Abstract
Annual MRI screening is recommended as an adjunct to mammography for BRCA1 and BRCA2 mutation carriers. Prophylactic oophorectomy has been shown to decrease breast cancer risk in BRCA1/2 mutation carriers. Here, we aimed to examine the combined effects of MRI and oophorectomy. For this purpose, 93 BRCA1/2 mutation carriers were screened with yearly mammograms and yearly MRI scans. Study endpoints were defined as date of breast cancer diagnosis, date of prophylactic mastectomy, or date of most recent contact. Of 93 women, with a median age of 47, 80 (86%) had prophylactic oophorectomy. Fifty-one women (55%) had BRCA1 mutations. A total of 283 MRI scans were performed. Eleven breast cancers (9 invasive, 2 ductal carcinoma in situ) were detected in 93 women (12%) with a median follow-up of 3.2 years (incidence 40 per 1,000 person-years). Six cancers were first detected on MRI, three were first detected by mammogram, and two were “interval cancers.” All breast cancers occurred in BRCA1 mutation carriers (incidence 67 per 1,000 person-years). Apart from BRCA1 vs. BRCA2 mutation status, there were no other significant predictors of breast cancer incidence. Most invasive breast cancers were estrogen receptor negative (7 of 9) and lymph node negative (7 of 9). There have been no systemic recurrences with a median follow-up of 19 months after cancer diagnosis. Finally, it was concluded that all breast cancers occurred in BRCA1 mutation carriers, in most cases despite oophorectomy. These data suggest that surveillance and prevention strategies may have different outcomes in BRCA1 and BRCA2 mutation carriers.
Keywords: BRCA1, BRCA2, Oophorectomy, MRI
Background
Women with BRCA1 and BRCA2 mutations are at greatly elevated risk of developing breast cancer and ovarian cancer, with lifetime risks of 40–80% and 10–45%, respectively [1, 2]. In addition, mutation carriers are at significantly increased risk of developing second primary breast cancers, with a lifetime risk estimated at 50% [3]. Management of these high-risk women includes prophylactic surgery, chemoprevention, and enhanced surveillance to aid in early detection [4, 5]. Bilateral prophylactic oophorectomy (BPO) is one of the most effective risk reduction strategies available for BRCA1 and BRCA2 mutation carriers. BPO reduces the risk of ovarian cancer by more than 85% [6–8], the risk of breast cancer by approximately 50% [6, 7, 9, 10], and may also decrease mortality [11]. Given the substantial impact of oophorectomy, women with BRCA1 and BRCA2 mutations are counseled to undergo oophorectomy once child bearing is complete and generally prior to age 40.
BRCA1 and BRCA2 mutations are associated with distinct breast cancer phenotypes; BRCA1-associated breast cancers are predominantly estrogen receptor (ER) negative, whereas the majority of BRCA2-associated breast cancers are ER positive [12]. Despite this, breast cancer risk reduction following BPO has been observed in studies examining BRCA1 carriers only [9, 13], as well as in studies including both groups together. However, more recently, a large prospective study demonstrated a significantly protective effect of BPO on breast cancer in BRCA2 carriers (HR = 0.28; CI 0.08–0.92; P = 0.036), while for BRCA1 carriers, such an effect was not statistically significant, with a hazard ratio of 0.61 (CI 0.30–1.22) [14]. Further studies with larger cohorts and longer follow-up are ongoing regarding this important issue.
The addition of annual MRI to annual mammography has become the standard of care for the early detection of breast cancer in BRCA1 and BRCA2 carriers, due to its significantly improved sensitivity [15, 16]. However, most studies of MRI surveillance of BRCA1/2 carriers either do not report [17–20] or have low rates [20, 21] of BPO. In addition, most published studies examining the breast cancer risk reduction conferred by BPO do not specify or did not control for surveillance practices [6, 7, 9, 11, 13, 22]. Here, we examined the combined effects of oophorectomy and intensive surveillance (including breast MRI) in a prospective cohort of BRCA1 and BRCA2 mutation carriers.
Methods
Study participants
Participants enrolled on University of Pennsylvania Protocol 708206, which was an IRB-approved protocol for MRI screening. For this analysis, we included all patients recruited onto protocol 708206 examining MRI screening, and only those patients. We did not include high-risk women who were involved in other studies in the Cancer Risk Evaluation Program if they were not enrolled on this specific Protocol. Women were recruited from the Cancer Risk Evaluation Programs at the University of Pennsylvania, Pennsylvania Hospital, or affiliated sites and provided written informed consent. All women had undergone genetic counseling and testing prior to enrollment on this study.
Eligibility criteria included the following: (1) women over the age of 25 and (2) known deleterious mutation in BRCA1 or BRCA2, or prior probability of a mutation of >75%. Those patients who were pregnant, had a contra-indication to MRI examination (presence of a pacemaker, magnetic aneurysm clip or other implanted magnetic device, severe claustrophobia), or had bilateral mastectomies were excluded. Women with unresolved actionable (recommended for biopsy or follow-up) clinical or mammogram findings prior to study entry were excluded until those findings were resolved. Patients with new or recurrent ovarian cancer within 4 years prior to recruitment were excluded. Patients were required to be at least 3 months from any breast biopsies, completion of lactation, radiation treatments, and chemotherapy treatments. Women with prior breast cancer were eligible for the study as long as all other entry criteria were met.
Study procedures
Upon study entry, a comprehensive medical history was obtained which included information on each patient's menopausal status, breast health, hormonal treatment history, and prior screening. In addition, patients' demographic information and genetic testing results were obtained, and baseline physical examinations were performed. At entry, women underwent mammogram and bilateral breast MRI within 3 months. Breast MRI with and without gadolinium was performed. The study paid for MRI between 2003 and 2005. Yearly exams were performed by a medical oncologist (SMD, RK). Beginning in January 2006, women were counseled that they should continue to obtain yearly MRI with coverage through their health insurance plan. At this time, MRI and mammogram were staggered at approximately 6 month intervals.
Follow-up
All study participants were followed from date of consent until individual study endpoints. Study endpoints were: breast cancer diagnosis, prophylactic mastectomy, or date of most recent disease-free patient contact through May 7, 2008.
Data collection
Demographic data was collected at baseline including age, type of BRCA1 or BRCA2 mutation, prior breast cancer history, oral contraceptive use, hormone replacement therapy use, and parity. Data collected during the study included the BIRADS classification for each mammogram and MRI, follow-up recommendations, the number of biopsies performed, and the characteristics of breast cancer which were diagnosed. Women with BIRADS 1 or 2 category studies underwent routine follow-up. Those with BIRADS 3 category studies underwent a 6 month follow-up with that modality (either mammogram or MRI). Those with BIRADS 4 or 5 category lesions were given a recommendation for biopsy. The study protocol is summarized in Fig. 1.
Fig. 1.
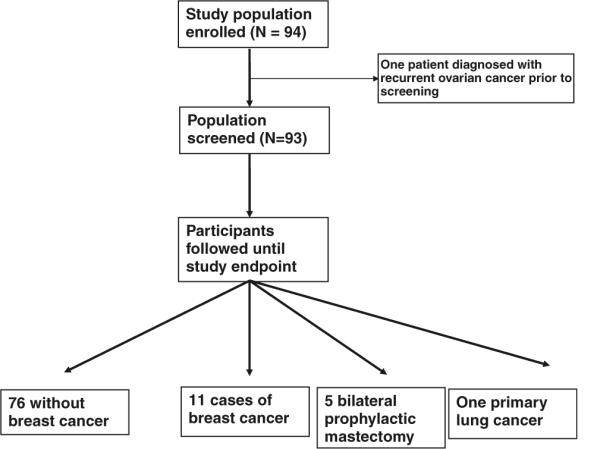
Study design
Statistical analysis
Data were analyzed using STATA v. 9.0 statistical software. Characteristics of those diagnosed with breast cancer were compared to those not diagnosed with breast cancer using chi-square tests for categorical variables and t-tests for continuous variables. Log rank tests comparing median length of disease-free survival between women with different characteristics were used for confirmation. Because there were no breast cancer cases in the BRCA2 mutation carriers, BRCA mutation status could not be included in multivariate regression models and other variables were not significant predictors of breast cancer incidence in univariate analyses. A P-value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
Between February 2003 and September 2005, 94 women between 28 and 72 years of age were consented for and enrolled in this study. One subject was diagnosed with recurrent ovarian cancer prior to her first scheduled MRI, and was therefore excluded. Ninety-three subjects with 171 intact breasts were followed. The median age of study participants at consent was 47. Eighty women (86%) had prophylactic oophorectomy (70% prior to study entry, 16% in follow-up). Fifty-one women (55%) had BRCA1 mutations; 41 (44%) had BRCA2 mutations; and 1 individual was not a known BRCA1/2 mutation carrier, however, her prior probability of mutation was 77% (she had a personal history of early onset breast cancer and family history of a breast/ovarian multiple primary). Forty women (43%) had a prior history of early-stage breast cancer. Participant characteristics, including oral contraceptive use, hormone replacement therapy use, and selective estrogen receptor modulator (SERM) use are summarized in Table 1.
Table 1.
Participant characteristics
| All (n = 93) | BRCA1 (n = 51) | BRCA2 (n = 41) | |
|---|---|---|---|
| Median age at study consent (years) | 47 | 44 | 48 |
| Oophorectomy | 80 (86.0%) | 45 (86.3%) | 35 (85.4%) |
| Median age at oophorectomy (years) | 45 | 42 | 47 |
| Median duration between oophorectomy and endpoint (years) | 4.0 | 4.0 | 4.3 |
| Personal history of breast CA prior to study entry | 40 (43.0%) | 22 (43.1%) | 17 (41.5%) |
| Hormonal contraception use | 70 (75.3%) | 41 (80.4%) | 28 (68.3%) |
| HRT use | 31 (33.3%) | 22 (43.1%) | 9 (22.0%) |
| SERM use | 43 (46.2%) | 17 (33.3%) | 25 (61.0%) |
The median follow-up from study entry was 3.2 years. During this time, 283 study MRIs and 282 study mammograms were performed. Eleven breast cancers were diagnosed in 11 participants. Five patients underwent prophylactic mastectomy and were censored at the time of their surgery. One participant developed and died of primary lung cancer. The remaining 76 patients were followed to most recent disease-free contact through May 7, 2008.
Characteristics of affected participants
Eleven breast cancers were detected in 93 women (12%). All cancers were diagnosed in BRCA1 mutation carriers, with a median age at diagnosis of 47 years (mean of 49, range 35–62 years). Nine of 11 patients were status post oophorectomy at the time of diagnosis with a median age at oophorectomy of 42. The median time from oophorectomy to cancer diagnosis was 3.6 years (range, 0.1–8.1 years). Five of the nine women diagnosed with breast cancer while on study had a personal history of breast cancer prior to study entry. Ten affected patients (91%) ever used hormonal contraception, five (45%) ever used hormone replacement therapy, and six (55%) used either tamoxifen or raloxifene prior to study endpoint. Only mutation status significantly differed between affected and unaffected patients, when comparing either the incidence of breast cancer at the end of follow-up (P-value 0.001) or the median disease-free survival (P-value 0.002) (Table 2). Similarly, there were no significant differences in the characteristics of BRCA1 mutation carriers who did, and did not, develop breast cancer.
Table 2.
Univariate analysis
| All patients |
BRCA1 mutation carriers |
|||||
|---|---|---|---|---|---|---|
| No breast cancer (n = 82) | Breast cancer (n = 11) | P-value | No breast cancer (n = 40) | Breast cancer (n = 11) | P-value | |
| BRCA1 | 40 (49.4%) | 11 (100.0%) | 0.002 | - | ||
| BRCA2 | 41 (50.6%) | - | ||||
| Oophorectomy | 71 (86.6%) | 9 (81.8%) | 0.754 | 36 (90.0%) | 9 (81.8%) | 0.884 |
| Oophorectomy ≤40 years | 22 (26.8%) | 3 (27.2%) | 0.777 | 15 (37.5%) | 3 (27.2%) | 0.531 |
| Personal history of breast cancer prior to study | 32 (39.0%) | 5 (45.5%) | 0.114 | 14 (35.0%) | 5 (45.5%) | 0.066 |
| Hormonal contraception use (ever) | 60 (73.2%) | 10 (90.9%) | 0.223 | 31 (77.5%) | 10 (90.9%) | 0.537 |
| HRT use (ever) | 26 (31.7%) | 5 (45.4%) | 0.338 | 17 (42.5%) | 5 (45.4%) | 0.894 |
| Post-ooph | 20 (76.9%) | 3 (60.0%) | 0.337 | 14 (35.0%) | 3 (60.0%) | 0.207 |
| SERM use (ever) | 37 (45.1%) | 6 (54.5%) | 0.990 | 11 (27.5%) | 6 (54.5%) | 0.364 |
Tumor characteristics
Characteristics of the breast cancers are detailed in Table 3. Nine cancers were invasive ductal; two were ductal carcinoma in situ (DCIS). The majority of invasive cancers was lymph node negative (7 of 9, 78%) and measured 1 cm or less (5 of 9, 56%). Seven of the nine invasive cancers were ER, progesterone receptor (PR), and her-2/neu (HER2) negative (78%). Two were ER positive and HER2 negative, although one ER-positive invasive cancer had low-level (10%) expression and was PR negative. Six of nine (67%) patients with invasive cancer received chemotherapy. One patient (#3 in Table 3) with a 3.0 cm tumor by physical exam and MRI had a complete pathologic response following preoperative chemotherapy with dose-dense adriamycin/cyclophosphamide followed by paclitaxel. Another patient (#5 in Table 3) with a 5.0 cm ipsilateral breast recurrence in the axillary tail by MRI received perioperative docetaxel with a clinical and pathological partial response. All 11 patients are alive and without recurrence, with a median 1.6 years of follow-up as of May 7, 2008 (Table 3).
Table 3.
Breast cancer characteristics
| Age at Dx (years) | Gene | Cancer type | Histology | Size (cm) | Grade | Lymph nodes | ER | PR | Her2/neu | Modality | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | B1 | P | Invasive | 0.9 | I | 0/2 | + | + | − | MRI |
| 2 | 40 | B1 | P | Invasive | 0.9 | III | 1/13 | − | − | − | MRI |
| 3 | 46 | B1 | P | Invasive | 3.0a | III | 0/26 | − | − | − | Interval |
| 4 | 55 | B1 | P | Invasive | 1.0 | II | 0/1 | − | − | − | MRI |
| 5 | 62 | B1 | Ipsi | Invasive | 5.0a | II | 2/5 | − | − | − | MRI |
| 6 | 35 | B1 | P | Invasive | 0.5 | II | 0/3 | − | − | − | MRI |
| 7 | 51 | B1 | Contra | Invasive | 0.5 | III | 0/3 | − | − | − | Mammo |
| 8 | 42 | B1 | Contra | Invasive | 1.3 | III | 0/2 | − | − | − | Mammo |
| 9 | 40 | B1 | P | Invasive | 1.9 | III | 0/3 | 10% | − | − | Interval |
| 10 | 47 | B1 | Ipsi | DCIS | 0.2 | III | 0/2 | + | + | N/a | Mammo |
| 11 | 61 | B1 | Ipsi | DCIS | 0.9 | III | − | − | N/a | MRI |
P primary, Ipsi ipsilatcral, Contra contralateral cancer
Tumor Size estimated by preoperative imaging characteristics
Of the 11 women who developed breast cancer during the study, 6 had no prior cancer history. Two women had prior contralateral breast cancer and developed a second primary cancer in the opposite breast. Three additional women had a prior breast cancer and developed breast cancer in the ipsilateral breast during the study. Two of these women developed DCIS (and therefore were considered to have developed a second primary cancer). The final patient (#5 in Table 3, also described above) developed an ipsilateral invasive breast cancer 5 years after her initial cancer in the same breast, which may have been an ipsilateral recurrence rather than a new primary.
Modes of cancer detection
Six of the 11 breast cancers were initially detected by MRI. Four of these patients then underwent mammograms (within 1 month), of which two were positive and two were falsely negative. Of the two cases of falsely negative mammography, one cancer was DCIS (Fig. 2a) and the other was invasive. Two patients did not have a concurrent mammogram and went directly to MRI-guided biopsy based on abnormal enhancement without a mass lesion. Three of the 11 cancers were initially detected by mammogram. Two of these cancers were detected on study entry screening; both were seen on MRI done within 1 month of abnormal mammogram and biopsy (BIRADS 6). Concurrent MRI was not performed in the third patient whose cancer was detected by mammography and was found to be DCIS. Both women who had breast cancer diagnosed at study entry had been undergoing routine yearly screening mammography before study enrollment.
Fig. 2.
a BIRADS 4 MRI (left) and BIRADS 2 mammogram (right) performed on the same day in a patient diagnosed with DCIS. b BIRADS 2 MRI (left) in a patient with an interval cancer; concurrent mammogram was read as BIRADS 1. Eight months later, the patient self-palpated a breast mass; diagnostic mammogram was read as BIRADS 4. MRI was also performed and demonstrated the lesion (right, BIRADS 6)
Two patients developed interval breast cancers despite intensive screening. One patient (#3 in Table 3), who had no prior history of breast cancer and was status post oophorectomy, had an abnormal breast self-exam 8 months after a BIRADS 2 MRI and a BIRADS 1 mammogram. At the time of palpable breast mass, the lesion was seen on mammogram (BIRADS 4). Following biopsy demonstrating a high-grade invasive ductal carcinoma, this lesion was also seen on MRI (BIRADS 6) (Fig. 2b). Based on imaging characteristics, the tumor size was estimated to be 3.0 cm, and as described above, she had a complete response to preoperative chemotherapy. A second woman (#9, Table 3) with no personal history of breast cancer and intact ovaries had an abnormal breast self-exam 4 months after a BIRADS 1 mammogram and 10 months after a BIRADS 1 MRI. A fine-needle aspiration demonstrated tumor, which was seen on MRI (BIRADS 6) done 1 week later. This patient had a 1.9-cm high-grade invasive ductal carcinoma with no nodal involvement and began treatment with docetaxel and cyclophosphamide.
Discussion
The present report represents an observational evaluation of several components of medical management in BRCA mutation carriers: primary prevention via prophylactic oophorectomy, prevention via SERMs, and intensive breast cancer surveillance. In the cohort of BRCA1 and BRCA2 mutation carriers studied, 86% of whom had oophorectomy, 46% of whom had used SERMs, and all of whom underwent screening with MRI, 11 (12%) of 93 study participants, and 11 of 51 (22%) of BRCA1 mutation carriers were diagnosed with breast cancer with a median 3.2 years of follow-up.
All breast cancers occurred in BRCA1 mutation carriers, despite the fact that our cohort included a significant proportion (44%) of BRCA2 mutation carriers. This finding suggests that there may be differential effects of oophorectomy on breast cancer risk in BRCA1 vs. BRCA2 mutation carriers and implies that the breast cancer risk reduction conferred by oophorectomy may be greater in BRCA2 than in BRCA1 mutation carriers during the first few years following surgery. This outcome is consistent with the findings of a recent multicenter, prospective study that examined 792 BRCA1 and BRCA2 mutation carriers with a median follow-up of 3.2 years [14]. In this study, oophorectomy was associated with a significant reduction in breast cancer risk in BRCA2 mutation carriers (HR = 0.28; 95% CI 0.08–0.92; P = 0.036). There was a suggested, but statistically nonsignificant risk reduction in BRCA1 mutation carriers (HR = 0.61; 95% CI 0.30–1.22; P = 0.16). In contrast, several studies have demonstrated a significant breast cancer risk reduction specifically in BRCA1 mutation carriers [9, 13, 23, 24]. In a small prospective study involving 98 BRCA1 mutation carriers, Kramer et al. observed breast cancer risk reduction associated with oophorectomy with a HR = 0.38 [13]. In a retrospective case–control study of over 3,000 mutation carriers, Eisen et al. observed breast cancer risk reduction associated with oophorectomy in BRCA1 (OR = 0.44; 95% CI 0.29–0.66) as well as BRCA2 mutation carriers (46% risk reduction, OR = 0.57; 95% CI 0.28–1.15). Limitations of this study are its case–control design and the potential for ascertainment bias [25].
An additional factor which may have contributed to absence of cancers in BRCA2 mutation carriers in this cohort is the high use of SERMs. Sixty-one percent of the BRCA2 mutation carriers in this study either used tamoxifen or raloxifene. It has been suggested that SERMS may be more effective in BRCA2 rather than BRCA1 mutation carriers, although data remain quite limited in the primary prevention setting [26]. Studies have demonstrated a reduction in contralateral breast cancer risk in both BRCA1 and BRCA2 mutation carriers with the use of tamoxifen, although the added benefit of tamoxifen following oophorectomy in this situation is uncertain [27, 28].
It has been well established that BRCA1 mutation-associated breast cancers are more likely to be hormone-receptor negative than non-BRCA1-associated breast cancers [12, 29, 30], while BRCA2 mutation-associated tumors are generally ER positive. Oophorectomy may decrease the risk of hormone receptor-positive breast cancers by primary prevention and also by treatment of subclinical cancers through estrogen deprivation, which may have contributed to the absence of ER positive, BRCA2-associated breast cancers found in our cohort. The mechanism by which oophorectomy contributes to breast cancer risk reduction in BRCA1 mutation carriers has not been well understood. It has been hypothesized that ovarian hormone ablation may inhibit BRCA1 mutation-associated, ER negative cancers at tumor-igenesis [31–33], thus the protective effect of oophorectomy on such cancers may require either a longer duration of follow-up or surgery at a younger age to become apparent. This hypothesis is consistent with data suggesting that oophorectomy is more protective when performed before age 40 [9, 13].
In this study, seven of nine invasive cancers were ER, PR, and HER2 negative (consistent with the basal pheno-type demonstrated in prior studies to be associated with BRCA1 mutations) [12, 34]. One additional tumor had low level of ER expression. The median duration between oophorectomy and study endpoint was relatively brief: 4 years for the entire cohort. The duration of follow-up may have been too short for the protective effect on BRCA1 mutation carriers to be observed. In addition, most study participants underwent oophorectomy later than age 40; median age at oophorectomy was 45 years. Thus, it is possible that oophorectomy was not performed early enough to have its optimal effect in these women. The other possibility is that, despite prior publications demonstrating otherwise, oophorectomy has a limited ability to decrease breast cancer risk in BRCA1 mutation carriers.
The rate of breast cancers in this study, 40 per 1,000 women years, is higher than in previously reported studies [18, 21] that have estimated rates of approximately 25 per 1,000. Our overall higher rate of new breast cancers could be due to the specifics of our study population such as the inclusion of women with prior breast cancer and the age of oophorectomy.
Evidence suggests that MRI allows for detection of cancer at earlier stages when cure is more likely [18, 21, 35] and appears to be cost effective, particularly for BRCA1 mutation carriers [36]. Here, the majority of women developed lymph node-negative tumors. However, two women developed interval cancers and 54% (6/11) women underwent chemotherapy. Although all affected participants are currently alive and without systemic recurrence, the morbidity associated with the diagnosis of cancer, including psychological distress and physical side effects of chemotherapy, must be considered as women choose between prophylactic mastectomy and nonsurgical screening and prevention strategies.
Despite the absence of breast cancer in BRCA2 mutation carriers in our study, it should not be assumed that MRI is not an important screening modality in these patients. Oophorectomy in BRCA2 mutation carriers may be particularly effective for short-term risk reduction. However, BRCA2 mutation carriers have an older age of onset of breast cancer compared to BRCA1 mutation carriers, with similar lifetime risk estimates [1, 2]. Annual MRI should continue to be a part of clinical management pending further studies, which ideally should involve large numbers of mutation carriers and be done prospectively.
This study has limitations. Our follow-up included a relatively small sample size (93) and events (11), with short follow-up (median 4.0 years after oophorectomy, and a median 3.2 years after consent to study surveillance procedures). The relatively short duration of follow-up limits conclusions about the longer-term effects of surveillance and oophorectomy in this population. In addition, not all study participants were screened on an annual basis, particularly after the study-funded MRI period ended. However, the deviations from surveillance schedules were largely due to insurance issues and patient compliance, issues commonly encountered in clinical practice, and thus our data are likely an accurate representation of the outcome of surveillance practices as clinically implemented.
This study provides confirmation that the current recommendation for annual breast MRI for carriers promotes increased early detection of breast cancers in this population, but interval cancers still occur. In addition, there appears to be a continued high risk of BRCA1-related breast cancer following oophorectomy in the short term. Larger prospective studies with longer follow-up periods, and randomized comparisons of various screening schedules, are necessary to further elucidate distinct screening needs in BRCA1 and BRCA2 mutation carriers who have undergone oophorectomy.
Acknowledgments
This research was supported by the Cancer Genetics Network (HHSN21620074400C to SMD), the Marjorie Cohen Foundation (to SMD), the QVC Network-Fashion Footwear Association of New York (SMD), and NIH P01-CA-82707 (MS).
Footnotes
This work was presented in part at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, 2008.
References
- 1.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King MC, Marks JH, Mandell JB, New York Breast Cancer Study Group Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 3.Pierce LJ, Strawderman M, Narod SA, Oliviotto I, Eisen A, Dawson L, et al. Effect of radiotherapy after breast-conserving treatment in women with breast cancer and germline BRCA1/2 mutations. J Clin Oncol. 2000;18(19):3360–3369. doi: 10.1200/JCO.2000.18.19.3360. [DOI] [PubMed] [Google Scholar]
- 4.Domchek SM, Weber BL. Clinical management of BRCA1 and BRCA2 mutation carriers. Oncogene. 2006;25(43):5825–5831. doi: 10.1038/sj.onc.1209881. [DOI] [PubMed] [Google Scholar]
- 5.Robson M, Offit K. Clinical practice. Management of an inherited predisposition to breast cancer [see comment] N Engl J Med. 2007;357(2):154–162. doi: 10.1056/NEJMcp071286. [DOI] [PubMed] [Google Scholar]
- 6.Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van't Veer L, Garber JE, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346(21):1616–1622. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 7.Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346(21):1609–1615. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 8.Finch A, Beiner M, Lubinski J, Lynch HT, Moller P, Rosen B, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 mutation. JAMA. 2006;296(2):185–192. doi: 10.1001/jama.296.2.185. [DOI] [PubMed] [Google Scholar]
- 9.Eisen A, Lubinski J, Klijn J, Moller P, Lynch HT, Offit K, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol. 2005;23(30):7491–7496. doi: 10.1200/JCO.2004.00.7138. [DOI] [PubMed] [Google Scholar]
- 10.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domchek SM, Friebel TM, Neuhausen SL, Wagner T, Evans G, Isaacs C, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol. 2006;7(3):223–229. doi: 10.1016/S1470-2045(06)70585-X. [DOI] [PubMed] [Google Scholar]
- 12.Lakhani SR, Reis-Filho JS, Fulford L, Penault-Llorca F, van der Vijver M, Parry S, et al. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res. 2005;11(14):5175–5180. doi: 10.1158/1078-0432.CCR-04-2424. [DOI] [PubMed] [Google Scholar]
- 13.Kramer JL, Velazquez IA, Chen BE, Rosenberg PS, Struewing JP, Greene MH. Prophylactic oophorectomy reduces breast cancer penetrance during prospective, long-term follow-up of BRCA1 mutation carriers. J Clin Oncol. 2005;23(34):8629–8635. doi: 10.1200/JCO.2005.02.9199. [DOI] [PubMed] [Google Scholar]
- 14.Kauff ND, Domchek SM, Friebel TM, Robson ME, Lee J, Garber JE, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med. 2008;148(9):671–679. doi: 10.7326/0003-4819-148-9-200805060-00007. [DOI] [PubMed] [Google Scholar]
- 16.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American cancer society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 17.Kuhl CK, Schmutzler RK, Leutner CC, Kempe A, Wardelmann E, Hocke A, et al. Breast MR imaging screening in 192 women proved or suspected to be carriers of a breast cancer susceptibility gene: preliminary results. Radiology. 2000;215(1):267–279. doi: 10.1148/radiology.215.1.r00ap01267. [DOI] [PubMed] [Google Scholar]
- 18.Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005;365(9473):1769–1778. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 19.Warner E, Plewes DB, Hill KA, Causer PA, Zubovits JT, Jong RA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292(11):1317–1325. doi: 10.1001/jama.292.11.1317. [DOI] [PubMed] [Google Scholar]
- 20.Lehman CD, Isaacs C, Schnall MD, Pisano ED, Ascher SM, Weatherall PT, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244(2):381–388. doi: 10.1148/radiol.2442060461. [DOI] [PubMed] [Google Scholar]
- 21.Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 22.Laki F, Kirova YM, This P, Plancher C, Asselain B, Sastre X, Stoppa-Lyonnet D, Salmon R, the IC-BOCRSG IC-BOCRSG: Institut Curie -Breast Ovary Cancer Risk Study Group Prophylactic salpingo-oophorectomy in a series of 89 women carrying a BRCA1 or a BRCA2 mutation. Cancer. 2007;109(9):1784–1790. doi: 10.1002/cncr.22603. [DOI] [PubMed] [Google Scholar]
- 23.Metcalfe K, Lynch HT, Ghadirian P, Tung N, Olivotto I, Warner E, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22(12):2328–2335. doi: 10.1200/JCO.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 24.Rebbeck TR, Levin AM, Eisen A, Snyder C, Watson P, Cannon-Albright L, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91(17):1475–1479. doi: 10.1093/jnci/91.17.1475. [DOI] [PubMed] [Google Scholar]
- 25.Klaren HM, van't Veer LJ, van Leeuwen FE, Rookus MA. Potential for bias in studies on efficacy of prophylactic surgery for BRCA1 and BRCA2 mutation [see comment] J Natl Cancer Inst. 2003;95(13):941–947. doi: 10.1093/jnci/95.13.941. [DOI] [PubMed] [Google Scholar]
- 26.King MC, Wieand S, Hale K, Lee M, Walsh T, Owens K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National surgical adjuvant breast and bowel project (NSABP-P1) breast cancer prevention trial. JAMA. 2001;286(18):2251–2256. doi: 10.1001/jama.286.18.2251. [DOI] [PubMed] [Google Scholar]
- 27.Narod SA, Brunet JS, Ghadirian P, Robson M, Heimdal K, Neuhausen SL, et al. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Hereditary breast cancer clinical study group. Lancet. 2000;356(9245):1876–1881. doi: 10.1016/s0140-6736(00)03258-x. [DOI] [PubMed] [Google Scholar]
- 28.Gronwald J, Tung N, Foulkes WD, Offit K, Gershoni R, Daly M, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118(9):2281–2284. doi: 10.1002/ijc.21536. [DOI] [PubMed] [Google Scholar]
- 29.Foulkes WD, Metcalfe K, Sun P, Hanna WM, Lynch HT, Ghadirian P, et al. Estrogen receptor status in BRCA1- and BRCA2-related breast cancer: the influence of age, grade, and histological type. Clin Cancer Res. 2004;10(6):2029–2034. doi: 10.1158/1078-0432.ccr-03-1061. [DOI] [PubMed] [Google Scholar]
- 30.Lakhani SR, Van De Vijver MJ, Jacquemier J, Anderson TJ, Osin PP, McGuffog L, et al. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol. 2002;20(9):2310–2318. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- 31.Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57(22):4987–4991. [PubMed] [Google Scholar]
- 32.Zeps N, Bentel JM, Papadimitriou JM, D'Antuono MF, Dawkins HJ. Estrogen receptor-negative epithelial cells in mouse mammary gland development and growth. Differentiation. 1998;62(5):221–226. doi: 10.1046/j.1432-0436.1998.6250221.x. [DOI] [PubMed] [Google Scholar]
- 33.Evers B, Jonkers J. Mouse models of BRCA1 and BRCA2 deficiency: past lessons, current understanding and future prospects. Oncogene. 2006;25(43):5885–5897. doi: 10.1038/sj.onc.1209871. [DOI] [PubMed] [Google Scholar]
- 34.Foulkes WD, Stefansson IM, Chappuis PO, Begin LR, Goffin JR, Wong N, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95(19):1482–1485. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 35.Tilanus-Linthorst MM, Bartels CC, Obdeijn AI, Oudkerk M. Earlier detection of breast cancer by surveillance of women at familial risk. Eur J Cancer. 2000;36(4):514–519. doi: 10.1016/s0959-8049(99)00337-8. [DOI] [PubMed] [Google Scholar]
- 36.Plevritis SK, Kurian AW, Sigal BM, Daniel BL, Ikeda DM, Stockdale FE, et al. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA. 2006;295(20):2374–2384. doi: 10.1001/jama.295.20.2374. [DOI] [PubMed] [Google Scholar]



