Abstract
Background:
Hypoxia inducible factor (HIF)-1 plays an important role in cellular adaptation to hypoxia by activating oxygen-regulated genes such as vascular endothelial growth factor (VEGF) and erythropoietin. Sputum VEGF levels are reported to be decreased in COPD, despite hypoxia. Here we show that patients with COPD fail to induce HIF-1α and VEGF under hypoxic condition because of a reduction in histone deacetylase (HDAC) 7.
Methods:
Peripheral blood mononuclear cells (PBMCs) were obtained from patients with moderate to severe COPD (n = 21), smokers without COPD (n = 12), and nonsmokers (n = 15). PBMCs were exposed to hypoxia (1% oxygen, 5% CO2, and 94% N2) for 24 h, and HIF-1α and HDAC7 protein expression in nuclear extracts were determined by sodium dodecyl sulfate poly acrylamide gel electrophoresis (SDS-PAGE)/Western blotting.
Results:
HIF-1α was significantly induced by hypoxia in each group when compared with the normoxic condition (12-fold induction in nonsmokers, 24-fold induction in smokers without COPD, fourfold induction in COPD), but induction of HIF-1α under hypoxia was significantly lower in patients with COPD than in nonsmokers and smokers without COPD (P < .05 and P < .01, respectively). VEGF messenger RNA detected by quantitative real-time polymerase chain reaction was correlated with HIF-1α protein in nuclei (r = 0.79, P < .05), and HDAC7 protein expression was correlated with HIF-1α protein in nuclei (r = 0.46, P < .05). HDAC7 knockdown inhibited hypoxia-induced HIF-1α activity in U937 cells, and HIF-1α nuclear translocation and HIF-1α binding to the VEGF promoter in A549 cells.
Conclusions:
HDAC7 reduction in COPD causes a defect of HIF-1α induction response to hypoxia with impaired VEGF gene expression. This poor cellular adaptation might play a role in the pathogenesis of COPD.
Hypoxia inducible factor (HIF)-1, which is a heterodimer composed of HIF-1α and HIF-1β, is an important transcription factor for cellular adaptation to hypoxia.1,2 In normoxic condition, HIF-1α protein is constantly degraded because of ubiquitination and subsequent proteasomal degradation. The ubiquitination of HIF-1α is caused by hydroxylation of prolyl residues by prolyl hydroxylase, which is an oxygen-sensitive enzyme. In hypoxic condition, HIF-1α is stabilized because of inactivation of prolyl hydroxylase. As a result, HIF-1α translocates into the nucleus and binds specific binding sites on promoters of oxygen-regulated genes, such as vascular endothelial growth factor (VEGF)3 and erythropoietin.4
Histone deacetylase (HDAC) is an enzyme that removes an acetyl group from lysine on a histone. Deacetylation of histone proteins results in unwinding of the chromatin structure, resulting in transcriptional inactivation. Eighteen HDAC molecules are known and they are divided into three groups, types I, II, and III. The HDAC enzymes also deacetylate nonhistone proteins such as glucocorticoid receptor5 and nuclear factor-κB (NF-κB).6,7 We reported previously that HDAC2 is decreased in COPD8 and is involved in steroid insensitivity.5,9
Hypoxic condition is seen in patients with severe and very severe COPD or during exacerbations.10 Hypoxic condition is also seen even in patients with moderate COPD after exercise.11 However, VEGF has been reported to decrease with increasing severity of COPD,12,13 despite hypoxic condition, although many proinflammatory cytokines and chemokines are reported to increase and correlate with the severity of COPD.14 It is speculated that the decreased VEGF levels in COPD affect the pathogenesis of emphysema.12 The molecular mechanisms of the paradoxic VEGF reduction are unknown.
We hypothesized that the impaired HIF-1α response to hypoxia in patients with COPD is caused by impaired HIF-1α nuclear translocation due to reduction of HDAC7. The aim of this study was to investigate HIF-1α, HDAC7, and VEGF expression in clinical samples and to explore the relationship of the molecules using molecular biology methods. Here, we show that a poor HIF-1α response to hypoxia is one of the features of patients with COPD and is caused by a reduction in HDAC7 protein expression.
Materials and Methods
Patient Recruitment
Patients and healthy control subjects were recruited from the outpatient department of the Royal Brompton Hospital, local general practice, and the National Heart and Lung Institute. Patients with COPD were given a diagnosis using the criteria of the Global Initiative for Obstructive Lung Disease (GOLD).10 Patients with COPD who had had an exacerbation during the 2 months prior to the visit were excluded. This study was reviewed and approved by the Hounslow and Hillingdon Research Ethics Committee, approval number 05/Q0407/91. Written informed consent was obtained from all subjects.
Peripheral Blood Mononuclear Cell Isolation and Peripheral Lung Tissue Collection
Peripheral blood mononuclear cells (PBMCs) were isolated using ACCUSPIN System-Histopaque (Sigma-Aldrich) according to the manufacturer’s instructions. Isolated PBMCs were resuspended in RPMI-1640 medium (Invitrogen) containing 10% fetal bovine serum and 2 mM l-glutamine. Peripheral lung tissue was obtained using a tissue bank linked to an established patient registry (e-Table 1).15
Sputum Processing and Oxidative Stress Marker Measurement
Induced sputum samples were solubilized with 0.005% dithiothreitol. The solution was centrifuged at 1,500 rpm for 10 min to obtain cell-free supernatants. Malondialdehyde, an oxidative stress marker in sputum supernatants, was measured with a commercially available thiobarbituric acid reactive substances assay kit (Cayman) according to the manufacturer’s instructions.
Hypoxia Treatment
The Modular Incubator Chamber (MIC-101; Billups-Rothenberg Inc), a tightly sealed chamber, was used to maintain hypoxic conditions. PBMCs were seeded in culture dishes with 0.5 × 106/mL and placed in the chamber. The hypoxia gas (1% oxygen, 5% CO2, and 94% N2) was flowed into the chamber at 20 L/min for 5 min, followed by incubation for 1 h at 37°C. This procedure was repeated three times prior to overnight incubation in the sealed chamber at 37°C. If necessary, U937 cells (American Type Culture Collection) were treated with trichostatin A (TSA) (type I and II HDAC inhibitor) (Sigma-Aldrich) and incubated for 12 h under hypoxia.
In some experiments, hypoxia treatment was performed with AnaeroPack-Anaero 5% (Mitsubishi Gas Chemical Company, Inc) according to the manufacturer’s instructions. This system maintains 0.1% oxygen and 5% CO2 for 24 h. With this system, HIF-1α nuclear translocation was observed (e-Fig 1) the same as with the Modular Incubator Chamber system.
Reverse Transcription and Real-Time Quantitative Polymerase Chain Reaction
Total RNA extraction and reverse transcription of total RNA was done using the RNeasy kit and the Omniscript RT kit (QIAGEN), respectively, according to the manufacturer’s instructions. The VEGF messenger RNA (mRNA) expression was determined by real-time quantitative polymerase chain reaction (PCR) on a Rotor-Gene 3000 (Corbett Research) with TaqMan universal master mix and TaqMan primer mix (Applied Biosystems). The PCR cycles were 95°C for 15 s for denaturing, and 60°C for 1 min for annealing and extension. Data analysis was performed using the Rotor-Gene software. The amount of transcripts in each sample was standardized with that of guanine nucleotide binding protein, β polypeptide 2-like 1 (GNB2L1).
Protein Detection
Nuclear proteins were extracted using a Nuclear Extraction kit (Active Motif) according to the manufacturer’s instructions. The protein samples were loaded onto the gel (10% Novex Tris-Glycine Gel [Invitrogen]) and electrophoresed at 150 V, 40 mA, 7.0 W for 90 min. The protein samples in the gels were blotted onto nitrocellulose membrane using the iBlot Dry Blotting System and iBlot Gel Transfer Stacks (Invitrogen) according to the manufacturer’s instructions. HIF-1α protein and HDAC7 protein were detected with anti-HIF-1α antibody (BD Biosciences) and HDAC7 antibody (Sigma-Aldrich), respectively.
RNA Interference
U937 cells and A549 cells (American Type Culture Collection) were grown in RPMI-1640 medium and Dulbecco modified eagle medium, respectively, containing 10% fetal bovine serum and 2 mM l-glutamine. Short interference RNAs (siRNAs) (HDAC1, 2, 3, 5, 7, and 8) or control siRNA (Applied Biosystems) (30 nM) were transfected to U937 using jetSI (Polyplus-Transfection Inc), and the cells were incubated for 48 h under normoxic condition, followed by 12 h incubation under hypoxic condition. Nuclear fraction was extracted and HIF-1 activities were measured using the TransAM kit (Active Motif). siRNA transfection to A549 cells was carried out with the lipofectamin RNAiMAX Transfection Agent (Invitrogen) and Silencer Select siRNA (Life Technologies) according to the manufacturer’s instructions.
Cigarette Smoke-Conditioned Medium
Commercially available tobacco (Marlboro light [Philip Morris]) smoke was bubbled in 10 mL phosphate buffered saline. The optical density (OD) of cigarette smoke-conditioned medium (CSM) was measured at 320 nm. CSM with an OD value of 0.5 was used for the experiments.
Chromatin Immunoprecipitation Assay in VEGF Promoter
A549 cells were cultured in a six-well plate at a density of 1 × 106 cells/well and transfected with 100 nM of siRNA of HDAC7 and HDAC2 by the Lipofectamine RNAiMAX Transfection Agent (Invitrogen). Twenty-four hours later, cells were treated with CoCl2 (50 μM) and incubated for a further 24 h. Cells were then treated with 1% formaldehyde and 0.125 M glycine. Soluble chromatins were isolated by using the EZ-Zyme chromatin prep kit (Millipore) and immunoprecipitated with anti-HIF-1α antibody. Histone/DNA crosslinks were reversed by adding 5 N NaCl at 65°C for 4 h, followed by phenol/chloroform extraction and ethanol precipitation. PCR was performed to amplify the VEGF promoter using chromatin immunoprecipitation primers (sense 5′-AGACTCCACAGTGCATACGTG-3′ and antisense 5′-AGTGTGTCCCTCTGACAATG-3′) as reported previously.16 The PCR product was detected by StepOnePlus (Applied Biosystems).
Statistics
Results were presented as mean ± SD of the mean. Multiple comparisons were performed by analysis of variance following Bonferroni multiple comparison test. When the data were not normally distributed, determination of variance was done by nonparametric statistical analyses, Kruskal-Wallis analysis followed by Dunn post test. The comparisons between the two groups were performed by Welch t test or the Mann-Whitney U test. A P value < .05 was considered statistically significant. These analyses were performed using Graph Pad Prism 4 Software (Graph Pad Prism).
Results
The Level of Nuclear HIF-1α Protein From Patients With COPD Is Lower Than That From Healthy Subjects Under Hypoxic Condition
A total of 48 subjects (21 patients with COPD aged 62 ± 9 y, 12 smokers without COPD aged 52 ± 10 y, and 15 healthy nonsmokers aged 51 ± 12 y) were recruited for this study (Table 1). FEV1 % predicted and FEV1/FVC of patients with COPD (59% ± 14% and 58% ± 10%, respectively) showed significant airflow limitation, whereas those of nonsmokers (97% ± 13% and 80% ± 5%, respectively) and smokers without COPD (94% ± 7% and 78% ± 4%, respectively) were in the normal range. The severity of COPD was stage II (n = 15), stage III (n = 5), or stage IV (n = 1). Eleven out of 21 patients with COPD were treated with inhaled corticosteroids, 11 patients were treated with long-acting muscarinic antagonists, and eight patients were administered long-acting β-agonists. No patient was given theophylline.
Table 1.
—Patient Characteristics
| Characteristic | Nonsmokers (n = 15) | Smokers Without COPD (n = 12) | COPD (n = 21) |
| Age, y | 51 ± 12 | 52 ± 10 | 62 ± 9a,b |
| Sex, female (male) | 6 (9) | 7 (5) | 8 (13) |
| Pack-y | 0 ± 0 | 24 ± 17 | 60 ± 36c |
| Smoking status, current smoker (ex-smoker) | NA | 12 (0) | 9 (12) |
| FEV1, L | 3.06 ± 0.72 | 2.74 ± 0.86 | 1.76 ± 0.58b,d |
| FEV, % predicted | 97 ± 13 | 94 ± 7 | 59 ± 14c,d |
| FEV1/FVC, % | 80 ± 5 | 78 ± 4 | 58 ± 10c,d |
NA = not applicable.
P < .01 compared with nonsmokers.
P < .01 compared with smokers without COPD.
P < .001 compared with smokers without COPD.
P < .001 compared with nonsmokers.
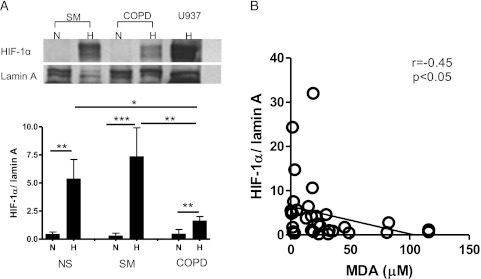
The level of nuclear HIF-1α protein in PBMCs exposed to normal oxygen levels overnight at 37°C showed no significant difference among the groups (ratio of HIF-1α to lamin A, 0.44 ± 0.20 in nonsmokers, 0.30 ± 0.23 in smokers without COPD, and 0.46 ± 0.40 in patients with COPD). However, hypoxia (1% oxygen) significantly increased HIF-1α expression in nuclear protein from PBMC 12-fold in nonsmokers and 24-fold in smokers, whereas the increase of HIF-1α protein in patients with COPD was only fourfold. The level of HIF-1α protein in the nuclei of PBMCs obtained from patients with COPD (ratio of HIF-1α to lamin A, 1.6 ± 0.38) was significantly lower than the levels from smokers without COPD (7.4 ± 2.6) and nonsmokers (5.4 ± 1.1) after exposure to hypoxia (Fig 1A). The level of HIF-1α protein in nuclei significantly correlated with sputum malondialdehyde, an oxidative stress marker (r = 0.45, P < .05) (Fig 1B).
Figure 1.
HIF-1α protein in nuclei of peripheral blood mononuclear cells (PBMCs). PBMCs were incubated in hypoxic condition (1% oxygen, 5% CO2, and 94% N2) for 24 h. Nuclear protein was extracted, and HIF-1α protein was detected. A, HIF-1α protein was normalized by lamin A. Representative Western blot images are also shown. B, Correlation between HIF-1α protein in nuclei and sputum malondialdehyde (an oxidative stress marker). *P < .05; **P < .01; ***P < .001. H = hypoxia; HIF-1α = hypoxia inducible factor-1α; MDA = malondialdehyde; N = normoxia; NS = nonsmokers; SM = smokers without COPD.
Nuclear HIF-1α Protein Correlated With VEGF mRNA
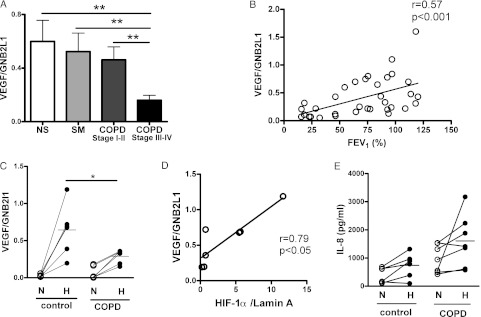
VEGF is a HIF-1α-responsive gene. We measured VEGF in peripheral lung tissue obtained from a tissue bank linked to an established patient registry (e-Table 1).15 As shown in Figure 2A, VEGF mRNA levels in the peripheral lung of patients with severe COPD (COPD stage III or IV, 0.16 ± 0.037) were significantly lower than those of any other groups (nonsmokers, 0.60 ± 0.16; smokers without COPD, 0.52 ± 0.14; COPD stage I or II, 0.46 ± 0.097). The levels also correlated with FEV1 (r = 0.57, P < .01) (Fig 2B).
Figure 2.
VEGF messenger RNA (mRNA) is correlated with HIF-1α protein. A, VEGF mRNA in lung tissue was quantified using real-time quantitative polymerase chain reaction (PCR). B, Correlation between FEV1 and VEGF mRNA expression in the lung tissue. C, PBMCs were incubated in the hypoxic chamber (1% oxygen, 5% CO2, and 94% N2) for 24 h, and then VEGF mRNA was quantified using quantitative real-time PCR. D, VEGF mRNA correlated with HIF-1α protein expression. E, PBMCs were incubated under hypoxia (1% oxygen, 5% CO2, and 94% N2) for 24 h, and then IL-8 in the supernatants was measured. VEGF mRNA values were normalized by GNB2L1 mRNA. HIF-1α values were normalized using lamin A. *P < .05; **P < .01. GNB2L1 = guanine nucleotide binding protein, β polypeptide 2-like 1; VEGF = vascular endothelial growth factor. See Figure 1 legend for expansion of other abbreviations.
Measuring HIF-1α in the lung tissue is not an appropriate method for comparing HIF-1α protein amounts among subjects because the samples are exposed to oxygen (room air) during surgical acquisition, leading to HIF-1α degradation. In fact, we could not detect HIF-1α protein in the peripheral lung tissue (data not shown). Therefore, we investigated the relationship between VEGF mRNA and HIF-1α protein in PBMCs under ex vivo hypoxic condition. VEGF mRNA was measured in PBMCs obtained from six patients with COPD and six healthy subjects who were selected randomly. VEGF mRNA in PBMC in normoxic condition was 0.032 ± 0.0079 in control subjects and 0.064 ± 0.035 in patients with COPD, showing no statistically significant difference between them. Hypoxia induced VEGF mRNA expression 20-fold in healthy control subjects (0.64 ± 0.13); in contrast, the elevation was only fivefold in patients with COPD (0.30 ± 0.037) (Fig 2C). Furthermore, VEGF mRNA was correlated with HIF-1α protein after hypoxia exposure (r = 0.79, P < .05) (Fig 2D). In contrast to VEGF, IL-8 release from PBMCs was induced by hypoxia in both patients with COPD (972 ± 286 pg/mL in normoxia and 1,607 ± 348 pg/mL in hypoxia) and control subjects (392 ± 112 pg/mL in normoxia and 740 ± 178 pg/mL in hypoxia) (Fig 2E), and the mean induction of IL-8 by hypoxia was 2.3 ± 0.7-fold in control subjects and 1.9 ± 0.6-fold in patients with COPD, showing no statistically significant difference between them (Fig 2E).
Nuclear HIF-1α Protein Correlated With HDAC7 Protein Levels
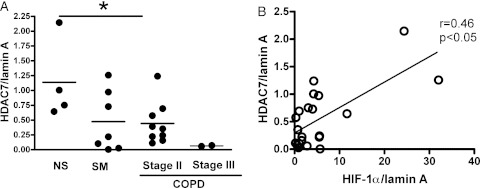
The nuclear levels of HDAC7 in patients with COPD after exposure to hypoxia (0.37 ± 0.11) were significantly lower than those of nonsmokers (1.1 ± 0.34) (Fig 3A), although the HDAC7 protein level in nuclei was not different under normoxic condition between groups and there was no difference in HDAC7 protein level in cytoplasmic protein (data not shown). In addition, the mean level of HDAC7 in patients with moderate COPD was significantly lower than that in nonsmoking healthy control subjects, and the level in patients with severe COPD was far lower than that in patients with moderate COPD (Fig 3A), although no significant difference was detected between smokers without COPD and patients with moderate COPD. In addition, HDAC7 protein correlated with HIF-1α protein in nuclei (r = 0.46, P < .05) (Fig 3B).
Figure 3.
Nuclear HDAC7 is correlated with HIF-1α protein in nuclei in PBMC. PBMCs were incubated in the hypoxic chamber (1% oxygen, 5% CO2, and 94% N2) for 24 h and nuclear protein was extracted. A, HDAC7 protein level in nuclei corrected to the lamin A protein expression. B, HDAC7 protein levels correlated with HIF-1α protein expression. *P < .05. HDAC = histone deacetylase. See Figure 1 legend for expansion of other abbreviations.
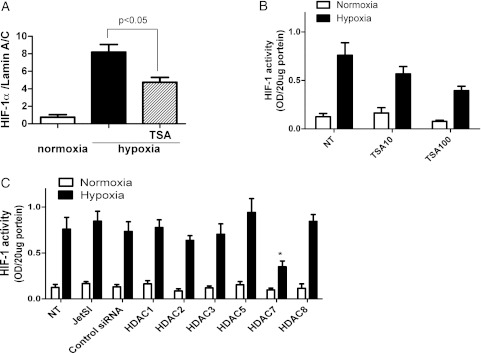
HDAC Inhibition Reduced HIF-1α Protein Expression
U937 cells were treated with TSA and incubated for 12 h under hypoxic condition. As shown in Figure 4A, hypoxia clearly increased the protein level of HIF-1α in nuclei. The HDAC inhibitor TSA inhibited HIF-1α expression by about 50% (Fig 4A). In addition, TSA also inhibited HIF-1 activity in nuclear protein in a concentration-dependent manner (0.76 ± 0.13 without TSA, 0.57 ± 0.08 with 10 ng/mL TSA, and 0.39 ± 0.05 with 100 ng/mL TSA) (Fig 4B). siRNAs of HDAC1, 2, 3, 5, 7, and 8 were transfected, and we confirmed by real-time quantitative PCR analysis a 60% to 80% reduction of mRNA level in each gene after 48 h of incubation. As shown in Figure 4C, HIF-1 activity under hypoxia was significantly inhibited by 58% with HDAC7 knockdown. However, HDAC1, 2, 3, 5, and 8 knockdown did not affect HIF-1 activity (HIF-1α activity, OD: control siRNA, 0.73 ± 0.10; HDAC1, 0.78 ± 0.08; HDAC2, 0.64 ± 0.05; HDAC3, 0.70 ± 0.11; HDAC5, 0.94 ± 0.15; HDAC8, 0.84 ± 0.08) (Fig 4C).
Figure 4.
HIF-1α nuclear translocation is inhibited by TSA and HDAC7 knockdown. U937 cells were incubated under hypoxia (1% oxygen, 5% CO2, and 94% N2) with or without TSA. A, HIF-1α protein in nuclei was quantified. B, HIF-1 activity in nuclei was quantified. U937 cells were treated with siRNAs (HDAC1, 2, 3, 5, 7, or 8) for 48 h under normoxic condition, followed by 12-h incubation under hypoxia. C, HIF-1 activity in nuclei was quantified. JetSI=cells incubated with jetSI transfection reagent without siRNA; NT=not treated; OD=optical density; siRNA = short interference RNA; TSA = trichostatin A. See Figure 1 and 3 legends for expansion of other abbreviations.
HDAC7 Knockdown Reduced HIF-1α Protein Nuclear Translocation and HIF-1α Binding to VEGF Promoter
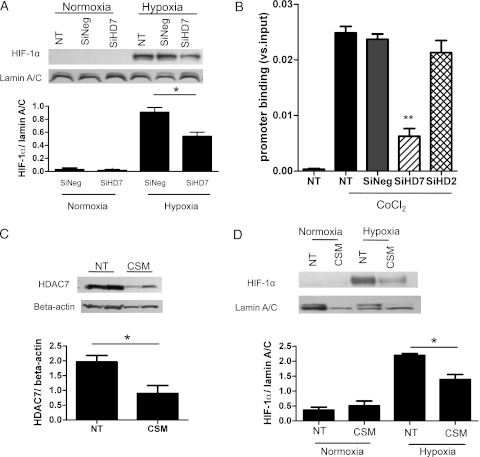
siRNA of HDAC7 or Silencer Select negative control siRNA was transfected to A549 cells, and then cells were incubated under hypoxia. HDAC7 siRNA transfection reduced HDAC7 protein expression by 74% (e-Fig 2). Hypoxia treatment markedly induced HIF-1α nuclear translocation (Fig 5A). The induced HIF-1α nuclear translocation was significantly (P < .05) abolished by transfection of HDAC7 siRNA (HIF-1α/lamin A/C, 0.91 ± 0.08 with SiNeg; 0.54 ± 0.06 with SiRNA of HDAC7) (Fig 5A).
Figure 5.
HDAC7 reduction by RNA interference or cigarette smoke extract affected HIF-1α nuclear translocation and VEGF promoter binding in A549 cells. siRNA of HDAC7 was transfected to A549, and then cells were incubated under hypoxia (0.1% oxygen, 5% CO2) for 24 h. A, HIF-1α nuclear translocation was evaluated. B, HIF-1α binding to the VEGF promoter under treatment with CoCl2 (50 μM) (mimic hypoxia) was also analyzed by chromatin immunoprecipitation (ChIP) assay. The ratio of the cycle threshold (Ct) value of HIF-1α ChIP product to input (total cells) is shown (**P < .01 vs SiNeg transfection). C, A549 cells were treated with cigarette smoke-conditioned media (CSM) for 24 h, and then HDAC7 protein expressions in whole cell extracts were determined. A549 cells were treated CSM for 24 h under hypoxia or normoxia. D, HIF-1α protein in nuclear extracts was evaluated. *P < .05; **P < .01. CSM = cigarette smoke-conditioned medium; SiHD2 = short interference RNA HDAC2; SiHD7 = short interference RNA HDAC7; SiNeg = Silencer Select negative control siRNA. See Figure 1, 3, and 4 legends for expansion of other abbreviations.
As shown in Figure 5B, the hypoxia mimic, CoCl2 treatment (50 μM, 24 h), clearly increased HIF-1α binding to the VEGF promoter by chromatin immunoprecipitation assay analysis. However, in HDAC7 knockdown cells, the level of HIF-1α binding to the VEGF promoter was reduced to 60% of control, but HDAC2 knockdown did not affect it.
HDAC7 Inhibition by CSM Reduced HIF-1α Protein Nuclear Translocation
A549 cells were treated with CSM, and HDAC7 protein expressions in whole cell extracts were evaluated. Treatment with CSM significantly reduced HDAC7 expression (HDAC7/β-actin, 1.96 ± 0.21 without CSM; 0.90 ± 0.27 with CSM; P < .05) (Fig 5C). Next, we investigated hypoxia-induced HIF-1α nuclear translocation in the presence of CSM. CSM significantly abolished HIF-1α nuclear translocation (HIF-1α/lamin A/C, 2.20 ± 0.06 without CSM; 1.39 ± 1.67 with CSM; P < .05) (Fig 5D).
Discussion
HIF-1α is degraded rapidly under normoxic condition after sampling specimens or during isolation of PBMCs. Therefore, it is difficult to monitor the actual HIF-1α levels in samples taken from subjects. To evaluate the HIF-1α response in patients with COPD, we exposed PBMCs collected from subjects to hypoxic condition ex vivo and investigated the response to hypoxia. PBMCs from healthy nonsmokers and smokers responded well to hypoxia, and the levels of HIF-1α protein in nuclei increased 12-fold and 24-fold compared with basal levels. However, the nuclear translocation of HIF-1α was lower in patients with COPD, suggesting PBMCs from patients with COPD respond poorly to hypoxia. This finding implies a defect in adaptation to hypoxia in patients with COPD due to poor activation of HIF-1α. The defect of HIF-1α nuclear translocation was observed equally in both moderate and severe patients with COPD (HIF-1α/lamin A in nuclei was 1.58 ± 0.43 and 1.47 ± 0.70 in moderate and severe patients with COPD, respectively). The mean age of the patients with COPD recruited in our study was higher than that of nonsmokers and smokers without COPD. However, HIF-1α protein levels were not correlated with age in patients with COPD (r = −0.011, P > .05) or control subjects (r = −0.084, P > .05) (data not shown).
VEGF and erythropoietin genes are known to be upregulated by HIF-1α activation to adapt to hypoxia by angiogenesis and stimulating erythrocytosis, respectively.1,2 In our results, hypoxia significantly induced VEGF mRNA expression, with a concomitant increase in nuclear HIF-1α protein in PBMCs from non-COPD volunteers. However, the induction level of VEGF mRNA by hypoxia was significantly lower in PBMCs from patients with COPD (Fig 2C). VEGF mRNA levels were also decreased in the peripheral lung of patients with severe COPD (Fig 2A). This is consistent with the findings of previous clinical investigations in which VEGF levels in the sputum of patients with severe COPD were lower than those of patients with other stages of COPD,13 despite the fact that patients with severe COPD usually have hypoxia, especially after exercise. The impaired VEGF induction response to hypoxia in patients with COPD is a result of the reduced HIF-1α response. In contrast to VEGF, the levels of IL-8 released from PBMC were increased by hypoxia in both groups, and the induction levels of the IL-8 response to hypoxia were not different between the groups (2.3 ± 0.7-fold in control subjects and 1.9 ± 0.6-fold in patients with COPD) (Fig 2E). It has been reported that hypoxia induces inflammatory cytokines17,18 via NF-κB activation.19 Thus, VEGF and IL-8 were induced by different transcription factors and this suggests that the hypoxia-induced HIF-1α response was impaired in the COPD but not in the NF-κB pathway.
In our data, the nuclear levels of HDAC7 in patients with COPD were significantly lower than those in nonsmokers (Fig 3A). Furthermore, the mean level of HDAC7 in patients with severe COPD after exposure to hypoxia was far lower than that in patients with moderate COPD (Fig 3A). HDAC7 is one of the class II HDACs20 and has an ability to shuttle between the cytoplasm and nucleus, which is regulated by phosphorylation and dephosphorylation of the HDAC protein. The phosphorylation of class II HDACs is known to be regulated by Ca2+/calmodulin-dependent kinase I and IV20 and protein kinase D, which is a downstream effector of protein kinase C.21‐23 Mutation of the phosphorylatable residues in the HDACs abolishes the nuclear-cytoplasmic shuttling.24,25 In this study, we showed a good correlation between nuclear HIF-1α and HDAC7 proteins in PBMC nuclei (Fig 3B). HIF-1 activity under hypoxia was reduced by knockdown of HDAC7 but not by knockdown of HDAC1, HDAC2, HDAC3, HDAC5, or HDAC8 (Fig 4C). Furthermore, knockdown of HDAC7 abolished HIF-1α nuclear translocation and HIF-1α binding to the VEGF promoter in A549 cells (Figs 5A, 5B). HIF-1α translocates to the nucleus together with HDAC7.26 Furthermore, it was demonstrated that cotransfection of HIF-1α and HDAC7 to human embryonic kidney cells enhanced the VEGF mRNA in hypoxia more than overexpression of HIF-1α in the cells, whereas HDAC7 overexpression did not change the amount of VEGF mRNA.26 Thus, HDAC7 is a prerequisite molecule to the accumulation of HIF-1α in the nucleus. Taken together, the decreased HIF-1α in nuclei of COPD under hypoxic condition is likely caused by the failure of the cotranslocation of HIF-1α and HDAC7 due to the decreased HDAC7 levels in patients with COPD.
In our data, CSM abolished HDAC7 protein expression (Fig 5C) and HIF-1α nuclear translocation (Fig 5D). Clinical data showed that HIF-1α in nuclei negatively correlated with the oxidative stress marker in sputum (Fig 1B). The facts suggest that oxidative stress has an association with HIF-1α nuclear translocation, which is a condition shown in patients with COPD. Thus, oxidative stress seems to be an important factor for HDAC7 depression and subsequent defect of HIF-1α nuclear translocation in COPD. Smoking status and pack-years did not have an association with HIF-1α in nuclei in our data. This may be because of the difference in endogenous antioxidant levels in each patient.
While we were preparing this manuscript, a group published data showing that HIF-1α depression is observed in COPD, and HIF-1α correlated positively with both HDAC2 and VEGF.27 The authors speculated that HDAC2 inhibition leads to HIF-1α depression. Shortly after, the authors in the same group published data suggesting that HDAC inhibition leads to reduction of HIF-1α expression via p53 upregulation, followed by reduction of VEGF.28 HIF-1α signaling is complex. Not only expressions of the mRNA and proteins, but also degradation, nuclear translocation, and DNA binding are potentially involved in the reduced HIF-1α in COPD. Here, we showed the defect of nuclear translocation of HIF-1α due to the reduction of HDAC7. In addition, we confirmed that HIF-1α binding to the VEGF promoter region was decreased in HDAC7 knockdown cells under the hypoxia mimic system, CoCl2 (Fig 5B). Both mechanisms possibly exist in COPD. One of the mechanisms might play a role in the pathogenesis of COPD, which is phenotype dependent.
The only limitation of this study was the lack of information on HDAC7 activity in clinical samples. HDACs activity is regulated by posttranslational modification, such as nitration and phosphorylation. We could not evaluate HDAC7 activity in PBMCs because of ethical issues because analysis of HDAC7 activity would require huge numbers of PBMCs. If HDAC7 activity could be measured, additional information would be added to HDAC7 protein in PBMC.
Conclusions
In conclusion, a defect of HDAC7 in COPD causes an impaired HIF-1α induction in response to hypoxia and subsequently reduces hypoxia-induced VEGF expression. This HIF-1α defect may result in poor cellular adaptation to hypoxia in patients with COPD and may play a significant role in COPD pathogenesis.
Supplementary Material
Acknowledgments
Author contributions: Dr To: contributed to the study concept and design; acquisition, analysis, and interpretation of data; recruitment of subjects; drafting of the manuscript; and approval of the final version of the submitted manuscript.
Dr Yamamura: contributed to the study concept and design; acquisition, analysis, and interpretation of data; and approval of the final version of the submitted manuscript.
Dr Akashi: contributed to the acquisition, analysis, and interpretation of data, and approval of the final version of the submitted manuscript.
Dr Charron: contributed to the acquisition, analysis, and interpretation of data; drafting of the manuscript; and approval of the final version of the submitted manuscript.
Dr Haruki: contributed to the acquisition, analysis, and interpretation of data; drafting of the manuscript; and approval of the final version of the submitted manuscript.
Dr Barnes: contributed to the drafting of the manuscript and approval of the final version of the submitted manuscript.
Dr Ito: contributed to the study concept and design; acquisition, analysis, and interpretation of data; drafting of the manuscript; and approval of the final version of the submitted manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr. Ito is an employee of RespiVert Ltd and a stock option holder of Johnson and Johnson. He received a research grant from Astra-Zeneca, not related to this study, in 2009. Drs To, Yamamura, Akashi, Charron, Haruki, and Barnes have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The funding sources provided research funding for this study but played no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Other contributions: The authors thank Angela Spencer, SRN; Lynda Walker, SRN; Arnell Colongon, SRN; and Meah Sally, SRN, for their support in the recruitments of volunteers.
Additional information: The e-Figures and e-Table can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/141/5/1233/suppl/DC1.
Abbreviations
- CSM
cigarette smoke-conditioned medium
- HDAC
histone deacetylase
- HIF
hypoxia inducible factor
- mRNA
messenger RNA
- NF-κB
nuclear factor-κB
- OD
optical density
- PBMC
peripheral blood mononuclear cell
- PCR
polymerase chain reaction
- siRNA
short interference RNA
- TSA
trichostatin A
- VEGF
vascular endothelial growth factor
Footnotes
Funding/Support: Financial support was provided by the National Heart and Lung Institute, Imperial College, London, England; Wellcome Trust [076472/Z/05/Z]; GlaxoSmithKline, England; and Teijin Pharma Limited, Japan.
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial License (http://creativecommons.org/licenses/by-nc/3.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Information for commercial entities is available online (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Ikeda E. Cellular response to tissue hypoxia and its involvement in disease progression. Pathol Int. 2005;55(10):603–610. doi: 10.1111/j.1440-1827.2005.01877.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Endler A, Shibasaki F. Hypoxia and angiogenesis: regulation of hypoxia-inducible factors via novel binding factors. Exp Mol Med. 2009;41(12):849–857. doi: 10.3858/emm.2009.41.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damert A, Ikeda E, Risau W. Activator-protein-1 binding potentiates the hypoxia-inducible factor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem J. 1997;327(pt 2):419–423. doi: 10.1042/bj3270419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12(12):5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito K, Yamamura S, Essilfie-Quaye S, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006;203(1):7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito K. Impact of post-translational modifications of proteins on the inflammatory process. Biochem Soc Trans. 2007;35(pt 2):281–283. doi: 10.1042/BST0350281. [DOI] [PubMed] [Google Scholar]
- 8.Ito K, Ito M, Elliott WM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352(19):1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 9.Ito K, Lim S, Caramori G, et al. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. FASEB J. 2001;15(6):1110–1112. [PubMed] [Google Scholar]
- 10.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, updated 2007. GOLD website. http://www.goldcopd.org. Accessed November 2010.
- 11.To Y, Takezawa T, Arai T, et al. The Effect of tiotropium bromide on exercise-induced oxygen desaturation in patients with COPD. Am J Respir Crit Care Med. 2009;179:A6177. [Google Scholar]
- 12.Kanazawa H, Asai K, Hirata K, et al. Possible effects of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Am J Med. 2003;114(5):354–358. doi: 10.1016/s0002-9343(02)01562-0. [DOI] [PubMed] [Google Scholar]
- 13.Kanazawa H, Yoshikawa J. Elevated oxidative stress and reciprocal reduction of vascular endothelial growth factor levels with severity of COPD. Chest. 2005;128(5):3191–3197. doi: 10.1378/chest.128.5.3191. [DOI] [PubMed] [Google Scholar]
- 14.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56(4):515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 15.Ding L, Quinlan KB, Elliott WM, et al. A lung tissue bank for gene expression studies in chronic obstructive pulmonary disease. COPD. 2004;1(2):191–204. doi: 10.1081/copd-120039810. [DOI] [PubMed] [Google Scholar]
- 16.Shin J, Lee HJ, Jung DB, et al. Suppression of STAT3 and HIF-1 alpha mediates anti-angiogenic activity of betulinic acid in hypoxic PC-3 prostate cancer cells. PLoS ONE. 2011;6(6):e21492. doi: 10.1371/journal.pone.0021492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zampetaki A, Mitsialis SA, Pfeilschifter J, et al. Hypoxia induces macrophage inflammatory protein-2 (MIP-2) gene expression in murine macrophages via NF-kappaB: the prominent role of p42/ p44 and PI3 kinase pathways. FASEB J. 2004;18(10):1090–1092. doi: 10.1096/fj.03-0991fje. [DOI] [PubMed] [Google Scholar]
- 18.Schmedtje JF, Jr, Ji YS, Liu WL, et al. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997;272(1):601–608. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- 19.Culver C, Sundqvist A, Mudie S, et al. Mechanism of hypoxia-induced NF-kappaB. Mol Cell Biol. 2010;30(20):4901–4921. doi: 10.1128/MCB.00409-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin M, Kettmann R, Dequiedt F. Class IIa histone deacetylases: conducting development and differentiation. Int J Dev Biol. 2009;53(2-3):291–301. doi: 10.1387/ijdb.082698mm. [DOI] [PubMed] [Google Scholar]
- 21.Matthews SA, Liu P, Spitaler M, et al. Essential role for protein kinase D family kinases in the regulation of class II histone deacetylases in B lymphocytes. Mol Cell Biol. 2006;26(4):1569–1577. doi: 10.1128/MCB.26.4.1569-1577.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parra M, Kasler H, McKinsey TA, et al. Protein kinase D1 phosphorylates HDAC7 and induces its nuclear export after T-cell receptor activation. J Biol Chem. 2005;280(14):13762–13770. doi: 10.1074/jbc.M413396200. [DOI] [PubMed] [Google Scholar]
- 23.Vega RB, Harrison BC, Meadows E, et al. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24(19):8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin M, Kettmann R, Dequiedt F. Class IIa histone deacetylases: regulating the regulators. Oncogene. 2007;26(37):5450–5467. doi: 10.1038/sj.onc.1210613. [DOI] [PubMed] [Google Scholar]
- 25.Kao HY, Verdel A, Tsai CC, et al. Mechanism for nucleocytoplasmic shuttling of histone deacetylase 7. J Biol Chem. 2001;276(50):47496–47507. doi: 10.1074/jbc.M107631200. [DOI] [PubMed] [Google Scholar]
- 26.Kato H, Tamamizu-Kato S, Shibasaki F. Histone deacetylase 7 associates with hypoxia-inducible factor 1alpha and increases transcriptional activity. J Biol Chem. 2004;279(40):41966–41974. doi: 10.1074/jbc.M406320200. [DOI] [PubMed] [Google Scholar]
- 27.Yasuo M, Mizuno S, Kraskauskas D, et al. Hypoxia inducible factor-1alpha in human emphysema lung tissue. Eur Respir J. 2011;37(4):775–783. doi: 10.1183/09031936.00022910. [DOI] [PubMed] [Google Scholar]
- 28.Mizuno S, Yasuo M, Bogaard HJ, et al. Inhibition of histone deacetylase causes emphysema. Am J Physiol Lung Cell Mol Physiol. 2011;300(3):L402–L413. doi: 10.1152/ajplung.00207.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.