Abstract
Background:
Comparisons of lung manifestations in primary pulmonary vs disseminated nontuberculous mycobacterial disease have not been well described. The clinical, histopathologic, and radiologic disease manifestations of primary pulmonary or disseminated nontuberculous mycobacterial disease were compared in an autopsy series.
Methods:
Medical and microbiologic records, autopsy reports, histopathologic slides of the lungs, and chest CT scans were reviewed on patients at the National Institutes of Health with nontuberculous mycobacterial disease who died between 1996 and 2010.
Results:
The 11 patients with primary pulmonary nontuberculous mycobacterial disease were predominantly female (n = 9), with symptom onset at median 50 (range 35, 71) years and time from onset until death of 12 (3, 34) years. Bronchiectasis with cavity formation and necrotizing bronchocentric granulomatous inflammation predominated but extrapulmonary infection was absent. The five patients with disseminated disease and systemic immune defects were all men with age at onset of 2 (0.33, 33) years and time from onset of disease until death of 9 (1, 31) years. Miliary nodules and/or consolidation with poorly formed granulomatous inflammation were noted in the three disseminated patients with mycobacterial lung involvement. Significant extrapulmonary infection was noted in all five with a relative paucity of lung findings.
Conclusions:
Nontuberculous mycobacteria can cause progressive, fatal disease. Primary pulmonary disease is bronchocentric and lacks extrathoracic infection consistent with impaired airway surface defenses. In contrast, fatal disseminated infections involving the lung have hematogenous spread, extensive extrathoracic disease, and a distinct pulmonary histopathology consistent with systemic immune dysfunction.
The nontuberculous mycobacteria (NTM) are ubiquitous environmental organisms which typically cause disease in patients with altered host defenses.1 Pulmonary disease is the most common type of involvement and occurs primarily in patients with preexisting structural lung damage such as emphysema or bronchiectasis. Pulmonary involvement is less common in the setting of systemic immune dysfunction, such as hematologic malignancies or primary immunodeficiencies, especially those involving the interferon-γ (IFN-γ)/IL-12 axis.2‐5 Nodular bronchiectasis associated with NTM, predominantly in postmenopausal women without apparent predisposing causes was initially described over 20 years ago and is increasing in the United States.6,7 The initial disease presentation with chronic cough, difficulty in distinguishing environmental contamination from true infection, and poor treatment tolerability contributes to delayed or missed diagnosis and deferred or inadequate treatment.1,8 However, these organisms can be associated with severe and fatal disease.9 Little has been written about the lung manifestations of fatal primary pulmonary disease and less about pulmonary manifestations in disseminated disease associated with primary immunodeficiencies. Comparing patterns of disease in patients autopsied with definitive NTM disease suggests distinct pulmonary and systemic mycobacterial controls.
Materials and Methods
Subjects
HIV-negative individuals were enrolled into approved natural history and treatment protocols of the National Institutes of Health (NIH). Family members provided consent for autopsy. Between 1996 and 2010, 16 patients had full autopsies available. Patients were considered primary pulmonary NTM disease (PNTM) if they fulfilled 2007 American Thoracic Society criteria.1 Patients classified as disseminated NTM disease (DNTM) had predisposing immunologic defects with proven mycobacterial infection.
Data Extraction and Statistics
Records were abstracted for (1) demographics, age at onset of mycobacterial disease symptoms, and age at death; (2) coexisting morbidities; (3) immediate and contributing causes of death (determined in the autopsy report by a combination of clinical history review and autopsy findings); (4) pulmonary function and BMIs; (5) microbiology; and (6) antibiotic use. Summary statistics are expressed as medians (ranges) and were calculated using Excel (Microsoft Corporation) or Graphpad Prism 5 (Graphpad Software Inc).
Histologic Examination
After cultures were obtained, the lungs (except patient 4 [Table 1], embalmed prior to autopsy) were inflated in formalin and fixed overnight. Lung sections were paraffin-embedded, cut at 4-micron thickness, and stained with hematoxylin and eosin. Selected histologic sections were examined with Fite and Gomori methenamine silver stains by an experienced pathologist (D. E. K.) and compared between PNTM and DNTM groups.
Table 1.
—Demographic, Microbiologic, and Clinical Characteristics
| No. | Sex/Race | Age Onset | Age at Death, y | NTM Species | Extrapulmonary NTM | Other Lung Microa | Cause of Death |
| P1 | F/W | 68 | 81 | M int | N | Yeast, S mal, P aer | Sepsis |
| P2 | F/W | 71 | 75 | M avi | N | Yeast | PNTM infection |
| P3 | F/W | 45 | 55 | M int | N | Yeast, B pet, P lil, Pen species, A ver, A fla, Ram species | PNTM infection |
| P4 | M/W | 71 | 76 | M int | N | Yeast, Kle species, P aer, C alb, C ind, A nig | PNTM infection |
| P5 | F/W | 50 | 62 | M int | N | Yeast, E der, P aer, A bas, Ach species, E clo, A xyl | PNTM infection |
| P6 | F/W | 50 | 53 | MAC | N | C alb, E clo, C fre, A fum, S mar, K pne, P aer | Acute pulmonary hemorrhage |
| P7 | F/W | 40 | 74 | M abs | N | Yeast, A fum, K pne | PNTM infection |
| P8 | F/W | 63 | 74 | MAC | N | C alb, A fum | PNTM infection |
| P9 | F/W | 43 | 72 | M kan, M abs | N | Yeast, A ver | PNTM infection |
| P10 | M/W | 35 | 61 | M abs | N | Yeast, S mal, P aer | Bronchopneumonia |
| P11 | F/B | 44 | 59 | M avi M muc | N | Yeast, A fum, A ust, Pen species, Cla species, A nig, P aer | Aspiration pneumonia |
| D12 | M/W | 2 | 9 | M for, M int | Bowel, liver, adrenal, bone marrow, aorta, kidney, nerve, muscle, LN | None | Respiratory arrest and tachycardia following seizure |
| D13 | M/W | 13 | 44 | MAC, “X” | LN, liver, skin, testes | C par | Disseminated and severe cutaneous NTM |
| D14 | M/W | 0.33 | 9 | M avi | Skin, bowel, pericardium, liver, pancreas | C gla, Sac species, | GI hemorrhage |
| D15 | M/W | 33 | 34 | M tri | LN, bowel, liver, pancreas | C tro, C alb | Acute hemorrhage |
| D16 | M/W | 1 | 25 | M avi, M abs | LN | E fae, P aer, C gla, C alb | Sepsis |
A bas = Arthroconidial basidiomycete; Ach species = Achromobacter species; A fla = Aspergillus flavus; A fum = Aspergillus fumigatus; A nig = Aspergillus niger; A ust = Apergillus ustus; A ver = Aspergillus versicolor; A xyl = Alcaligenes xylosoxidans; B = black; B pet = Bordetella petrii; C alb = Candida albicans; C fre = Citrobacter freundii; C gla = Candida glabrata; C ind = Chryseobacterium indologenes; Cla species = Cladosporium species; C par = Candida parapsilosis; C tro = Candida tropicalis; D = disseminated nontuberculous mycobacterial disease; F = female; E clo = Enterobacter cloacae; E der = Exophiala dermatiditis; E fae = Enterococcus faecium; K pne = Klebsiella pneumoniae; Kle species = Klebsiella species; LN = lymph node; M = male; M abs = Mycobacterium abscessus; MAC = Mycobacterium avium complex; MAC “X” = Mycobacterium avium complex “X” cluster; M avi = Mycobacterium avium; M for = Mycobacterium fortuitum; M int = Mycobacterium intracellular; M kan = Mycobacterium kansasii; M muc = Mycobacterium mucogenicum; M tri = Mycobacterium triplex; N = none; NTM = nontuberculous mycobacteria; P = pulmonary nontuberculous mycobacterial disease; P aer = Pseudomonas aeruginosa; Pen species = Penicillium species; P lil = Paecilomyces lilacinus; PNTM = pulmonary nontuberculous mycobacteria; Ram species = Ramicholoridium species; Sac species = Saccharomyces species; S mal = Stenotrophomonas maltophilia; S mar = Serratia marcescens; W = white.
Microbiology obtained within the 6 months prior to death.
Radiologic Examination
CT images were acquired helically (Siemens, Siemens Healthcare USA; Phillips, Phillips Healthcare; or GE, GE Healthcare). Reconstruction and viewing were by an experienced radiologist (L. R. F.) in 5 × 5 mm slices in lung and soft-tissue windows on a PACS workstation (Kodak Carestream; Centricity-PACS, GE Healthcare IT).
Results
Clinical and Microbiologic Data
The 11 patients with PNTM were predominantly female (one black and eight white women, two white men) with onset of symptoms at 50 (35, 71) years. Diagnosis of PNTM disease occurred at 54 (45, 71) years, and age at death was 72 (53, 81) years (Table 1). The time from disease onset to death was 12 (3, 34) years. BMI was 17.8 (13.5, 32.9) kg/m2 (n = 11). All had clinically severe lung disease at the time of death: FEV1 within 2 years of death (n = 9) was 43% predicted (22%-66% predicted), supplemental oxygen use at home was required by 80% (n = 10), and pulmonary hypertension was demonstrated in seven patients. Six were prior smokers and five were nonsmokers. Four received systemic steroids and four were on inhaled steroids within the year before death. At mycobacterial disease presentation, all patients had bronchiectasis. Associations were Sjögren syndrome (n = 2), sarcoidosis (n = 1), cystic fibrosis (CF) diagnosed at time of adult mycobacterial disease presentation (n = 1), and idiopathic bronchiectasis (n = 7).
Eight individuals had Mycobacterium avium complex (MAC); three had Mycobacterium abscessus; one each had M abscessus plus Mycobacterium kansasii, and MAC plus Mycobacterium mucogenicum. In the 6 months prior to death, all patients with PNTM had positive respiratory mycobacterial smears and cultures. Other organisms included yeast (n = 11), Aspergillus species (n = 7), and Pseudomonas aeruginosa (n = 6). Three of the 10 patients with lung tissue mycobacterial cultures obtained at autopsy were culture positive. All patients were taking three or more antibiotics for treatment of their NTM in the year prior to death.
Comorbid conditions included chronic hyponatremia, cardiovascular disease, and chronic malnutrition (four individuals required enteral feeding tubes) (e-Table 1). All patients had comprehensive immune evaluations; one patient had selective IgA deficiency and two had idiopathic CD4 lymphocytopenia. Three patients had hypothyroidism, two had Sjögren syndrome, one had discoid lupus, and one had Evans syndrome requiring splenectomy.
Progressive respiratory failure due to mycobacterial disease was determined at autopsy as the cause of death in seven patients with PNTM. The other four patients with extensive lung disease died of sepsis after a ruptured duodenal ulcer (patient 1), acute pulmonary hemorrhage likely related to locally invasive Aspergillus (patient 6), acute bronchopneumonia with only heavy growth of Stenotrophomonas maltophilia at the time of death (patient 10), and aspiration pneumonia (patient 11).
The patients with DNTM were all white men with symptom onset at 2 (0.33, 33) years, diagnosis of disseminated disease at 13 (0.33, 33) years, and death at 25 (9, 44) years. Death occurred 9 (1, 31) years after disease onset. The BMI was 19.9 (6.9, 24.9) kg/m2. Four of the five individuals had histories of MAC; one each had both Mycobacterium intracellulare and Mycobacterium fortuitum; M avium and M abscessus; and one had Mycobacterium simplex. All patients with DNTM had positive mycobacterial cultures and/or acid fast bacilli smears within 6 months prior to death. Three of the five had active mycobacterial lung infection with positive lung tissue cultures at autopsy; patient 15 had positive blood cultures for Candida glabrata and Candida tropicalis and positive lung tissue cultures for C tropicalis and Candida albicans at autopsy; and patient 16 had only lower lobe congestion, consolidation, hemorrhage, atelectasis, and subpleural fibrosis without evidence of active lung infection at autopsy. None used tobacco or received steroids.
In contrast to patients with PNTM, patients with DNTM had relatively little comorbidity not directly caused by infections or their treatments. All had immune defects involving the IFN-γ/IL-12 axis: autosomal-recessive IFN-γ receptor (IFNγR) 2 (patient 12); nuclear factor (NF)-κB essential modulator (patients 13, 14, 16); and undefined combined immunodeficiency (patient 15).
All patients with DNTM died of complications of chronic infections; no deaths were related to mycobacterial lung involvement. Patient 12 died of respiratory arrest following a generalized seizure; patient 13 died of progressive cutaneous mycobacterial disease; patient 14 died of gastrointestinal hemorrhage in the setting of disseminated intravascular coagulopathy; patient 15 died of acute hemorrhage following a central line placement; and patient 16 died of myocardial infarction in the setting of calciphylaxis.
Pulmonary Histopathology
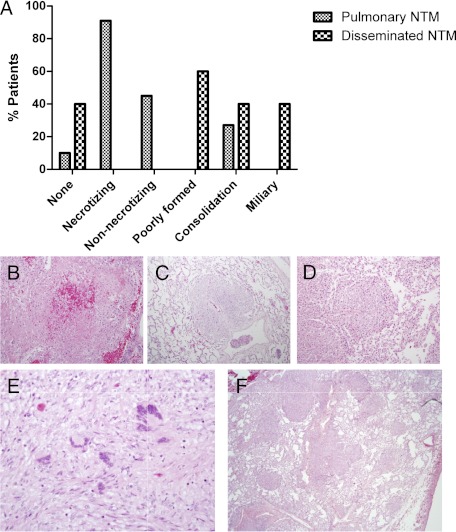
All patients with PNTM except patient 10 had well-organized necrotizing granulomatous inflammation in the lung (Fig 1). Five patients also exhibited nonnecrotizing granulomas and three had diffuse granulomatous consolidation. Mycobacteria were seen in scant amounts in the bronchial walls, predominantly at the edge of the granulomas in five patients (Fig 2A). Patients 5 and 6 also had fungi in the bronchial wall granulomatous inflammation (Fig 2B). Only three patients (60%) with DNTM had pulmonary granulomatous reaction with tissue invasion by mycobacteria. The granulomas were poorly organized: Two had diffuse consolidation, and two had miliary nodules (Figs 1E, 1F). Mycobacteria were seen predominantly within macrophages and multinucleated giant cells and were generally more numerous than in patients with PNTM.
Figure 1.
A-F, Granulomatous inflammation. Distribution of patterns of granulomatous inflammation by type of nontuberculous mycobacterial disease (A) include both necrotizing (B, hematoxylin and eosin, original magnification × 400) and nonnecrotizing (C, hematoxylin and eosin, original magnification × 100) granulomas seen in pulmonary NTM disease and poorly formed granulomas (D, hematoxylin and eosin, original magnification × 200) seen in disseminated NTM disease. A pattern of diffuse granulomatous consolidation (E, hematoxylin and eosin, original magnification × 400) was seen in both, and miliary distribution (F, hematoxylin and eosin, original magnification × 40) of granulomas was seen in disseminated disease involving the lung. NTM = nontuberculous mycobacteria.
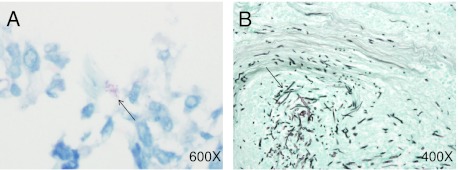
Figure 2.
Microbial invasion. A, Fite stain showing mycobacteria (black arrow) at the edge of a necrotizing granuloma (patient 8) (original magnification × 600). B, Gomori methenamine silver stain showing fungi (black arrow) invading tissue (patient 6) (original magnification × 400).
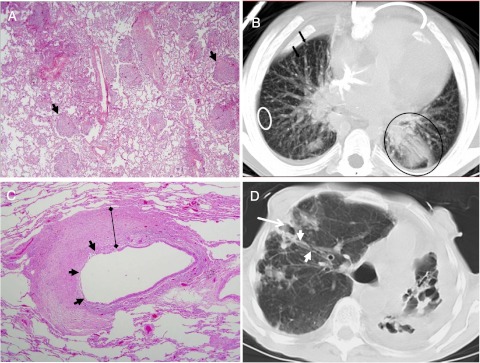
PNTM pulmonary pathology was characterized by bronchocentric chronic inflammation and bronchiectasis in 10 patients with bronchial obliteration by granulomatous inflammation in eight (Fig 3C, Table 2). Cavities (73%) and nodules (36%) were common in PNTM. Fibrosis was seen in all patients with PNTM with emphysema in 73% and diffuse alveolar damage in 36%. There were fewer pulmonary histopathologic findings in patients with DNTM. Miliary nodules scattered throughout the lung parenchyma without aggregation around the airways (n = 2) and acute pneumonia (n = 2) predominated in the three patients who had evidence of lung involvement (Fig 3A).
Figure 3.
A, B, Histopathology and CT scan findings. The panels show poorly formed granulomas (black arrowheads) (A, hematoxylin and eosin, original magnification × 40) and both a miliary radiographic pattern (white circle and black arrows) and consolidation (black circle) (B) from a patient (patient 12) with an interferon-γ receptor defect and disseminated NTM disease. Patterns typical of pulmonary NTM disease from a woman (patient 8) with longstanding pulmonary Mycobacterium avium complex show bronchocentric pathology (C, hematoxylin and eosin, original magnification × 40) with sloughing of the epithelial lining (black arrowheads) and circumferential replacement of the bronchial wall architecture with granulomatous inflammation (black bar) that correlates with bronchiectasis and bronchial wall thickening (white arrowheads) ending in distal cavitary type changes (white arrow) (D). See Figure 1 legend for expansion of abbreviations.
Table 2.
—Histology and Chest CT Scan Findings
| Findings | PNTM, n = 11 (%) | DNTM, n = 5 (%) |
| Histopathology | ||
| Bronchiectasis | 10 (91) | 0 |
| Obliterated bronchi | 8 (73) | 1 (20) |
| Cavities | 8 (73) | 1 (20) |
| Nodules | 4 (36) | 2 (40) |
| Acute pneumonia | 1 (10) | 2 (40) |
| Diffuse alveolar damage | 4 (36) | 0 |
| Pulmonary edema | 3 (27) | 1 (20) |
| Emphysema | 8 (73) | 0 |
| Fibrosis | 11 (100) | 1 (20) |
| CT scan | ||
| Bronchiectasis | 10 (91) | 0 |
| Cavities | 7 (63) | 0 |
| Nodules | 10 (91) | 2 (40) |
| Miliary pattern | 0 | 2 (40) |
| Calcifications | 5 (45) | 0 |
| Consolidation | 5 (45) | 3 (60) |
| Ground glass opacity | 6 (55) | 1 (20) |
| Atelectasis | 3 (27) | 0 |
| Emphysema | 3 (27) | 0 |
| Reticular pattern | 4 (36) | 0 |
DNTM = disseminated nontuberculous mycobacteria. See Table 1 legend for expansion of other abbreviation.
Chest Radiography
The time between the last CT scan and death was 2.7 ± 5.8 months. The PNTM CT scan findings reflected the nodular (91%), bronchiectatic (91%), and cavitary (64%) predominance of the disease (Fig 3B, Table 2). Ground glass opacities (55%), consolidation (45%), and calcifications (45%) were also common. Longstanding, progressive disease was reflected in atelectasis (27%), emphysema (27%), and a reticular pattern (ie, architectural distortion, intralobular lines, parenchymal bands) (36%). There were fewer types of pulmonary radiographic findings in patients with DNTM than in patients with PNTM (Table 2). Miliary nodules and consolidation were the predominant radiographic findings in the three patients with DNTM with lung involvement (Fig 3D).
Discussion
Fatal PNTM was associated with extensive bronchiectasis and cavity formation with bronchocentric granulomatous inflammation. Most patients with PNTM had mycobacterial disease limited to the chest. In contrast, fatal DNTM was accompanied by a relative paucity of lung findings. When the lung was involved, it was with poorly formed miliary granulomas or consolidated mycobacterial pneumonia. These patterns of disease suggest impaired airway surface defenses either as a predisposing factor for bronchiectasis or as a result of mycobacterial-induced damage in PNTM and impaired systemic immunity in DNTM. The lung has a large surface area exposed to the environment and a large vascular surface area in close proximity, providing the potential for environmental microbes to encounter both airway surface and systemic immune defenses. The prototypical bronchiectatic diseases, such as CF and primary ciliary dyskinesia, are characterized by dysfunctional mucociliary clearance and an exceedingly high prevalence of NTM.10,11 High prevalences of CFTR mutations and abnormal ion transport have been described in patients with idiopathic bronchiectasis.12,13 Tomashefski et al14 reviewed autopsies in 18 patients with CF with at least one positive NTM culture and compared them to 18 patients with CF with repeated negative sputum mycobacterial cultures. Only six had more than one positive NTM culture and only three were considered to have clinically significant disease. All patients had end-stage lung disease characterized by chronic bronchitis, purulent bronchiectasis, bronchiolitis obliterans, acute bronchopneumonia, and chronic organizing pneumonia. Ulcerative, “semigranulomatous” bronchitis was seen in approximately half the patients in both groups. However, mycobacterial invasion was associated with necrotizing granulomatous inflammation and granulomatous organizing pneumonia. None of these patients had disease outside the thorax. The bronchocentric nature of the radiographic and histologic findings in the patients with PNTM is similar to those described in earlier stage disease in immunocompetent patients.15,16
Hafeez et al17 reported three cases of pulmonary deterioration from presumed chronic necrotizing pulmonary aspergillosis occurring in PNTM that temporally improved with corticosteroids. All had normal IgE levels without eosinophilia and none had responded to itraconazole. The potential role of fungi in their deterioration is of concern given the high prevalence of positive fungal cultures in the patients (Table 1). Steroids are well known to predispose to invasive fungal disease. Two of the patients with PNTM (5, 6) had fatal lung disease associated with antemortem recovery of filamentous fungi with confirmed tissue invasion at autopsy. Neither had eosinophilia or an elevated IgE. Patient 5 had received a course of moderate dose prednisone 1 to 2 months prior to her death after a decline in lung function, weight, and functional status without improvement.
DNTM disease is rare, but often caused by defects in IFN-γ/IL-12 signaling.18 IFN-γ is diffusely involved in intracellular killing of mycobacteria, ranging from upregulating major histocompatibility complex class 2 antigens, to stimulating differentiation of lymphocytes into T helper 1 cells and stimulation of release of proinflammatory cytokines. Tumor necrosis factor-α signals through intracellular NF-κB to drive granuloma formation.2 The defects in patients with DNTM involve disruption of IFN-γ signaling and NF-κB signaling, causing marked impairment in mycobacterial control. Dorman et al4 reported organ-specific mycobacterial involvement in patients with either recessive complete IFNγR1 deficiency (n = 22) or dominant partial IFNγR1 deficiency (n = 38). Pulmonary MAC was seen in approximately 75% of recessive complete deficiency vs approximately 30% in patients with dominant partial defects. In NF-κB essential modulator deficiency mycobacterial infections tended to develop later in childhood than infections from pyogenic bacteria. Two of the patients in this report who died of disseminated MAC had miliary nodules in the lung and other organs.5 The pathologic findings in the lung in the patients with DNTM are similar to autopsy series of MAC in AIDS. There lung pathology included miliary, poorly formed granulomas with numerous mycobacteria in 20% to 30% of cases.19,20 In patients with AIDS who died with MAC bacteremia only 30% had histopathologic evidence of end-organ disease. In contrast, all of the patients with DNTM had histologic evidence of mycobacterial disease in other organs. Patients with DNTM generally had relatively prolonged survival compared with autopsy reports in patients with AIDS. In AIDS, the likelihood of histopathologic findings correlated with the length of survival.19,20 All of the patients received aggressive antibiotic therapy against their mycobacteria for prolonged courses with the potential for significant toxicity.8 Patient 6 had clofazimine crystal deposition in the spleen, mesenteric lymph nodes, and small bowel.21 Patient 16 developed end stage renal disease years following nephrotoxic drug exposures (eg, amikacin, amphotericin). He subsequently developed widespread calcific uremic arteriolopathy involving arteries of almost every organ. This poorly understood condition, also known as calciphylaxis, involves calcium-phosphate homeostasis and has a very high associated mortality.22
A limitation of this study is our inability to assess potential factors that might predict more severe disease. Systematic long term follow-up with the ability to assess overall mortality has been reported in association with recent treatment trials. The British Thoracic Society noted a 5-year mortality of 36% among a cohort of 75 patients with moderately severe MAC disease treated for 2 years.9 The patients in that study were predominantly men with cavitary disease. However, the mortality attributable to the NTM was only 4%. In our cohort, a high percentage of patients required supplemental oxygen and had pulmonary hypertension, both previously associated with fatal disease.23 Some patients had very low BMIs and some needed enteral gastrostomy tubes.
In summary, fatal PNTM occurred in patients with extensive bronchiectasis, cavity formation, lung volume loss and scarring, and many had evidence of chronic hypoxemia, pulmonary hypertension, and malnutrition. Concomitant airway pathogens, especially the filamentous fungi, may contribute to onset or progression of terminal disease. Most of these patients had significant comorbidities not directly related to their infections but had no infection outside the chest. In contrast, fatal DNTM occurred in patients with widespread infection which in some cases also involved the lungs with less differentiated granulomas. These contrasting pathologic features reflect the profound differences between isolated pulmonary disease due to abnormalities only apparent in the airways, and disseminated disease due to defects in cell mediated immunity, which generally spares the airways. More detailed understanding of the mycobacterial pathology of the airway and the systemic antimycobacterial immune response will illuminate the mechanisms by which the same organisms elicit such disparate responses.
Supplementary Material
Acknowledgments
Author contributions: Dr Olivier verified, and is responsible for, the accuracy of the data reported.
Ms O’Connell: contributed equally with Ms Birkenkamp to searching records to identify patients who had available autopsy material, abstracted clinical data from medical records, organized and abstracted joint reviewing sessions with pathology and radiology, and drafted the data tables and initial versions of the manuscript.
Ms Birkenkamp: contributed equally with Ms O’Connell to searching records to identify patients who had available autopsy material, abstracted clinical data from medical records, organized and abstracted joint reviewing sessions with pathology and radiology, and drafted the data tables and initial versions of the manuscript.
Dr Kleiner: contributed to the autopsy service at the NIH Clinical Center, reviewed all of the autopsy slides, provided the pathologic images, and wrote/edited the histopathologic sections of the manuscript.
Dr Folio: contributed to the review of all CT scans, provided CT images, and wrote/edited the radiologic sections of the manuscript.
Dr Holland: contributed as the principal investigator of the clinical research protocols under which these patients were followed at the NIH, provided valuable input on the clinical history of these patients, and edited the manuscript.
Dr Olivier: initiated this research project, supervised its progress, and wrote the final version of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: This work was performed at the Clinical Center of the National Institutes of Health, Bethesda, MD.
Additional information: The e-Table can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/141/5/1203/suppl/DC1.
Abbreviations
- CF
cystic fibrosis
- DNTM
disseminated nontuberculous mycobacteria
- IFN-γ
interferon-γ
- IFNγR
interferon-γ receptor
- MAC
Mycobacterium avium complex
- NF
nuclear factor
- NIH
National Institutes of Health
- NTM
nontuberculous mycobacteria
- PNTM
pulmonary nontuberculous mycobacteria
Footnotes
Ms Birkenkamp is currently at the University of Minnesota Medical School (Minneapolis, MN).
Mss O’Connell and Birkenkamp contributed equally to this study.
Funding/Support: This research was supported by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, and the National Institutes of Health Clinical Center.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, et al. ATS Mycobacterial Diseases Subcommittee American Thoracic Society Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [erratum published in: Am J Respir Crit Care Med. 2007;175(7):744-745] Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 2.Sexton P, Harrison AC. Susceptibility to nontuberculous mycobacterial lung disease. Eur Respir J. 2008;31(6):1322–1333. doi: 10.1183/09031936.00140007. [DOI] [PubMed] [Google Scholar]
- 3.Afessa B, Green W, Chiao J, Frederick W. Pulmonary complications of HIV infection: autopsy findings. Chest. 1998;113(5):1225–1229. doi: 10.1378/chest.113.5.1225. [DOI] [PubMed] [Google Scholar]
- 4.Dorman SE, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet. 2004;364(9451):2113–2121. doi: 10.1016/S0140-6736(04)17552-1. [DOI] [PubMed] [Google Scholar]
- 5.Orange JS, Jain A, Ballas ZK, Schneider LC, Geha RS, Bonilla FA. The presentation and natural history of immunodeficiency caused by nuclear factor kappaB essential modulator mutation. J Allergy Clin Immunol. 2004;113(4):725–733. doi: 10.1016/j.jaci.2004.01.762. [DOI] [PubMed] [Google Scholar]
- 6.Prince DS, Peterson DD, Steiner RM, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321(13):863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 7.Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182(7):970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballarino GJ, Olivier KN, Claypool RJ, Holland SM, Prevots DR. Pulmonary nontuberculous mycobacterial infections: antibiotic treatment and associated costs. Respir Med. 2009;103(10):1448–1455. doi: 10.1016/j.rmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Research Committee of the British Thoracic Society Pulmonary disease caused by Mycobacterium avium-intracellulare in HIV-negative patients: five-year follow-up of patients receiving standardised treatment. Int J Tuberc Lung Dis. 2002;6(7):628–634. [PubMed] [Google Scholar]
- 10.Olivier KN, Weber DJ, Wallace RJ, Jr, et al. Nontuberculous Mycobacteria in Cystic Fibrosis Study Group Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med. 2003;167(6):828–834. doi: 10.1164/rccm.200207-678OC. [DOI] [PubMed] [Google Scholar]
- 11.Noone PG, Leigh MW, Sannuti A, et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169(4):459–467. doi: 10.1164/rccm.200303-365OC. [DOI] [PubMed] [Google Scholar]
- 12.Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178(10):1066–1074. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziedalski TM, Kao PN, Henig NR, Jacobs SS, Ruoss SJ. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest. 2006;130(4):995–1002. doi: 10.1378/chest.130.4.995. [DOI] [PubMed] [Google Scholar]
- 14.Tomashefski JF, Jr, Stern RC, Demko CA, Doershuk CF. Nontuberculous mycobacteria in cystic fibrosis. An autopsy study. Am J Respir Crit Care Med. 1996;154(2 pt 1):523–528. doi: 10.1164/ajrccm.154.2.8756832. [DOI] [PubMed] [Google Scholar]
- 15.Fujita J, Ohtsuki Y, Shigeto E, et al. Pathological findings of bronchiectases caused by Mycobacterium avium intracellulare complex. Respir Med. 2003;97(8):933–938. doi: 10.1016/s0954-6111(03)00120-3. [DOI] [PubMed] [Google Scholar]
- 16.Jeong YJ, Lee KS, Koh WJ, Han J, Kim TS, Kwon OJ. Nontuberculous mycobacterial pulmonary infection in immunocompetent patients: comparison of thin-section CT and histopathologic findings. Radiology. 2004;231(3):880–886. doi: 10.1148/radiol.2313030833. [DOI] [PubMed] [Google Scholar]
- 17.Hafeez I, Muers MF, Murphy SA, Evans EG, Barton RC, McWhinney P. Non-tuberculous mycobacterial lung infection complicated by chronic necrotising pulmonary aspergillosis. Thorax. 2000;55(8):717–719. doi: 10.1136/thorax.55.8.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haverkamp MH, van Dissel JT, Holland SM. Human host genetic factors in nontuberculous mycobacterial infection: lessons from single gene disorders affecting innate and adaptive immunity and lessons from molecular defects in interferon-gamma-dependent signaling. Microbes Infect. 2006;8(4):1157–1166. doi: 10.1016/j.micinf.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Klatt EC, Jensen DF, Meyer PR. Pathology of Mycobacterium avium-intracellulare infection in acquired immunodeficiency syndrome. Hum Pathol. 1987;18(7):709–714. doi: 10.1016/s0046-8177(87)80242-3. [DOI] [PubMed] [Google Scholar]
- 20.Torriani FJ, McCutchan JA, Bozzette SA, Grafe MR, Havlir DV. Autopsy findings in AIDS patients with Mycobacterium avium complex bacteremia. J Infect Dis. 1994;170(6):1601–1605. doi: 10.1093/infdis/170.6.1601. [DOI] [PubMed] [Google Scholar]
- 21.Jadhav MV, Sathe AG, Deore SS, Patil PG, Joshi NG. Tissue concentration, systemic distribution and toxicity of clofazimine—an autopsy study [erratum published in Indian J Pathol Microbiol. 2004;47(4):590] Indian J Pathol Microbiol. 2004;47(2):281–283. [PubMed] [Google Scholar]
- 22.Rogers NM, Teubner DJ, Coates PT. Calcific uremic arteriolopathy: advances in pathogenesis and treatment. Semin Dial. 2007;20(2):150–157. doi: 10.1111/j.1525-139X.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamada H, Kohyama T, Terashi K, et al. Fatal pulmonary infection due to multidrug-resistant Mycobacterium kansasii which developed in an immunocompetent young man. Intern Med. 1997;36(4):298–300. doi: 10.2169/internalmedicine.36.298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.