Abstract
We assessed the post-blood meal flight distance of four mosquito species in a unique environment using blood meal analysis. Mosquitoes were trapped at the Rio Grande Zoo in Albuquerque, NM, and the blood source of blood-engorged mosquitoes was identified. The distance from the enclosure of the animal serving as a blood source to the trap site was then determined. We found that mosquitoes captured at the zoo flew no more than 170 m with an average distance of 106.7 m after taking a blood meal. This is the first study in which the flight distance of wild mosquitoes has been assessed using blood meal analysis and the first in which zoo animals have served as the exclusive source of blood meals.
Keywords: Aedes, Culex, blood meal, mosquito, flight distance
INTRODUCTION
Flight range has long been recognized as a factor in the importance of a given mosquito species in disease transmission (Reeves 1965, Rowley et al. 1968). The ability to disperse, along with its host preferences, biting frequency, and transmission rates are crucial components of vector capacity and the ability to efficiently transmit a particular pathogen (Saegerman et al. 2008). Traditionally, the primary method used to determine flight range in mosquitoes has been to mark them in some way, release them at a known point, and then try to recapture the marked mosquitoes at trap sites a known distance from the release point. This technique has been attempted repeatedly, but only a small fraction of the released mosquitoes are often recaptured (Russell et al. 1944, Wolfinsohn and Galun 1953, Quraishi et al. 1966, Muir and Kay 1998, Russell et al. 2005, Midega et al. 2007). A flight mill system developed in 1968 and subsequently improved by incorporating computer analysis in 1984 has been used successfully to determine the flight range of flying insects (Rowley et al. 1968, Clarke III et al. 1984). This system has been effective in assessing flight range, speed, and duration of flight. It did not, however, mimic the natural environmental conditions to which mosquitoes are normally exposed. In 1984, an alternative method utilizing rubidium-marked eggs was developed (Kimsey and Kimsey 1984). Its efficacy was first assessed with Aedes aegypti in 1995 where the flight distance post-blood meal was found to be a maximum of 441 m (Reiter et al. 1995). Most investigations of mosquito flight distance have utilized labreared mosquitoes. While such mosquitoes give us an idea of flight range, they do not, however, necessarily behave as wild-type mosquitoes would.
The importance that zoo animals can play as indicators of recent, emerging infectious disease was highlighted by the emergence of West Nile Virus in 1999. The deaths of a Chilean flamingo and other exotic bird species at the Bronx Zoo were important early indicators that a new pathogen was spreading in North America (Lanciotti et al. 1999). Upon further testing, West Nile virus, previously only found in Africa and Europe, was identified as the infecting virus (Anderson et al. 1999). Other vector-borne pathogens have also been recognized as problematic in zoological parks. Avian malaria in particular has been identified in captive birds in zoos around the world, including the United States, New Zealand, Brazil, and Japan (Cranfield et al.1994, Derraik 2004, Ejiri et al. 2009, Bueno et al. 2010). Penguins, which have no natural resistance to Plasmodium, are at especially elevated risk, and penguin deaths due to avian malaria have been reported from zoos in the United States and Brazil (Cranfield et al. 1994, Bueno et al. 2010).
The sources of mosquito blood meals have been identified by a variety of methods (Irby and Apperson 1988, Gingrich and Williams 2005, Kay et al. 2007, Molaei et al. 2006, 2007, Sawabe et al. 2010, Barrera et al. 2011). None of these studies, however, incorporated a trapping area that encompassed a zoo containing many exotic species. Here we report on a blood-meal analysis of mosquitoes trapped at the Rio Grande Zoo, in Albuquerque, NM. Many of the blood meal sources have been identified as exotic species that were housed at the zoo. Because we can precisely measure the distance from an animal’s enclosure to the site where a particular mosquito was trapped, we have been able to estimate the post-blood meal flight distance for several mosquito species, some of which transmit arboviruses of veterinary or medical significance. The presence of a variety of relatively confined hosts within the zoo, along with many readily available water sources for oviposition, make zoos a unique and interesting setting for the study of mosquitoes. In this study, we report on the post-blood meal flight distance of mosquitoes located in a zoo habitat. Our findings indicate that the Rio Grande Zoo may be acting as a self-sustaining mosquito habitat. Limited dispersal within this habitat has important implications for arbovirus transmission.
MATERIALS AND METHODS
Collections
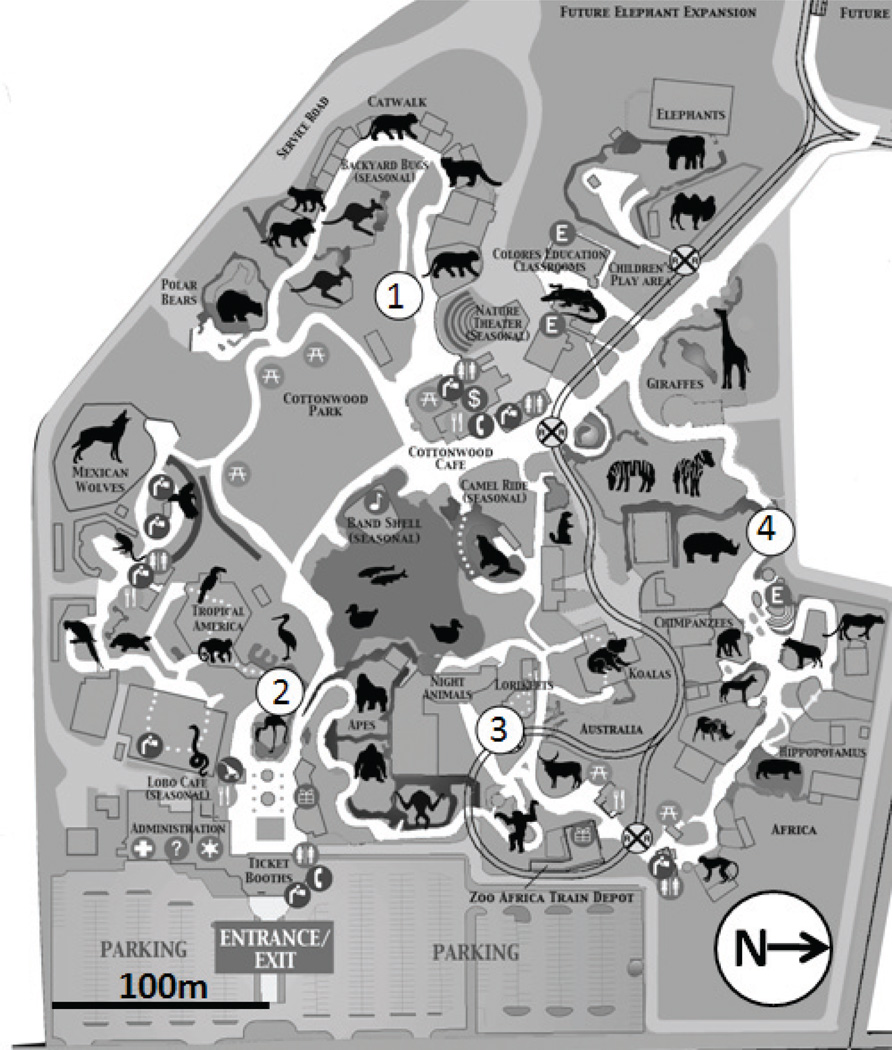
Mosquitoes were collected between 2006 and 2010 at the Rio Grande Zoo in Albuquerque, NM (Figure 1). The Rio Grande Zoo is located in a riparian zone adjacent to the Rio Grande River. As such, there are many standing water pools serving as ideal locations for mosquito oviposition. Furthermore, standing water in the form of water features and drinking containers is ubiquitous throughout the zoo. In 2006, only one trap site was present at the zoo. The number of trap sites was increased to two in 2008 and to four in 2010. Mosquitoes were trapped once a week throughout the mosquito season, May through mid-October, for approximately 24 consecutive weeks. Both a CDC light trap and gravid trap were placed at each trapping site for each trapping occasion. Two control trapping sites were placed in nearby areas where mosquito activity is known to be high: one to the north and one on the adjacent west side of the Rio Grande River, each approximately 3.5 km from the Rio Grande Zoo. CDC light traps were suspended 1.5 m from the ground and baited with approximately 1.5 kg of dry ice, and gravid traps were baited with dechlorinated water infused with horse manure, grass clippings, and bacterial culture (Propump Liquid Live Bacteria High Count, Ecological Laboratories, Freeport, NY, U.S.A.), which was allowed to ferment for two weeks. Traps were set in the afternoon and were collected the following morning. The collected mosquitoes were immediately placed on dry ice until they could be stored in a −80° C freezer. Mosquitoes were identified to species according to dichotomous keys (Carpenter and LaCasse 1955, Pratt and Barnes 1959, Darsie and Ward 1981). Mosquitoes containing blood meals were set aside for blood meal analysis.
Figure 1.
Map of the Rio Grande Zoo. Numbers indicate the location of mosquito trapping sites within the zoo.
Blood meal analysis
Mosquitoes with a visible blood meal were individually placed on a microscope slide. The abdomen and midgut were removed using a razor blade and sterilized forceps under a dissecting microscope. Forceps were sterilized and a new slide and razor blade were used for each mosquito. Genomic DNA was then extracted from the midgut and abdomen using a modified DNAzol BD (Molecular Research Center, Cincinnati, OH, U.S.A.) protocol (Molaei et al. 2006).
The blood meal source was determined by subjecting each genomic DNA sample to two subsequent PCR reactions. Each reaction employed primers specific for either an avian or a mammalian portion of the cytochrome b gene (see Table 1 for primer sequences and cycling conditions). If the first set of reactions did not amplify the target DNA with either avian or mammalian primers, a second set of reactions was attempted. The sample was archived after two failed sets of reactions. Each 50 µl reaction contained 200–400 ng of genomic DNA serving as template, 5 µl of 10× buffer (Roche Applied Science Indianapolis, IN, U.S.A.), 8 µl dNTPs (200 µL of each, Applied Biosystems, Foster City, CA, U.S.A.), 8 µL MgCl2 (4 mM, Roche Applied Science Indianapolis, IN, U.S.A.), 5µl forward primer (0.5 µM), 5 µl reverse primer (0.5 µM), and 0.25 µl TaqGold Polymerase (1.25 U per reaction, Roche Applied Science Indianapolis, IN, U.S.A.). Sterile water (Sigma-Aldrich Irvine, CA, U.S.A.) was added to bring the total reaction volume to 50 µl. All reactions also included a positive control containing either mammalian (Mus musculus) or avian (Zenaida macroura) genomic DNA serving as a template. A negative water control, lacking template, was included with all PCR reactions. A second negative control was also included and consisted of a DNAzol extract, lacking mosquito midgut and abdomen.
Table 1.
PCR primers and temperature regime.
| Mammalian specific primers 5’-CGAAGCTTGATATGAAAAACCATCGTTG-3’(f) 5’-TGTAGTTRTCWGGGTCHCCTA-3’ (r) |
Avian-specific primers 5’-GACTGTGACAAAATCCCNTTCCA-3’(f) 5’-GGTCTTCATCTYHGGYTTACAAGAC-3’(r) |
|||
|---|---|---|---|---|
| Initial Denaturation | 10 min 95° C | 10 min 95° C | ||
| 36 cycles | Denature | 30 sec 95° C | 30 sec 95° C | |
| Anneal | 40 sec 55° C | 40 sec 56° C | ||
| Extension | 50 sec 72° C | 50 sec 72° C | ||
| Final extension | 3 min 72° C | 3 min 72° C | ||
Amplified PCR products were purified with one of several methods including E-Gel® SizeSelect™ gels (Invitrogen, Carlsbad, CA, U.S.A.), PCR purification kit (Qiagen, Valencia, CA, U.S.A.), minielute column (Millipore, Billerica, MA, U.S.A.), or exo-sap (Affymetrix, Cleveland, OH, U.S.A.). Amplicons were directly sequenced with a Big Dye 3.1 sequencing kit, using the big dye step protocol PCR regime (Platt et al. 2007). Samples were then sequenced on an ABI 3130 DNA Sequencer (Applied Biosystems, Foster City, CA, U.S.A.) at the University of New Mexico, Department of Biology Molecular Facility. Sequences were edited using Sequencher version 4.10.1 (Gene Codes, Ann Arbor, MI, U.S.A.) and identified to species through a blast search comparison with the GenBank DNA database (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi).
Measuring flight range
If the source of a blood meal was identified as an exotic species not normally present in New Mexico but found at the Rio Grande Zoo, it was included in our analysis. The distance from the center of the animal’s enclosure to the mosquito trap site was determined to the nearest meter using GPS. Flight distances of the different species were compared for significance using a t-test. Mosquitoes that took blood meals from native species that may or may not have been within the zoo were excluded from analysis.
RESULTS
A total of 4,917 mosquitoes, representing 12 different species, was recovered from the four zoo trap sites (Table 2). Of these mosquitoes, 70.8% were trapped with CDC light traps, while 29.2% were recovered from in gravid traps.
Table 2.
Summary of mosquitoes trapped at the Rio Grande Zoo, Albuquerque, NM, from 2006 through 2010. The number and percentages of mosquito caught in each trap type is indicated.
| Mosquito species | CDC light traps non-blood fed |
Gravid traps non-blood fed |
|---|---|---|
| Aedes dorsalis N=4 | 4 (100%) | 0 |
| Aedes melanimon N=8 | 8 (100%) | 0 |
| Aedes nigromaculis N=2 | 2 (100%) | 0 |
| Aedes trivittatus N=1 | 1 (100%) | 0 |
| Aedes vexans N=2,216 | 2,208 (99.6%) | 8 (0.4%) |
| Anopheles franciscanus N=1 | 1 (100%) | 0 |
| Anopheles freeborni N=9 | 6 (66.7%) | 3 (33.3%) |
| Culex quinquefasciatus N=2,250 | 844 (37.5%) | 1,406 (62.5%) |
| Culex tarsalis N=387 | 371 (95.9%) | 16 (4.1%) |
| Culiseta inornata N=37 | 35 (94.6%) | 2 (5.4%) |
| Aedes dorsalis N=1 | 1 (100%) | 0 |
| Orthopodomyia signifera N=1 | 1 (100%) | 0 |
| Total mosquitoes N=4,917 | 3,482 (70.8%) | 1,435 (29.2%) |
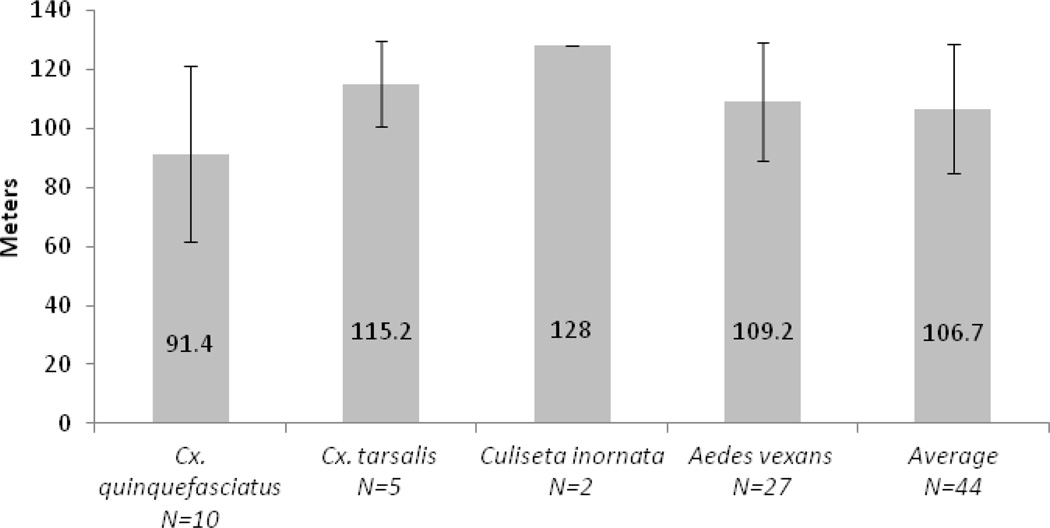
A total of 106 mosquito blood meal sources were identified from the zoo (Table 3). Of these, 44 (41.5%) were unambiguously from animals in exhibits. Only these mosquitoes were utilized in the flight distance analysis. Other identified blood meal sources were non-exotic birds, coyotes, small mammals, or domestic animals such as dogs, which are common in the area immediately surrounding the zoo. Of the 44 mosquitoes included in our study, 27 (61.4%) were Aedes vexans, ten (22.7%) were Culex quinquefasciatus, five (11.4%) were Cx. tarsalis, and two (4.5%) were Culiseta inornata (see Table 3). The average post-blood meal flight distance was 109.2 m for Ae. vexans (Figure 2). For Cx. quinquefasciatus this value was 91.4 m, while for Cx. tarsalis and Cu. inornata it was 115.2 m and 128 m, respectively. The difference in flight distance between Cx. quinquefasciatus and Ae. vexans was not significant. Because of low sample size, Cx. tarsalis and Cu. inornata were not included in the statistical analysis. The average flight distance for all mosquito species was 106.7 m. No mosquitoes engorged on exotic species were collected from control-trapping sites outside the grounds of the Rio Grande Zoo.
Table 3.
Mosquito flight distances measured to the nearest meter. An (*) denotes mosquito trapped at a different trap site. Flight distance for non-exotic species was not assessed.
| Species | Blood meal source | Common name (number) | Distance in meters |
|---|---|---|---|
| Culex quinquefasciatus N=43 | Hylobates syndactylus | Siamang gibbon (1) | 37 |
| Panthera tigris | Tiger (1) | 18 | |
| Dromaius novaehollandiae | Emu (1) | 67 | |
| Trichoglossus haematodus | Rainbow Lorikeet (1) | 3 | |
| Ceratotherium simum | White Rhino (2) | 140 | |
| Potamochoerus porcus | Red River Hog (1) | 122 | |
| Struthio camelus | Ostrich (1) | 168 | |
| Unica unica | Snow Leopard (1) | 72 | |
| Vultur gryphis | Andean Condor (1) | 147 | |
| Accipiter Cooperi | Cooper’s Hawk (1) | ||
| Anas platyrhynchos | Muscovy Duck (1) | ||
| Canis latrans | Coyote (1) | ||
| Canis lupis familiaris | Dog (2) | ||
| Carpodacus mexicanus | Finch (3) | ||
| Columba livia | Pigeon (1) | ||
| Geococcyx califoriaus | Roadrunner (1) | ||
| Mus musculus | Mouse (1) | ||
| Passer domesticus | House sparrow (7) | ||
| Pheucticus melanocephalus | Black-headed Grosbeak (1) | ||
| Turdus migratorius | American Robin (8) | ||
| Zenaida macroura | Mourning Dove (6) | ||
| Culex tarsalis N=5 | Lynx candensis | Lynx (1) | 64 |
| Lama guanaco | Guanaco (4) | 128 | |
| Culiseta inornata N=3 | Lama pacos | Alpaca (2) | 128 |
| Bos taurus | Cow (1) | ||
| Aedes vexans N=55 | Hippopotamus amphibious | Hippo (1) | 140 |
| Lama glama | Llama (5) | 128 | |
| Lama pacos | Alpaca(4) | 128 | |
| Lama guanaco | Guanaco (5) | 128 | |
| Alopex lagopus | Arctic Fox (1) | 76 | |
| Ailurus fulgens | Red Panda (1) | 65 | |
| Camelus bacteranus | Bactran Camel (1) | 133 | |
| Camelus dromedaries | Dromedary camel (1) | 96 | |
| Ceratotherium simum | White Rhino (1) | 161 | |
| Ceratotherium simum | White Rhino*(1) | 12 | |
| Dromaius novaehollandiae | Emu (1) | 54 | |
| Equus grevyi | Zebra (1) | 167 | |
| Macropus rufus | Red Kangaroo (1) | 49 | |
| Panthera leo | Lion (1) | 63 | |
| Panthera tigris | Tiger (1) | 18 | |
| Potamochoerus porcus | Red River Hog (1) | 122 | |
| Bos taurus | Cow (6) | ||
| Canis lupis familiaris | Dog (2) | ||
| Equus caballus | Horse (1) | ||
| Homo sapiens | Human (9) | ||
| Sylvilagus audobonii | Cotton tail (10) | ||
Figure 2.
Average distances of each individual species and average distance for all species combined.
DISCUSSION
We have estimated the post-blood meal flight distance for four mosquito species, utilizing blood meal analysis of mosquitoes trapped in a zoological park, where mosquito flight distance from blood meal source to trap site could be easily determined. The majority of the mosquitoes collected were caught in CDC light traps (Table 2). However, for one important species in our study, Cx. quinquefasciatus, gravid traps were found to be the more effective trap type. This highlights the need to use more than one trap type to more accurately describe a particular area’s mosquito fauna.
The mosquito species that we assessed flew an average of less than 107 m following a blood meal. Because mosquitoes vary their flight paths (Bidlingmayer 1975, Bidlingmayer and Hem 1979) it cannot be assumed that the mosquitoes in our study flew directly in a straight line to trap sites. Thus, our data represents flight dispersal distance as opposed to an actual linear measurement of flight. Furthermore, once trapped, mosquitoes are obviously unable to fly further. Because untrapped mosquitoes might ordinarily alight repeatedly, we may be underestimating the actual distance traversed by a blood-fed mosquito.
Several other factors that limit the precision of our data must be addressed. Our measurements of flight distance were made from the center of an animal enclosure. Obviously, animals may have been anywhere within their enclosure when fed upon. This would, however, alter our results by, at the most, only several meters. We also have no knowledge of how many mosquitoes fed upon zoo animals and likewise how many mosquitoes were not trapped. Finally, we were unable to account for the effects of wind, rainfall, or other phenomena that may affect mosquito flight.
Our results suggest that a blood meal imposes a heavy burden on a mosquito in terms of its dispersal ability. Non-engorged mosquitoes often fly far greater distances. Provost (1957) noted that Aedes taeniorhynchus are capable of flying over 24 miles. Anopheles gambiae and An. atroparvus were found to fly as far as 10 km (Kauffman and Briegel 2004). Because we lack data on the flight distances of blood-seeking mosquitoes, we are unable to make a direct comparison between engorged and non-engorged mosquitoes in our study.
Our results, however, are similar to those in other studies in which post-blood meal flight distance was assessed. Laboratory-reared Ae. aegypti were found to fly an average of 181 m following a blood meal, utilizing rubidium-marked eggs (Reiter et al. 1995). Russell et al. (2005), using a mark-release-recapture-technique, estimated that blood fed mosquitoes flew an average distance of 77.8 m. It is difficult, however, to draw meaningful conclusions from this study, as only 3.4% of the released mosquitoes were recaptured.
The short distances traveled by the mosquitoes may reflect to some degree the unique conditions found within the zoo environment. Although blood-fed mosquitoes might disperse further in other situations, it is entirely possible that within the zoo, flight distances are reduced. A large number of easily accessible hosts are available at the zoo in relatively confined enclosures, and various water features, ponds, and drinking water containers provide ample habitat for oviposition in close proximity to the animals. The vast majority of the non-exotic species upon which zoo mosquitoes fed are abundant within the confines of the zoo (see Table 3). Even those non-exotics not found within the zoo property, such as horses and cows, are common in the semi-rural habitat immediately adjacent to the zoo. Thus, the zoo may serve as a sort of “closed system,” in which mosquitoes complete their entire life cycle without leaving the zoo premises. If this is the case, it has obvious implications for the control of arbovirus transmission within the confines of the zoo. More specifically, it suggests that focal control within the zoo may be of greater value than previously suspected, even if mosquito populations outside the zoo remain uncontrolled.
Of the 109 blood meals we identified, nine (8.49%) were identified as human. We have no conclusive means to demonstrate that these individuals were at the zoo while fed upon. Yet our results, which indicate that mosquitoes travel less than 107 m following a blood meal, as well as the high concentration of people at the zoo, suggest that the zoo may be a likely place for mosquitoes to feed upon humans. All nine human blood meals were taken by Ae. vexans. Molaei and Andreadis (2006) have suggested that Ae. vexans may be involved in bridge transmission of West Nile virus to humans, which currently presents a risk in central New Mexico for humans as well as other mammals. This mosquito species may occasionally vector other arboviruses as well such as western equine encephalitis virus, which, while rarely infecting humans in New Mexico, may occasionally present an important health risk (Clark et al. 1986).
Additionally, our data suggest that mosquitoes native to the southwest United States feed readily on exotic species. While the tendency of mosquitoes to feed on exotic species has been found before (Lanciotti et al. 1999), this is the first data set obtained entirely from zoo animals. The species we have investigated, specifically Cx. quinquifasciatus and Cx. tarsalis, are known arbovirus vectors and may play an especially important role in the transmission of West Nile virus to birds and mammals (Sardelis et al. 2001, Goddard et al. 2002, Turell et al. 2001, 2005). Our results may therefore be of value to those interested in zoo veterinary medicine. Exotic species in zoos have already proven to be excellent sentinels of newly emerging zoonotic diseases and they may suffer high morbidity and mortality as a result of infection (Lanciotti et al. 1999). Additionally, the Rio Grande Zoo has suffered significant losses of some species to West Nile virus, most notably Rainbow Lorikeets (Trichoglossus haematodus) in previous years (DiMenna, personal communication). The importance of vector control for the maintenance of zoo animal health has been previously noted (Derraik 2005). A better understanding of vector behavior, including post-blood meal flight, might be utilized to enhance known mosquito control methods in zoos, resulting in improved protection of zoo animals and the human population visiting these attractions.
Acknowledgments
We thank Goudarz Molaei and Coen Adema for their expertise in molecular lab techniques. The blood meal analysis was conducted in the laboratories of Robert Miller and Charles Cunningham of the University of New Mexico Biology Department. This project was supported in part by a grant from the National Institute of General Medical Sciences award number T34GM00851. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institute of Health. Additional support was provided by the New Mexico Horse Council and by the University of New Mexico, Research Allocation Committee, Grant # 06-32. Mosquito surveillance is funded in part by the Centers for Disease Control and Prevention funds administrated through the New Mexico Department of Health and the taxpayers of the City of Albuquerque and Bernalillo County. Support was also provided by the UNM Center for Evolutionary and Theoretical Immunology (CETI) and the UNM Molecular Biology Facility, supported by NIH grant 1P20RR18754 from the Institute Development Award Program of the National Center for Research Resources.
REFERENCES CITED
- Anderson JF, Andreadis TG, Vossbrinck CR, Tirrell S, Wakem EM, French RA, Garmendia AE, Kruiningen HJ. Isolation of West Nile virus from mosquitoes, crows, and a Cooper’s hawk in Connecticut. Science. 1999;286:2331–2333. doi: 10.1126/science.286.5448.2331. [DOI] [PubMed] [Google Scholar]
- Barrera R, Amador M, Young G, Komar N. Mosquito (Diptera:Culicidae) bloodmeal sources during a period of West Nile virus transmission in Puerto Rico. J. Med. Entomol. 2011;48:701–704. doi: 10.1603/ME10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmayer WL. Mosquito flight paths in relation to the environment. Effect of vertical and horizontal visual barriers. Ann. Entomol. Soc. Am. 1975;68:51–57. [Google Scholar]
- Bidlingmayer WL, Hem DG. Mosquito (Diptera: Culicidae) flight behavior near conspicuous objects. Bull. Entomol. Res. 1979;69:691–700. [Google Scholar]
- Bueno MG, Lopez RPG, Menezes RMTD, Costa-Nascimento MDJ, Araújo RADS, Guida FJV, Kirchgatter K. Identification of Plasmodium relictum causing mortality in penguins (Spheniscus magellanicus) from Sao Paulo Zoo, Brazil. Vet. Parasitol. 2010;173:123–127. doi: 10.1016/j.vetpar.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Carpenter HJ, Lacasse WJ. Mosquitoes of North America (North of Mexico) Berkeley and Los Angeles, CA: University of California Press; 1955. 360 pp. [Google Scholar]
- Clark GG, Crabs CL, Bailey CL, Calisher CH, Craig GB., Jr Identification of Aedes campestris from New Mexico: with notes on the isolation of western equine encephalitis and other arboviruses. J. Am. Mosq. Contr. Assoc. 1986;2:529–534. [PubMed] [Google Scholar]
- Clarke JL, III, Rowley WA, Christiansen S, Jacobson DW. Microcomputer-based monitoring and data acquisition system for a mosquito flight mill. Ann. Entomol. Soc. Am. 1984;77:119–122. [Google Scholar]
- Cranfield MR, Graczyk TK, Beall FB, Ialeggio DM, Shaw ML, Skjoldager ML. Subclinical avian malaria infections in African black-footed penguins (Spheniscus demersus) and induction of parasite recrudescence. J. Wildl. Dis. 1994;30:372–376. doi: 10.7589/0090-3558-30.3.372. [DOI] [PubMed] [Google Scholar]
- Darsie RF, Ward RA. Identification and geographical distribution of the mosquitoes of North America, north of Mexico. Mosq. Syst. Suppl. 1981;1:1–313. [Google Scholar]
- Derraik JGB. A survey of the mosquito (Diptera: Culicidae) fauna of the Auckland Zoological Park. New Zealand Entomol. 2004;27:51–55. [Google Scholar]
- Derraik JGB. Recommendations for mosquito control in zoological parks to reduce disease transmission. Weta. 2005;29:16–20. [Google Scholar]
- Ejiri H, Sato Y, Sawai R, Sasaki E, Matsumoto R, Ueda M, Higa Y, Tsuda Y, Omori S, Murata K, Yukawa M. Prevalence of avian malaria parasite in mosquitoes collected at a zoological garden in Japan. Parasitol. Res. 2009;105:629–633. doi: 10.1007/s00436-009-1434-9. [DOI] [PubMed] [Google Scholar]
- Gingrich JB, Williams GM. Host-feeding patterns of suspected West Nile virus mosquito vectors in Delaware, 2001–2002. J. Am. Mosq. Contr. Assoc. 2005;21:194–200. doi: 10.2987/8756-971X(2005)21[194:HPOSWN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Emerg. Infect. Dis. 2002;8:1385–1391. doi: 10.3201/eid0812.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irby WS, Apperson CS. Hosts of mosquitoes in the coastal plain of North Carolina. J. Med. Entomol. 1988;25:85–93. doi: 10.1093/jmedent/25.2.85. [DOI] [PubMed] [Google Scholar]
- Kay BH, Boyd AM, Ryan PA, Hall RA. Mosquito feeding patterns and natural infection of vertebrates with Ross River and Barmah Forest viruses in Brisbane, Australia. Am. J. Trop. Med. Hyg. 2007;76:417–423. [PubMed] [Google Scholar]
- Kauffman C, Briegel H. Flight performance of the malaria vectors Anopheles gambiae and Anopheles atroparvus. J. Vector Ecol. 2004;29:140–153. [PubMed] [Google Scholar]
- Kimsey RB, Kimsey PB. Identification of arthropod blood meals using rubidium as a marker: a preliminary study. J. Med. Entomol. 1984;21:714–719. doi: 10.1093/jmedent/21.6.714. [DOI] [PubMed] [Google Scholar]
- Lanciotti R, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- Midega JT, Mbogo CM, Mwambi H, Wilson MD, Ojwang G, Mwangangi JM, Nzovu JG, Githure JI, Yan G, Beier JC. Estimating dispersal and survival of Anopheles gambiae and Anopheles funestus along the Kenyan coast by using mark–release–recapture methods. J. Med. Entomol. 2007;44:923–929. doi: 10.1603/0022-2585(2007)44[923:edasoa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG. Identification of avian- and mammalian-derived bloodmeals in Aedes vexans and Culiseta melanura (Diptera: Culicidae) and its implication for West Nile virus transmission in Connecticut, U.S.A. J. Med. Entomol. 2006;43:1088–1093. doi: 10.1603/0022-2585(2006)43[1088:IOAAMB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Vossbrinck C. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, Northeastern United States. Emerg. Infect. Dis. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Bueno R, Jr, Dennett JA, Real SV, Sargent C, Bala A, Randle Y, Guzman H, Travassos da Rosa A, Wuithiranyagool T, Tesh RB. Host feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of West Nile virus in Harris County, Texas. Am. J. Trop. Med. Hyg. 2007;77:73–81. [PubMed] [Google Scholar]
- Muir LE, Kay BH. Aedes aegypti survival and dispersal estimated by mark-release-recapture in northern Australia. Am. J. Trop. Med. Hyg. 1998;58:277–282. doi: 10.4269/ajtmh.1998.58.277. [DOI] [PubMed] [Google Scholar]
- Platt AR, Woodhall RW, George AL., Jr Improved DNA sequencing quality and efficiency using an optimized fast cycle sequencing protocol. BioTechniques. 2007;43:58–62. doi: 10.2144/000112499. [DOI] [PubMed] [Google Scholar]
- Pratt HD, Barnes RC. Identification keys for common mosquitoes of United States. CDC training guide U.S. Department of Health, Education and Welfare. Atlanta, GA: Public Health Service; 1959. 40 pp. [Google Scholar]
- Provost MW. The dispersal of Aedes taeniorhynchus: II. The second experiment. Mosq. News. 1957;17:433–436. [Google Scholar]
- Quraishi M, Faghih S, Esghi N. Flight range, lengths of gonotrophic cycles, and longevity of P32-labeled Anopheles stephensi mysorensis. J. Econ. Entomol. 1966;59:50–55. doi: 10.1093/jee/59.1.50. [DOI] [PubMed] [Google Scholar]
- Reeves WC. Ecology of mosquitoes in relation to arboviruses. Annu. Rev. Entomol. 1965;10:25–46. [Google Scholar]
- Reiter P, Amador MA, Anderson RA, Clark GG. Short report: dispersal of Aedes aegypti in an urban area after blood feeding as marked by rubidium-marked eggs. Am. J. Trop. Med. Hyg. 1995;52:177–179. doi: 10.4269/ajtmh.1995.52.177. [DOI] [PubMed] [Google Scholar]
- Rowley WA, Graham CL, Williams RE. A flight mill system for the laboratory study of mosquito flight. Ann. Entomol. Soc. Am. 1968;61:1507–1514. [Google Scholar]
- Russell PF, Knipe FW, Ramachandra Rao T, Putnam P. Some experiments on flight range of Anopheles culicifacies. J. Exp. Zool. 1944;97:135–163. [Google Scholar]
- Russell RC, Webb CE, Williams CR, Ritchie SA. Mark-release–recapture study to measure dispersal of the mosquito Aedes aegypti in Cairns, Queensland, Australia. Med. Vet. Entomol. 2005;19:451–457. doi: 10.1111/j.1365-2915.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- Saegerman C, Berkvens D, Mellor PS. Bluetongue epidemiology in the European Union. Emerg. Infect. Dis. 2008;14:539–544. doi: 10.3201/eid1404.071441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Dohm DJ, O’Guinn M. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg. Infect. Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawabe K, Isawa H, Hoshino K, Sasaki T, Roychoudhury S, Higa Y, Kasai S, Tsuda Y, Nishiumi I, Hisai N, Hamao S, Kobayashi M. Host-feeding habits of Culex pipiens and Aedes albopictus (Diptera: Culicidae) collected at the urban and suburban residential areas of Japan. J. Med. Entomol. 2010;47:442–450. doi: 10.1603/ME09256. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J. Med. Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Turrell MJ, Dohm DJ, Sardelis MR, O’Guinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 2005;42:57–63. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Wolfinsohn M, Galun R. A method for determining flight range of Aedes aegypti. Bull. Res. Council Israel. 1953;2:433–436. [Google Scholar]