Abstract
‘Endothelial cells lining vascular luminal surface represent an important site of signaling and injurious effects of reactive oxygen species (ROS) produced by other cells and endothelium itself in ischemia, inflammation and other pathological conditions. Targeted delivery of ROS modulating enzymes conjugated with antibodies to endothelial surface molecules (vascular immunotargeting) provides site-specific interventions in the endothelial ROS, unattainable by other formulations including PEG-modified enzymes. Targeting of ROS generating enzymes (e.g., glucose oxidase) provides ROS- and site-specific models of endothelial oxidative stress, whereas targeting of antioxidant enzymes SOD and catalase offers site-specific quenching of superoxide anion and H2O2. These targeted antioxidant interventions help to clarify specific role of endothelial ROS in vascular and pulmonary pathologies and provide basis for design of targeted therapeutics for treatment of these pathologies. In particular, antibody/catalase conjugates alleviate acute lung ischemia/reperfusion injury, whereas antibody/SOD conjugates inhibit ROS-mediated vasoconstriction and inflammatory endothelial signaling. Encapsulation in protease-resistant, ROS-permeable carriers targeted to endothelium prolongs protective effects of antioxidant enzymes, further diversifying the means for targeted modulation of endothelial ROS.
Keywords: Endothelial cells, Drug delivery, Vascular immunotargeting, Oxidative stress, Antioxidant enzymes
1. Introduction: vascular oxidative stress and antioxidant interventions in the endothelium
Abnormally high influx of reactive oxygen species (ROS) that exceeds normal cellular antioxidant capacity, collectively termed “oxidative stress”, causes many pathological processes including inflammation, cellular dysfunction and tissue damage. Endothelial cell monolayer lining the vascular lumen controls vital integral functions (transport between organs, vascular permeability and tone, blood fluidity, host defense, angiogenesis and carcinogenesis) and represents arguably one of the most sensitive and important targets for oxidative stress [1,2]. Excessive ROS cause pathological activation of endothelium including exposure of cell adhesion molecules (such as ICAM-1 and VCAM-1) and inhibitors of fibrinolysis [3–5], loss of transmembrane glycoprotein thrombomodulin that normally exerts anti-thrombotic and anti-inflammatory functions [6], disruption of the endothelial barrier and, in severe cases, cell death. These pathological changes lead to and propagate thrombosis, edema, inflammation, ischemia, abnormal vascular growth and functions. Further, ROS superoxide anion quenches NO• produced by endothelium, thereby aggravating vasoconstriction and thrombosis (Fig. 1). Endothelial disorders and injury caused by ROS are implicated in ischemia, inflammation, stroke, acute lung injury, myocardial infarction, atherosclerosis, hypertension and diabetes, among other maladies [7,8].
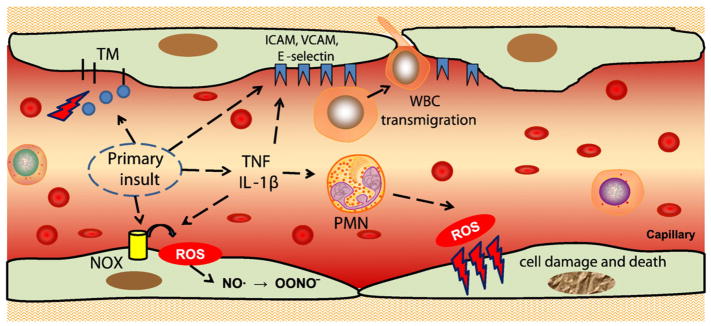
Fig. 1.
Vascular oxidative stress. Pro-inflammatory insults cause endothelial exposure of cell adhesion molecules (selectins, ICAM or VCAM) and cytokine production. Cell adhesion molecules facilitate white blood cell (WBC) adhesion and transmigration. Activation of Nox (for example by angiotensin II) leads to generation of superoxide that quenches NO and thus causes vasoconstriction. Activated WBCs bind to endothelium via cell adhesion molecules and produce reactive oxygen species (ROS) and other aggressive molecules that can result in oxidative damage and death of endothelial cells. ICAM, intercellular adhesion molecule; Nox, NADPH oxidase; PMN, polymorphonuclear neutrophils; TM, thrombomodulin; ICAM, intercellular cell adhesion molecule; VCAM, vascular cell adhesion molecule; TNF, tumor necrosis factor, IL, interleukin.
Therefore, design of effective and safe means for specific interventions in endothelial ROS, produced by abnormally activated endothelial cells or released by leukocytes, represents an important biomedical problem [9]. In acute settings, such interventions can be achieved by administration of antioxidant therapeutics. In theory, enzymatic antioxidants can provide highly specific and effective detoxification of endothelial ROS, on the condition that the formulations are properly delivered to the target cells. This specific aspect of vascular drug delivery and targeting attracts a considerable attention for several decades and has been reviewed in this journal ten years ago [10]. This article offers an updated analysis of the problem, focused on recent achievements in targeted delivery of antioxidant enzymes to endothelial cells.
2. Reactive oxygen species (ROS), antioxidant defense and spatiotemporal requirements for antioxidant interventions
Resident and migrant cells in the vasculature including macrophages, white blood cells, smooth muscle cells and endothelial cells produce the ROS superoxide anion O2•− from oxygen using enzymes including mitochondrial respiratory chain [11], xanthine oxidase [8] and NADPH oxidase [12]. O2•− forms a strong oxidant, peroxinitrate (ONOO−), in a fast reaction with NO•, thereby inactivating this vasodilatory and anti-thrombotic mediator, or spontaneously transforms into H2O2. By accelerating the latter transformation, a family of enzymes superoxide dismutase (SOD) including mitochondrial MnSOD (86–88 kD), cytosolic CuZnSOD (32 kD) and extracellular SOD (135 kD) preserves NO• and blocks ONOO− formation [13]. Freely diffusible H2O2 is more stable than O2•−, yet, in reactions with transition metals, myeloperoxidase, superoxide and NO• it forms strong oxidants including •OH radical and HOCl [14]. A highly potent enzyme catalase consisting of four identical 60 kD subunits localized predominantly in the cytosol and specific vacuoles (peroxisomes) decomposes H2O2 into water. However, when ROS detoxification and repair of oxidized biomolecules are insufficient, cellular and tissue abnormality ensues, leading to pathology (Fig. 2).
Fig. 2.
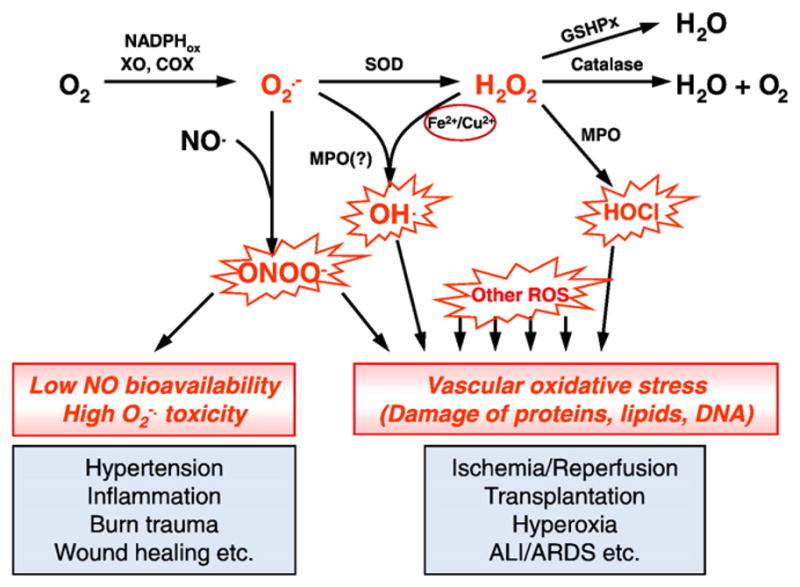
Reactive oxygen species pathways, antioxidant enzymes and their role in vascular oxidative stress. Superoxide is produced by several cellular enzyme systems including NADPH-oxidases, xanthine oxidase, etc. It can react with NO producing aggressive peroxynitrite anion ONOO− and decreasing NO pool. Superoxide spontaneously or by action of superoxide dismutase may be reduced into hydrogen peroxide H2O2. Hydrogen peroxide can produce extremely reactive hydrogen radical •OH in the presence of transition metals or hypochlorous acid by myeloperoxidase. Catalase and glutathione peroxidases protect cells against hydrogen peroxide. ALI/ARDS, acute lung injury/acute respiratory distress syndrome; COX, cyclooxygenase; GSHPx, glutathione peroxidases; MPO, myeloperoxidase; ROS, reactive oxygen species; SOD, superoxide dismutase; XO, xanthine oxidase.
Direct effects of ROS, especially labile and poorly diffusible O2•− are compartmentalized within nanometers of generation site. Therefore, interventions in ROS mediated processes should be precisely controlled spatiotemporally, ideally at subcellular level. For example, unguided O2•− dismutation may be inefficient or even aggravate the injury if local H2O2 reduction is not sufficient [15,16]. These considerations emphasize the need for site-specific delivery of antioxidants. For example, treatment of oxidative stress caused by excessive ROS flux in mitochondria may be optimally served by mitochondrial interventions [17]. Membrane-permeable and mitochondria-directed SOD quenchers and mimetic consuming reducing cofactors to catalyze O2•− dismutation show promising protective effects in cell culture and animal models [18,19]. Mutant SOD2/3 chimera binding to cell surface via negatively charged glycocalyx and cell transfection by SOD confer protective antioxidant and anti-inflammatory effects [20–24].
However, these antioxidant interventions including delivery using membrane permeating peptides [25], provide no endothelial targeting and act in diverse tissue and cellular compartments [26]. Such spatially promiscuous effects may be beneficial in treatment generalized forms of oxidative stress and inflammation, such as sepsis and radiation injury, but insufficiently targeted for site-specific endothelial interventions. As a result, our understanding of where antioxidant interventions are required, which specific ROS need to be decomposed and means to achieve this goal are acutely incomplete, in part due to insufficiently effective and precise targeted delivery of antioxidants [27,28]. Further, translation of many of these antioxidant delivery means into therapeutic domain is hindered by concerns of practicality, efficacy, specificity, safety and spatiotemporal control of the interventions. For example, the feasibility, expedience, and safety of gene therapy are sub-optimal for use in most acute settings, whereas practicality of enzyme infusion is similarly limited for management of chronic oxidative stress. This article is focused on endothelial delivery of antioxidant enzymes for treatment of acute oxidative stress in the vasculature.
2.1. Endothelial delivery of antioxidants: requirements and challenges
Prolonged and prophylactic administration of non-enzymatic anti-oxidants including ROS scavengers (e.g., vitamin E), reducing agents (e.g., N-acetylcysteine) and antioxidant inducers (e.g., curcumin), may confer some extent of alleviation of subtle and modest forms of chronic oxidative stress, but provides no tangible benefits in acute conditions (Table 1) [29]. Antioxidant enzymes can potentially afford more potent and specific effects. For example, intratracheal delivery of catalase and SOD and their transgenic expression alleviated oxidative stress occurring in the airways of animals [30–35]. Unfortunately, the adequate delivery of enzymes to the endothelium, that is not readily accessible from the airways, has not yet being achieved [36].
Table 1.
Classes of antioxidants tested for therapeutic use.
| Class of antioxidants | Examples | Mechanism of action |
|---|---|---|
| Antioxidant enzymes | Superoxide dismutase | Catalytic dismutation of superoxide anion into O2 and H2O2 |
| Catalase | Disproportionation of H2O2 into O2 and H2O | |
| Flavonoids | Catechins, flavonols, flavons | Both transition metal chelators and ROS scavengers |
| Isothiocyanates | Sulforaphane, oltipraz | Phase II enzyme enhancers |
| Phenolic compounds | BHA, t-BHQ, curcumin | Enhancers of phase II enzymes and conjugating enzymes |
| Thiols | N-acetylcysteine, cysteine, dithiols, reduced glutathione | Intracellular redox buffer, metal chelators, radical quenchers |
| Vitamins | Tocopherol (vitamin E), ascorbic acid (vitamin C) | ROS scavengers |
| Others | Melatonin | ROS scavengers |
BHA, butylated hydroxyanisole; t-BHQ, t-butylhydroquinone 5; ROS, reactive oxygen species.
Endothelial delivery of antioxidant enzymes via the vascular route seems more suitable for this goal. Of note, the pulmonary vasculature represents about 20–25% of the total endothelial surface in the body, receives the entire venous blood ejected by the heart (i.e., 50% of the total cardiac blood output, whereas all other organs including pulmonary bronchial circulation share arterial blood) and thus represents the privileged vascular target [37,38]. Therefore, compounds with high affinity to endothelium injected in the bloodstream quickly accumulate in the lungs [39]. Local infusion of such compounds including antibodies and antibody fragments directed to endothelial antigens and natural ligands of endothelial receptors, in a conduit artery greatly enhances uptake in the downstream vasculature of an organ of interest, whereas pulmonary vasculature takes up the lion share of the leftovers entering the systemic circulation [40–42].
The liver and kidney eliminate naked catalase and SOD within minutes after IV injection [10]. Conjugation with polyethylene glycol, PEG [43], encapsulation in PEG-liposomes [44] or PEG-coated polymeric carriers [45–47], conjugation with PEG-based pluronics [48,49] and other modifications such as conjugation with compounds favoring binding to plasma albumin prolong the circulation time of catalase and SOD, thereby enhancing their potency in some forms of systemic oxidative stress in animals [50–55]. Due to enhanced aqueous solubility affording high doses in cell culture medium, PEG-enzymes enter intracellular vesicles via non-specific uptake of fluid phase after prolonged incubations in cell cultures [56]. However, efficacy of this pathway for intracellular delivery is limited in vivo due to lack of endothelial affinity [10,38]. In fact, inhibition of interactions with cells provided by PEG corona is one of the key features of this stealth technology. Tracing of radiolabeled PEG-catalase and PEG-SOD showed no better uptake by endothelial cells in culture and delivery to endothelium in vivo than achieved by naked enzymes [57].
Some SOD and catalase formulations bind to and enter cells due to hydrophobic or electrostatic interactions (e.g., enzymes coupled to cationic membrane-permeating peptides such as TAT) [52,53,58–60]. Further, constructs fusing cytosolic CuZnSOD with glycocalyx-binding peptides showed promising protective effects in animal models of inflammation [22]. However, endothelial targeting of these derivatives has yet to be proven in animal studies. They do not accumulate in the pulmonary vasculature and provide rather modest protective effects in animal models of acute endothelial oxidative stress [10,38]. Using carriers with affinity to endothelial surface molecules enables more effective and specific targeting of antioxidant enzymes [2,9].
2.2. Vascular immunotargeting to endothelial surface molecules
In order to achieve specific targeting of antioxidant enzymes to endothelial cells, we and other labs devised a “vascular immunotargeting” strategy that employs conjugation of cargoes with antibodies (or their fragments) that bind to specific endothelial surface epitopes [2,61–68]. Epitopes tested for this goal include constitutively expressed angiotensin-converting enzyme, ACE [39,61,69], amino-peptidase P [70], pan-endothelial Platelet-Endothelial Cell Adhesion Molecule-1 (PECAM) [2] and transferrin receptor [71] or Intercellular Adhesion Molecule-1 (ICAM-1) [72] (Table 2). ICAM-1 is constitutively exposed on endothelium in the vasculature and further up-regulated by inflammatory agents, abnormal blood flow and oxidative stress [73,74]. Molecules exposed exclusively on activated endothelium (e.g., E- and P-selectins and VCAM-1) represent attractive targets for delivery of drugs and imaging probes to pathological sites in the vasculature [75–79].
Table 2.
Targets for endothelial drug delivery.
| Endothelial target | MM | Functions | Vascular expression, EC density, internalization |
|---|---|---|---|
| PECAM-1 | 130 kDa | Component of intercellular junctions, interaction with cytoskeleton, ITIM-mediated inhibitory function, mechanoreceptor | Constitutively expressed by EC of all blood and lymphatic vessels at high density. Internalizable upon clustering. |
| ICAM-1 | 85–110 kDa | Leukocyte adhesion to endothelium | Ubiquitously expressed in vasculature, upregulated in activated EC. Internalizable upon clustering. |
| VCAM-1 | 110 kDa | Monocyte adhesion to endothelium | Expressed at a low level, upregulated on sites of inflammation |
| E-selectin | 150 kDa | Leukocyte rolling and adhesion | Expressed at a low level, upregulated on sites of inflammation |
| P-selectin | 146–160 kDa | Leukocyte rolling and adhesion | Constitutively expressed by EC, localized in Weibel-Palade bodies, released upon cell activation |
| ACE | 150–180 kDa | Peptidase, converts AngI into AngII, cleaves bradykinin | Constitutively expressed. Enriched in lung endothelium |
| APP-2 | Trimer of 75 kDa | Membrane-bound metalloprotease, plays a role in inflammation, metabolizes bradykinin | Enriched in lung endothelium. Transcytosed along with caveolae. |
| TfR | 180 kDa | Cellular uptake of iron occurs via receptor-mediated endocytosis of ligand-occupied transferrin receptor into specialized endosomes | Ubiquitously distributed. Internalized by receptor-mediated endocytosis. |
| TM | 105 kDa | EC receptor, forms a complex with thrombin, which activate protein C producing anticoagulant APC | Constitutively expressed. Enriched in lung endothelium |
ACE, angiotensin-converting enzyme; APP-2, aminopeptidase 2; EC, endothelial cell; ICAM, intercellular adhesion molecule; MM, molecular mass; PECAM, platelet-endothelial cell adhesion molecule; TfR, transferrin receptor; TM, thrombomodulin; VCAM, vascular cell adhesion molecule.
Binding of antibodies and carriers carrying these antibodies may activate or inhibit target molecules, for example via their cross-linking, blocking or induced disappearance from the plasmalemma (shedding or internalization) [80–82]. This may lead to either beneficial or adverse side effects in the context of the therapeutic intervention. For example, antibodies to the constitutive endothelial protein thrombomodulin (TM) accumulate in the pulmonary vasculature [68,83], but cannot be used for therapies, since TM inhibition leads to thrombosis and inflammation [84]. However, targeting of H2O2-generating enzyme glucose oxidase conjugated with anti-TM (anti-TM/GOX) provides useful animal models of acute oxidative stress in the pulmonary vasculature described in the next section [6,85–87].
Inhibition of ACE leading to reduction of the level of AngII, a potent vasoconstricting, pro-inflammatory and pro-oxidant mediator activating ROS production in endothelium, may provide beneficial effects in the context of treatment conditions associated with hypertension, ischemia, inflammation and oxidative stress [88]. On the other hand, inhibition of bradykinin metabolizing enzymes ACE and aminopeptidase P leads to elevated levels of bradykinin, which may cause hypotension, enhanced vascular permeability and edema [89,90]. Thus, ACE inhibitory antibodies and ACE-targeted conjugates can be important ways to modulate ACE activity in lab animals and pilot human studies [91]. Cell adhesion molecules ICAM and PECAM are transmembrane glycoproteins involved in WBC adhesion and transmigration, cellular recognition and signaling [92,93]. Pulmonary accumulation of WBC is generally viewed as a pro-inflammatory process implicated in pathogenesis of ALI, hyperoxia, ischemia and other diseases [94–96]. Cell adhesion blockade inhibits WBC transmigration and inflammation [97,98]. Thus, drug targeting to ICAM and PECAM may suppress inflammation. Studies in diverse animal species revealed no harmful effects of drug targeting directed to ICAM-1 [99–101] and PECAM-1 [38,81,85,102–106].
Endothelium constitutively stably expresses approximately 1–3×105 copies of ACE and ICAM-1 and 0.5–1.5×106 copies of PECAM-1, respectively, on the surface of one cell [39,99,102]. These endothelial determinants are among the most extensively studied as anchors for vascular immunotargeting and drug delivery [9,38,107,108]. Endothelial cells internalize ACE antibodies, likely via the clathrin-related endocytosis [82], and multivalent conjugates carrying multiple copies of ICAM and PECAM antibodies [42,102,109], via an unusual endocytic pathway, CAM-mediated endocytosis distinct from phagocytosis, caveolar and clathrin endocytosis and remotely resembling macropinocytosis, in some aspects [110]. Of note, endothelial cells internalize relatively large conjugates and nanocarriers directed to ICAM and PECAM, with maximal dimension of several microns [111].
Enzymes, genetic materials, nanocarriers, liposomes and other cargoes and carriers conjugated or fused with antibodies to ACE, ICAM and PECAM (anti-ACE, anti-ICAM and anti-PECAM) bind to endothelial cells in cultures and, more importantly, in the vasculature in intact animals [38,39,99,104,112]. As a result, drugs, enzymes, DNA, viruses, liposomes and diverse nanocarriers conjugated with anti-ACE, anti-ICAM and anti-PECAM accumulate in the lungs and other highly vascularized organs after intravascular injection, providing drug delivery to the endothelium [38,81,85,102–106,112–114]. Most studies of vascular immunotargeting of enzymes controlling ROS level in the endothelial cells employed antibodies to ACE, ICAM and PECAM [46,108].
2.2.1. Vascular immunotargeting of ROS generating enzymes: modeling of endothelial oxidative stress
Studies of endothelial oxidative stress and testing of antioxidant interventions require well controlled models of elevated ROS influx in this cell type. Treatment of cells in culture with ROS or ROS generating enzymes offers a simple and straightforward approach. For example, exposure to glucose oxidase/glucose system generating H2O2 or to xanthine oxidase/xanthine system generating both superoxide and H2O2 can be employed in model studies [86,115]. Further, abnormally high and low levels of oxygen and chemicals including quinones and paraquat cause intracellular ROS production in endothelial mitochondria [6]. Inflammatory agonists including AngII, VEGF and cytokines, as well as abrupt changes in perfusion rate, cause endothelial ROS production via enzymatic systems including transmembrane NADPH oxidase [1,116].
Of course, these agents cause very complex changes in animal studies, not limited to ROS influx in endothelial cells. Vascular immunotargeting of ROS generating enzymes helps to achieve this specific effect, useful for modeling and delineating the role of endothelial oxidative stress and testing means for its specific treatment. Thus, glucose oxidase (GOX) conjugated with anti-ACE, anti-PECAM and anti-TM binds to and enters endothelial cells, causing acute oxidative stress [87,117,118]. Further, these GOX conjugates accumulate in the pulmonary endothelium after intravenous injection and cause acute vascular oxidative stress manifested by dose-dependent pulmonary edema, thrombosis, inflammation, accumulation of oxidized molecules and, in most severe cases, mortality [6,86,115,118]. Specific manifestations of vascular oxidative stress in these animal models vary depending on endothelial surface molecule or even epitope selected for GOX targeting. For example, anti-TM/GOX causes more severe, heavily thrombotic pulmonary injury than anti-PECAM/GOX, likely due to thrombomodulin inhibition unleashing thrombin [87]. Anti-TM/GOX conjugates with higher avidity to endothelium provide more potent injury, whereas hyperoxia further augment oxidative stress caused by anti-TM/GOX by providing enhanced supply of the rate-limiting GOX substrate, oxygen [6]. Therefore, it is possible to fine-tune the extent and mechanism of acute vascular oxidative stress in models using antibody/GOX conjugates, by selecting optimal endothelial epitopes, conjugate avidity, dose and oxygen supply. Of interest, a model of acute pulmonary oxidative stress caused by GOX targeting to endothelium in the presence of elevated oxygen level imitates clinical settings of acute lung injury in the patients on mechanical ventilation.
These features of endothelial targeting of ROS generating enzymes lend themselves to use for modeling specific endothelial components of acute endothelial oxidative stress in animals, which may have mechanistic value. In theory, this strategy might also prove useful for targeted eradication of certain types of endothelial cells such as tumor endothelium. However, animal models of anti-TM/GOX induced vascular oxidative stress found their primary application in testing protective effects of endothelial targeting of antioxidant enzymes, catalase and SOD.
2.2.2. Endothelial delivery and protective effects of antibody conjugated catalase and SOD
SOD and catalase have been conjugated with anti-ACE [69], anti-ICAM [72] and anti-PECAM [119] using diverse cross-linking chemistries including streptavidin–biotin [102] and SATA-SMCC [120]. These supramolecular conjugates (which will be called hereafter collectively Ab/SOD or Ab/catalase, unless specified otherwise), but not control IgG/SOD or IgG/catalase conjugates as well as PEG-modified enzymes, specifically bound to and entered endothelial cells, but not control cell types lacking the target antigen [57,102,115]. Accordingly, Ab/catalase and Ab/SOD, but not untargeted formulations protect endothelial cells against toxic effects of H2O2 [102,121] and O2•− [115] flux in the medium, thereby inhibiting ROS-induced cellular necrosis and apoptosis [115] (Fig. 3). Further, these conjugates protect from oxidative stress induced by ROS produced intracellularly in endothelial cells treated with paraquat [115].
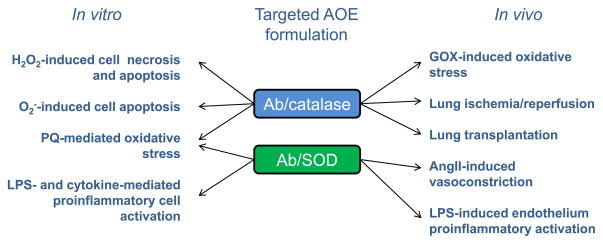
Fig. 3.
Protective effects of targeted formulations of AOEs in models of oxidative stress in vitro and in vivo. Catalase and SOD were conjugated to antibodies against endothelial target. AngII, angiotensin II; GOX, glucose oxidase; LPS, lipopolysaccharide; PQ, paraquat; SOD, superoxide dismutase.
Radiolabeled Ab/AOE targeted to ACE, ICAM and PECAM, but not IgG/AOE or PEG/AOE accumulate in the pulmonary vasculature in rats, mice, pigs and dogs after intravenous injection [57,69,72]. Confocal fluorescent microscopy revealed that Ab/SOD conjugate accumulates in endothelial intracellular vesicles in the pulmonary vasculature after systemic injection [57]. Optimal size of the Ab/AOE conjugates for highly specific endothelial targeting in vivo is within the range 30–500 nm diameter, which does not cause excessive non-specific retention in the capillaries (and coincides with the optimal size range for endothelial avidity and uptake of conjugates). In particular, anti-PECAM/AOE conjugates with diameter close to 300 nm show optimal efficacy and specificity of targeting to the pulmonary vasculature [120].
Functional activity of the injected conjugates has been initially validated in animal studies involving artificial influx of ROS in the pulmonary vasculature. In the first model, Ab/catalase conjugates detoxify H2O2 infused in lung isolated from rats pre-injected with the conjugate, thus protecting the pulmonary vasculature against oxidative injury and affirming the functionality of delivered antioxidant [85,102,106]. In the second, more challenging and physiologically relevant model, Ab/catalase, but not PEG-catalase or IgG/catalase, co-injected in mice with anti-TM/GOX (a conjugate that accumulates and generates H2O2 in the pulmonary vasculature) attenuated oxidative stress in lungs, markedly attenuated edema and reduced lethality from 100% to <20% [85]. As expected, Ab/SOD did not protect against anti-TM/GOX induced pulmonary injury, since H2O2 is the injurious ROS directly produced by GOX [122].
Anti-PECAM/catalase, but not control formulations injected in donor rats prior to lung harvest, markedly attenuated acute oxidative stress, edema, tissue injury and leukocyte sequestration in lungs transplanted to recipient rats after 18 h of cold ischemia and improved blood oxygenation [106]. This encouraging result has been independently reproduced using anti-ACE/catalase in rat model of lung transplantation in heart beating and nonheart beating donor rats [123,124]. Furthermore, anti-PECAM/catalase alleviated lung ischemia–reperfusion injury in situ in ventilated mice [122] (Fig. 3).
These animal studies affirmed targeted delivery to and protective effects of antioxidant enzyme conjugates in the pulmonary endothelium and provided means to define selectively role of given ROS in animal models of acute oxidative stress. For example, Ab/catalase, but not Ab/SOD conferred protection in models of pulmonary ischemia/reperfusion injury implicating H2O2 as a main damaging ROS [122]. However, the acuteness and severity of tissue injury in this type of animal model could overshadow more subtle effects of Ab/SOD.
Indeed, Ab/SOD, but not Ab/catalase or untargeted SOD formulations, inhibited vasoconstriction induced by angiotensin II in mice, thereby confirming the key role of superoxide produced by endothelial NADPH oxidase in quenching NO [122]. Furthermore, Ab/SOD, but not Ab/catalase or untargeted SOD formulations including PEG-SOD, inhibited pathological endothelial activation induced by cytokines and manifested by expression of VCAM-1 in the pulmonary endothelium in mice [57]. Studies in cell culture revealed that anti-PECAM/SOD accumulating in the endosomes quenches superoxide anion produced into vesicular lumen by NADPH oxidase, thereby intercepting specific pro-inflammatory signaling by intracellular superoxide inaccessible to other SOD formulations [57].
2.2.3. Control of duration of effects and endothelial delivery of antioxidant enzymes loaded into protective carriers
Endothelial cells internalize AOE conjugates anchored to ACE, ICAM and PECAM. This enables site-specific quenching of ROS in endosomal compartment, critically important for interception of pro-inflammatory signaling [57]. However, due to vesicular trafficking involving a series of sodium–proton exchangers, within few hours internalized conjugates reach lysosomes and proteolytic degradation limits duration of antioxidant effects [125]. Of note, ICAM molecules delivering anchored conjugates dissociate from them in the endosomes and recycle to the cell surface, thereby allowing sustained intracellular delivery and effect of circulating anti-ICAM conjugates [126]. Further, auxiliary drugs disrupting microtubules and lysosome maturation prolong antioxidant effects of the internalized catalase conjugates [100,126]. These findings provide a basis for pharmaceutical regulation of the duration of therapeutic effects of targeted AOE.
Using nanocarriers offers an additional approach to modulate endothelial delivery and effects of AOE. First, geometry (size and shape) of nanocarriers affects their circulation, interaction with targets and intracellular processing [46,127,128]. This, elongated carriers offer higher degree of endothelial targeting specificity, while decelerating arrival of the lysosomes, thereby prolonging effect of ICAM-targeted catalase formulations [111].
An alternative approach is to encapsulate AOE into nanocarriers permeable for small molecules of ROS, but not proteases (proteins with MW in tens of kD). For example, a freeze-thawing double emulsion technique allows fairly efficient (~10%) encapsulation of active catalase into spherical polymer nanocarriers (200–400 nm diameter) based on PEG–PLGA and similar di-block copolymers, permeable for H2O2 and protecting catalase from proteases [129]. Using PEG-catalase further enhances the encapsulation efficacy and protection against proteases [45], whereas modulating molar ratio and size of PEG and PLGA chains in copolymer allows to produce catalase-loaded nanocarriers of spherical or filamentous shape [47]. Isotope-labeled catalase encapsulated in PEG–PLGA nanocarriers is protected from proteolysis and circulates for a prolonged period of time in mice, similarly to PEG-catalase, yet PEG-catalase is degraded by proteases [45].
Coating of catalase-loaded PEG–PLGA nanocarriers by anti-PECAM conjugated with PEG end groups provides highly specific and effective endothelial targeting of isotope-labeled cargo in vitro and in vivo and prolonged antioxidant protection of the endothelium [130]. Of note, PEG–PLGA polymer matrix is readily diffusible for H2O2, but not superoxide; hence encapsulation into PEG–PLGA nanocarriers practically obliterates effective enzymatic activity of SOD [130]. In contrast, encapsulation of either catalase or SOD into micelles formed by controlled precipitation of magnetic nanoparticles using calcium and oleate provides composite nanocarriers (200–300 nm diameter) containing active catalase or SOD accessible for either H2O2 of superoxide and protected from proteases [131]. Targeting this formulation to endothelial cells using magnetic delivery [131] or anti-PECAM conjugated to surface of the micelles confers endothelial targeting and antioxidant protection.
3. Conclusion
There was an impressive progress in the design of antioxidant interventions targeted to the vascular endothelium in the last decade. A series of animal studies has demonstrated superiority of targeting catalase and SOD to endothelial markers including ACE and cell adhesion molecules over non-targeted formulations. Identification of alternative markers including those typical of specific endothelial phenotypes is underway and will even further diversify our arsenal of new means for targeted antioxidant interventions. These new means will help to dissect mechanisms of vascular oxidative stress and, hopefully, eventually translate into the clinical domain, thereby improving management of disease conditions involving this pathological mechanism. Ischemia–reperfusion injury in organ transplantation represents a clinical setting especially well posed to this development. Of course, industrial development of such complex drug delivery system will be modulated by commercial, regulatory and practical aspects. In this context, treatment of acute oxidative stress represents a more plausible therapeutic target than prolonged management of subtle chronic conditions.
Acknowledgments
This work was supported, in whole or in part, by the National Institutes of Health Grants RO1 HL073940 and HL087036 and Project 4 of PO1 HL079063.
References
- 1.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Anti-oxid Redox Signal. 2008;10(10):1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 2.Simone E, Ding BS, Muzykantov V. Targeted delivery of therapeutics to endothelium. Cell Tissue Res. 2009;335(1):283–300. doi: 10.1007/s00441-008-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka KA, Key NS, Levy JH. Blood coagulation: hemostasis and thrombin regulation. Anesth Analg. 2009;108(5):1433–1446. doi: 10.1213/ane.0b013e31819bcc9c. [DOI] [PubMed] [Google Scholar]
- 4.Boffa MB, Koschinsky ML. Curiouser and curiouser: recent advances in measurement of thrombin-activatable fibrinolysis inhibitor (TAFI) and in understanding its molecular genetics, gene regulation, and biological roles. Clin Biochem. 2007;40(7):431–442. doi: 10.1016/j.clinbiochem.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Cook-Mills JM, Marchese M, Abdala-Valencia H. VCAM-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal. doi: 10.1089/ars.2010.3522. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shuvaev VV, Christofidou-Solomidou M, Scherpereel A, Simone E, Arguiri E, Tliba S, Pick J, Kennel S, Albelda SM, Muzykantov VR. Factors modulating the delivery and effect of enzymatic cargo conjugated with antibodies targeted to the pulmonary endothelium. J Control Release. 2007;118(2):235–244. doi: 10.1016/j.jconrel.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pratico D. Antioxidants and endothelium protection. Atherosclerosis. 2005;181(2):215–224. doi: 10.1016/j.atherosclerosis.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 8.McCord JM, Roy RS, Schaffer SW. Free radicals and myocardial ischemia. The role of xanthine oxidase. Adv Myocardiol. 1985;5:183–189. [PubMed] [Google Scholar]
- 9.Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR. Advanced drug delivery systems that target the vascular endothelium. Mol Interv. 2006;6(2):98–112. doi: 10.1124/mi.6.2.7. [DOI] [PubMed] [Google Scholar]
- 10.Muzykantov VR. Targeting of superoxide dismutase and catalase to vascular endothelium. J Control Release. 2001;71(1):1–21. doi: 10.1016/s0168-3659(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 11.Ichimura H, Parthasarathi K, Quadri S, Issekutz AC, Bhattacharya J. Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries. J Clin Invest. 2003;111(5):691–699. doi: 10.1172/JCI17271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res. 2004;95(5):532–539. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]
- 13.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271(5 Pt 1):C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 14.Fridovich I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann NY Acad Sci. 1999;893:13–18. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- 15.McCord JM. Superoxide dismutase: rationale for use in reperfusion injury and inflammation. J Free Radic Biol Med. 1986;2(5–6):307–310. doi: 10.1016/s0748-5514(86)80029-0. [DOI] [PubMed] [Google Scholar]
- 16.Traber DL. Systemic cardiovascular changes with acute lung injury. Crit Care Med. 1995;23(1):7. doi: 10.1097/00003246-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Nagata K, Iwasaki Y, Yamada T, Yuba T, Kono K, Hosogi S, Ohsugi S, Kuwahara H, Marunaka Y. Overexpression of manganese superoxide dismutase by N-acetylcysteine in hyperoxic lung injury. Respir Med. 2007;101(4):800–807. doi: 10.1016/j.rmed.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Chang LY, Subramaniam M, Yoder BA, Day BJ, Ellison MC, Sunday ME, Crapo JD. A catalytic antioxidant attenuates alveolar structural remodeling in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2003;167(1):57–64. doi: 10.1164/rccm.200203-232OC. [DOI] [PubMed] [Google Scholar]
- 19.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, Samulski TV, Fridovich I, Dewhirst MW, Anscher MS. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33(6):857–863. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 20.Matsui T, Yamagishi S, Nakamura K, Inoue H. Bay w 9798, a dihydropyridine structurally related to nifedipine with no calcium channel-blocking properties, inhibits tumour necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in endothelial cells by suppressing reactive oxygen species generation. J Int Med Res. 2007;35(6):886–891. doi: 10.1177/147323000703500617. [DOI] [PubMed] [Google Scholar]
- 21.Yamagishi S, Nakamura K, Matsui T. Role of oxidative stress in the development of vascular injury and its therapeutic intervention by nifedipine. Curr Med Chem. 2008;15(2):172–177. doi: 10.2174/092986708783330557. [DOI] [PubMed] [Google Scholar]
- 22.Gao B, Flores SC, Leff JA, Bose SK, McCord JM. Synthesis and anti-inflammatory activity of a chimeric recombinant superoxide dismutase: SOD2/3. Am J Physiol Lung Cell Mol Physiol. 2003;284(6):L917–L925. doi: 10.1152/ajplung.00374.2002. [DOI] [PubMed] [Google Scholar]
- 23.Lin SJ, Shyue SK, Shih MC, Chu TH, Chen YH, Ku HH, Chen JW, Tam KB, Chen YL. Superoxide dismutase and catalase inhibit oxidized low-density lipoprotein-induced human aortic smooth muscle cell proliferation: role of cell-cycle regulation, mitogen-activated protein kinases, and transcription factors. Atherosclerosis. 2007;190(1):124–134. doi: 10.1016/j.atherosclerosis.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 24.Epperly MW, Guo HL, Jefferson M, Nie S, Gretton J, Bernarding M, Bar-Sagi D, Archer H, Greenberger JS. Cell phenotype specific kinetics of expression of intratracheally injected manganese superoxide dismutase-plasmid/liposomes (MnSOD-PL) during lung radioprotective gene therapy. Gene Ther. 2003;10(2):163–171. doi: 10.1038/sj.gt.3301852. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe N, Iwamoto T, Bowen KD, Dickinson DA, Torres M, Forman HJ. Bio-effectiveness of Tat-catalase conjugate: a potential tool for the identification of H2O2-dependent cellular signal transduction pathways. Biochem Biophys Res Commun. 2003;303(1):287–293. doi: 10.1016/s0006-291x(03)00335-8. [DOI] [PubMed] [Google Scholar]
- 26.Kim DW, Kim SY, Lee SH, Lee YP, Lee MJ, Jeong MS, Jang SH, Park J, Lee KS, Kang TC, Won MH, Cho SW, Kwon OS, Eum WS, Choi SY. Protein transduction of an antioxidant enzyme: subcellular localization of superoxide dismutase fusion protein in cells. BMB Rep. 2008;41(2):170–175. doi: 10.5483/bmbrep.2008.41.2.170. [DOI] [PubMed] [Google Scholar]
- 27.Yao H, Yang SR, Kode A, Rajendrasozhan S, Caito S, Adenuga D, Henry R, Edirisinghe I, Rahman I. Redox regulation of lung inflammation: role of NADPH oxidase and NF-kappaB signalling. Biochem Soc Trans. 2007;35(Pt 5):1151–1155. doi: 10.1042/BST0351151. [DOI] [PubMed] [Google Scholar]
- 28.Oakley FD, Abbott D, Li Q, Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal. 2009;11(6):1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christofidou-Solomidou M, Muzykantov VR. Antioxidant strategies in respiratory medicine. Treat Respir Med. 2006;5(1):47–78. doi: 10.2165/00151829-200605010-00004. [DOI] [PubMed] [Google Scholar]
- 30.Bowler RP, Arcaroli J, Crapo JD, Ross A, Slot JW, Abraham E. Extracellular superoxide dismutase attenuates lung injury after hemorrhage. Am J Respir Crit Care Med. 2001;164(2):290–294. doi: 10.1164/ajrccm.164.2.2011054. [DOI] [PubMed] [Google Scholar]
- 31.Davies R, Edwards RE, Green JA, Legg RF, Snowden RT, Manson MM. Antioxidants can delay liver cell maturation which in turn affects gamma-glutamyltranspeptidase expression. Carcinogenesis. 1993;14(1):47–52. doi: 10.1093/carcin/14.1.47. [DOI] [PubMed] [Google Scholar]
- 32.Engelhardt JF. Redox-mediated gene therapies for environmental injury: approaches and concepts. Antioxid Redox Signal. 1999;1(1):5–27. doi: 10.1089/ars.1999.1.1-5. [DOI] [PubMed] [Google Scholar]
- 33.Epperly MW, Kagan VE, Sikora CA, Gretton JE, Defilippi SJ, Bar-Sagi D, Greenberger JS. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) administration protects mice from esophagitis associated with fractionated radiation. Int J Cancer. 2001;96(4):221–231. doi: 10.1002/ijc.1023. [DOI] [PubMed] [Google Scholar]
- 34.Erzurum SC, Lemarchand P, Rosenfeld MA, Yoo JH, Crystal RG. Protection of human endothelial cells from oxidant injury by adenovirus-mediated transfer of the human catalase cDNA. Nucleic Acids Res. 1993;21(7):1607–1612. doi: 10.1093/nar/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest. 1999;103(7):1055–1066. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danel C, Erzurum SC, Prayssac P, Eissa NT, Crystal RG, Herve P, Baudet B, Mazmanian M, Lemarchand P. Gene therapy for oxidant injury-related diseases: adenovirus-mediated transfer of superoxide dismutase and catalase cDNAs protects against hyperoxia but not against ischemia–reperfusion lung injury. Hum Gene Ther. 1998;9(10):1487–1496. doi: 10.1089/hum.1998.9.10-1487. [DOI] [PubMed] [Google Scholar]
- 37.Muzykantov VR. Delivery of antioxidant enzyme proteins to the lung. Antioxid Redox Signal. 2001;3(1):39–62. doi: 10.1089/152308601750100489. [DOI] [PubMed] [Google Scholar]
- 38.Muzykantov VR. Biomedical aspects of targeted delivery of drugs to pulmonary endothelium. Expert Opin Drug Deliv. 2005;2(5):909–926. doi: 10.1517/17425247.2.5.909. [DOI] [PubMed] [Google Scholar]
- 39.Danilov SM, Gavrilyuk VD, Franke FE, Pauls K, Harshaw DW, McDonald TD, Miletich DJ, Muzykantov VR. Lung uptake of antibodies to endothelial antigens: key determinants of vascular immunotargeting. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1335–L1347. doi: 10.1152/ajplung.2001.280.6.L1335. [DOI] [PubMed] [Google Scholar]
- 40.Danielyan K, Ding BS, Gottstein C, Cines DB, Muzykantov VR. Delivery of anti-platelet-endothelial cell adhesion molecule single-chain variable fragment-urokinase fusion protein to the cerebral vasculature lyses arterial clots and attenuates postischemic brain edema. J Pharmacol Exp Ther. 2007;321(3):947–952. doi: 10.1124/jpet.107.120535. [DOI] [PubMed] [Google Scholar]
- 41.Garnacho C, Albelda SM, Muzykantov VR, Muro S. Differential intra-endothelial delivery of polymer nanocarriers targeted to distinct PECAM-1 epitopes. J Control Release. 2008;130(3):226–233. doi: 10.1016/j.jconrel.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scherpereel A, Wiewrodt R, Christofidou-Solomidou M, Gervais R, Murciano JC, Albelda SM, Muzykantov VR. Cell-selective intracellular delivery of a foreign enzyme to endothelium in vivo using vascular immunotargeting. FASEB J. 2001;15(2):416–426. doi: 10.1096/fj.00-0022com. [DOI] [PubMed] [Google Scholar]
- 43.White CW, Jackson JH, Abuchowski A, Kazo GM, Mimmack RF, Berger EM, Freeman BA, McCord JM, Repine JE. Polyethylene glycol-attached antioxidant enzymes decrease pulmonary oxygen toxicity in rats. J Appl Physiol. 1989;66(2):584–590. doi: 10.1152/jappl.1989.66.2.584. [DOI] [PubMed] [Google Scholar]
- 44.Corvo ML, Boerman OC, Oyen WJ, Van Bloois L, Cruz ME, Crommelin DJ, Storm G. Intravenous administration of superoxide dismutase entrapped in long circulating liposomes. II. In vivo fate in a rat model of adjuvant arthritis. Biochim Biophys Acta. 1999;1419(2):325–334. doi: 10.1016/s0005-2736(99)00081-4. [DOI] [PubMed] [Google Scholar]
- 45.Simone EA, Dziubla TD, Arguiri E, Vardon V, Shuvaev VV, Christofidou-Solomidou M, Muzykantov VR. Loading PEG-catalase into filamentous and spherical polymer nanocarriers. Pharm Res. 2009;26(1):250–260. doi: 10.1007/s11095-008-9744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simone EA, Dziubla TD, Muzykantov VR. Polymeric carriers: role of geometry in drug delivery. Expert Opin Drug Deliv. 2008;5(12):1283–1300. doi: 10.1517/17425240802567846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simone EA, Dziubla TD, Discher DE, Muzykantov VR. Filamentous polymer nanocarriers of tunable stiffness that encapsulate the therapeutic enzyme catalase. Biomacromolecules. 2009;10(6):1324–1330. doi: 10.1021/bm900189x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82(2–3):189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 49.Yi X, Zimmerman MC, Yang R, Tong J, Vinogradov S, Kabanov AV. Pluronic-modified superoxide dismutase 1 attenuates angiotensin II-induced increase in intracellular superoxide in neurons. Free Radic Biol Med. 2010;49(4):548–558. doi: 10.1016/j.freeradbiomed.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barnard ML, Baker RR, Matalon S. Mitigation of oxidant injury to lung microvasculature by intratracheal instillation of antioxidant enzymes. Am J Physiol. 1993;265(4 Pt 1):L340–L345. doi: 10.1152/ajplung.1993.265.4.L340. [DOI] [PubMed] [Google Scholar]
- 51.Freeman BA, Turrens JF, Mirza Z, Crapo JD, Young SL. Modulation of oxidant lung injury by using liposome-entrapped superoxide dismutase and catalase. Fed Proc. 1985;44(10):2591–2595. [PubMed] [Google Scholar]
- 52.Igarashi R, Hoshino J, Ochiai A, Morizawa Y, Mizushima Y. Lecithinized superoxide dismutase enhances its pharmacologic potency by increasing its cell membrane affinity. J Pharmacol Exp Ther. 1994;271(3):1672–1677. [PubMed] [Google Scholar]
- 53.Inoue M, Ebashi I, Watanabe N, Morino Y. Synthesis of a superoxide dismutase derivative that circulates bound to albumin and accumulates in tissues whose pH is decreased. Biochemistry. 1989;28(16):6619–6624. doi: 10.1021/bi00442a013. [DOI] [PubMed] [Google Scholar]
- 54.Koyama S, Kobayashi T, Kubo K, Sekiguchi M, Ueda G. Recombinant-human superoxide dismutase attenuates endotoxin-induced lung injury in awake sheep. Am Rev Respir Dis. 1992;145(6):1404–1409. doi: 10.1164/ajrccm/145.6.1404. [DOI] [PubMed] [Google Scholar]
- 55.Viau AT, Abuchowski A, Greenspan S, Davis FF. Safety evaluation of free radical scavengers PEG-catalase and PEG-superoxide dismutase. J Free Radic Biol Med. 1986;2(4):283–288. doi: 10.1016/s0748-5514(86)80011-3. [DOI] [PubMed] [Google Scholar]
- 56.Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest. 1982;47(5):412–426. [PubMed] [Google Scholar]
- 57.Shuvaev VV, Han J, Yu KJ, Huang S, Hawkins BJ, Madesh M, Nakada M, Muzykantov VR. PECAM-targeted delivery of SOD inhibits endothelial inflammatory response. FASEB J. 2011;25(1):348–357. doi: 10.1096/fj.10-169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boissinot M, Kuhn LA, Lee P, Fisher CL, Wang Y, Hallewell RA, Tainer JA. Rational design and expression of a heparin-targeted human superoxide dismutase. Biochem Biophys Res Commun. 1993;190(1):250–256. doi: 10.1006/bbrc.1993.1038. [DOI] [PubMed] [Google Scholar]
- 59.Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci USA. 1991;88(22):10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin LH, Bahn JH, Eum WS, Kwon HY, Jang SH, Han KH, Kang TC, Won MH, Kang JH, Cho SW, Park J, Choi SY. Transduction of human catalase mediated by an HIV-1 TAT protein basic domain and arginine-rich peptides into mammalian cells. Free Radic Biol Med. 2001;31(11):1509–1519. doi: 10.1016/s0891-5849(01)00734-1. [DOI] [PubMed] [Google Scholar]
- 61.Danilov SM, Muzykantov VR, Martynov AV, Atochina EN, Sakharov I, Trakht IN, Smirnov VN. Lung is the target organ for a monoclonal antibody to angiotensin-converting enzyme. Lab Invest. 1991;64(1):118–124. [PubMed] [Google Scholar]
- 62.Jacobson BS, Schnitzer JE, McCaffery M, Palade GE. Isolation and partial characterization of the luminal plasmalemma of microvascular endothelium from rat lungs. Eur J Cell Biol. 1992;58(2):296–306. [PubMed] [Google Scholar]
- 63.McIntosh DP, Tan XY, Oh P, Schnitzer JE. Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: a pathway to overcome cell barriers to drug and gene delivery. Proc Natl Acad Sci USA. 2002;99(4):1996–2001. doi: 10.1073/pnas.251662398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pasqualini R, McDonald DM, Arap W. Vascular targeting and antigen presentation. Nat Immunol. 2001;2(7):567–568. doi: 10.1038/89704. [DOI] [PubMed] [Google Scholar]
- 65.Rajotte D, Arap W, Hagedorn M, Koivunen E, Pasqualini R, Ruoslahti E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J Clin Invest. 1998;102(2):430–437. doi: 10.1172/JCI3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schnitzer JE. Vascular targeting as a strategy for cancer therapy. N Engl J Med. 1998;339(7):472–474. doi: 10.1056/NEJM199808133390711. [DOI] [PubMed] [Google Scholar]
- 67.Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci USA. 1999;96(23):13203–13207. doi: 10.1073/pnas.96.23.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson A, Zhou W, Champion HC, Alber S, Tang ZL, Kennel S, Watkins S, Huang L, Pitt B, Li S. Targeted delivery of oligodeoxynucleotides to mouse lung endothelial cells in vitro and in vivo. Mol Ther. 2005;12(3):510–518. doi: 10.1016/j.ymthe.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Muzykantov VR, Atochina EN, Ischiropoulos H, Danilov SM, Fisher AB. Immunotargeting of antioxidant enzyme to the pulmonary endothelium. Proc Natl Acad Sci USA. 1996;93(11):5213–5218. doi: 10.1073/pnas.93.11.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE, Schnitzer JE. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429(6992):629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 71.Wang J, Tian S, Petros RA, Napier ME, Desimone JM. The complex role of multivalency in nanoparticles targeting the transferrin receptor for cancer therapies. J Am Chem Soc. 2010;132(32):11306–11313. doi: 10.1021/ja1043177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Atochina EN, Balyasnikova IV, Danilov SM, Granger DN, Fisher AB, Muzykantov VR. Immunotargeting of catalase to ACE or ICAM-1 protects perfused rat lungs against oxidative stress. Am J Physiol. 1998;275(4 Pt 1):L806–L817. doi: 10.1152/ajplung.1998.275.4.L806. [DOI] [PubMed] [Google Scholar]
- 73.Hopkins AM, Baird AW, Nusrat A. ICAM-1: targeted docking for exogenous as well as endogenous ligands. Adv Drug Deliv Rev. 2004;56(6):763–778. doi: 10.1016/j.addr.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 74.Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28(9):1379–1386. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 75.Harari OA, Wickham TJ, Stocker CJ, Kovesdi I, Segal DM, Huehns TY, Sarraf C, Haskard DO. Targeting an adenoviral gene vector to cytokine-activated vascular endothelium via E-selectin. Gene Ther. 1999;6(5):801–807. doi: 10.1038/sj.gt.3300898. [DOI] [PubMed] [Google Scholar]
- 76.Keelan ET, Harrison AA, Chapman PT, Binns RM, Peters AM, Haskard DO. Imaging vascular endothelial activation: an approach using radiolabeled monoclonal antibodies against the endothelial cell adhesion molecule E-selectin. J Nucl Med. 1994;35(2):276–281. [PubMed] [Google Scholar]
- 77.Kiely JM, Cybulsky MI, Luscinskas FW, Gimbrone MA., Jr Immunoselective targeting of an anti-thrombin agent to the surface of cytokine-activated vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1995;15(8):1211–1218. doi: 10.1161/01.atv.15.8.1211. [DOI] [PubMed] [Google Scholar]
- 78.Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104(17):2107–2112. doi: 10.1161/hc4201.097061. [DOI] [PubMed] [Google Scholar]
- 79.Spragg DD, Alford DR, Greferath R, Larsen CE, Lee KD, Gurtner GC, Cybulsky MI, Tosi PF, Nicolau C, Gimbrone MA., Jr Immunotargeting of liposomes to activated vascular endothelial cells: a strategy for site-selective delivery in the cardiovascular system. Proc Natl Acad Sci USA. 1997;94(16):8795–8800. doi: 10.1073/pnas.94.16.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Danilov S, Atochina E, Hiemisch H, Churakova T, Moldobayeva A, Sakharov I, Deichman G, Ryan U, Muzykantov VR. Interaction of mAb to angiotensin-converting enzyme (ACE) with antigen in vitro and in vivo: antibody targeting to the lung induces ACE antigenic modulation. Int Immunol. 1994;6(8):1153–1160. doi: 10.1093/intimm/6.8.1153. [DOI] [PubMed] [Google Scholar]
- 81.Garnacho C, Shuvaev V, Thomas A, McKenna L, Sun J, Koval M, Albelda S, Muzykantov V, Muro S. RhoA activation and actin reorganization involved in endothelial CAM-mediated endocytosis of anti-PECAM carriers: critical role for tyrosine 686 in the cytoplasmic tail of PECAM-1. Blood. 2008;111(6):3024–3033. doi: 10.1182/blood-2007-06-098657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muzykantov VR, Atochina EN, Kuo A, Barnathan ES, Notarfrancesco K, Shuman H, Dodia C, Fisher AB. Endothelial cells internalize monoclonal antibody to angiotensin-converting enzyme. Am J Physiol. 1996;270(5 Pt 1):L704–L713. doi: 10.1152/ajplung.1996.270.5.L704. [DOI] [PubMed] [Google Scholar]
- 83.Kennel SJ, Falcioni R, Wesley JW. Microdistribution of specific rat monoclonal antibodies to mouse tissues and human tumor xenografts. Cancer Res. 1991;51(5):1529–1536. [PubMed] [Google Scholar]
- 84.Esmon CT. Thrombomodulin as a model of molecular mechanisms that modulate protease specificity and function at the vessel surface. FASEB J. 1995;9(10):946–955. doi: 10.1096/fasebj.9.10.7615164. [DOI] [PubMed] [Google Scholar]
- 85.Christofidou-Solomidou M, Scherpereel A, Wiewrodt R, Ng K, Sweitzer T, Arguiri E, Shuvaev V, Solomides CC, Albelda SM, Muzykantov VR. PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2003;285(2):L283–L292. doi: 10.1152/ajplung.00021.2003. [DOI] [PubMed] [Google Scholar]
- 86.Christofidou-Solomidou M, Pietra GG, Solomides CC, Arguiris E, Harshaw D, Fitzgerald GA, Albelda SM, Muzykantov VR. Immunotargeting of glucose oxidase to endothelium in vivo causes oxidative vascular injury in the lungs. Am J Physiol Lung Cell Mol Physiol. 2000;278(4):L794–L805. doi: 10.1152/ajplung.2000.278.4.L794. [DOI] [PubMed] [Google Scholar]
- 87.Christofidou-Solomidou M, Kennel S, Scherpereel A, Wiewrodt R, Solomides CC, Pietra GG, Murciano JC, Shah SA, Ischiropoulos H, Albelda SM, Muzykantov VR. Vascular immunotargeting of glucose oxidase to the endothelial antigens induces distinct forms of oxidant acute lung injury: targeting to thrombomodulin, but not to PECAM-1, causes pulmonary thrombosis and neutrophil transmigration. Am J Pathol. 2002;160(3):1155–1169. doi: 10.1016/S0002-9440(10)64935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Messadi-Laribi E, Griol-Charhbili V, Gaies E, Vincent MP, Heudes D, Meneton P, Alhenc-Gelas F, Richer C. Cardioprotection and kallikrein–kinin system in acute myocardial ischaemia in mice. Clin Exp Pharmacol Physiol. 2008;35(4):489–493. doi: 10.1111/j.1440-1681.2008.04902.x. [DOI] [PubMed] [Google Scholar]
- 89.Fryer RM, Segreti J, Banfor PN, Widomski DL, Backes BJ, Lin CW, Ballaron SJ, Cox BF, Trevillyan JM, Reinhart GA, von Geldern TW. Effect of bradykinin metabolism inhibitors on evoked hypotension in rats: rank efficacy of enzymes associated with bradykinin-mediated angioedema. Br J Pharmacol. 2008;153(5):947–955. doi: 10.1038/sj.bjp.0707641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moreau ME, Dubreuil P, Molinaro G, Chagnon M, Muller-Esterl W, Lepage Y, Marceau F, Adam A. Expression of metallopeptidases and kinin receptors in swine oropharyngeal tissues: effects of angiotensin I-converting enzyme inhibition and inflammation. J Pharmacol Exp Ther. 2005;315(3):1065–1074. doi: 10.1124/jpet.105.088005. [DOI] [PubMed] [Google Scholar]
- 91.Skirgello OE, Balyasnikova IV, Binevski PV, Sun ZL, Baskin II, Palyulin VA, Nesterovitch AB, Albrecht RF, II, Kost OA, Danilov SM. Inhibitory antibodies to human angiotensin-converting enzyme: fine epitope mapping and mechanism of action. Biochemistry. 2006;45(15):4831–4847. doi: 10.1021/bi052591h. [DOI] [PubMed] [Google Scholar]
- 92.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418(6894):200–203. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 93.Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;99(1):3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meyrick B. Pathology of the adult respiratory distress syndrome. Crit Care Clin. 1986;2(3):405–428. [PubMed] [Google Scholar]
- 95.Tasaka S, Qin L, Saijo A, Albelda SM, DeLisser HM, Doerschuk CM. Platelet endothelial cell adhesion molecule-1 in neutrophil emigration during acute bacterial pneumonia in mice and rats. Am J Respir Crit Care Med. 2003;167(2):164–170. doi: 10.1164/rccm.2202011. [DOI] [PubMed] [Google Scholar]
- 96.Ware LB, Camerer E, Welty-Wolf K, Schultz MJ, Matthay MA. Bench to bedside: targeting coagulation and fibrinolysis in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L307–L311. doi: 10.1152/ajplung.00157.2006. [DOI] [PubMed] [Google Scholar]
- 97.Mulligan MS, Miyasaka M, Tamatani T, Jones ML, Ward PA. Requirements for L-selectin in neutrophil-mediated lung injury in rats. J Immunol. 1994;152(2):832–840. [PubMed] [Google Scholar]
- 98.Vaporciyan AA, DeLisser HM, Yan HC, Mendiguren II, Thom SR, Jones ML, Ward PA, Albelda SM. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science. 1993;262(5139):1580–1582. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- 99.Murciano JC, Muro S, Koniaris L, Christofidou-Solomidou M, Harshaw DW, Albelda SM, Granger DN, Cines DB, Muzykantov VR. ICAM-directed vascular immunotargeting of antithrombotic agents to the endothelial luminal surface. Blood. 2003;101(10):3977–3984. doi: 10.1182/blood-2002-09-2853. [DOI] [PubMed] [Google Scholar]
- 100.Muro S, Dziubla T, Qiu W, Leferovich J, Cui X, Berk E, Muzykantov VR. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule 1. J Pharmacol Exp Ther. 2006;317(3):1161–1169. doi: 10.1124/jpet.105.098970. [DOI] [PubMed] [Google Scholar]
- 101.Muro S, Schuchman EH, Muzykantov VR. Lysosomal enzyme delivery by ICAM-1-targeted nanocarriers bypassing glycosylation- and clathrin-dependent endocytosis. Mol Ther. 2006;13(1):135–141. doi: 10.1016/j.ymthe.2005.07.687. [DOI] [PubMed] [Google Scholar]
- 102.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci USA. 1999;96(5):2379–2384. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Park HS, Kim KY, Lee JS, Choi C, Bae YS, Lee BI, Rhee SG, Kang SW. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435(7040):347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 104.Ding BS, Hong N, Murciano JC, Ganguly K, Gottstein C, Christofidou-Solomidou M, Albelda SM, Fisher AB, Cines DB, Muzykantov VR. Prophylactic thrombolysis by thrombin-activated latent prourokinase targeted to PECAM-1 in the pulmonary vasculature. Blood. 2008;111(4):1999–2006. doi: 10.1182/blood-2007-07-103002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li S, Tan Y, Viroonchatapan E, Pitt BR, Huang L. Targeted gene delivery to pulmonary endothelium by anti-PECAM antibody. Am J Physiol Lung Cell Mol Physiol. 2000;278(3):L504–L511. doi: 10.1152/ajplung.2000.278.3.L504. [DOI] [PubMed] [Google Scholar]
- 106.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, Muro S, Buerk DG, Solomides CC, Albelda SM, Patterson GA, Muzykantov VR. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol. 2003;21(4):392–398. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 107.Muro S, Muzykantov VR. Targeting of antioxidant and anti-thrombotic drugs to endothelial cell adhesion molecules. Curr Pharm Des. 2005;11(18):2383–2401. doi: 10.2174/1381612054367274. [DOI] [PubMed] [Google Scholar]
- 108.Carnemolla R, Shuvaev VV, Muzykantov VR. Targeting antioxidant and antithrombotic biotherapeutics to endothelium. Semin Thromb Hemost. 2010;36(3):332–342. doi: 10.1055/s-0030-1253455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Muro S, Cui X, Gajewski C, Murciano JC, Muzykantov VR, Koval M. Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress. Am J Physiol Cell Physiol. 2003;285(5):C1339–C1347. doi: 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- 110.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116(Pt 8):1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 111.Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol Ther. 2008;16(8):1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ding BS, Gottstein C, Grunow A, Kuo A, Ganguly K, Albelda SM, Cines DB, Muzykantov VR. Endothelial targeting of a recombinant construct fusing a PECAM-1 single-chain variable antibody fragment (scFv) with prourokinase facilitates prophylactic thrombolysis in the pulmonary vasculature. Blood. 2005;106(13):4191–4198. doi: 10.1182/blood-2005-05-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ding BS, Hong N, Christofidou-Solomidou M, Gottstein C, Albelda SM, Cines DB, Fisher AB, Muzykantov VR. Anchoring fusion thrombomodulin to the endothelial lumen protects against injury-induced lung thrombosis and inflammation. Am J Respir Crit Care Med. 2009;180(3):247–256. doi: 10.1164/rccm.200809-1433OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reynolds PN, Nicklin SA, Kaliberova L, Boatman BG, Grizzle WE, Balyasnikova IV, Baker AH, Danilov SM, Curiel DT. Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nat Biotechnol. 2001;19(9):838–842. doi: 10.1038/nbt0901-838. [DOI] [PubMed] [Google Scholar]
- 115.Shuvaev VV, Tliba S, Nakada M, Albelda SM, Muzykantov VR. Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of super-oxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J Pharmacol Exp Ther. 2007;323(2):450–457. doi: 10.1124/jpet.107.127126. [DOI] [PubMed] [Google Scholar]
- 116.van der Vliet A. NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic Biol Med. 2008;44(6):938–955. doi: 10.1016/j.freeradbiomed.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gow AJ, Branco F, Christofidou-Solomidou M, Black-Schultz L, Albelda SM, Muzykantov VR. Immunotargeting of glucose oxidase: intracellular production of H(2) O(2) and endothelial oxidative stress. Am J Physiol. 1999;277(2 Pt 1):L271–L281. doi: 10.1152/ajplung.1999.277.2.L271. [DOI] [PubMed] [Google Scholar]
- 118.Muzykantov VR, Martynov AV, Puchnina EA, Danilov SM. In vivo administration of glucose oxidase conjugated with monoclonal antibodies to angiotensin-converting enzyme. The tissue distribution, blood clearance, and targeting into rat lungs. Am Rev Respir Dis. 1989;139(6):1464–1473. doi: 10.1164/ajrccm/139.6.1464. [DOI] [PubMed] [Google Scholar]
- 119.Shuvaev VV, Dziubla T, Wiewrodt R, Muzykantov VR. Streptavidin–biotin crosslinking of therapeutic enzymes with carrier antibodies: nanoconjugates for protection against endothelial oxidative stress. Methods Mol Biol. 2004;283:3–19. doi: 10.1385/1-59259-813-7:003. [DOI] [PubMed] [Google Scholar]
- 120.Shuvaev VV, Tliba S, Pick J, Arguiri E, Christofidou-Solomidou M, Albelda SM, Muzykantov VR. Modulation of endothelial targeting by size of antibody–antioxidant enzyme conjugates. J Control Release. 2011;149(3):236–241. doi: 10.1016/j.jconrel.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sweitzer TD, Thomas AP, Wiewrodt R, Nakada MT, Branco F, Muzykantov VR. PECAM-directed immunotargeting of catalase: specific, rapid and transient protection against hydrogen peroxide. Free Radic Biol Med. 2003;34(8):1035–1046. doi: 10.1016/s0891-5849(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 122.Shuvaev VV, Christofidou-Solomidou M, Bhora F, Laude K, Cai H, Dikalov S, Arguiri E, Solomides CC, Albelda SM, Harrison DG, Muzykantov VR. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J Pharmacol Exp Ther. 2009;331(2):404–411. doi: 10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nowak K, Hanusch C, Nicksch K, Metzger RP, Beck G, Gebhard MM, Hohenberger P, Danilov SM. Pre-ischaemic conditioning of the pulmonary endothelium by immunotargeting of catalase via angiotensin-converting-enzyme antibodies. Eur J Cardiothorac Surg. 2010;37(4):859–863. doi: 10.1016/j.ejcts.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 124.Nowak K, Weih S, Metzger R, Albrecht RF, II, Post S, Hohenberger P, Gebhard MM, Danilov SM. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia–reperfusion injury of the lung in vivo. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L162–L169. doi: 10.1152/ajplung.00001.2007. [DOI] [PubMed] [Google Scholar]
- 125.Muro S, Mateescu M, Gajewski C, Robinson M, Muzykantov VR, Koval M. Control of intracellular trafficking of ICAM-1-targeted nanocarriers by endothelial Na+/H+ exchanger proteins. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L809–L817. doi: 10.1152/ajplung.00311.2005. [DOI] [PubMed] [Google Scholar]
- 126.Muro S, Gajewski C, Koval M, Muzykantov VR. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105(2):650–658. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]
- 127.Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297(5583):967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 128.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 129.Dziubla TD, Karim A, Muzykantov VR. Polymer nanocarriers protecting active enzyme cargo against proteolysis. J Control Release. 2005;102(2):427–439. doi: 10.1016/j.jconrel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 130.Dziubla TD, Shuvaev VV, Hong NK, Hawkins BJ, Madesh M, Takano H, Simone E, Nakada MT, Fisher A, Albelda SM, Muzykantov VR. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials. 2008;29(2):215–227. doi: 10.1016/j.biomaterials.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chorny M, Hood E, Levy RJ, Muzykantov VR. Endothelial delivery of anti-oxidant enzymes loaded into non-polymeric magnetic nanoparticles. J Control Release. 2010;146(1):144–151. doi: 10.1016/j.jconrel.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]