factor 6 in primary hepatocellular carcinoma and hepatoma cell lines. J Exp Clin Cancer Res. 2007;26:117–124. [PubMed] [Google Scholar]
- Wilson LA, Gemin A, Espiritu R, Singh G. Ets-1 is transcriptionally up-regulated by H2O2 via an antioxidant response element. FASEB J. 2005;19:2085–2087. doi: 10.1096/fj.05-4401fje. [DOI] [PubMed] [Google Scholar]