Summary
The pathogenesis of alcoholic liver injury involves interactions of several intracellular signalling pathways in different cell types of the liver. Alcohol-induced sensitization of liver macrophages to portal endotoxin/lipopolysaccharide (LPS) is considered a hallmark of alcoholic liver disease (ALD). Intracellular mechanisms associated with LPS-induced signalling play a crucial role in the initiation and progression of alcoholic liver injury, and are being extensively explored. LPS recognition by Toll-like receptor 4 (TLR4) on macrophages and other cell types in the liver, activation of downstream signalling pathways culminating in activation of transcription factors such as NFκB, AP-1 leads to increased inflammatory cytokine production in ALD. In addition, LPS-induced MAPK such as ERK and p38 also contribute to liver injury. The importance of alcohol-induced reactive oxygen species and interactions with TLR pathways in macrophages leading to inflammation is becoming increasingly evident. Collectively, these signalling pathways induce pro- and anti-inflammatory cytokines that play an important role in ALD. In this review we describe the key signalling intermediates leading to alcohol-induced inflammation in alcoholic liver disease.
Keywords: macrophages, Kupffer cells, TLRs, MAP kinases, transcription factors
1.0 Introduction
Alcohol consumption is associated with a spectrum of diseases in the liver ranging from steatosis, steatohepatitis to cirrhosis and hepatocellular carcinoma. The pathogenesis of acute and chronic alcohol consumption is multi-factorial with diverse consequences in different cell types. Alcohol-induced injury occurs at multiple levels ranging from the innate immune cells to the liver parenchymal cells, hepatocytes. The innate immune cells including hepatic macrophages (Kupffer cells) play a pivotal role in early alcohol-induced liver injury via recognition of endotoxin/lipoplysaccharide in the portal circulation. The progression of alcohol-induced liver damage involves parenchymal cells and macrophages through the direct effects of alcohol as well as indirect effects of metabolites, oxidative stress, immunologic and inflammatory events. In macrophages, alcohol directly induces oxidative stress and sensitizes to LPS-induced inflammatory cytokine production. Inflammatory cytokines particularly TNFα, contributes to the development of alcoholic liver disease (ALD). Alcohol sensitizes hepatocytes to TNFα-induced apoptosis. A complete understanding of alcohol-mediated intracellular signalling mechanisms leading to inflammatory cytokine induction in macrophages will provide new insights into the development of new potential targets for therapeutic intervention.
The goal of this concise article is to review the alcohol-mediated signalling pathways, particularly Toll-like receptors and their adaptors in macrophages. The importance of transcription factors such as NFκB, AP-1, Egr-1 and STATs, intracellular kinases such as MAP kinases and pro- and anti-inflammatory cytokines in ALD will also be reviewed.
2.0 Interactions of immune and parenchymal cells of the liver in ALD
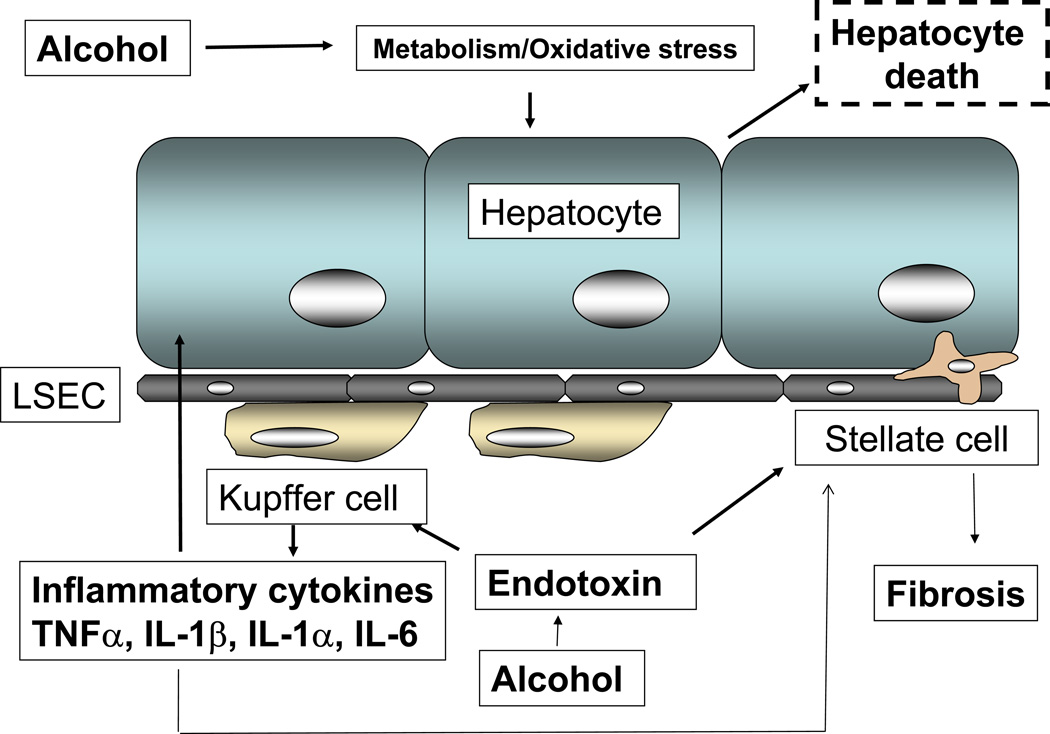
Innate immune responses activated in the resident liver macrophages, Kupffer cells play a key role in the early pathogenesis of alcohol-induced liver injury (1). Increased levels of circulating LPS in alcoholic patients have been shown (2). The currently accepted model of alcoholic liver injury elucidates that LPS promotes hepatic injury via induction of Kupffer cell activation resulting in production of TNFα and other inflammatory mediators. The Kupffer cells respond to stimulation by gut-derived endotoxins and apoptotic dead cells in the tissue resulting in increased inflammatory responses. Circulating TNFα is increased in chronic alcoholics as well as in mouse chronic alcohol feeding models (3, 4). In addition to hepatocytes, abnormal stellate cell activity and induction of fibrosis is also dependent on Kupffer cells via production of reactive oxygen species and pro-inflammatory cytokines (5). Liver natural killer (NK) cells exposed to alcohol contribute to fibrosis and inflammation via inhibition of NK cell accumulation and reduced NK cell killing of hepatic stellate cells (6). Thus, modulation of the innate immune system is an important mechanism contributing to liver inflammation, hepatocyte death and liver fibrosis (Fig1).
Fig 1.
Cells involved in alcoholic liver injury. Alcohol-mediated increase in gut-derived endotoxin with oxidative stress mechanisms sensitizes hepatic macrophages to release inflammatory cytokines such as TNFα, IL-1β, IL-1α and IL-6 that affects stellate cells and hepatocyte functions. Endotoxin also affects stellate cell and endothelial cell activation and contributes to liver injury.
3.0 Receptor-mediated signalling pathways affected by alcohol in liver inflammatory cells
3.0.1 Toll-like receptors, adapters and signalling
Recent discoveries of pattern recognition receptors focused attention on Toll-like receptors (TLRs) that sense pathogen-derived molecules as well as host-derived damage signals (7, 8). Among ten different TLRs described in humans, the functional significance of TLR4 and its downstream signalling in alcoholic liver disease is extensively elucidated (Fig 2). TLR4 recognizes the lipid A motif of the lipopoysaccharide (LPS), a suggested cofactor in the pathogenesis of ALD (9). TLR4 is a major component of the LPS recognition receptor complex, which also involves the co-receptors CD14 and MD-2, and LPS binding protein (LBP) (10, 11). LBP is a soluble shuttle protein that directly binds LPS and facilitates the association between LPS and CD14 (12, 13).
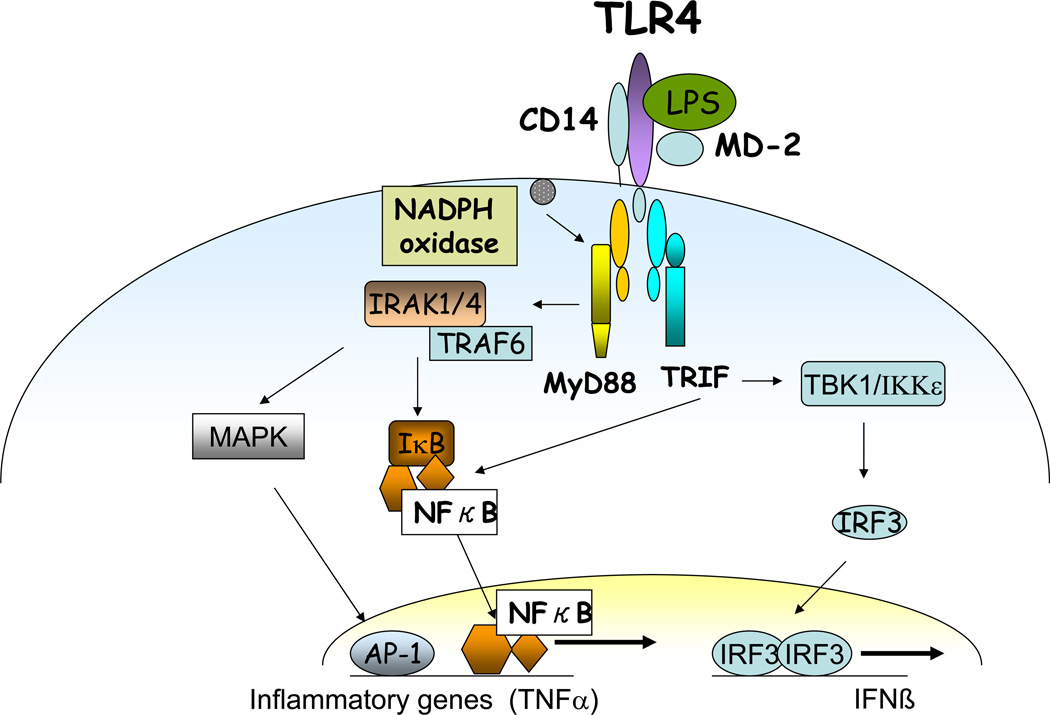
Fig 2.
TLR4 mediated signalling in alcohol-exposed macrophages. Alcohol alters functions of Toll-like receptors TLR4 in the liver resulting in regulation of MyD88 dependent activation of downstream signalling molecules such as IRAK kinase, IKK and NFκB. MyD88-independent, TRIF-dependent activation of IRF3 is also regulated alcohol exposure. Alcohol also induces NADPH oxidase via reactive oxygen species leading to inflammation.
CD14 is a glycosylphosphatidylinositol-anchored protein, which also exists in a soluble form. CD14 facilitates the transfer of LPS to the TLR4/MD2 receptor complex and modulates LPS recognition (14). CD14 facilitates TLR4 induced responses (7, 15) and appears to be required for MyD88-independent signalling (16).
MD2 is a soluble protein that non-covalently associates with TLR4 and binds LPS directly to form a complex with LPS in the absence of TLRs (17, 18, 19). Although no evidence suggests that TLR4 can bind LPS directly, TLR4 can enhance the binding of LPS to MD2 (20).
In the liver, TLR4 is expressed not only on innate immune cells such as Kupffer cells and recruited macrophages, but also on hepatocytes, sinusoidal endothelial cells, biliary epithelial cells and stellate cells. Indeed, LPS activation was shown to result in activation and functional changes in all of these cell types in different liver diseases (21, 22).
A major role for LPS-induced, TLR4 mediated signalling via its ligand, endotoxin, in alcoholic liver disease (ALD) was established by studies of Thurman and colleagues (23, 24). Studies in knockout mouse models have shown that chronic alcohol feeding in mice deficient of CD14, TLR4 and LPS-binding protein (LBP) results in alleviation of alcohol-induced liver injury indicating an important role for the TLR4 pathway (24–27). Recent studies suggested that LPS recognition by TLR4 expressed on hepatic stellate cells and sinusoidal epithelial cells may further contribute to the progression of ALD (28, 29).
Alcohol sensitizes Kupffer cells and monocytes/macrophages to produce increased TNFα in response to endotoxin (30). Studies investigating mechanisms of alcohol-induced sensitization of Kupffer cells to endotoxin focused on intermediates of the TLR4 induced signalling pathway. Reports on the effects of alcohol on membrane proximal events using mutant and knock out mice have shown an important role for CD14 (26) and TLR4 (24). In another report, hepatic expression of TLR1, 2, 4, 6, 7, 8 and 9 mRNA was increased in wild-type mice using the Leiber-DeCarli chronic alcohol feeding model (31). Alcohol feeding also resulted in sensitization to liver damage and inflammation because administration of TLR1, 2, 4, 6, 7, 8 and 9 ligands resulted in increased expression of TNFα mRNA (31). Other investigations found that deficiency in TLR2 had no protective effect on ALD in a mouse model of chronic alcohol feeding (32).
TLR4 triggers signalling from the cytoplasmic TIR domain via recruitment of different intracellular adaptors and culminate in the production of pro-inflammatory cytokines or Type I IFN to activate innate immune responses (33–34) (Fig 2). The myeloid differentiation factor 88 (MyD88) is a common adapter to all known TLRs except for TLR3 which exclusively utilizes TIR-domain containing adaptor inducing TGFβ (TRIF) (35). However, TLR4 is unique in utilization of both MyD88 and TRIF (MyD88-independent) downstream signalling (36). Upon activation of TLR4, signalling intermediates IRAK1 and 4 are recruited to the TLR4 complex via interaction with MyD88 leading to IKK kinase activation and induction of pro-inflammatory cytokines. Recruitment of the TRIF adapter activates IRF3 phsophorylation that results in Type I IFN production (35, 36). Whether chronic alcohol affects activation and recruitment of the IRAK family members is currently not known. However, acute alcohol-induced tolerance and sensitization has been attributed to changes in IRAK-1 expression (37) and kinase activity in in-vitro models in macrophages (38). The role of MyD88, the common TLR adaptor molecule was recently evaluated in chronic alcohol induced liver injury in a mouse model (32). These studies showed that MyD88 knockout mice were highly susceptible to alcohol-induced fatty liver (32), indicating a role for a MyD88-independent pathway in alcoholic liver injury. TLR4-induced MyD88-independent signalling leads to activation of IKKε, NFκB as well as interferon regulatory factor 3 (IRF3) and downstream Type I IFN activation (33, 34). While the nuclear levels of IRF3, that would indicate activation, were not significantly increased in the livers of alcohol-fed mice, other investigators found that acute alcohol administration suppressed TLR3 downstream signalling in macrophages (39). Recent investigations suggest that TRIF-regulated IRF3 binds to the promoter region of the TNFα gene and upregulates transcription in chronic alcohol-exposed macrophages contributing to alcohol-induced steatosis (40).
In contrast to chronic alcohol consumption, acute alcohol exposure inhibited TLR4 signalling in monocytes and macrophages after in vitro as well as in vivo alcohol treatment in mice leading to decreased LPS-induced TNFα production (41, 42). In-vitro studies show that acute ethanol exposure leads to decreased LPS-induced IRAK-1 phosphorylation (38). The role of membrane and endosomal TLRs and their intracellular adapter molecules is increasingly evident with TLR4 playing a “gate-keeper” role in ALD.
3.0.2 Alcohol, TLRs and ROS: an emerging link
Oxidative stress-induced cellular responses play an important role in innate immune cell activation. In the liver, Kupffer cells produce reactive oxygen species (ROS) in response to chronic alcohol exposure as well as endotoxin (43, 44). Alcohol-induced sensitization to LPS has been recently attributed to ROS production. Evidence shows that direct interaction of NADPH oxidase isozyme 4 with TLR4 is involved in LPS-mediated ROS generation and NFκB activation in neutrophils (45). Furthermore, NADPH oxidase induces TLR2 and TLR4 expression in human monocytic cells (46). In chronic alcohol fed rats, pretreatment with diphenyliodonium (DPI), which inhibits NADPH oxidase, normalized ROS production, decreased LPS-induced ERK1/2 phosphorylation and inhibited increased TNFα production in Kupffer cells (43,44). In a recent study, inhibition of NADPH oxidase prevented steatosis, up-regulation of TLR2, 4, 6 and 9 mRNA, and sensitization to respective ligand- induced liver injury (31), indicating a cross talk between oxidative stress and TLR pathways in ALD. Previous reports indicate that p47 phox −/− mice are resistant to alcohol-induced liver injury, further suggesting an important role for NADPH oxidase in not only inflammatory responses but also liver injury (43). In another study, dilinoleoylphosphotidylcholine (DPC), an anti-oxidant, prevented LPS-induced NFκB and ERK1/2 activation and TNFα production in Kupffer cells of chronic alcohol-fed rats (47). It is now widely accepted that ROS not only plays a critical role in direct hepatocyte injury but also contributes to increased inflammatory responses contributing to liver injury.
4.0 Transcription factors in alcoholic liver injury
4.0.1 NFkB
The nuclear regulatory factor κB (NFκB) is a central regulator of cellular stress in all cell types in the liver. The family of NFκB proteins such as RelA/p65, RelB, c-Rel and p50, reside in the cytosol of resting cells as dimers in a complex with inhibitory κB molecules (48). These dimers are activated and translocated to the nucleus upon stress signals that could be pathogen-derived, oxidative stress, etc. Danger signals lead to activation of the IKK kinase complex consisting of IKK/IKKβ/NEMO and phosphorylation of IκB. Phosphorylated IκB is then ubiquitinated and degraded by the proteosomal pathway (48). Dissociation of IκB exposes the nuclear translocation sites of NFκB allowing nuclear translocation and DNA binding. NFκB forms p65/p50 heterodimers in macrophages and binds to the promoter region of various pro-inflamamatory genes to result in gene transactivation.
Hepatic macrophage expression of pro-inflammatory mediators is largely regulated by NFκB. Chronic alcohol mediated liver injury is associated with activation of TLR4 by circulating LPS on liver macrophages culminating in NFκB activation and pro-inflammatory cytokine production. Murine models of chronic alcohol administration show increased NFκB DNA binding in the liver (49). It is believed that chronic alcohol primes the liver by sustained NFκB activation and induction of basal and LPS-stimulated TNFα (50). Similar to resident liver macrophages, monocytes from chronic alcoholic patients also showed increased NFκB activation compared to controls (51).
In contrast to increased NFκB activation by chronic alcohol, acute alcohol exposure decreased nuclear translocation and activation of NFκB p65/p50 heterodimer (52) whereas NFκB p50 homodimer binding to DNA was increased by acute alcohol in human monocytic cells (53). It is evident from these studies that differential regulation of NFκB is based on the length of alcohol exposure and is pivotal in the regulation of TNFα induction in monocytes and macrophages in ALD.
4.0.2 AP-1
Transcription factors AP-1 are homodimers and heterodimers composed of basic region-leucine zipper (hZIP) protein that belongs to the Jun (c-Jun, Jun B and JunD), Fos (c-Fos, FosB, Fra-1, Fra-2), Jun dimerization partners (JDP1, JDP2) and the closely related activating transcription factors (ATFs) (54). AP-1 regulates cellular proliferation and death through induction of cell cycle modulators such as cyclin D1 and p53 (55). While AP-1 is activated by pro-inflammatory cytokines, oxidative stress, growth factors and endotoxin, the MAPK kinase cascade system seems to be the most important intracellular mediator (55). Acute alcohol-induced increased sensitization of macrophages was associated with an upregulation of AP-1 activity, increased CD14 mRNA and pro-inflammatory cytokine production (56). Inhibition of ROS production using a recombinant adenovirus overexpressing antioxidant Cu,Zn superoxide dismutase (SOD) in the liver abrogated both AP-1 activity and CD14 expression (56). Furthermore, acute alcohol-induced AP-1 activation in human monocytes is dependent on Src kinase activity and regulates IL-10 production (57). On the other hand, chronic alcohol-induced AP-1 mediated transcription through a PKC-dependent pathway via increased expression of c-jun and c-fos in hepatocytes, thus influencing proliferative activity (58) and possibly contributing to malignant transformation. Interestingly, LPS-stimulated activation of AP-1 binding to DNA was not increased in isolated Kupffer cells by chronic ethanol feeding (59).
4.0.3 EGR-1
Early growth response-1 (Egr-1) is a transcription factor regulated by the MAPK signalling cascade and induced by LPS (60). Egr-1, an immediate early gene/zinc-finger transcription factor is required for induction of TNFα, adhesion molecules, basic fibroblast growth factor, transforming growth factor-β, MCP-1 and MIP-2 (61). Studies have shown that chronic alcohol feeding increases Egr-1 binding to the promoter of the TNFα promoter and this is MAPK-ERK dependent in hepatic macrophages (62). In fact, it has been suggested that Egr-1 contributes to the increased sensitivity of macrophages to LPS-stimulated TNFα production after chronic alcohol exposure (59). Furthermore, the requirement for Egr-1 in chronic alcohol-induced liver injury was shown by a lack steatosis or increased ALT and TNFα levels in the liver of chronic alcohol-fed Egr-1 knockout mice (63). Whether Egr-1 contributes to induction of other inflammatory cytokine genes such as MCP-1, MIP-2 and ICAM-1, all enhanced during alcoholic liver injury, requires further investigation.
4.0.4 STAT
The Janus-kinase (JAK) associated signal transducer and activator transcription factor (STAT) is activated by cytokines and growth factors and has been implicated in immune and hepatic cell functions (64, 65). Activation of STATs via interleukin-6 (IL-6) and interferons (IFN) play an important role in hepatic regeneration, antiviral activity and gene expression (66). Acute alcohol activates LPS-induced Src-dependent STAT3 activation to modulate IL-10 production in monocytes (57). On the other hand, IL-6-activated STAT3 and IFN-induced STAT1 was blocked by acute alcohol exposure in monocytes (67) and freshly isolated but not in cultured hepatocytes (68). Immunoinhibitory molecules such as SOCS1 and SOCS3 were upregulated in acute alcohol exposed monocytes, resulting in down-modulation of cytokine-induced STAT1/3 signalling (67). Activation of STAT3 was reportedly decreased in alcoholic liver disease patients with cirrhosis (69). Recent studies suggest that chronic alcohol-fed hepatocyte-specific STAT3 knockout mice are more susceptible to alcohol-induced fatty liver injury with less pronounced inflammatory cytokine induction whereas macrophage-specific STAT3 knockout mice exhibit reduced liver inflammation (70). Thus, the pro- or anti-inflammatory role of STAT3 after chronic alcohol feeding is dependent on the cell-type involved in the liver. Chronic alcohol consumption attenuates IFN-induced STAT1 activation and results in loss of NK cell activity in the liver leading to acceleration of hepatic fibrosis (6). It appears that while STAT1 activity is abrogated by acute and chronic alcohol exposure, chronic alcohol enhances STAT3 and modulates alcohol-induced steatosis and inflammation based on the cell-type in which it is expressed.
5.0 MAPK signalling
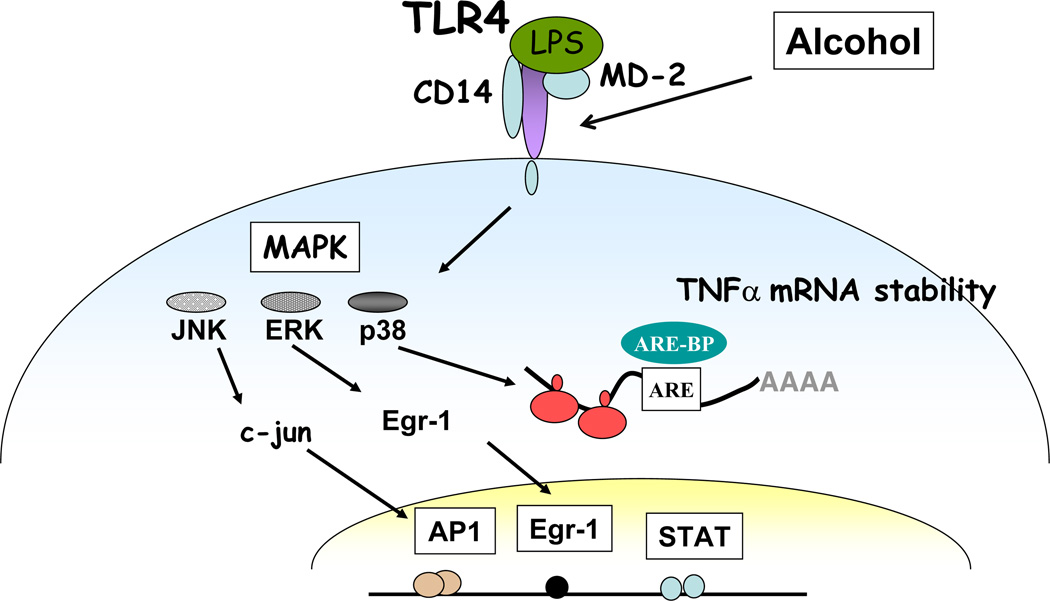
LPS recognition activates the MAPK family members including extracellular receptor activated kinases 1/2 (ERK1/2), p38 and c-jun-N-terminal kinase (JNK) resulting in TNFα production (71). It has been shown that chronic alcohol induces LPS-induced ERK1/2 activation and subsequent transcription of Egr-1, an immediate early gene transcription factor, which contributes to TNFα expression in murine hepatic macrophages (59, 72). Similarly, in vitro LPS stimulation of Kupffer cells exposed to chronic alcohol showed increased p38 activity whereas decreased JNK activity as observed in liver after chronic alcohol feeding (73). Activation of p38 MAPK by LPS contributes to TNFα mRNA stability via interaction with tristetraprolin (TTP) (74). Inhibition of p38 activation completely abrogated alcohol-mediated stabilization of TNFα mRNA (73). On the other hand, ERK1/2 inhibition did not alter TNFα mRNA stability but affected its transcription in chronic alcohol-exposed macrophages (59). LPS stimulation of JNK leads to phosphorylation of c-jun and subsequent binding of c-jun to CRE/AP-1 site in the TNFα promoter (71). Although chronic alcohol feeding decreased JNK activity without any effect on TNFα mRNA, acute alcohol exposure increased JNK phosphorylation as well as AP-1 binding in the presence of combined TLR4 plus TLR2 stimulation (38) in human monocytes. Furthermore, LPS-induced ERK1/2 phosphorylation was decreased in acute in vitro alcohol-exposed monocytes (38), whereas p38 MAPK activity was increased contributing to anti-inflammatory mediators such as IL-10 after acute alcohol exposure in monocytes (75). A single dose of in vivo alcohol exposure inhibited IL-6 and TNFα induced by TLR2, TLR4 and TLR9 ligands due to impaired p38 and ERK 1/2 activation (76). Thus, chronic and acute alcohol modulate MAP kinases differentially depending on the cell type and duration of alcohol exposure and ERK regulation appears to contribute to ALD.
6.0 Cytokines and cytokine receptors in ALD
6.0.1 TNFα and pro-inflammatory cytokines
Among the pro-inflammatory cytokines produced during alcoholic liver injury, TNFα has been well characterized in animal models and human studies (4, 77–80). The most compelling evidence documenting the pivotal role of TNFα in alcohol-induced liver injury came from experiments that used anti-TNFα antibody to prevent liver injury in alcohol-fed rats (81, 82) and the observation that mice lacking TNF type I receptor do not develop alcoholic liver injury (83). The role of TNFα in human alcoholic hepatitis is evident from elevated serum levels of TNFα and increased immunohistochemical staining of TNFα in the liver (82, 84, 85). Chronic alcohol not only increases TNFα in resident hepatic macrophages but also circulating monocyte/macrophages contributing to development and progression of liver disease. Recent studies have reported that higher bioactive TNFα and increased expression of Th1-type cytokines IL-6, IFN-γ and IL-12 was observed in the liver of alcohol of fed rats and contributes to alcohol- induced steatosis (85).
6.0.2 Anti-inflammatory cytokines and negative regulators
Resolution of inflammation is a hallmark to dampen the increased inflammatory gene expression and curb the inflammatory response. Various cytokines such as IL-10, prostaglandins, TGF-beta (86, 87) and intracellular signalling molecules such as IRAK-M, ST2, PI3-K, SOCS1, A20 and SIGIRR (88) have been shown to contribute to the anti-inflammatory pathway. Increased injury in chronic alcohol fed IL-10 knockout mice indicate a role for IL-10 in alcohol-induced liver sensitization to LPS, (89) due to increase in pro-inflammatory cytokines. It is thus tempting to speculate that the antiinflammatory cytokine, IL-10, could counter-regulate the sustained pro-inflammatory activation in the chronic alcoholic liver. However, increased expression of IL-10 by adiponectin, a adipokine with potent anti-inflammatory properties, suppressed TLR4-mediated signalling and TNFα production in Kupffer cells (90, 91). In contrast to chronic alcohol, acute alcohol exposure increases IL-10 and TGF-beta production in monocytes (92, 93). Acute alcohol mediated augmentation of IL-10 was regulated by hemeoxygenase-1 (HO-1) via increase p38 MAP kinase activity in human monocytes (75). Furthermore, it was shown that acute alcohol activates Src/STAT3, AP-1 and Sp-1 pathways to modulate IL-10 production in monocytes (57). Immunoinhibitory molecules such as SOCS1 and SOCS3 were also upregulated in acute alcohol exposed monocytes, resulting in modulation of cytokine-induced STAT1/3 signalling (67). Additional studies on specific anti-inflammatory mechanisms will provide a better understanding of the contribution of negative regulation of inflammation to alcohol-induced liver injury.
7.0 Conclusion
In this review, we have discussed the signalling molecules affected during acute and chronic alcohol exposure of immune cells in the liver. Interactions of parenchymal and the non-parenchymal cells of the liver are mediated by inflammatory cytolines and alcohol-induced oxidative stress. Emerging evidence suggests a pivotal role for various signalling molecules including the Toll-like receptors, transcription factors such as NFκB, AP-1, Egr-1 and STATs. Studies so far indicate that a number of signalling pathways are involved in alcoholic liver disease. An integrative systems biology approach to characterize disease-specific pathways and their coordination within each cell type and between cells in the liver will provide a better understanding of the disease-phenotype and aid in drug discovery for treatment of alcoholic liver damage.
Fig 3.
TLR4 mediated MAPK signalling in alcohol-exposed macrophages. Alcohol alters functions of Toll-like receptors TLR4 in the liver resulting in activation of MAP kinases culminating in alteration of binding to transcription factors such as Egr-1, AP-1 and STAT1. Alcohol also affects cytokine mRNA stability via modulation of MAP kinase activity.
Acknowledgement
The central theme and content of this review encompasses information presented at the Satellite meeting supported by NIH AA017357 and AA015283 conference grant.
Abbreviations
- ALD
alcoholic liver disease
- Egr-1
early growth responae factor-1
- HSF
heat shock transcription factor
- IRF
interferon-responsive factor
- NF-kB
nuclear factor kappa B
- MAPK
mitogen activated protein kinase
- MyD88
myeloid differentiation primary response gene (88)
- STAT
signal transducers and activators of transcription
- SOCS
suppressor of cytokine signalling
- TLR
Toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hines IN, Wheeler MD. Recent advances in alcoholic liver disease III. Role of innate immune responses in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G310–G314. doi: 10.1152/ajpgi.00094.2004. [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, Tamagawa T, et al. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of Liver disturbance. Alcohol Clin Exp Res. 2000;24:48S–54S. [PubMed] [Google Scholar]
- 3.McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- 4.Khoruts A, Stahnke L, McClain CJ, Logan G, Allen J. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267–276. [PubMed] [Google Scholar]
- 5.Thakur V, McMullen MR, Ptritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J Gastroenterol Hepatol. 2007;22(suppl 1):S53–S56. doi: 10.1111/j.1440-1746.2006.04650.x. [DOI] [PubMed] [Google Scholar]
- 6.Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 8.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Saitoh S, Akashi S, Yamada T, Tanimura N, Kobayashi M, Konno K, et al. Lipid A antagonist, lipid IVa, is distinct from lipid A in interaction with Toll-like receptor 4 (TLR4)-MD-2 and ligand-induced TLR4 oligomerization. Int Immunol. 2004;16:961–969. doi: 10.1093/intimm/dxh097. [DOI] [PubMed] [Google Scholar]
- 10.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10682. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 11.Visintin A, Mazzoni A, Spitzer JA, Segal DM. Secreted MD-2 is a large polymeric protein that efficiently confers lipopolysaccharide sensitivity to Toll-like receptor 4. Proc Natl Acad Sci USA. 2001;98:12156–12161. doi: 10.1073/pnas.211445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobias PS, Soldau K, Ulevitch RJ. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med. 1986;164:777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright SD, Tobias PS, Ulevitch RJ, Ramos RA. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J Exp Med. 1989;170:1231–1241. doi: 10.1084/jem.170.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 15.Dunne A, O’Neill LA. Adaptor usage and Toll-like receptor signaling specificity. FEBS Lett. 2005;579:3330–3335. doi: 10.1016/j.febslet.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Jang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, et al. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 17.Simazu R, Akashi S, Ogata H, Nagai Y, Fukodome K, Miyake K, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 19.Giovanni TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, et al. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation of picomolar concentrations. Proc Natl Acad Sci USA. 2004;101:4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitsuzawa H, Nishitani C, Hyakushima N, Shimzu T, Sano H, Matsushima N, et al. Recombinant soluble forms of extracellular TLR4 domain and MD-2 inhibit lipopolysaccharide binding on cell surface and dampen lipopolysaccharide-induced pulmonary inflammation in mice. J Immunol. 2006;177:8133–8139. doi: 10.4049/jimmunol.177.11.8133. [DOI] [PubMed] [Google Scholar]
- 21.Szabo G, Dolganiuc A, Mandrekar P. Pattern recognition receptors. Hepatology. 2006;44:287–298. doi: 10.1002/hep.21308. [DOI] [PubMed] [Google Scholar]
- 22.Seki E, Brenner DA. TLRs and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 23.Enomoto N, Ikejima K, Bradford B, Rivera C, Kono H, Brenner DA, et al. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology. 1998;115:443–451. doi: 10.1016/s0016-5085(98)70211-2. [DOI] [PubMed] [Google Scholar]
- 24.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 25.Uesugi T, Froh M, Arteel GE, Bradford BU, Wheller MD, Gabele E, et al. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol. 2002;168:2963–2969. doi: 10.4049/jimmunol.168.6.2963. [DOI] [PubMed] [Google Scholar]
- 26.Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, et al. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4747–4742. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- 27.Romics L, Jr, Mandrekar P, Kodys K, Velayudham A, Drechsler Y, Dolganiuc A, et al. Increased lipopolysaccharide sensitivity in alcoholic fatty livers is independent of leptin deficiency and Toll-like receptor 4 (TLR4) or TLR2 mRNA expression. Alcohol Clin Exp Res. 2005;29:1018–1026. doi: 10.1097/01.alc.0000167744.60838.4a. [DOI] [PubMed] [Google Scholar]
- 28.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 29.Deaciuc IV, Spitzer JJ. Hepatic sinusoidal endothelial cell in alcoholemia and endotoxemia. Alcohol Clin Exp Res. 1996;20:607–614. doi: 10.1111/j.1530-0277.1996.tb01662.x. [DOI] [PubMed] [Google Scholar]
- 30.Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med. 2003;228:882–890. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- 31.Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, et al. Differential liver sensitization to Toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- 32.Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kurt-Jones E, et al. The critical role of Toll-like receptor 4 in alcoholic liver disease is independent of the common TLR adaptor, MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, et al. LPS-TLR4 signaling to IRF-3 3/7 and NF-kappaB involves the Toll adaptors TRAM and TRIF. J Exp Med. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawai T, Takeuchi O, Fujita T, Inoue J, Mühlradt PF, Sato S, et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 35.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto M, Sata S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 37.Yamashina S, Wheeler MD, Rusyn I, Ikejima K, Sato N, Thurman RG. Tolerance and sensitization to endotoxin in Kupffer cells caused by acute ethanol involve interleukin-1 receptor-associated kinase. Biochem Biophys Res Commun. 2000;277:686–690. doi: 10.1006/bbrc.2000.3738. [DOI] [PubMed] [Google Scholar]
- 38.Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol. 2006;176:7628–7635. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- 39.Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Acute exposure to ethanol affects Toll-like receptor signaling and subsequent responses: an overview of recent studies. Alcohol. 2004;33:235–239. doi: 10.1016/j.alcohol.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Zhao XJ, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, et al. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181:3049–3056. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandrekar P, Dolganiuc A, Bellerose G, Kodys K, Romics L, Nizamani R, et al. Acute alcohol inhibits the induction of nuclear regulatory factor kappa B activation through CD14/Toll-like receptor 4, interleukin-1, and tumor necrosis factor receptors: a common mechanism independent of inhibitory kappa B alpha degradation? Alcohol Clin Exp Res. 2002;26:1609–1614. doi: 10.1097/01.ALC.0000036926.46632.57. [DOI] [PubMed] [Google Scholar]
- 42.Mandrekar P, Catalano D, White B, Szabo G. Moderate alcohol intake in humans attenuates monocyte inflammatory responses: inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcohol Clin Exp Res. 2006;30:135–139. doi: 10.1111/j.1530-0277.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 43.Kono H, Rusyn I, Yin M, Gäbele E, Yamashina S, Dikalova A, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thakur V, McMullen MR, Wang Q, Nagy LE. Enhanced LPS-stimulated ERK ½ activation and TNF alpha secretion by rat Kupffer cells after chnic ethanol feeding is mediated via increased production of reactive oxygen species (ROS) by NADPH oxidase. Hepatology. 2005;42:571A. [Google Scholar]
- 45.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NADPH oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 46.Dasu MR, Devaraj S, Ling Z, Hwang DH, Jialal I. High glucose induces Toll-like receptor expression in human monocytes: mechanisms of activation. Diabetes. 2008;57:3090–3098. doi: 10.2337/db08-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao Q, Mak KM, Leiber CS. Dilinoleoylphosphatidylcholine decreases LPS-induced TNF-alpha generation in Kupffer cells of ethanol-fed rats: respective roles of MAPKs and NF-kappaB. Biochem Biophy Res Comm. 2002;294:849–853. doi: 10.1016/S0006-291X(02)00586-7. [DOI] [PubMed] [Google Scholar]
- 48.Ghosh S. Regulation of inducible gene expression by the transcription factor NF-kappaB. Immunol Res. 1999;19:183–189. doi: 10.1007/BF02786486. [DOI] [PubMed] [Google Scholar]
- 49.Wheeler MD, Yamashina S, Froh M, Rusyn I, Thurman RG. Adenoviral gene delivery can inactivate Kupffer cells: role of oxidants in NF-kappaB activation and cytokine production. J Leukoc Biol. 2001;69:622–630. [PubMed] [Google Scholar]
- 50.Nanji AA, Jokelainen K, Rahemtulla A, Miao L, Fogt F, Matsumoto H, et al. Activation of nuclear factor kappa B and cytokine imbalance in experimental alcoholic liver disease in the rat. Hepatology. 1999;30:934–943. doi: 10.1002/hep.510300402. [DOI] [PubMed] [Google Scholar]
- 51.Hill DB, Barve S, Joshi-Barve S, McClain C. Increased monocyte nuclear factor-kappaB activation and tumor necrosis factor production in alcoholic hepatitis. J Lab Clin Med. 2000;135:387–395. doi: 10.1067/mlc.2000.106451. [DOI] [PubMed] [Google Scholar]
- 52.Mandrekar P, Catalano D, Szabo G. Inhibition of lipopolysaccharide-mediated NFkappaB activation by ethanol in human monocytes. Int Immunol. 1999;11:1781–1790. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- 53.Mandrekar P, Catalano D, Szabo G. Alcohol-induced regulation of nuclear regulatory factor-kappa beta in human monocytes. Alcohol Clin Exp Res. 1997;21:988–994. [PubMed] [Google Scholar]
- 54.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell proliferation and transformation. Biochem Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 55.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 56.Wheeler MD, Thurman RG. Upregulation of CD14 in liver due to acute ethanol involves oxidant-dependent AP-1 pathway. J Biol Chem. 2003;278:8345–8351. doi: 10.1074/jbc.M212076200. [DOI] [PubMed] [Google Scholar]
- 57.Norkina O, Dolganiuc A, Shapiro T, Kodys K, Mandrekar P, Szabo G. Acute alcohol activates STAT3, AP-1, and Sp-1 transcription factors via the family of Src kinases to promote IL-10 production in human monocytes. J Leukoc Biol. 2007;82:752–762. doi: 10.1189/jlb.0207099. [DOI] [PubMed] [Google Scholar]
- 58.Casini A, Galli G, Salzano R, Ceni E, Franceschelli F, Rotella CM, et al. Acetaldehyde induces c-Fos and c-Jun proto-oncogenes in fat-storing cell cultures through protein kinase C activation. Alcohol Alcohol. 1994;29:303–314. [PubMed] [Google Scholar]
- 59.Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr-1 contribute to increased TNF-alpha production in rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2002;282:G6–G15. doi: 10.1152/ajpgi.00328.2001. [DOI] [PubMed] [Google Scholar]
- 60.Gashler A, Sukhatme VP. Early growth response protein 1 (Egr-1) prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol. 1995;50:191–224. doi: 10.1016/s0079-6603(08)60815-6. [DOI] [PubMed] [Google Scholar]
- 61.Yan SF, Fujita T, Lu J, Okada K, Zou YS, Mackman N, et al. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med. 2000;6:1355–1361. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- 62.Pritchard MT, Nagy LE. Etahnol-induced liver injury: potential roles for egr-1. Alcohol Clin Exp Res. 2005;29:146S–150S. doi: 10.1097/01.alc.0000189286.81943.51. [DOI] [PubMed] [Google Scholar]
- 63.McMullen MR, Pritchard MT, Wang Q, Millward CA, Croniger CM, Nagy LE. Early growth response-1 transcription factor is essential for ethanol-induced fatty liver injury in mice. Gastroenterology. 2005;128:2066–2076. doi: 10.1053/j.gastro.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 65.Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- 66.Kerr IM, Costa-Pereira AP, Lillemeier BF, Strobl B. Of JAKs, STATs, blind watchmakers, jeeps and trains. FEBS Lett. 2003;3(546):1–5. doi: 10.1016/s0014-5793(03)00411-3. [DOI] [PubMed] [Google Scholar]
- 67.Norkina O, Dolganiuc A, Catalano D, Kodys K, Mandrekar P, Syed A, et al. Acute Alcohol Intake Induces SOCS1 and SOCS3 and Inhibits Cytokine-Induced STAT1 and STAT3 Signaling in Human Monocytes. Alcohol Clin Exp Res. 2008;32:1565–1573. doi: 10.1111/j.1530-0277.2008.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J, Clemens DL, Cederbaum AI, Gao B. Ethanol inhibits the JAK-STAT signaling pathway in fresh isolated rat hepatocytes but not in cultured hepatocytes or HepG2 cells: evidence for a lack of involvement of ethanol metabolismk. Clin Biochem. 2001;34:203–209. doi: 10.1016/s0009-9120(01)00216-8. [DOI] [PubMed] [Google Scholar]
- 69.Horiguchi N, Ishac EJ, Gao B. Liver regeneration is suppressed in alcoholic cirrhosis: correlation with decreased STAT3 activation. Alcohol. 2007;41:271–280. doi: 10.1016/j.alcohol.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, Lafdil F, et al. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148–1158. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells: regulation by Egr-1, c-jun and NF-kappaB transcription factors. J Biol Chem. 1997;272:17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- 72.Shi L, Kishore R, McMullen MR, Frenkel J, Nagy LE. Chronic ethanol increases lipopolysaccharide stimulated Egr-1 expression in RAW 264.7 macrophages: contribution to enhanced tumor necrosis factor alpha production. J Biol Chem. 2002;277:14777–14785. doi: 10.1074/jbc.M108967200. [DOI] [PubMed] [Google Scholar]
- 73.Kishore R, McMullen MR, Nagy LE. Stabilization of tumor necrosis factor alpha mRNA by chronic ethanol: role of A + U-rich elements and p38 mitogen-activated protein kinase signaling pathways. J Biol Chem. 2001;276:41930–41937. doi: 10.1074/jbc.M107181200. [DOI] [PubMed] [Google Scholar]
- 74.Mahtani KR, Brook M, Dean JL, Sully G, Saklatvala J, Clark AR. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol Cell Biol. 2001;21:6461–6469. doi: 10.1128/MCB.21.9.6461-6469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drechsler Y, Dolganiuc A, Norkina O, Romics L, Li W, Kodys, et al. Heme oxygenase-1 mediates the anti-inflammatory effects of acute alcohol on IL-10 induction involving p38 MAPK activation in monocytes. J Immunol. 2006;177:2592–2600. doi: 10.4049/jimmunol.177.4.2592. [DOI] [PubMed] [Google Scholar]
- 76.Goral J, Kovacs EJ. In vivo ethanol exposure down-regulations TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J Immunol. 2005;174:456–463. doi: 10.4049/jimmunol.174.1.456. [DOI] [PubMed] [Google Scholar]
- 77.Song Z, Zhou Z, Uriarte S, Wang S, Kang YJ, Chen T, et al. S-denosylhomocysteine sensitizes to TNF-alpha hepatotoxicity in mice and liver cells: a possible etiological factor in alcoholic liver disease. Hepatology. 2004;40:989–997. doi: 10.1002/hep.20412. [DOI] [PubMed] [Google Scholar]
- 78.McClain C, Hill D, Schmidt J, Diehl AM. Cytokines and alcoholic liver disease. 1993;13:170–182. doi: 10.1055/s-2007-1007347. [DOI] [PubMed] [Google Scholar]
- 79.Honchel R, Ray M, Marsano L, Cohen D, Lee E, Shedlofsky S, et al. Tumor necrosis factor in alcohol-enhanced endotoxin liver injury. Alcohol Clin Exp Res. 1992;16:665–669. doi: 10.1111/j.1530-0277.1992.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 80.Thurman RG. Mechanisms of hepatic toxicity. III. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 81.Tilg H, Jalan R, Kaser A, Davies NA, Offner FA, Hodges SI, et al. Anti-tumor necrosis factor-alpha monoclonal antibody therapy in severe alcoholic hepatitis. J Hepatol. 2003;38:419–425. doi: 10.1016/s0168-8278(02)00442-7. [DOI] [PubMed] [Google Scholar]
- 82.Imuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alpha attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 83.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, et al. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 84.McClain CJ, Barve S, Barve S, Deaciuc I, Hill DB. Tumor necrosis factor and alcoholic liver disease. Alcohol Clin Exp Res. 1998;22(Suppl 5):248S–252S. doi: 10.1097/00000374-199805001-00006. [DOI] [PubMed] [Google Scholar]
- 85.Olleros ML, Martin ML, Vesin D, Fotio AL, Santiago-Rober ML, Rubbia-Brandt L, et al. Fat diet and alcohol-induced steatohepatitis after LPS challenge in mice: role of bioactive TNF and Th1 type cytokines. Cytokine. 2008;44:118–125. doi: 10.1016/j.cyto.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 86.Schmidt-Weber CB, Blaser K. Regulation and role of transforming growth factor-beta in immune tolerance induction and inflammation. Curr Opin Immunol. 2004;16:709–716. doi: 10.1016/j.coi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 87.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy–review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 88.Liew FY, Xu D, Brint EK, Oneill LA. Negative regulation of Toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 89.Hill DB, D’Souza NB, Lee EY, Burikhanov R, Deaciuc IV, deVilliers WJ. A role for interleukin-10 in alcohol-induced liver sensitization to bacterial lipopolysaccharide. Alcohol Clin Exp Res. 2002;26:74–82. [PubMed] [Google Scholar]
- 90.Thakur V, Prtichard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF-alpha production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2006;290:G998–G1007. doi: 10.1152/ajpgi.00553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang H, Park PH, McMullen MR, Nagy LE. Mechanisms for the anti-inflammatory effects of adiponectin in macrophages. J Gastroenterol Hepatol. 2008;23(Suppl 1):S850–S853. doi: 10.1111/j.1440-1746.2007.05284.x. [DOI] [PubMed] [Google Scholar]
- 92.Mandrekar P, Catalano D, Girouard L, Szabo G. Human monocyte IL-10 production is increased by acute ethanol treatment. Cytokine. 1996;8:567–577. doi: 10.1006/cyto.1996.0076. [DOI] [PubMed] [Google Scholar]
- 93.Szabo G, Mandrekar P, Girouard L, Catalano D. Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-alpha, interleukin-1 beta and elevated interleukin-10, and transforming growth factor-beta production. Alcohol Clin Exp Res. 1996;20:900–907. doi: 10.1111/j.1530-0277.1996.tb05269.x. [DOI] [PubMed] [Google Scholar]