Abstract
Purpose.
The goal in this study was to compare rates of visual field (VF) change before and after the initiation of treatment in participants originally randomized to the observation arm of the Ocular Hypertension Treatment Study (OHTS).
Methods.
We included OHTS participants originally randomized to observation and excluded those who reached non-POAG endpoints. VF progression was determined using trend analysis. Global and localized rates of VF change were calculated based on linear regression over time of mean deviation (MD) and threshold sensitivity values for each test location. MD rates (MDR) and pointwise linear regression (PLR) analysis were also assessed using six VF tests before and after the initiation of treatment. A PLR endpoint was defined as a VF test location progressing faster than −0.5 dB/year at P < 0.01.
Results.
We included 780 eyes from 432 OHTS participants. Following the initiation of treatment, the mean MDR decreased from −0.23 ± 0.6 to −0.06 ± 0.5 dB/year (P < 0.01) and the number of VF locations reaching a PLR endpoint decreased from 2.13 ± 6.0 to 1.00 ± 4.0 (P < 0.01). The benefit of treatment was significant both among participants who did not convert (−0.17 ± 0.6 vs. −0.01 ± 0.5 dB/year, P < 0.01) and among those who converted to glaucoma (−0.51 ± 0.8 vs. −0.27 ± 0.7 dB/year, P < 0.01) based on the OHTS event-based endpoint.
Conclusions.
The initiation of ocular hypotensive medication among OHTS participants originally randomized to observation significantly reduced the velocity of VF progression. (ClinicalTrials.gov number, NCT00000125.)
The initiation of ocular hypotensive medication among the Ocular Hypertension Treatment Study participants originally randomized to the observation arm significantly reduced the velocity of visual field progression as measured using trend analysis.
Introduction
The Ocular Hypertension Treatment Study (OHTS), a randomized clinical trial (RCT) of the safety and efficacy of topical ocular hypotensive treatment in eyes with ocular hypertension without glaucomatous optic neuropathy or visual field (VF) damage, demonstrated that lowering intraocular pressure (IOP) decreased the incidence of primary open angle glaucoma (POAG) by 50%.1,2 Along with this finding, the OHTS identified baseline demographic and clinical variables that were associated with an increased risk of developing glaucomatous optic neuropathy and/or VF loss.3,4
Standard achromatic perimetry (SAP) was used in the OHTS and other glaucoma RCTs sponsored by the National Eye Institute to assess visual function and determine if functional progression had taken place.1,5–10 SAP is a widely available method to assess the vision status of glaucoma patients, and its results are well correlated with quality of life.11 Nevertheless, detection of VF change over time remains a challenging task due to the high variability observed, especially at VF locations with moderate to severe damage,12 and a variety of algorithms have been employed to detect progression.
To overcome these challenges, the major RCTs formulated different criteria for classifying the cross-sectional status of VF tests in order to detect longitudinal change more objectively.1,5–10 In the OHTS, for instance, the definition of “change” was based on the comparison between the follow-up data of enrolled participants and predefined boundaries of normality. In other words, change was not defined based on intrasubject, longitudinal variability but, rather, on how follow-up VF results differed compared to the VF results of age-matched controls. This would affect the observed incidence of VF progression in the OHTS, as those participants with baseline tests closer to the boundaries of abnormality were more likely to develop confirmed VF abnormality even with small amounts of subsequent deterioration. The converse is true for eyes or test locations with greater sensitivity at baseline. Alternatively, VF change can be determined using other forms of event-based analyses that account for longitudinal variability. Glaucoma progression analysis (GPA, Carl Zeiss Meditec, Inc., Dublin, CA) is an example of a method that is often used in practice and in clinical research.5,9 One of the disadvantages of event-based methods is that interim VF data that fail to meet an endpoint are less influential. Depending on the significance of the change between final and baseline results of the same subject, progression is defined as a binary outcome (progressing or stable).
Trend-based analysis provides another method of determining and measuring VF progression.13 This technique can use all available VF data in the calculation of regression parameters that describe the rate of VF change for individual test locations or the entire field. Using linear regression allows the calculation of a slope that numerically represents the velocity of progression in decibels per year (dB/year), as well as whether the change is significantly different from a given value, most commonly zero (the P value).14 Trend analysis can be applied to global VF indices (e.g., mean deviation [MD] and pattern standard deviation [PSD]) or localized to individual test locations (pointwise linear regression [PLR] analysis).14–17 Thus, trend analysis can provide not only a binary definition of progression but, also, a continuous variable that correlates with the velocity of VF progression over time.
In contrast to the original OHTS design, which examined the incidence of glaucomatous VF damage using event analysis, the goal of the present study was to use trend analysis to examine the velocity of global and localized VF progression in the observation group to (1) determine the incidence of VF progression using PLR compared to the OHTS event-based VF endpoint, (2) determine the effect of topical hypotensive treatment on the velocity of VF progression, and (3) detect differences in the velocity of VF progression between participants who developed POAG and those who did not.
Methods
The baseline data and design of the OHTS have been described elsewhere.1,2 All OHTS participants signed a statement of informed consent prior to study entry after having the risks and benefits of participation explained to them. The study followed the tenets of the Declaration of Helsinki, and the institutional review boards at all participating clinical sites approved their respective informed consent statements and procedures.
All participants enrolled in the OHTS were required to have at least two reliable (fixation losses and false-negative and false-positive responses <33%), achromatic, automated VFs (Humphrey Visual Field Analyzer, program 30-2, Carl Zeiss Meditec, Inc.) that were within normal limits during the qualifying period. The OHTS analysis dataset available for this study contained all VF tests and endpoint determinations in the OHTS database as of March 2009. Several inclusion and exclusion criteria were then applied as outlined below.
The full OHTS dataset (n = 1636) was filtered to include only those participants who were originally randomized to the observation arm of the study (n = 819). We excluded eyes that reached an endpoint that was determined by the endpoint committee to be due to causes other than POAG (261 eyes from 202 participants). We then selected only those follow-up VF tests that were considered reliable (false positives, false negatives, and fixation loss all <33% for full threshold testing and false positives <15%, false negatives, and fixation loss <33% for Swedish interactive threshold algorithm [SITA]-SAP) and only those eyes that had a change in management from observation to treatment at some point during follow-up. Trend analysis was performed using PLR and linear regression of the MD, providing rates of change (MDR, dB/year). Due to the different termination criteria used in SITA and full threshold testing, a correction of +1.0 dB was applied to all thresholds measured using the full threshold algorithm,18,19 in an attempt to maintain equivalence when the algorithm changed at the commencement of the second phase of the OHTS.
The incidence of progression using PLR endpoints was determined by utilizing the entire sequence of eligible VF tests and was then compared to the OHTS event-based VF endpoints. This analysis included eyes that began treatment either as a result of an OHTS POAG endpoint or at the transition between the first phase of OHTS and the second phase of OHTS, at which time treatment was offered to all participants in the observation arm that remained untreated at the end of phase 1.
The visit at which an eye was first noted to be on treatment was defined as the “treatment point” for that eye. Given the focus of the present study on the effect of treatment on the velocity of VF progression, we focused our analysis on the VF examinations before and after the treatment point. Therefore, two sequences of VF tests were analyzed per participant; the six VF tests prior to the treatment point (“before”) and the first six VF tests beginning at least 9 months after the treatment point (“after”). Sequences of six fields were chosen to standardize the sequence length for all participants and so that the sequences would be sufficiently long to derive a robust measure of the rate of VF change.16,20 A 9-month gap was left between commencing treatment and the start of the after sequence to ensure that the treatment regimen and its effects had time to stabilize. Participants with less than six VF tests before and after the treatment point were excluded. We did not require treatment to be continuous once commenced. The velocities of VF progression in the before and after sequences were calculated and compared to determine the effect of treatment for all participants meeting the above-described criteria. To detect differences in the velocity of VF progression between participants who reached an OHTS POAG endpoint and those who did not, PLR and MDR calculations were performed for each subgroup.
An effect of the initiation of treatment on the global and localized velocities of progression was sought using data from both eyes of each individual (where available). First, a nonparametric Wilcoxon signed-rank test was used to compare the MDRs in the before and after sequences. Second, a comparison was performed using the generalized estimating equations (GEE) technique to account for correlation between the fellow eyes of an individual. Specifically, the change in MDR given by the formula MDR after treatment point − MDR before treatment point was set as the outcome of a GEE regression with no independent variables. The resulting slope and P value from the GEE regression were used as estimates of the MD change and its level of significance, respectively.
In order to determine whether the amount of IOP reduction influenced the magnitude of the treatment effect, a further GEE model was constructed. The change in MDR at the split-point (MDR after − MDR before) was set as the outcome of a GEE regression, with the predictor being the reduction in IOP (IOP after − IOP before [Δ IOP]). The IOP for this analysis was taken as being either the mean or the maximum of the six measurements in the before and after sequences of visits. This was repeated in the subgroup of participants that reached a POAG endpoint.
For PLR progression criteria, a VF location was considered “progressing” if it was deteriorating more rapidly than −0.5 dB/year and if this change was significant at P < 0.01. An eye was considered progressing by PLR if at least two neighboring locations in the same hemifield were progressing based on the above-described criteria. A criterion of −0.5 dB/year was used rather than −1.0 dB/year, as has been more commonly used by other investigators in studies of eyes with established glaucomatous VF loss,14–17 since very rapid deterioration might be less likely in eyes at such an early stage of disease. The number of progressing VF locations in the before and after sequences for each eye were compared using the same statistic approaches described above. For the PLR analyses, all test locations within the 30-2 test pattern were used except locations at the blind spot.
Details of treatment modalities have been described elsewhere.21 In brief, the most commonly prescribed class of drugs in this group of participants were prostaglandin analogs (approximately 65% of patients), followed by beta blockers (30%).
Results
Of the total number of OHTS participants randomized (n = 1636), 819 participants were randomized to the observation arm of the study. Of these 819 participants, 780 eyes from 432 participants met the entry criteria for this report.
Incidence of Progression Using PLR Endpoints Compared to the OHTS Event-Based Endpoint
Of the 780 eyes, 139 (17.8%) reached an OHTS event analysis POAG endpoint. When trend analysis was performed over the entire testing sequence for the 780 eyes, the total number of eyes meeting the predefined PLR criteria (−0.5 dB/year, P < 0.01) was 141 (18.1%, P = 0.93 compared to the OHTS event-based POAG endpoint, McNemar's test).
The Velocity of VF Progression before and after Initiation of Ocular Hypotensive Treatment
The MDR of the six VF tests prior to the initiation of treatment was significantly different from the MDR of the six VF tests after treatment initiation (mean ± SD, −0.23 ± 0.6 vs. −0.06 ± 0.5 dB/year, respectively, P < 0.001 for both Wilcoxon's and GEE). Similarly, using our PLR criteria, the number of progressing locations per eye decreased significantly after the treatment point, from 2.13 ± 6.0 to 1.00 ± 4.0 locations, respectively (P < 0.001 for both Wilcoxon's and GEE analyses, Table 1).
Table 1.
Effect of Treatment on Rates of VF Change for All Observed Participants Meeting Eligibility Criteria*
|
Before |
After |
P Value |
|
| MDR (dB/y) | −0.23 ± 0.6 | −0.06 ± 0.5 | <0.01 |
| No. of VF test points meeting PLR criteria | 2.13 ± 6.0 | 1.00 ± 4.0 | <0.01 |
n = 780 eyes from 432 participants.
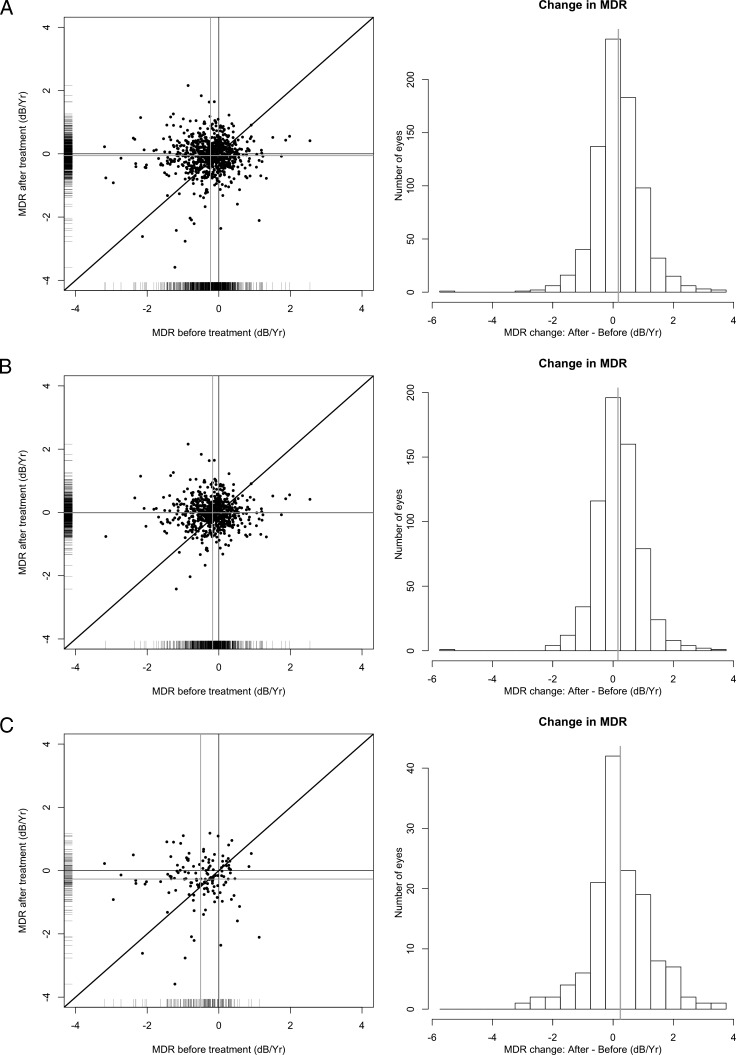
The total number of eyes defined as progressing using PLR criteria (i.e., at least two adjacent locations in the same hemifield) decreased from 192 eyes before treatment to 124 eyes after treatment was initiated (P < 0.001, McNemar's test). The difference in the number of progressing eyes detected by PLR for the entire VF sequence and for the six VF sequence subsets is addressed in the Discussion section. Figure 1A shows the distribution of MDR values for each participant before and after treatment.
Figure 1.
Comparison of changes in MDRs before (x-axis) and after (y-axis) treatment. (A) All participants are included. (B) Only participants who did not reach a POAG endpoint are included. (C) Only participants who reached a POAG endpoint are included. The dark grey lines show flat slopes (0.0 dB/year) in both axes, and the light grey lines show the average change in MDR before and after treatment.
The Velocity of VF Progression in Eyes That Developed an OHTS POAG Endpoint Compared to Those That Did Not
Among eyes that did not reach a POAG endpoint during follow-up (641 eyes of 380 participants), the MDR prior to the treatment point was significantly different from the MDR after the treatment point (−0.17 ± 0.6 vs. −0.01 ± 0.5 dB/year respectively, P < 0.001 for both Wilcoxon's and GEE). Similarly, using our PLR criteria, the number of progressing locations per eye over the sequence of six VF tests decreased significantly, from 1.53 ± 5.1 locations before treatment to 0.64 ± 2.7 locations after treatment initiation (P < 0.001 for both Wilcoxon's and GEE analyses). Figure 1B shows the distribution of MDR values for each participant before and after treatment.
For the subset of eyes that reached a POAG endpoint (n = 139 eyes from 109 participants), there was a significant improvement in the MDR after the treatment point (−0.51 ± 0.8 vs. −0.27 ± 0.7 dB/year, respectively, P < 0.01 for both Wilcoxon's and GEE). Similarly, using our PLR definition, the number of progressing locations per eye decreased significantly after the treatment point (4.90 ± 8.5 vs. 2.71 ± 7.2, respectively, P < 0.01 for Wilcoxon's, P = 0.03 for GEE, Tables 2 and 3). Figure 1C shows the distribution of MDR values for each participant before and after treatment.
Table 2.
Effect of Treatment on Rates of VF Change for Subset That Did Not Reach a POAG Endpoint*
|
Before |
After |
P Value |
|
| MDR (dB/y) | −0.17 ± 0.6 | −0.01 ± 0.5 | <0.01 |
| No. of VF test points meeting PLR criteria | 1.53 ± 5.1 | 0.64 ± 2.7 | <0.01 |
n = 641 eyes of 380 participants.
Table 3.
Effect of Treatment on Rates of VF Change for Subset That Reached a POAG Endpoint
|
Before |
After |
P Value |
|
| MDR (dB/y) | −0.51 ± 0.8 | −0.27 ± 0.7 | <0.01 |
| No. of VF test points meeting PLR criteria | 4.90 ± 8.5 | 2.71 ± 7.2 | <0.01 |
n = 139 eyes from 109 participants.
A significant relation was found between the magnitude of IOP reduction and the change in MDR. For the entire dataset, the improvement in MDR was given by (intercept, slope, P value) 0.0240, −0.03 × (Δ IOP), P = 0.005 when the mean IOP within each period was used, and 0.01, −0.02 × (Δ IOP), P = 0.003 when the maximum IOP within each period was used. For the POAG eyes only, the change in MDR was given by −0.60, −0.11 × (Δ IOP), P < 0.0001 using the mean IOP and −0.21, −0.05 × (Δ IOP), P = 0.0004 when using the maximum IOP. This means that among POAG eyes, for instance, a 10-mm Hg IOP reduction led to an MDR improvement of 0.50 dB/year on average (Fig. 2).
Figure 2.
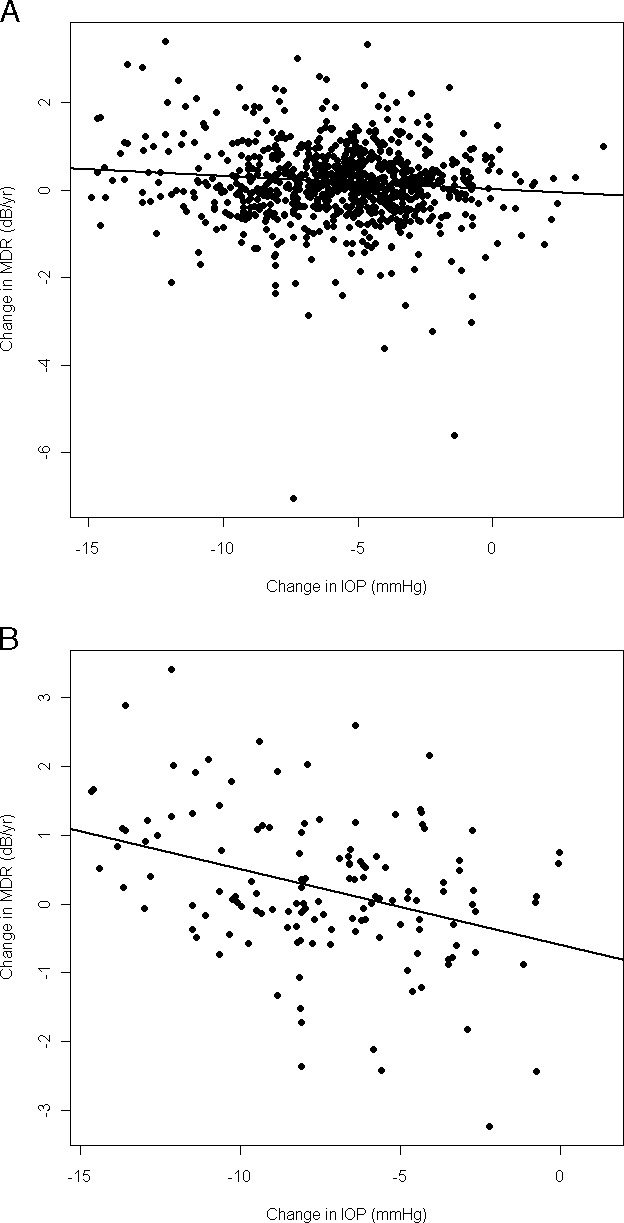
Comparison between changes in absolute IOP (x-axis) and changes in MDRs of decline (y-axis) following treatment. The black line corresponds to the best-fit linear regression line. (A) All participants are included (P = 0.005). (B) Only participants who reached a POAG endpoint are included (P < 0.0001).
Discussion
This study investigated the impact of topical ocular hypotensive treatment on OHTS participants initially randomized to observation. Using trend analysis, we found that treatment decreased the rate of VF change (dB/year) by 74% and the localized rate of change (number of progressing locations) by 53%. To our knowledge, this is the first report to measure the effect of treatment on velocities of VF progression in ocular hypertensive subjects. The effect of treatment in slowing the velocity of VF loss was observed over the entire sample of 780 eyes (432 participants) included in this report, as well as among those who reached an OHTS POAG endpoint (139 eyes of 109 participants) and among those participants that did not reach a POAG endpoint (641 eyes of 380 participants) during the course of the study.
For all eyes, the mean velocity of MD change found in our study following the initiation of treatment was −0.06 ± 0.5 dB/year. This velocity of deterioration was significantly less than zero (P = 0.008, GEE). Since the MD index is designed to be age adjusted, one would expect eyes that are stable and only deteriorating at the normal aging rate to display a flat slope of MD over time. The negative slope in treated ocular hypertensive patients demonstrates that the average velocity of VF deterioration remained significantly faster than expected compared to the VF results of normal subjects, suggesting that progression still occurs, albeit more slowly, if the IOP is reduced in ocular hypertensive patients by 20% (on average).
The present study confirms that lowering IOP is beneficial in ocular hypertension and slows the velocity of VF progression after conversion from ocular hypertension to POAG. However, the rate of MD change after treatment in eyes that reached a POAG endpoint was still meaningful (−0.27 ± 0.7 dB/year), suggesting that once the disease is established (POAG), the target IOP reduction should be greater than that at earlier stages (ocular hypertension). This posttreatment rate was considerably higher than the rate of the cohort as a whole and that of the subset of eyes that did not reach an OHTS POAG endpoint.
Kass et al.21 recently reported the effect of delayed treatment in OHTS by comparing VF outcomes in participants initially randomized to treatment versus observation. The authors stratified the baseline risk of the study participants using a risk calculator developed for ocular hypertensive subjects.22 They observed that initiation of treatment was effective in preventing VF progression both among patients originally randomized to observation (late treatment) and those in the treatment arm (early treatment). They also found that early treatment was particularly advantageous among high-risk patients. Our study provides additional information that supports these findings. First, we were able to objectively measure the efficacy of treatment on rates of VF change among participants undergoing late treatment (average 74% reduction). Second, by showing that treatment was also effective among patients who converted to POAG—which may comprise the majority of high-risk patients at baseline evaluation—we showed a significant reduction in the velocity of VF progression even among participants that had been progressing at much higher rates.
Greater amounts of IOP reduction are thought to be more effective for preserving visual function than less aggressive IOP lowering. In the initial OHTS report investigating risk factors for glaucoma onset among ocular hypertensives, IOP reduction decreased the 5-year risk of conversion to POAG by roughly 50%. Moreover, each 1-mm Hg-higher mean IOP increased the risk by 10%.3 The European Glaucoma Prevention Study (EGPS) suggested a similar effect attributable for each 1-mm Hg-higher IOP (11%),8 and the Diagnostic Innovations in Glaucoma Study (DIGS) reported that each 1-mm Hg-higher mean follow-up IOP increased the risk of conversion from OHT to glaucoma by 20%.23 Using trend analysis, Folgar et al.24 recently reported that an average IOP reduction of 40% following glaucoma surgery resulted in a 70% reduction in global rates of VF change and that locations progressing significantly before intervention had their progression either halted or slowed significantly. They also found that for each 1-mm Hg-lower postoperative IOP, the velocity of VF deterioration was slowed by 0.1 dB/year. While the OHTS outcomes publication demonstrated that lowering the IOP decreased the risk of conversion to POAG in treated in comparison to untreated participants,2 we showed that there is a significant and positive relationship between the amount of IOP reduction and its effect on the rate of VF decay and that each additional 1 mm Hg reduction lowered the rate of VF change by 0.02 to 0.10 dB/year on average. That is, the greater the amount of absolute IOP reduction, the more substantial the improvement in the rate of VF change.
Although our results can only be applied to ocular hypertensive patients, some similarities can be identified with the results of other clinical trials that evaluated patients with established glaucoma. The Early Manifest Glaucoma Trial (EMGT), for example, showed that IOP reduction halved the risk of VF progression in patients with newly diagnosed, established glaucoma.5 In a recent report, the median rate of MD change in untreated EMGT patients was −0.40 dB/year.25 This velocity is similar to what we have found in progressing, untreated OHTS participants (−0.51 dB/year) prior to commencing treatment who were later determined to convert to POAG (i.e., they had progressive glaucoma leading up to a POAG endpoint determination). This observation shows that the untreated participants who went on to reach a POAG endpoint in the OHTS progressed at a velocity similar to that of untreated glaucoma patients in the EMGT and confirms our current understanding that in some eyes, ocular hypertension can be seen as a potential precursor to OAG in the glaucoma continuum.
The importance of using trend analysis in the present study is 2-fold. First, we demonstrated that ocular hypertensive eyes that reached an OHTS POAG endpoint indeed progressed more rapidly than those that did not reach an event analysis endpoint. Therefore, our results do not support the hypothesis that eyes reached POAG endpoints in the OHTS merely because they had VF sensitivities closer to the boundaries of abnormality at baseline. Second, by knowing the rates of VF change before and after treatment initiation, one can estimate visual function and vision-related quality of life outcomes in ocular hypertensive patients seen in clinical practice. For instance, in the Los Angeles Latino Eye Study Survey,26 the investigators found that even small amounts of VF loss as measured by MD may lead to significant functional disability. If a patient's VF MD is progressing linearly,27 and assuming that an MD of 4 to 5 dB represents a clinically meaningful difference,26 the average untreated OHTS participant would develop significantly worse vision-related quality of life in 17 to 22 years. On the other hand, those who reached a POAG endpoint would take approximately 8 to 10 years to develop this level of damage. Using a much more conservative approach and assuming advanced visual impairment when the MD reaches −15 dB, an average subject in the OHTS observation arm would take approximately 65 years for his or her visual function to become severely affected if treatment was never initiated. On the other hand, those who reached a POAG endpoint would take approximately 28 years to become severely visually impaired if they were never treated, which is probably within the lifespan of the average OHTS patient (mean age at study entry, 56 years). The commencement of treatment in those patients trebles the projected time to advanced visual impairment. These estimates, of course, are based on mean values and assume linearity of VF progression, and one should take into consideration the large variability in individual patient behavior in this and other populations.
In conclusion, our results demonstrate that, while the average velocity of VF change in untreated ocular hypertensives is slow, IOP reduction significantly alters the rate of VF change in a beneficial manner. Reduction in the velocity of VF progression following the initiation of topical ocular hypotensive medication was statistically significant among participants who did and did not reach an OHTS POAG endpoint, and this effect was correlated with the magnitude of IOP reduction. VF trend analysis is a quantitative and objective method that can measure the effectiveness of IOP-lowering therapy and has potential applications in clinical practice.
Footnotes
Supported by grants EY09307 and EY09341 from National Institutes of Health; National Center on Minority Health and Health Disparities; Merck, Inc., Whitehouse Station, New Jersey; Pfizer, Inc., New York, New York; Research to Prevent Blindness, New York, New York; Gildor Research Fund of the New York Glaucoma Research Institute, New York, New York; Glaucoma Research and Education Fund of Lenox Hill Hospital (CGDM), New York, New York; Edith C. Blum Foundation (CGDM), New York, New York; and Legacy Good Samaritan Foundation, Portland, Oregon.
Presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2010.
Disclosure: C.G. De Moraes, None; S. Demirel, None; S.K. Gardiner, None; J.M. Liebmann, None; G.A. Cioffi, None; R. Ritch, None; M.O. Gordon, None; M.A. Kass, None
References
- 1. Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–583. [DOI] [PubMed] [Google Scholar]
- 2. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. [DOI] [PubMed] [Google Scholar]
- 3. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. [DOI] [PubMed] [Google Scholar]
- 4. Gordon MO, Torri V, Miglior S, et al. Ocular Hypertension Treatment Study Group; European Glaucoma Prevention Study Group. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. [DOI] [PubMed] [Google Scholar]
- 6. Investigators. AGIS. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:490–491. [DOI] [PubMed] [Google Scholar]
- 7. Musch DC, Gillespie BW, Lichter PR, et al. Visual field progression in the Collaborative Initial Glaucoma Treatment Study: the impact of treatment and other baseline factors. Ophthalmology. 2009;116:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miglior S, Torri V, Zeyen T, et al. Intercurrent factors associated with the development of open-angle glaucoma in the European Glaucoma Prevention Study. Am J Ophthalmol. 2007;144:266–275. [DOI] [PubMed] [Google Scholar]
- 9. Medeiros FA, Sample PA, Zangwill LM, et al. Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol. 2003;136:805–813. [DOI] [PubMed] [Google Scholar]
- 10. Drance S, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708. [DOI] [PubMed] [Google Scholar]
- 11. Parrish RK, II, Gedde SJ, Scott IU, et al. Visual function and quality of life among patients with glaucoma. Arch Ophthalmol. 1997;115:1447–1455. [DOI] [PubMed] [Google Scholar]
- 12. Henson DB, Chaudry S, Artes PH, et al. Response variability in the visual field: comparison of optic neuritis, glaucoma, ocular hypertension, and normal eyes. Invest Ophthalmol Vis Sci. 2000;41:417–421. [PubMed] [Google Scholar]
- 13. Caprioli J. The importance of rates in glaucoma. Am J Ophthalmol. 2008;145:191–192. [DOI] [PubMed] [Google Scholar]
- 14. Fitzke FW, Hitchings RA, Poinoosawmy D, et al. Analysis of visual field progression in glaucoma. Br J Ophthalmol. 1996;80:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manassakorn A, Nouri-Mahdavi K, Koucheki B, et al. Pointwise linear regression analysis for detection of visual field progression with absolute versus corrected threshold sensitivities. Invest Ophthalmol Vis Sci. 2006;47:2896–2903. [DOI] [PubMed] [Google Scholar]
- 16. Gardiner SK, Crabb DP. Examination of different pointwise linear regression methods for determining visual field progression. Invest Ophthalmol Vis Sci. 2002;43:1400–1407. [PubMed] [Google Scholar]
- 17. Spry PG, Bates AB, Johnson CA, et al. Simulation of longitudinal threshold visual field data. Invest Ophthalmol Vis Sci. 2000;41:2192–2200. [PubMed] [Google Scholar]
- 18. Budenz DL, Rhee P, Feuer WJ, et al. Comparison of glaucomatous visual field defects using standard full threshold and Swedish interactive threshold algorithms. Arch Ophthalmol. 2002;120:1136–1141. [DOI] [PubMed] [Google Scholar]
- 19. Wild JM, Pacey IE, Hancock SA, et al. Between-algorithm, between-individual differences in normal perimetric sensitivity: full threshold, FASTPAC, and SITA. Swedish Interactive Threshold algorithm. Invest Ophthalmol Vis Sci. 1999;40:1152–1161. [PubMed] [Google Scholar]
- 20. Chauhan BC, Garway-Heath DF, Goñi FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92:569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kass MA, Gordon MO, Gao F, et al. Delaying treatment of ocular hypertension: The Ocular Hypertension Treatment Study. Arch Ophthalmol. 2010;128:276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gordon MO, Torri V, Miglior S, et al. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Medeiros FA, Weinreb RN, Zangwill LM, et al. Long-term intraocular pressure fluctuations and risk of conversion from ocular hypertension to glaucoma. Ophthalmology. 2008;115:934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Folgar FA, De Moraes CG, Prata TS, et al. Glaucoma surgery decreases the velocity of localized and global visual field progression. Am J Ophthalmol. 2010;149:258–264. [DOI] [PubMed] [Google Scholar]
- 25. Heijl A, Bengtsson B, Hyman L, et al. Natural history of open-angle glaucoma. Ophthalmology. 2009;116:2271–2276. [DOI] [PubMed] [Google Scholar]
- 26. McKean-Cowdin R, Varma R, Wu J, et al. Severity of visual field loss and health-related quality of life. Am J Ophthalmol. 2007;143:1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bengtsson B, Patella VM, Heijl A. Prediction of glaucomatous visual field loss by extrapolation of linear trends. Arch Ophthalmol. 2009;127:1610–1615. [DOI] [PubMed] [Google Scholar]