Abstract
Purpose.
The goals of this study were to determine if oxidative stress on human trabecular meshwork (HTM) cells influences the stability of key mRNAs containing AU rich elements (AREs) known to be associated with glaucoma progression, and if the presence of topographic cue alters the stability of these mRNAs.
Methods.
HTM cells were treated with 300 μM hydrogen peroxide (H2O2) for 1 hour in the presence of 5 μg/mL actinomycin D and compared with untreated cells. The selected mRNAs (IL-6, IL-8, myocilin, SPARC [secreted protein, acidic and rich in cysteine], matrix metalloproteinase [MMP]-3, and MMP-9) from the cells were analyzed by using relative quantitative PCR. Immunohistochemistry for Hu antigen R (HuR) was performed in addition to Western blots of HuR. HTM cells were also grown on topographically patterned surfaces, and IL-6 mRNA was analyzed by quantitative PCR.
Results.
H2O2 increased IL-6 mRNA stability 0.145 (0.095–0.27) to 0.345 (0.2–0.48) (normalized ratio, median [interquartile range]) (n = 5), while IL-8 mRNA was increased from 0.565 (0.408–0.6) to 0.775 (0.486–0.873) (n = 5). These differences were statistically significant (P = 0.0313, for both IL-6 and IL-8; Wilcoxon signed-rank test). The mRNAs of myocilin, SPARC, and MMP-3, which do not have AREs, wer7e more stable after actinomycin D treatment and were not altered with oxidation. Western blot and immunohistochemistry demonstrated that H2O2 treatment induces the translocation of HuR from the nucleus to the cytoplasm. Nanopatterned surfaces did not alter IL-6 mRNA stability.
Conclusions.
Oxidative stress stabilizes IL-6 and IL-8 mRNAs significantly. The decay of certain mRNAs associated with glaucoma may be altered in the trabecular meshwork of glaucoma patients.
Oxidative stress stabilized IL-6 and IL-8 mRNA in HTM cells. Our data suggest that the decay of certain mRNAs associated with glaucoma is altered in the trabecular meshwork of glaucoma patients in a manner consistent with the presence of AU rich elements and HuR (Hu antigen R) stabilization.
Introduction
Glaucoma is a major cause of blindness throughout the world. Despite the devastating consequences of this disease, the exact mechanisms leading to the onset and progression of glaucoma remain unknown. However, it is known that human trabecular meshwork (HTM) cells play a central role in the regulation of intraocular pressure (IOP) under both normal and disease conditions.
The antioxidative capacity of the aqueous humor of individuals with glaucoma is decreased compared with aqueous humor from normal individuals,1 and this decrease in antioxidative capacity has been experimentally verified in a rabbit model of glaucoma.2 In addition, increased oxidative stress in calf eyes resulted in a decrease in outflow facility.3 Reactive oxygen species (ROS) were implicated in studies demonstrating the amount of oxidative DNA damage in the HTM paralleled IOP increases and visual field defects in patients with glaucoma.4–6 Oxidative stress is present in glaucoma, and certain cytokines, specifically IL-6 and IL-8, have been shown to be increased in porcine trabecular meshwork (TM) cells as a result of oxidative challenge.7 The concentration of IL-8 is elevated in the aqueous humor of primary open angle glaucoma (POAG) patients.8,9 There is no direct evidence that IL-6 level is also elevated in POAG patients; however, IL-6 has been reported to be up-regulated in response to experimentally elevated IOP in human donor eyes.10 In addition, the IL-6 level in the aqueous humor has been reported to be elevated in pseudoexfoliation syndrome11 and in patients with neovascular glaucoma.12 It is unclear why these cytokines are elevated, but the mRNAs for these cytokines have an AU-rich element (ARE) on the 3′ untranslated regions (UTRs) that targets the mRNAs for rapid degradation.13,14 Hu antigen R (HuR) is a member of the Hu family of proteins and binds to AREs.14–16 HuR has been shown to stabilize mRNAs with AREs. HuR shuttles from the nucleus to the cytoplasm with these mRNAs, thereby protecting the mRNA associated with them from degradation.14 At least 57 mRNAs, including IL-6, IL-8, and matrix metalloproteinase [MMP]-9, have been reported to be stabilized by HuR.17–21 The increased levels of certain cytokines in patients with glaucoma suggest the normal mRNA degradation pathway for these cytokines in HTM cells is altered with disease.
Work in our laboratory has established that biophysical cues of nano to submicron topographic features play a fundamental role in determining HTM cellular behavior and gene expression.22 Recent studies employing topographically patterned substrates consisting of anisotropically ordered grooves and ridges in the submicron range (mimicking fibers present in basement membrane) dramatically altered cell behaviors and protein expression in HTM cells. However, the influence of topographic cues on the stabilization of cytokine mRNAs has never been investigated for any cell type.
The purpose of this study was to determine if the oxidative stress on HTM cells associated with glaucoma influences the stability of key ARE-containing mRNAs. Since topography has been shown to influence a number of HTM cell behaviors, experiments were also conducted to explore the effects of this ubiquitous biophysical cue on the mRNA stabilization of mRNAs for cytokines reported to be upregulated in glaucoma. In addition, we examined the mRNAs of SPARC (secreted protein, acidic and rich in cysteine), myocilin, and MMP-3, which are known to be altered in glaucoma; however, their mRNAs lack AREs.
Materials and Methods
Cell Cultures
HTM cells were obtained from corneal buttons that were deemed unsuitable for transplant (aged from 18 to 70 years; from the Heartland Lions Eye Bank, St. Louis, MO), as previously described.23 All work was conducted according to the tenets of the Declaration of Helsinki. HTM cells were maintained in Dulbecco's modified Eagle's medium (DMEM)/F-12 1:1 medium (HyClone, Logan, UT) with 10% fetal bovine serum and antibiotics. All HTM cells were used before the sixth passage. All cells were maintained in a 95% air/5% CO2 atmosphere at 37°C.
To introduce oxidative stress, cells were washed with serum-free medium and subsequently incubated with serum-free medium for 2 hours followed by 1 hour incubation with 300 μM H2O2 (Fisher Scientific, Fair Lawn, NJ). In control cultures, the medium without H2O2 was changed at the same time points.
Real-Time PCR
The mRNA levels for IL-6, IL-8, MMP-9, MMP-3, SPARC, and myocilin were analyzed by real-time PCR. We cultured 300,000 to 500,000 cells in 6-cm dishes. Cells were processed routinely for analysis by PCR 3 days after plating.
For oxidative stress, HTM cells were washed with serum-free medium and subsequently incubated with serum-free medium for 1.5 hours. Then, the cells were treated with 5 μg/mL actinomycin D that prevents additional mRNA synthesis (t = −30 min) so that effects of H2O2 on mRNA stability could be investigated independently from new mRNA transcription. After 30 min, cells were treated with or without 300 μM hydrogen peroxide (t = 0) and incubated for 1 hour (t = 1 hour). The mRNA was extracted at t = −30 min and t = 1 hour with RNeasy kits according to the manufacturer's protocol (Qiagen, Valencia, CA).
The amount of mRNA was measured with the NanoDrop Spectrophotometer (ND-1000, Thermo Scientific, Rockford, IL), and 70 ng was used with the OneStep kit for real-time PCR (TaqMan; Applied Biosystems, Inc., [ABI] Foster City, CA). Individual reactions with the real-time PCR machine (StepOne; ABI) were performed with a total volume of 10 μL. The reverse transcription reaction was performed for 30 minutes at 50°C followed by PCR enzyme activation for 10 minutes at 95°C. Forty cycles of 60°C for 1 minute followed by 95°C for 15 seconds were performed. GAPDH served as the reference RNA. We obtained the following probes from ABI: GAPDH (Mm99999905_m1), 18S (Hs99999901_s1), IL-6 (Hs00985639_m1), IL-8 (Hs01567912_g1), SPARC (Hs00277762_m1), MMP-3 (Hs00968308_m1), MMP-9 (Hs00234579_m1), and myocilin (Hs00165345_m1). The samples were done in triplicate and then normalized to the mean of t = −30 minute samples.
Immunofluorescence
HTM cells were plated on glass-bottom dishes treated with FNC coating mix (Athena Enzyme Systems, Baltimore, MD). After plating, the cells were allowed to attach to the dishes in normal media overnight. Next the cells were incubated for 2 hours in serum-free media and then treated with 300 μM H2O2 in serum-free medium for an additional 1 hour. The cells were fixed in 4% paraformaldehyde for 20 minutes. After washing with PBS, the cells were permeabilized with 0.5% Triton X-100 for 10 minutes. Then, the cells were blocked with 1% BSA in PBS for 30 minutes and washed again. The cells were incubated with anti-HuR antibody (sc-5261; 1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at room temperature and secondary antibody (1:400 dilution; Alexa Fluor 488, Molecular Probe, Eugene, OR). Alexa Fluor 567 phalloidin (1:200 dilution; Molecular Probe) and 4′,6′-diamino-2-phenylindole (DAPI; Molecular Probe) were used for fluorescent staining of the actin filaments and the cell nucleus, respectively. Images were obtained with the LSM 5 Pascal/Axiovert 200M microscope (Carl Zeiss Microscopy Japan, Tokyo, Japan).
Western Blotting
HTM cells were cultured in T-75 flasks incubated for 2 hours in serum-free media after which they were treated with or without 300 μM H2O2 for 1 hour in serum-free medium. The cells were then lysed with NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL) containing protease inhibitor cocktail (1:100 dilution; Pierce). Protein concentration was assessed using the BCA protein assay. Samples of 20 μg were electrophoresed on a 10% polyacrylamide gel (Invitrogen, Carlsbad, CA) and the protein was transferred to nitrocellulose membrane. The membrane then was blocked in SuperBlock blocking buffer (Thermo Scientific) for 1 hour. The primary antibodies (anti-HuR diluted 1:1000; anti-lamin B1, ab16048, diluted 1:4000, Abcam, Cambridge, MA) or the antibody to GAPDH conjugated with horse radish peroxidase (diluted 1:20,000, ab9482, Abcam) were allowed to react for 1 hour at room temperature. Following washes in PBS containing 0.1% Tween 20 (PBST), we incubated the membrane with secondary antibody for 1 hour and washed again in PBST. A chemiluminescent detection kit (Immobilon Western; Millipore, Billerica, MA) was used to detect bands. The banding pattern was taken with the ImageQuant 350 (GE Healthcare, Piscataway, NJ) and quantified with accompanying software (ImageQuant TL7.0; GE Healthcare). Background signal was subtracted by the software. The HuR bands intensities were normalized for the GAPDH or lamin B1 loading controls, and then the ratios of oxidatively stressed sample to control sample were calculated for both cytoplasmic and nuclear fractions.
Topographically Patterned Substrates
The topographically patterned and planar control substrates using optical adhesive (NOA 81; Norland Products Inc., Cranbury, NJ) were fabricated as previously reported.24 Three-square-centimeter nanogrooved surfaces of 1400-nm pitch (700 nm equal ridge and groove widths with grooves having a 300-nm depth) were used. The substrates were coated with FNC coating mix prior to cell seeding.
Statistical Analysis
The Wilcoxon signed-rank test was used to compare the mRNA stability with or without H2O2 treatment. For Western blots, a two-sided t-test was used. The null hypothesis was that there would be no difference of the ratio of stressed sample to control sample. A P value of <0.05 was considered statistically significant. The statistical package used was JMP version 8.0 (SAS Institute, Cary, NC).
Results
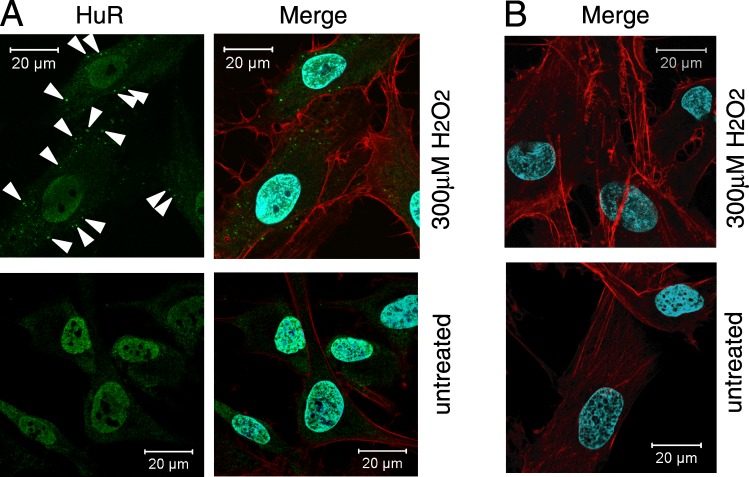
H2O2 induced translocation of HuR from the nucleus to the cytoplasm in primary HTM cells. Localization of HuR was detected faintly in the cytoplasm of untreated cells by laser confocal microscopy but was abundantly present in the nucleus. However, HuR in the cytoplasm increased following H2O2 treatment, and abundant HuR signal was still seen in the nucleus after H2O2 treatment (Fig. 1A). Negative control staining without anti-HuR antibody showed no reaction with the secondary antibody (Fig. 1B).
Figure 1.
Subcellular localization of HuR in HTM cells. (A) HuR (green) was visualized by laser confocal microscopy on cultured cells that were either left untreated or treated with 300 μM H2O2 (1 hour). DAPI (blue) staining served to visualize the nucleus and phalloidin (red) staining visualized actin in the cytoplasm. Note the distinct overlap of DAPI and HuR signals; while H2O2-treated cells also exhibited abundant nuclear HuR, the treatment caused an increase in the cytoplasmic HuR (arrow heads). (B) Negative control without primary antibody to HuR showed no reaction with the secondary antibody.
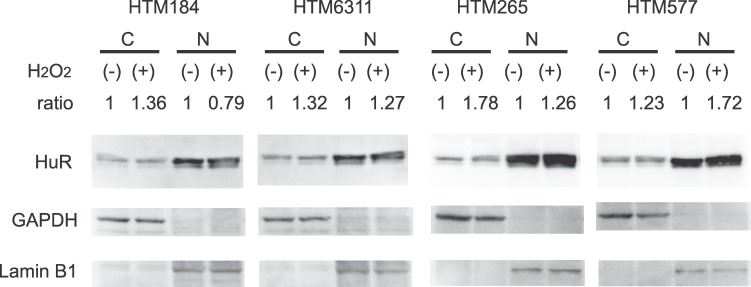
Protein lysates from the cytoplasm and the nucleus of HTM cells of four donors were analyzed by Western blot. Intensity analyses of the Western blots showed that HuR in the cytoplasm increased following H2O2 treatment consistent with the immunofluorescent data. The analysis of HuR showed that it was increased 42% (n = 4, average ratio 1.42, 95% confidential interval 1.04–1.81, P = 0.04, t-test) in the cytoplasm of the H2O2-treated cells compared to the control cells (Fig. 2). In contrast, there was no statistical difference in HuR band intensities in the nucleus (n = 4, average ratio 1.26, 95% confidential interval 0.66–1.86, P = 0.26, t-test) (Fig. 2).
Figure 2.
Increased cytoplasmic HuR after exposure to oxidative stress. HTM cells were harvested 1 hour after incubation with or without 300 μM H2O2. Cytoplasmic and nuclear lysates were prepared, and HuR levels were determined by Western blotting. GAPDH and lamin B1 show definitive fractionation of the cytoplasm (C) and nucleus (N). GAPDH and lamin B1 were used as loading controls for cytoplasmic and nuclear samples, respectively. Cytoplasmic and nuclear HuR and were normalized for GAPDH or lamin B1 and the intensities of oxidatively stressed samples were compared with control samples. Cytoplasmic HuR was increased after exposure to H2O2 (average ratio 1.42, 95% confidential interval 1.04–1.81, P = 0.04, Student's t-test). In contrast, there was no statistical difference in nuclear HuR (n = 4, average ratio 1.26, 95% confidential interval 0.66–1.86, P = 0.26, Student's t-test).
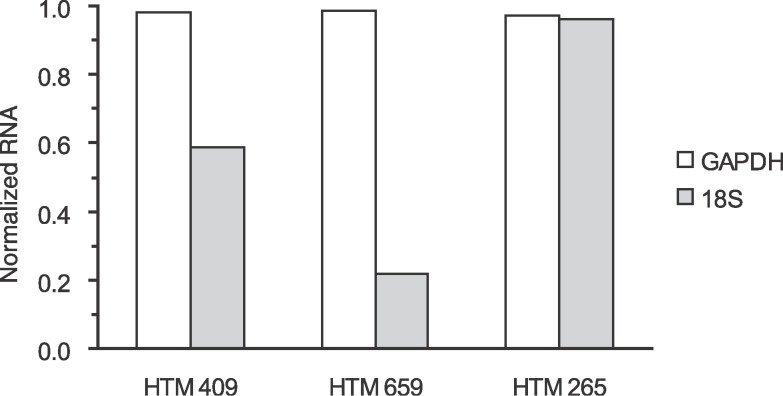
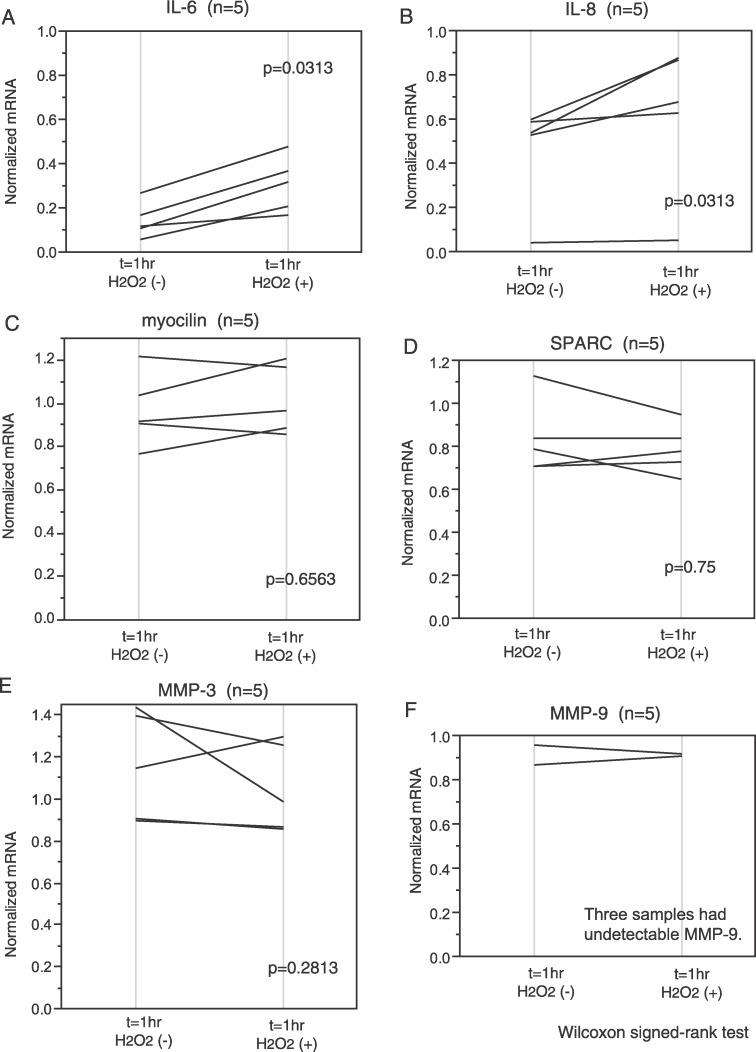
To select a proper internal control gene for real-time PCR analysis, we compared the 18S rRNA to the GAPDH mRNA expression. Samples from two out of three different individuals showed that GAPDH was less variable than the 18S after actinomycin D and H2O2 treatment (Fig. 3). Therefore, GAPDH mRNA was used as an internal control gene in all experiments. As already stated, use of actinomycin D allowed us to follow mRNA stabilization without new transcription. Cell cultures derived from five different individuals were analyzed (n = 5) for IL-6 mRNA stability. Exposure to H2O2 increased IL-6 mRNA compared to controls. One hour after treatment, the normalized values for the remaining IL-6 were 0.145 (0.095–0.27) (normalized ratio, median [interquartile range]) for the control cultures and 0.345 (0.2–0.48) for the cultures treated with H2O2 (Fig. 4A). Similarly IL-8 mRNA increased after H2O2 treatment. In control cultures, the normalized value of IL-8 mRNA was 0.565 (0.408–0.6) compared with the value 0.775 (0.486–0.873) with H2O2 treatment (n = 5; Fig. 4B). Both increases were statistically significant (P = 0.0313, both for IL-6 and IL-8; Wilcoxon signed-rank test).
Figure 3.
Determination of endogenous control for mRNA stabilization. To select a proper internal control gene for real-time PCR analysis, we compared 18S rRNA to GAPDH mRNA expression in our experiments. Cell cultures from three different individuals were analyzed. HTM cells were treated with 5 μg/mL actinomycin D to suppress transcription (t = −30 minutes). After 30 minutes, cells were treated with 300 μM hydrogen peroxide (t = 0) and incubated for 1 hour (t = 1 hour). They were harvested at t = −30 minutes and t = 1 hour. The same amount of total cellular RNA was used to standardize the samples. Each real-time PCR amplification was run in triplicate. Data are shown as mean and are normalized to t = −30 minutes. GAPDH RNA appeared more consistent than 18S RNA. Therefore, we used GAPDH mRNA as an internal control in subsequent experiments.
Figure 4.
Stability change of mRNA with H2O2 treatment in HTM cells. HTM cells were treated with 5 μg/mL actinomycin D to suppress transcription (t = −30 minutes). After 30 minutes, cells were treated with or without 300 μM H2O2 (t = 0) and incubated for 1 hour (t = 1 hour). They were harvested at t = −30min and t = 1 hour. Each real-time PCR amplification was run in triplicate. Data are shown as mean of the triplicate and normalized to t = −30 minutes. H2O2 increased IL-6 (A) and IL-8 (B) mRNA stability. The change of myocilin (C), SPARC (D), and MMP-3 (E) mRNA stability varied with each individual but was not significantly different between the treated and untreated cells. MMP-9 (F) did not vary much in the two samples.
The change of myocilin, SPARC, and MMP-3 mRNA levels after H2O2 treatment varied in each individual (n = 5), but the differences were not statistically significant (for myocilin; without H2O2, 0.98 [0.875–1.22]; with H2O2, 1.07 [0.883–1.18]; P = 0.6563) (for SPARC; without H2O2, 0.75 [0.71–0.913]; with H2O2, 0.755 [0.71–0.868]; P = 0.75) (for MMP-3; without H2O2, 1.275 [0.908–1.41]; with H2O2, 1.125 [0.868–1.27]; P = 0.2813) (Fig. 4C–E).
MMP-9, which also has AREs in its 3′ UTR, did not vary much in the two samples in which it could be detected. The amounts of MMP-9 mRNA were low with Ct values around 35 cycles. MMP-9 mRNA were undetectable in three out of five samples (Fig. 4F).
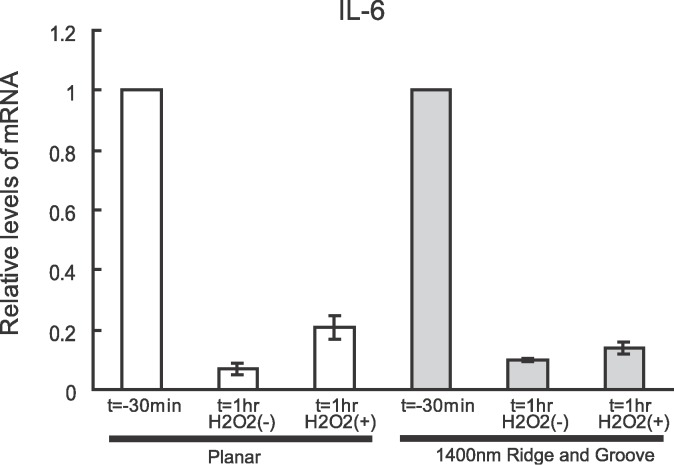
To determine if topographic cues in the biomimetic range would influence this mRNA stabilization, HTM cells from two different individuals were analyzed for IL-6 mRNA stability when cultured on 1400-nm pitch surfaces. Stabilization of the mRNA was similar to the planar substrates. One representative result from a 26-year-old donor is shown in Figure 5.
Figure 5.
Stability of mRNA for IL-6 with H2O2 treatment in HTM cells grown on 1400-nm pitch topography compared with planar surfaces. The result of one representative culture of two, obtained from a 26-year-old donor, is shown. HTM cells were treated with 5 μg/mL actinomycin D to suppress transcription (t = −30 minutes). Data are shown as mean ± standard deviation of the triplicate and normalized to t = −30 minutes of planar or topographic surfaces. No difference in stabilization of mRNA was noted between the cells on topography and planar substrates. Although nanopatterned surfaces influence a number of cellular behaviors, nanopatterned surfaces did not alter IL-6 mRNA stability. This result suggests topographic cues do not affect ARE stabilization by HuR.
Discussion
This is the first report to demonstrate HuR is present in HTM cells and that with oxidative stress, the HuR protein moves from the nucleus to the cytoplasm of the HTM cells. In a previous report, oxidative stress simulated by H2O2 exposure induced IL-6 and IL-8 in porcine TM cells.7 Our results demonstrate oxidative stress increased the levels of IL-6 and IL-8 mRNA in HTM cells as well. The observed increase in mRNA induced by oxidative stress suggests that the dynamics of mRNA degradation pathways for these mediators may be altered in the HTM cells by glaucoma-associated oxidative stress. HuR is known to stabilize mRNAs with AREs during H2O2 treatment in a variety of cell types.25–28 The altered stabilization of mRNA in other cell types suggests HuR has a similar role for certain cytokines in HTM cells.
HuR has been reported to shuttle from the nucleus to the cytoplasm.14 By Western blots, we found HuR to increase in the cytoplasm after oxidative stress. However, increased levels of HuR in the cytoplasm were not as large in the primary HTM cells compared with changes previously reported in cell lines. Moreover, the abundance of HuR in the nucleus in the HTM cells may have made it difficult to detect mild to moderate decreases of HuR in the nucleus in our four samples.
We selected the inflammatory mediators IL-6 and IL-8 for investigation because an increased expression of these inflammatory markers in the outflow pathways has been linked to glaucoma. IL-6 level is elevated in the aqueous humor in pseudoexfoliation syndrome11 and neovascular glaucoma,12 though a direct link with POAG has not been made. It has been reported that increasing IOP in ex vivo perfused human anterior segments increased IL-6 mRNAs10 and that induced IL-6 increased outflow facility in perfused anterior segments of porcine eyes.29 In aggregate, our findings and previous reports suggest a scenario whereby an increase in oxidative stress associated with glaucoma would lead to increased HuR in the cytoplasm thereby protecting IL-6 mRNA and a concomitant increase in protein expression with the associated beneficial effect on IOP control.
The concentration of IL-8 is elevated in the aqueous humor of glaucoma patients,8,9 and HTM cells have been shown to secrete IL-8.30 It has also been reported that IL-8 modulates the permeability of the Schlemm's canal endothelial cells.31 These reports suggest that IL-8 plays an important role in the outflow pathway. As with IL-6, it may be that the increased levels of these cytokines are a means by which the HTM cells attempt to ameliorate the change in outflow resistance associated with glaucoma.
A consensus does not exist regarding the relationship between these inflammatory mediators and the onset/progression of glaucoma. While an increase in these cytokines can have beneficial effects on outflow, it is also possible that chronic activation of these inflammatory mediators could induce pathologic changes in the TM contributing to the progression of glaucoma. IL-6 and IL-8 have been implicated in induction of cellular senescence,32 known to occur in the outflow pathway in glaucoma.33 Thus while these two cytokines may have valuable effects in the short term, their continued presence may ultimately have deleterious effects to the HTM cells.
MMP-3, MMP-9, SPARC, and myocilin were investigated because they have been associated with the meshwork and changes in the amounts of these proteins are associated with glaucoma. Many MMPs have been detected in TM cells.34 MMP-3 has been reported to be up-regulated in response to a variety of stimuli,35–40 and to be increased in aqueous samples from pseudoexfoliation eyes.41 A mixture of MMPs including MMP-3 and MMP-9 increased outflow facility in human anterior segment organ culture.42 In addition, MMP-9 has multiple AUUA motifs in the 3′ UTR. SPARC is distributed through out the TM43 and has been demonstrated in aqueous humor of humans.44 SPARC-null mice had lower IOP values compared to wild-type mice.45 Myocilin was the first gene to be associated with glaucoma.46 Although a consensus has not been reached regarding the physiological function of myocilin, it has been linked to elevations in IOP.47,48 Thus each of these proteins may be modulated by glaucoma associated oxidative stress, but only MMP-9 has AREs that would bind to HuR.
The expression of the mRNAs for inflammatory cytokines is normally tightly controlled as a result of having the AREs on their 3′ UTRs to ensure a short half-life. This is consistent with our results. Even with oxidative stress, the mRNAs of IL-6 and IL-8 were decreased compared with SPARC, myocilin, and the MMPs at the 1-hour time point. These data suggest that IL-6 and IL-8 mRNAs have a rapid turnover in the HTM cells. The observed lack of effect on stability of matricellular proteins, myocilin, SPARC, and MMP-3 mRNAs that do not have AREs and their high levels of expression during the course of our experiments indicate H2O2 treatment does not influence their mRNA stability.
Although HuR has been reported to stabilize rat MMP-9 mRNA,49 we could not detect any stabilization of HTM-associated MMP-9 mRNA despite the documented increase in cytosolic HuR associated with oxidative stress. MMP-9 mRNA expression in our experiments was minimal and near the limits of detection with quantitative PCR. This low level of expression may have prevented us from detecting any stability difference in MMP-9. Oh et al.40 reported an inability to detect MMP-9 mRNA in several samples of fresh HTM, and our findings would be consistent with their results.
This mRNA stabilization might also participate in the induction of inflammatory mediators by mechanical stress as well. Liton et al.50 observed that p38 mitogen-activated protein kinase (MAPK) contributes to increased IL-6 with mechanical stress on HTM cells. They suggested that p38 promoted IL-6 expression through mRNA stabilization. The p38 MAPK pathway leads to the inactivation of mRNA decay-promoting proteins such as TTP,51–53 and also regulates HuR's nuclear–cytoplasmic shuttling.25,54
Topographic cues have been shown to modulate many cellular behaviors, including proliferation, adhesion, and migration.55–58 Moreover, increased mRNA levels of myocilin and versican were observed in HTM cells grown on topographically patterned substrates.22 Growth on 1400-nm pitch topographically patterned substrates did not alter the stability of mRNAs for IL-6 in our experiments compared with planar substrates. These results suggest topographic features do not influence the mRNA stabilities in HTM cells explored in our experiments; the impact of this important and ubiquitous biophysical cue on the stability of other mRNAs remains unexplored. Thus, while biomimetic topographic cues profoundly influence multiple characteristics of HTM cells, our results suggest that the mRNA stabilization of specific mRNAs investigated are not affected by this biophysical cue.
In conclusion, oxidative stress stabilized IL-6 and IL-8 mRNA in HTM cells. Our data suggests that the decay of certain mRNAs associated with glaucoma is altered in the TM of glaucoma patients in a manner consistent with the presence of AREs and HuR stabilization. While induction of both of these cytokines would appear to be useful in counteracting the effects of high IOP, their continued presence may ultimately have deleterious effects on HTM cells. As a consequence of the oxidative stress associated with glaucoma, the stabilization of these cytokines may actually contribute to the progression of the disease.
Footnotes
Supported by grants from the National Institutes of Health R01EY019475 and P30EY12576; an unrestricted grant from the Research to Prevent Blindness; and a grant from National Glaucoma Research, a program of the American Health Assistance Foundation. HM is the recipient of the Hammond Glaucoma Research Fellowship of the University of California, Davis; the Alcon Japan Hida Memorial Award; and Imai Memorial Glaucoma Research Foundation, Japan.
Disclosure: H. Mochizuki, None; C.J. Murphy, None; J.D. Brandt, None; Y. Kiuchi, None; P. Russell, None
References
- 1. Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004;137:62–69. [DOI] [PubMed] [Google Scholar]
- 2. Beit-Yannai E, Trembovler V, Solomon AS. Decrease in reducing power of aqueous humor originating from glaucomatous rabbits. Eye. 2007;21:658–664. [DOI] [PubMed] [Google Scholar]
- 3. Kahn MG, Giblin FJ, Epstein DL. Glutathione in calf trabecular meshwork and its relation to aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 1983;24:1283–1287. [PubMed] [Google Scholar]
- 4. Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458–463. [DOI] [PubMed] [Google Scholar]
- 5. Sacca SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007;84:389–399. [DOI] [PubMed] [Google Scholar]
- 6. Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612:105–114. [DOI] [PubMed] [Google Scholar]
- 7. Li G, Luna C, Liton PB, Navarro I, Epstein DL, Gonzalez P. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol Vis. 2007;13:2282–2288. [PMC free article] [PubMed] [Google Scholar]
- 8. Kuchtey J, Rezaei KA, Jaru-Ampornpan P, Sternberg P, Jr, Kuchtey RW. Multiplex cytokine analysis reveals elevated concentration of interleukin-8 in glaucomatous aqueous humor. Invest Ophthalmol Vis Sci. 2010;51:6441–6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takai Y, Tanito M, Ohira A. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma. Exfoliation Glaucoma, and Cataract. Invest Ophthalmol Vis Sci. 2012;53:241–247. [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez P, Epstein DL, Borras T. Genes upregulated in the human trabecular meshwork in response to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2000;41:352–361. [PubMed] [Google Scholar]
- 11. Zenkel M, Lewczuk P, Jünemann A, Kruse FE, Naumann GOH, Schlötzer-Schrehardt U. Proinflammatory cytokines are involved in the initiation of the abnormal matrix process in pseudoexfoliation syndrome/glaucoma. Am J Pathol. 2010;176:2868–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen KH, Wu CC, Roy S, Lee SM, Liu JH. Increased interleukin-6 in aqueous humor of neovascular glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2627–2632. [PubMed] [Google Scholar]
- 13. Ma WJ, Cheng S, Campbell C, Wright A, Furneaux H. Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J Biol Chem. 1996;271:8144–8151. [DOI] [PubMed] [Google Scholar]
- 14. Abdelmohsen K, Kuwano Y, Kim HH, Gorospe M. Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: implications for cellular senescence. Biol Chem. 2008;389:243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu J, Dalmau J, Szabo A, Rosenfeld M, Huber J, Furneaux H. Paraneoplastic encephalomyelitis antigens bind to the AU-rich elements of mRNA. Neurology. 1995;45:544–550. [DOI] [PubMed] [Google Scholar]
- 16. Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998;17:3448–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hershko DD, Robb BW, Wray CJ, Luo GJ, Hasselgren PO. Superinduction of IL-6 by cycloheximide is associated with mRNA stabilization and sustained activation of p38 map kinase and NF-kappaB in cultured caco-2 cells. J Cell Biochem. 2004;91:951–961. [DOI] [PubMed] [Google Scholar]
- 18. Wong S, Schwartz RC, Pestka JJ. Superinduction of TNF-alpha and IL-6 in macrophages by vomitoxin (deoxynivalenol) modulated by mRNA stabilization. Toxicology. 2001;161:139–149. [DOI] [PubMed] [Google Scholar]
- 19. Witowski J, Jorres A, Coles GA, Williams JD, Topley N. Superinduction of IL-6 synthesis in human peritoneal mesothelial cells is related to the induction and stabilization of IL-6 mRNA. Kidney Int. 1996;50:1212–1223. [DOI] [PubMed] [Google Scholar]
- 20. Holtmann H, Winzen R, Holland P, et al. Induction of interleukin-8 synthesis integrates effects on transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol Cell Biol. 1999;19:6742–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nabors LB, Gillespie GY, Harkins L, King PH. HuR, a RNA stability factor, is expressed in malignant brain tumors and binds to adenine- and uridine-rich elements within the 3′ untranslated regions of cytokine and angiogenic factor mRNAs. Cancer Res. 2001;61:2154–2161. [PubMed] [Google Scholar]
- 22. Russell P, Gasiorowski JZ, Nealy PF, Murphy CJ. Response of human trabecular meshwork cells to topographic cues on the nanoscale level. Invest Ophthalmol Vis Sci. 2008;49:629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rhee DJ, Tamm ER, Russell P. Donor corneoscleral buttons: a new source of trabecular meshwork for research. Exp Eye Res. 2003;77:749–756. [DOI] [PubMed] [Google Scholar]
- 24. Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J Cell Sci. 2003;116:1881–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tran H, Maurer F, Nagamine Y. Stabilization of urokinase and urokinase receptor mRNAs by HuR is linked to its cytoplasmic accumulation induced by activated mitogen-activated protein kinase-activated protein kinase 2. Mol Cell Biol. 2003;23:7177–7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuwano Y, Kim HH, Abdelmohsen K, et al. MKP-1 mRNA stabilization and translational control by RNA-binding proteins HuR and NF90. Mol Cell Biol. 2008;28:4562–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin-Garrido A, Gonzalez-Ramos M, Griera M, et al. H2O2 regulation of vascular function through sGC mRNA stabilization by HuR. Arterioscler Thromb Vasc Biol. 2011;31:567–573. [DOI] [PubMed] [Google Scholar]
- 28. Dong R, Lu JG, Wang Q, He XL, Chu YK, Ma QJ. Stabilization of Snail by HuR in the process of hydrogen peroxide induced cell migration. Biochem Biophys Res Commun. 2007;356:318–321. [DOI] [PubMed] [Google Scholar]
- 29. Liton PB, Luna C, Bodman M, Hong A, Epstein DL, Gonzalez P. Induction of IL-6 expression by mechanical stress in the trabecular meshwork. Biochem Biophys Res. Commun. 2005;337:1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shifera AS, Trivedi S, Chau P, Bonnemaison LH, Iguchi R, Alvarado JA. Constitutive secretion of chemokines by cultured human trabecular meshwork cells. Exp Eye Res. 2010;91:42–47. [DOI] [PubMed] [Google Scholar]
- 31. Alvarado JA, Alvarado RG, Yeh RF, Franse-Carman L, Marcellino GR, Brownstein MJ. A new insight into the cellular regulation of aqueous outflow: how trabecular meshwork endothelial cells drive a mechanism that regulates the permeability of Schlemm's canal endothelial cells. Br J Ophthalmol. 2005;89:1500–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuilman T, Michaloglou C, Vredeveld LC, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. [DOI] [PubMed] [Google Scholar]
- 33. Liton PB, Challa P, Stinnett S, Luna C, Epstein DL, Gonzalez P. Cellular senescence in the glaucomatous outflow pathway. Exp Gerontol. 2005;40:745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keller KE, Aga M, Bradley JM, Kelley MJ, Acott TS. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009;88:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luna C, Li G, Liton PB, Epstein DL, Gonzalez P. Alterations in gene expression induced by cyclic mechanical stress in trabecular meshwork cells. Mol Vis. 2009;15:534–544. [PMC free article] [PubMed] [Google Scholar]
- 36. Kelley MJ, Rose AY, Song K, et al. Synergism of TNF and IL-1 in the induction of matrix metalloproteinase-3 in trabecular meshwork. Invest Ophthalmol Vis Sci. 2007;48:2634–2643. [DOI] [PubMed] [Google Scholar]
- 37. Fleenor DL, Pang IH, Clark AF. Involvement of AP-1 in interleukin-1alpha-stimulated MMP-3 expression in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003;44:3494–3501. [DOI] [PubMed] [Google Scholar]
- 38. Pang IH, Hellberg PE, Fleenor DL, Jacobson N, Clark AF. Expression of matrix metalloproteinases and their inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003;44:3485–3493. [DOI] [PubMed] [Google Scholar]
- 39. Parshley DE, Bradley JM, Fisk A, et al. Laser trabeculoplasty induces stromelysin expression by trabecular juxtacanalicular cells. Invest Ophthalmol Vis Sci. 1996;37:795–804. [PubMed] [Google Scholar]
- 40. Oh DJ, Martin JL, Williams AJ, Russell P, Birk DE, Rhee DJ. Effect of latanoprost on the expression of matrix metalloproteinases and their tissue inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47:3887–3895. [DOI] [PubMed] [Google Scholar]
- 41. Schlötzer-Schrehardt U, Lommatzsch J, Küchle M, Konstas AG, Naumann GO. Matrix metalloproteinases and their inhibitors in aqueous humor of patients with pseudoexfoliation syndrome/glaucoma and primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2003;44:1117–1125. [DOI] [PubMed] [Google Scholar]
- 42. Bradley JM, Vranka J, Colvis CM, et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998;39:2649–2658. [PubMed] [Google Scholar]
- 43. Rhee DJ, Fariss RN, Brekken R, Sage EH, Russell P. The matricellular protein SPARC is expressed in human trabecular meshwork. Exp Eye Res. 2003;77:601–607. [DOI] [PubMed] [Google Scholar]
- 44. Yan Q, Clark JI, Sage EH. Expression and characterization of SPARC in human lens and in the aqueous and vitreous humors. Exp Eye Res. 2000;71:81–90. [DOI] [PubMed] [Google Scholar]
- 45. Haddadin RI, Oh DJ, Kang MH, et al. SPARC-null mice exhibit lower intraocular pressures. Invest Ophthalmol Vis Sci. 2009;50:3771–3777. [DOI] [PubMed] [Google Scholar]
- 46. Polansky JR, Fauss DJ, Chen P, et al. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica. 1997;211:126–139. [DOI] [PubMed] [Google Scholar]
- 47. Resch ZT, Fautsch MP. Glaucoma-associated myocilin: a better understanding but much more to learn. Exp Eye Res. 2009;88:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360:1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alkool el S, Kleinert H, Hamada FM, et al. Nitric oxide increases the decay of matrix metalloproteinase 9 mRNA by inhibiting the expression of mRNA-stabilizing factor HuR. Mol Cell Biol. 2003;23:4901–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liton PB, Li G, Luna C, Gonzalez P, Epstein DL. Cross-talk between TGF-beta1 and IL-6 in human trabecular meshwork cells. Mol Vis. 2009;15:326–334. [PMC free article] [PubMed] [Google Scholar]
- 51. Stoecklin G, Stubbs T, Kedersha N, et al. MK2-induced tristetraprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 2004;23:1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hitti E, Iakovleva T, Brook M, et al. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol Cell Biol. 2006;26:2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Winzen R, Thakur BK, Dittrich-Breiholz O, et al. Functional analysis of KSRP interaction with the AU-rich element of interleukin-8 and identification of inflammatory mRNA targets. Mol Cell Biol. 2007;27:8388–8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lin FY, Chen YH, Lin YW, et al. The role of human antigen R, an RNA-binding protein, in mediating the stabilization of toll-like receptor 4 mRNA induced by endotoxin: a novel mechanism involved in vascular inflammation. Arterioscler Thromb Vasc Biol. 2006;26:2622–2629. [DOI] [PubMed] [Google Scholar]
- 55. Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J Cell Sci. 2003;116:1881–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Karuri NW, Liliensiek S, Teixeira AI, et al. Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. J Cell Sci. 2004;117:3153–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Teixeira AI, McKie GA, Foley JD, Bertics PJ, Nealey PF, Murphy CJ. The effect of environmental factors on the response of human corneal epithelial cells to nanoscale substrate topography. Biomaterials. 2006;27:3945–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liliensiek SJ, Campbell S, Nealey PF, Murphy CJ. The scale of substratum topographic features modulates proliferation of corneal epithelial cells and corneal fibroblasts. J Biomed Mater Res A. 2006;79:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]