Abstract
Purpose.
The purpose of this study was to determine whether clinical signs of infectious keratitis can be used to identify the causative organism.
Methods.
Eighty photographs of eyes with culture-proven bacterial keratitis or smear-proven fungal keratitis were randomly selected from 2 clinical trials. Fifteen cornea specialists from the F. I. Proctor Foundation and the Aravind Eye Care System assessed the photographs for prespecified clinical signs of keratitis, and they identified the most likely causative organism.
Results.
Clinicians were able to correctly distinguish bacterial from fungal etiology 66% of the time (P < 0.001). The Gram stain, genus, and species were accurately predicted 46%, 25%, and 10% of the time, respectively. The presence of an irregular/feathery border was associated with fungal keratitis, whereas a wreath infiltrate or an epithelial plaque was associated with bacterial keratitis.
Conclusions.
Cornea specialists correctly differentiated bacterial from fungal keratitis more often than chance, but in fewer than 70% of cases. More specific categorization led to less successful clinical distinction. Although certain clinical signs of infectious keratitis may be associated with a bacterial or fungal etiology, this study highlights the importance of obtaining appropriate microbiological testing during the initial clinical encounter. (ClinicalTrials.gov number, NCT00324168.)
This survey asked corneal specialists to evaluate photographs of infectious keratitis. The purpose of the study was to determine if clinical appearance can be used to identify the causative organism.
Introduction
Microbiological studies following the culture of corneal infiltrates are the gold standard for determining the etiology of infectious keratitis caused by bacteria or fungi; however, even if a culture of the corneal ulcer is obtained, subsequent growth and identification of microorganisms occurs in only 40% to 60% of cases.1–4 Corneal ulcers are often treated empirically without the benefit of microbiological data and, even in cases where a specimen is collected, it is generally recommended that treatment be initiated as soon as possible before obtaining the results and continued even if no microorganism is identified.5–8
The rationale for empirical treatment is based on the assumption that most cases of bacterial keratitis will respond to modern broad-spectrum antibiotics5,9; however, it is acknowledged that the success of such empirical therapy rests on the ability of the clinician to identify, through clinical history, signs, and symptoms, the nonbacterial and atypical organisms such as fungi, Acanthamoeba spp, and viruses. Failure to identify such causative agents increases the likelihood of advancing corneal infiltration and a poor therapeutic outcome.10 Despite its critical role within the therapeutic decision-making process, it is unclear how accurate clinicians are in predicting the causative organism in cases of infectious keratitis based solely on appearance. It is also unclear which clinical signs are used to make this determination, and whether these signs are actually predictive of the etiology. Here, we report the results of a photograph-based survey of cornea specialists at the F. I. Proctor Foundation and Aravind Eye Care System that evaluated the ability of clinicians to differentiate between bacterial and fungal etiology when examining photographs of corneal ulcers. We also describe the clinical signs most commonly used to reach their decision.
Methods
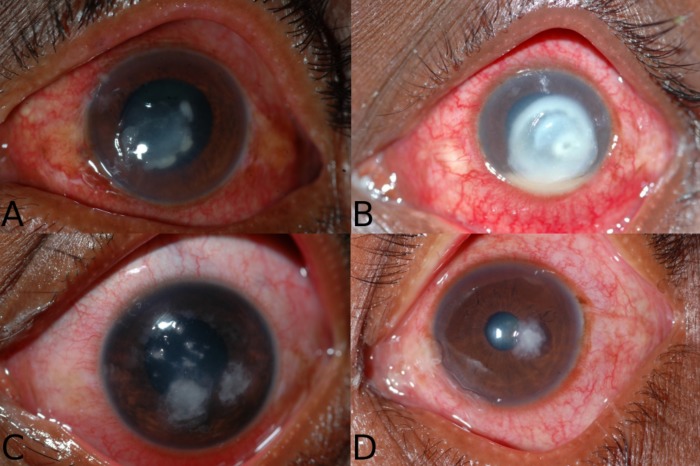
This is an ancillary study using data from two clinical trials of infectious keratitis that were conducted collaboratively by the Aravind Eye Care System, F. I. Proctor Foundation, and the Dartmouth-Hitchcock Medical Center from 2006 to 2010.11,12 For the Steroids for Corneal Ulcers Treatment (SCUT) trial, which consisted only of bacterial corneal ulcers, patients were included if they had documented bacterial growth on culture. For the fungal ulcer trial, patients were enrolled if they had fungal elements on a KOH smear. Corneal photography was performed using a standard protocol on initial presentation in both studies. All photographs were taken by a certified ophthalmic photographer using a Nikon D series digital single lens reflex camera with a 105-mm f/2.8D AF Micro Nikon autofocus lens and a modified SB29s electronic flash or equivalent with 1:1 magnification (Nikon, Tokyo, Japan) (Figs. 1A–D).
Figure 1.
These photographs are representative of the survey given to clinicians as they were asked to differentiate fungal versus bacterial etiologies. The causative organism and the proportion of clinicians who correctly identified the organism as fungal versus bacterial was (A) Streptococcus pneumoniae, 60% correctly identified as bacterial; (B) Pseudomonas aeruginosa, 93% correctly identified as bacterial; (C) Aspergillus flavus, 93% correctly identified as fungal; and (D) Fusarium spp, 67% correctly identified as fungal.
Using study numbers from the entire database of patients in the two trials, we randomized and selected 40 photographs of culture-proven bacterial corneal ulcers from the bacterial keratitis trial, and 40 photographs of smear-proven fungal corneal ulcers from the fungal keratitis trial (causative organisms listed in Table 1). This number of photographs was felt to be the maximum that our clinicians could be reasonably expected to evaluate in a detailed manner. The desired number of photographs was determined before randomization, and no individual selection of photographs based on content or picture quality was allowed. We chose a sample size of 15 raters for the 80 photographs (40 bacterial and 40 fungal) to provide approximately 90% power to detect a 57.5% accuracy for correct grading (where chance would allow 50% correct). The power calculation was performed using simulation based on the Fleiss multirater kappa statistic, using the statistical package R (version 2.12 for Macintosh, package irr). A group of 8 fellowship-trained cornea specialists from the F. I. Proctor Foundation and 7 fellowship-trained cornea specialists and fellows from the Aravind Eye Care System assessed each of the 80 photographs. Graders were informed that half of the photographs were taken from eyes with bacterial keratitis, and half from eyes with fungal keratitis. There was only one photograph for each case of keratitis. For each photograph, the clinician indicated whether the corneal ulcer was most likely fungal or bacterial in etiology, and clinicians listed the three traits most helpful in reaching their conclusion. Clinicians documented the likely Gram stain result, genus, and species for suspected bacterial infections, and the likely genus for suspected fungal infections. The species was also recorded for suspected Aspergillus infections, as species data were available for Aspergillus based on laboratory protocol. In addition, all cornea specialists from the Proctor group indicated the presence or absence of 23 clinical signs for each photograph (listed in Table 2). Because of the substantial amount of time required to complete the survey, clinicians of the Aravind group recorded the suspected organism but not the clinical signs. We defined a clinical sign as present if it was noted by more than 50% of graders.
Table 2.
Presence of Clinical Signs
|
Trait |
Fungal Consensus* (39 Photos) |
Bacterial Consensus* (40 Photos) |
| Central location | 37 | 34 |
| No plaque seen | 36 | 39 |
| Moderate haze | 21 | 17 |
| Well-delineated border | 19 | 20 |
| Hypopyon | 17 | 16 |
| Irregular/Feathery border† | 14 | 4 |
| Minimal haze | 11 | 17 |
| Pronounced haze | 7 | 6 |
| Stromal necrosis | 7 | 6 |
| Nonspecific infiltrate border | 6 | 6 |
| Purulent stromal infiltrate | 6 | 7 |
| Multifocal infiltrate | 4 | 2 |
| Satellite lesions | 4 | 2 |
| Endothelial plaque | 3 | 1 |
| Ring infiltrate | 2 | 1 |
| Dry appearance | 2 | 2 |
| Wreath infiltrate‡ | 1 | 3 |
| Pigmented infiltrate | 1 | 1 |
| Serpiginous infiltrate | 0 | 1 |
| Epithelial plaque§ | 0 | 0 |
| Stuck-on appearance | 0 | 0 |
| Intact epithelium | 0 | 1 |
| Hyphema | 0 | 0 |
Bolding indicates traits that were found to be statistically significant.
Presence defined as agreement in >50% of raters.
Favors fungal etiology, P = 0.002.
Favors bacterial etiology, P = 0.005.
Favors bacterial etiology, P = 0.02.
We computed the fraction of photos correctly identified as representing bacterial infections or fungal infections. We then calculated these separately for the Aravind and Proctor medical groups. We used permutation testing to assess (1) whether the raters performed better than chance, (2) whether the fraction correct was the same for bacterial and fungal ulcers, and (3) whether the two clinician groups had the same success rates. Confidence intervals (CIs) were computed using standard bootstrap percentile intervals.13 Inter-rater reliability was assessed using Fleiss' kappa statistic.14 We also examined which photographic features were associated with fungal etiology, using logistic regression based on the fraction of raters who reported the presence of each trait. All analyses were conducted using the statistical software R (www.R-project.org, Vienna, Austria). Approval from the Committee on Human Research at the University of California, San Francisco and Aravind was received. No patient-identifying information was used, and the study was performed using results already collected from existing studies.
Results
Seventy-nine of 80 photographs were included for analysis. We excluded one photograph from the fungal trial because it depicted a mixed bacterial and fungal infection. One of the clinicians from the Aravind group did not complete the survey and was excluded from the analysis regarding differentiation of Gram stain, genus, and species.
Of the 39 smear-proven fungal ulcers, 30 grew in culture and could be further identified. The most common causative organisms were Fusarium spp (n = 18, 45%) and Aspergillus spp (n = 10, 25%). Of the 40 bacterial ulcers, the most common causative organisms were Streptococcus pneumoniae (n = 20, 50%) and Pseudomonas aeruginosa (n = 10, 25%) (Table 1). As not all organisms can be speciated based on laboratory protocol, species data were included whenever available.
Table 1.
Distribution of Organisms
|
Bacteria |
N
= 40 |
| Streptococcus pneumoniae | 20 |
| Pseudomonas aeruginosa | 10 |
| Nocardia spp | 3 |
| Staphylococcus, coagulase negative | 2 |
| Streptococcus, viridans group | 2 |
| Bacillus spp | 1 |
| Mycobacterium spp | 1 |
| Corynebacterium xerosis | 1 |
| Fungi | N = 39 |
| Fusarium spp | 18 |
| Fungal—not classified | 9 |
| Aspergillus flavus | 6 |
| Aspergillus fumigatus | 3 |
| Bipolaris spp | 2 |
| Aspergillus terreus | 1 |
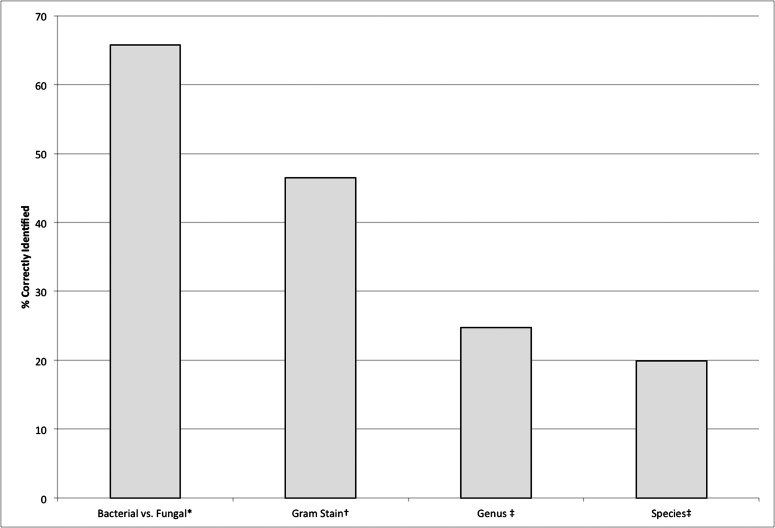
Clinicians were able to accurately distinguish bacterial from fungal etiology 66% (95% confidence interval, 63% to 68%) of the time, a result significantly better than chance alone (P < 0.001, permutation test) (Fig. 2). The clinicians from the Proctor were able to differentiate the etiologies in 63% (95% CI, 59% to 68%) of photographs, whereas the Aravind group was successful in 68% (95% CI, 64% to 73%) of cases. The two clinical groups (Aravind and Proctor) had a different success rate in distinguishing bacterial from fungal keratitis (P = 0.041, permutation test). Examining photographs of bacterial infections only, the probability of correct identification was 69% (95% CI, 62% to 76%), whereas for fungal infections, the probability of correct identification was 62% (95% CI, 55% to 69%).
Figure 2.
The aggregate results when looking at all clinicians and applicable photographs. * Based on all photographs, n = 79 photos. † Gram stain was examined for bacterial ulcers only, n = 40 photos. ‡ Based on photographs of both fungal and bacterial ulcers with available microbiologic data. For genus, n = 70 photos. For species, n = 44 photos.
Data were available regarding the Gram stain for all 40 bacterial ulcers, the genus for 40 bacterial ulcers and 30 fungal ulcers, and the species for 34 bacterial ulcers and 10 Aspergillus ulcers. Clinicians accurately predicted Gram stain results of the 40 bacterial ulcers 46% (95% CI, 40% to 53%) of the time, and were able to correctly identify the genus and species of these bacterial ulcers in 23% (95% CI, 17% to 30%) and 24% (95% CI, 16% to 31%) of cases, respectively (Fig. 2). When examining the 39 cases of fungal keratitis, the clinicians predicted genus in 27% of cases (95% CI, 21% to 33%) and species in 7.9% of cases (95% CI, 1.7% to 16.0%). When analyzing all available data for bacterial and fungal ulcers together, clinicians accurately predicted genus for 25% (95% CI, 20% to 29%) of 70 ulcers and species for 20% (95% CI, 15% to 25%) of 44 ulcers.
We analyzed the results of the aggregated bacterial and fungal ulcers for both the Proctor and Aravind groups. The Proctor group accurately predicted Gram stain results, genus, and species in 47% (95% CI, 37% to 56%), 22% (95% CI, 17% to 28%), and 15% (95% CI, 8.7% to 22%) of cases, respectively. The clinicians from the Aravind Eye Hospital identified Gram stain results, genus, and species accurately 46% (95% CI, 37% to 56%), 29% (95% CI, 22% to 35%), and 27% (95% CI, 17% to 36%) of the time, respectively.
We found no evidence that the two groups differed with respect to the ability to predict Gram stain results for bacterial ulcers (P > 0.99, permutation test) or genus for fungal ulcers (P = 0.31). The Aravind group was significantly better at predicting bacterial genus (P = 0.013), bacterial species (P = 0.0024), and Aspergillus species (P = 0.041).
The clinical signs most commonly used by clinicians to differentiate ulcers were infiltrate border appearance, surrounding stromal haze, and the presence (or absence) of hypopyon. The same clinical signs were listed as most helpful in the graders' clinical decision regardless of whether the photograph was correctly identified as bacterial or fungal.
The clinical signs noted most often were central location of the infiltrate, the absence of a plaque, well-delineated borders, and moderate surrounding stromal haze. The predictive abilities of clinical signs were analyzed with respect to the known etiology based on laboratory testing, not the clinical determination. The presence of irregular/feathery borders was strongly associated with fungal keratitis (P = 0.002, logistic regression) (Table 2). This clinical sign was present in 14 of 39 fungal photographs and 4 of 40 bacterial photographs. Although a wreath infiltrate was rarely seen, its presence was associated with bacterial keratitis (P = 0.005). The presence of an epithelial plaque was never agreed on by most raters as being present in any one photograph, yet it remained statistically associated with an underlying bacterial etiology (P = 0.02).
Discussion
In our study, although cornea specialists performed better than chance, they correctly differentiated bacterial from fungal corneal ulcers less than 70% of the time. As expected, more specific categorization led to less successful clinical distinction. Within bacterial ulcers and fungal ulcers, certain clinical signs are thought to be more common with specific organisms. For example, reports have suggested that diffuse corneal edema may occur with P. aeruginosa ulcers, whereas more localized edema is suggestive of S. pneumoniae, and that satellite lesions and irregular borders may be associated with filamentous fungi.6–8,15–18 In a study by Dahlgren et al.,19 clinicians completed a survey during a live examination of patients with suspected infectious keratitis. The examiners successfully distinguished among bacterial, fungal, and amebic keratitis in 73% of 74 culture-positive infections. The clinicians correctly identified 79% (41 of 52) of bacterial keratitis cases, but only 38% (5 of 13) of the cases of fungal keratitis. In addition, they found that the correct prediction of Acanthamoeba keratitis occurred significantly more often when a ring infiltrate was also present. The findings of this study support the notion that clinicians may do relatively well in predicting the underlying cause in infectious keratitis with more common organisms (i.e., bacterial rather than fungal), or with specific organisms when they demonstrate distinctive features.19 Unfortunately, clinicians in this study, as well as those in a study by Sun et al., were not accurate in cases of uncommon organisms or infections without the classic presentation.19,20 As geography influences the prevalence of bacterial and fungal keratitis, clinicians may not have equivalent clinical experience. For instance, the prevalence of fungal keratitis was found to be only 8% in a review of corneal ulcers at the Proctor Foundation, and is more prevalent in humid areas of the United States than in temperate climates.1,21 In addition, infectious keratitis is 10 times more common in India than the United States, with a much higher incidence of fungal infection.22,23
Despite the large list of clinical signs available, clinicians consistently ranked only a small subset as most important in predicting the underlying etiology. A difficulty in this study was establishing the true presence of a clinical sign in a clinical photograph. Because there was no objective measurement available, a trait was defined as present when more than 50% of clinicians were in agreement that it was depicted in the photograph. Certain clinical signs were found in most photographs, such as central location of the infiltrate, but were not found to be helpful in distinguishing bacterial and fungal organisms. The most robust statistical association was between irregular/feathery borders of the infiltrate and fungal keratitis. The presence of a wreath infiltrate was not commonly noted but, when present, was associated with bacterial keratitis.
The chief limitation of the project was that the photographic depiction of a corneal ulcer does not capture the entirety of a slit-lamp examination with a patient history. A three-dimensional and dynamic view, using a variety of illumination techniques, may be more informative. With regard to the information available to study participants, this study was designed to address the specific question of the usefulness of “classic” distinguishing clinical signs in making the distinction between bacterial and fungal corneal infection. Historical factors are often thought to be associated with bacterial versus fungal etiology, but no clinical history was provided in this survey.2–4,24 Different but equally important questions include the usefulness of clinical history alone in the prediction of a causative organism, and the interaction of history and clinical signs in making this prediction.
To decrease the possibility that graders based their clinical impressions on perceived prevalence (for example, that fungal ulcers may be less common), we selected an equal number of bacterial and fungal ulcers and disclosed this to the clinicians. Although randomly selected, the genus of the organisms did reflect the relative incidence of ulcers in southern India, as most ulcers enrolled in the clinical trials came from this region.25 We used kappa statistics during the analysis to address the potential bias of clinicians changing their answers based on their knowledge of the likely distribution of organisms.
Although the study has limitations, a photograph-based survey provides unique advantages. Many physicians can examine the same ulcer independently, in a masked manner, and without variability in examination technique.
Overall, this study suggests the importance of obtaining appropriate microbiological testing during the initial clinical encounter. Although experts were statistically better than chance at distinguishing bacterial from fungal ulcers, they were accurate only 60% to 70% of the time, and they were less successful in identifying Gram stain results, genus, and species. Because the treatment of fungal and bacterial keratitis is distinctly different, and individual organisms may be best treated by tailored therapy, appropriate microbiologic evaluation should be strongly considered during the initial clinical encounter.
Footnotes
Supported by Grant U10 EY015114 from the National Eye Institute (TML); That Man May See Foundation at the University of California, San Francisco; Grant K23 EY017897 from the National Eye Institute and a Research to Prevent Blindness Award (NRA); and core Grant EY02162 from the National Eye Institute. The sponsors did not have a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosure: C. Dalmon, None; T.C. Porco, None; T.M. Lietman, None; N.V. Prajna, None; L. Prajna, None; M.R. Das, None; J.A. Kumar, None; J. Mascarenhas, None; T.P. Margolis, None; J.P. Whitcher, None; B.H. Jeng, None; J.D. Keenan, None; M.F. Chan, None; S.D. McLeod, None; N.R. Acharya, None
References
- 1. Varaprasathan G, Miller K, Lietman T, et al. Trends in the etiology of infectious corneal ulcers at the F. I. Proctor Foundation. Cornea. 2004;23:360–364. [DOI] [PubMed] [Google Scholar]
- 2. Norina TJ, Raihan S, Bakiah S, Ezanee M, Liza-Sharmini AT. Wan Hazzabah WH. Microbial keratitis: aetiological diagnosis and clinical features of patients admitted to Hospital Universiti Sains Malaysia. Singapore Med J. 2008;49:67–71. [PubMed] [Google Scholar]
- 3. Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br J Ophthalmol. 2009;93:1319–1324. [DOI] [PubMed] [Google Scholar]
- 4. Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McLeod SD, Kolahdouz-Isfahani A, Rostamian K, Flowers CW, Lee PP, McDonnell PJ. The role of smears, cultures, and antibiotic sensitivity testing in the management of suspected infectious keratitis. Ophthalmology. 1996;103:23–28. [DOI] [PubMed] [Google Scholar]
- 6. Rodman RC, Spisak S, Sugar A, Meyer RF, Soong HK, Musch DC. The utility of culturing corneal ulcers in a tertiary referral center versus a general ophthalmology clinic. Ophthalmology. 1997;104:1897–1901. [DOI] [PubMed] [Google Scholar]
- 7. Benson WH, Lanier JD. Current diagnosis and treatment of corneal ulcers. Curr Opin Ophthalmol. 1998;9:45–49. [DOI] [PubMed] [Google Scholar]
- 8. McDonnell PJ. Empirical or culture-guided therapy for microbial keratitis? A plea for data. Arch Ophthalmol. 1996;114:84–87. [DOI] [PubMed] [Google Scholar]
- 9. Shah VM, Tandon R, Satpathy G, et al. Randomized clinical study for comparative evaluation of fourth-generation fluoroquinolones with the combination of fortified antibiotics in the treatment of bacterial corneal ulcers. Cornea. 2010;29:751–757. [DOI] [PubMed] [Google Scholar]
- 10. Qian Y, Meisler DM, Langston RH, Jeng BH. Clinical experience with Acanthamoeba keratitis at the Cole Eye Institute, 1999–2008. Cornea. 2010;29:1016–1021. [DOI] [PubMed] [Google Scholar]
- 11. Prajna NV, Mascarenhas J, Krishnan T, et al. Comparison of natamycin and voriconazole for the treatment of fungal keratitis. Arch Ophthalmol. 2010;128:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steroids for Corneal Ulcers Trial (SCUT). Identifier: NCT00324168. Bethesda, MD: National Institutes of Health; Available at: ClinicalTrials.gov. Accessed January 11, 2011. [Google Scholar]
- 13. Efron B. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall/CRC; 1993. [Google Scholar]
- 14. Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull. 1971;76:378–382. [Google Scholar]
- 15. Hyndiuk RA, Snyder RW. Bacterial keratitis. : Smolin G, Thoft RA. The Cornea. Scientific Foundations and Clinical Practice. 2nd ed. Boston/Toronto: Little, Brown and Company; 1987: 193– 204. [Google Scholar]
- 16. Forster RK. Fungal diseases. : Smolin G, Thoft RA. The Cornea. Scientific Foundations and Clinical Practice. 2nd ed. Boston/Toronto: Little, Brown and Company; 1987: 228– 233. [Google Scholar]
- 17. Srinivasan M, Mascarenhas J, Prashanth CN. Distinguishing infective versus noninfective keratitis. Indian J Ophthalmol. 2008;56:203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thomas PA, Leck AK, Myatt M. Characteristic clinical features as an aid to the diagnosis of suppurative keratitis caused by filamentous fungi. Br J Ophthalmol. 2005;89:1554–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dahlgren MA, Lingappan A, Wilhelmus K. The clinical diagnosis of microbial keratitis. Am J Ophthalmol. 2007;143:940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun RL, Jones DB, Wilhelmus KR. Clinical characteristics and outcome of Candida keratitis. Am J Ophthalmol. 2007;143:1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanure MA, Cohen EJ, Sudesh S, Rapuano CJ, Laibson PR. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea. 2000;19:307–312. [DOI] [PubMed] [Google Scholar]
- 22. Ou JI, Acharya NR. Epidemiology and treatment of fungal corneal ulcers. Int Ophthalmol Clin. 2007;47:7–16. [DOI] [PubMed] [Google Scholar]
- 23. Gonzales CA, Srinivasan M, Whitcher JP, Smolin G. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiol. 1996;3:159–166. [DOI] [PubMed] [Google Scholar]
- 24. Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis: predisposing factors and morbidity. Ophthalmology. 2006;113:109–116. [DOI] [PubMed] [Google Scholar]
- 25. Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81:965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]