Abstract
Culex pipiens quinquefasciatus were fed blood meals from a live chicken (LC), chicken blood in Alsever’s (AC) solution, defibrinated bovine blood (DB), or bovine blood in citrate (CB) and incubated at 28°C. The effects of different blood meal sources were evaluated with respect to rates of blood feeding and reproduction (i.e., fecundity and fertility) over two gonotrophic cycles. Mosquitoes that fed on the first blood meal were subjected to a second blood meal as follows (first blood meal/second blood meal): LC/LC, LC/DB, DB/DB, CB/CB, AC/AC. Fecundity and fertility of Cx. p. quinquefasciatus were significantly (P < 0.05) greater in mosquitoes fed LC blood; however, fecundity and fertility in different treatment groups varied by gonotrophic cycle. These results contribute to our understanding of the impact of blood meal source on feeding and reproduction in Cx. p. quinquefasciatus. The potential impacts of blood meal source on virus transmission experiments are discussed.
Keyword index: Culex pipiens quinquefasciatus, blood feeding, reproduction
INTRODUCTION
Culex pipiens quinquefasciatus Say is opportunistic, feeding on available avian and mammalian hosts (Jansen et al. 2009, MacKay et al. 2010). Culex p. quinquefasciatus is a vector of parasites throughout the world, including Dirofilaria immitis Leidy (Labarthe et al. 1998, Lai et al. 2001) and Wuchereria bancrofii Cobbold (Kasili et al. 2009). This mosquito species is also one of the primary vectors of the arboviruses St. Louis encephalitis virus (Chamberlain et al. 1959) and West Nile virus (Sardelis et al. 2001). Studies of Cx. p. quinquefasciatus are important because of its impact on both public and veterinary health.
Anautogenous female mosquitoes require the protein from blood to develop eggs (Clements 1992). Laboratory mosquito colonies are often maintained using animals as a blood source; however, this method is expensive, time consuming, and subject to government regulation and inspection, thereby limiting its use for some laboratory experiments (Turell 1988). Laboratory studies of biology and/or mosquito-pathogen interactions largely employ artificial blood delivery systems to feed mosquitoes, particularly in virus infection studies where the use of animals is restricted. Laboratories with limited access to blood sources (e.g., avian, bovine, etc.) must consider the effects of these sources, as well as colonization effects, on reproductive parameters in their mosquitoes. Little is known about how different blood sources affect the life table characteristics of mosquitoes. This must be considered when comparing the results of experiments that use either animals or blood provided by alternative methods. If there are physiological changes that occur in mosquitoes as a result of different blood sources, this variation could influence experimental results.
A previous study with blood fed Ae. aegypti Linnaeus showed that avian blood results in greater fecundity (number of eggs produced) than mammalian blood (Bennett 1970). Conversely, Suleman and Shirin (1981) showed that mammalian blood results in greater fecundity than avian blood in Cx. p. quinquefasciatus. These studies demonstrate variation in fecundity between species fed different blood sources (Bennett 1970, Suleman and Shirin 1981). Despite fecundity differences, no variation was seen in fertility (number of larvae hatched from eggs) for Cx. p. quinquefasciatus provided different blood sources (Suleman and Shirin 1981). These studies illustrate that the blood source affects mosquito reproduction; however, no known studies have investigated the extent to which defibrinated blood, or blood containing anticoagulants, affects mosquito reproduction.
Consequently, the current study investigates the extent to which reproduction (here, fertility and fecundity) in Cx. p. quinquefasciatus is affected by four different blood sources during two sequential gonotrophic cycles. Differences in reproduction observed in mosquitoes imbibing blood from live animals or treated blood through an artificial delivery system are evaluated. Blood feeding rates are also discussed in the framework of mosquito preference for different blood sources and blood delivery methods. The utility of conducting laboratory studies using blood delivered through artificial means as an alternative to animals is discussed.
MATERIALS AND METHODS
Mosquitoes
Five-day-old female Cx. p. quinquefasciatus from a colony established in Alachua County, FL, in 1995 (generation > F69) and maintained under a 14:10 (light:dark) cycle were used. Mosquitoes were reared using standard conditions (Richards et al. 2009) to generate similar-sized individuals. Adult mosquitoes were housed in 0.5 liter cardboard cages (Instawares, Kennesaw, GA) with mesh screening on top, provided a 20% sugar solution and water ad libitum, and maintained at 28° C and 70–80% humidity. A hand-held aspirator was used to access mosquitoes through a plugged hole in the side of the cage.
Mosquito feeding
Female Cx. p. quinquefasciatus were starved of sugar 24 h prior to feeding experiments to induce hunger. Two feeding trials were conducted and three replicate cages containing 27–95 (range) mosquitoes/cage were used for each trial and treatment. Live chickens are commonly used to maintain Culex spp. in colony and artificial feeding methods are often used in our laboratory experiments to evaluate mosquito-virus interactions. Experiments may require using mosquitoes that have completed more than one gonotrophic cycle and the reproductive effects of these feeding regimens are currently unknown. Hence, the treatments (first feed/second feed) were as follows: live chicken/live chicken (LC/LC), live chicken/defibrinated bovine blood (LC/DB), defibrinated bovine blood/defibrinated bovine blood (DB/DB), bovine blood in citrate/bovine blood in citrate (CB/CB), and chicken blood in Alsever’s solution/chicken blood in Alsever’s solution (AC/AC). Defibrinated blood has the fibrin protein mechanically removed during the clotting process, while both sodium citrate and Alsever’s solution are added anticoagulants. The only treatment group fed on two different types of blood was the LC/DB group. These two types of blood are commonly used for mosquito colony maintenance (LC) and in our laboratory experiments (DB), hence, we were interested in the reproductive effects of these specific blood sources.
Defibrinated bovine blood, as well as bovine and chicken blood containing anticoagulants, were acquired commercially (Hemostat, Dixon, CA). Live chickens were acquired locally (Vero Beach Feed and Farm Supply, Vero Beach, FL) and are used routinely for mosquito colony feeding under a protocol approved by the University of Florida Institutional Animal Care and Use Committee.
Similar methods were used for both blood feeding trials except that mosquitoes were 5 d old during the first blood feeding and 14 d old during the second feeding. These ages were selected based on the feeding and oviposition regimens known for this colony and allowed sufficient time for the completion of two gonotrophic cycles. Mosquitoes in different treatment groups were either allowed to feed on a LC or a pledget containing 3 ml of warm (35° C) DB, CB, or AC. Subsequent to feeding, mosquitoes were immobilized with cold and numbers of fully engorged, partially fed, or unfed were determined and recorded. Partially fed and unfed mosquitoes were discarded. Fully engorged specimens from each treatment group were transferred to 0.5 liter cardboard cages with mesh screening, placed in an incubator held at 28° C and 70–80% humidity, and provided 20% sucrose ad libitum. These cages contained an empty 100 ml plastic cup affixed to the bottom to be used later as an oviposition substrate.
Mosquito oviposition
Four days after the first blood feeding (mosquitoes 9 d old), 50 ml of tap water was added to each oviposition cup and mosquitoes were allowed to oviposit overnight. We and others have observed this time period to be sufficient for Cx. p. quinquefasciatus to complete the gonotrophic cycle prior to oviposition (Begum et al. 1985, S. Richards unpublished). Five days post-blood feeding, all adult mosquitoes were aspirated from cages and transferred according to treatment group to new cages containing an empty 100 ml plastic cup affixed to the bottom. As previously described, the plastic cup would be used later to contain an oviposition substrate. Four days after the second blood feeding (mosquitoes 18 d old), this oviposition process was repeated for all groups. For each blood feeding trial, after adults were removed from cages, the number of egg rafts was recorded for each cage. A fine tip paintbrush was used to carefully transfer single egg rafts to separate wells of flat-bottom 12-well polystyrene plates (Becton Dickinson, Franklin Lakes, NJ). A subset of egg rafts from each cage (N = 1–12) was transferred to these plates. If <12 egg rafts were transferred, mosquitoes in that cage laid <12 egg rafts. Egg rafts not included in subsets were discarded. A photograph of each egg raft was taken, transferred to a computer, printed, and the number of eggs/raft counted. After the photograph was taken, 3 ml of tap water and a 30 μl slurry of larval food diluted in water (1:1 yeast:liver powder) was added to each well for nutrition. Plates were returned to an incubator held at 28° C and 70–80% humidity. For each blood feeding trial, eight days post-feeding (three days post-oviposition), 1 ml 95% ethanol was added to the wells of each plate to kill larvae. Larvae were counted using an Olympus SZX12 stereoscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
Rates of feeding and oviposition were calculated for all female mosquitoes in each treatment group for each blood feeding trial. Feeding rates were characterized as follows: unfed (percentage of all mosquitoes tested that did not feed), partially fed (percentage of all mosquitoes tested that imbibed a partial blood meal), and fully engorged (percentage of all mosquitoes tested that were fully engorged). Oviposition rate was calculated as the percentage of egg rafts laid by fully engorged mosquitoes. Definitions of fecundity and fertility were adapted from Clements (1992). Fecundity and fertility were calculated for each subset of egg rafts in each treatment group for each blood feeding trial. Fecundity was calculated as the number of eggs/raft. Fertility was the percentage of larvae that hatched/raft (i.e., hatch rate).
All statistical analyses were completed using SAS (P < 0.05) (SAS Institute 2002, Cary, NC). χ2 tests were used to determine if there were significant differences between treatments in rates of feeding, oviposition, and hatch for each blood feeding trial. χ2 tests were also used to analyze significant differences in rates of feeding, oviposition, and hatch for each treatment group (LC/LC, LC/DB, DB/DB, CB/CB, AC/AC) between blood feeding trials. Analysis of variance was used to determine significant differences in fecundity and fertility in subsets of rafts between treatments and feeding trials. Means comparisons in SAS cannot be done on interactions, so each treatment group was coded differently with a dummy variable (Richards et al. 2009). If significant differences were observed, then Duncan means comparison tests were used to determine which means were significantly different.
RESULTS
Effects of blood meal source and gonotrophic cycle on egg raft appearance
In all groups, fecundity declined from the first to second gonotrophic cycle. This was most evident in groups fed bovine blood (i.e., defibrinated or citrated) during the second blood feeding.
Effects of blood meal source and gonotrophic cycle on rates of blood feeding, oviposition, hatch, fecundity, and fertility
For both the first and second blood meals, the number of partial and fully engorged female mosquitoes was significantly affected by treatment (Table 1) (partially engorged: first blood meal: χ2= 89.33, df = 4, P < 0.0001, second blood meal: χ2= 9.70, df = 4, P = 0.046; fully engorged: first blood meal: χ2= 69.59, df = 4, P < 0.0001, second blood meal: χ2= 17.50, df = 4, P < 0.0001). Mosquitoes offered a LC blood meal for the first feeding (i.e., LC/DB and LC/LC groups) showed the highest rates of both partial and full engorgement (Table 1). The second blood meal showed the AC/AC and LC/LC groups with the greatest number of fully engorged mosquitoes, while rates of partial engorgement were similar in all groups (Table 1). The DB/DB group showed low feeding rates for both the first and second blood meal compared to other groups (Table 1).
Table 1.
Results from first and second blood feeding trials. Feeding and reproduction for Cx. p. quinquefasciatus fed different types of blood meals and incubated at 28° C. Summarized results from three replicates are shown for each treatment.
| Treatment group | Total no. Females | No. unfed (% of total) | No. partially fed (% of total) | No. engorged (% of total) | No. egg rafts (% of fed that oviposited) | Mean no. eggs/raft (no. rafts in subset) | Mean no. larvae/raft (% hatched in subset) |
|---|---|---|---|---|---|---|---|
| First blood feeding trial
|
|||||||
| LC/LC | 251 | 48 (19) | 49 (20) | 154 (61) | 71 (46) | 122 ± 5.9 (34) | 101 ± 6.0 (83) |
| LC/DB | 183 | 34 (19) | 28 (15) | 121 (66) | 72 (60) | 125 ± 6.1 (35) | 97 ± 5.4 (78) |
| DB/DB | 261 | 174 (67) | 5 (2) | 82 (31) | 33 (40) | 60 ± 4.5 (22) | 40 ± 4.2 (70) |
| CB/CB | 243 | 127 (52) | 0 | 116 (48) | 77 (66) | 51 ± 2.2 (36) | 40 ± 2.6 (79) |
| AC/AC | 234 | 91 (39) | 16 (7) | 127 (54) | 95 (75) | 106 ± 3.7 (36) | 88 ± 3.5 (80) |
| Second blood feeding trial
|
|||||||
| LC/LC | 127 | 52 (41) | 19 (15) | 56 (44) | 34 (61) | 109 ± 8.6 (26) | 87 ± 7.4 (77) |
| LC/DB | 107 | 84 (79) | 8 (7) | 15 (14) | 6 (40) | 41 ± 2.4 (6) | 34 ± 5.5 (80) |
| DB/DB | 79 | 48 (61) | 9 (11) | 22 (28) | 13 (59) | 48 ± 6.9 (13) | 30 ± 6.3 (56) |
| CB/CB | 110 | 66 (60) | 4 (4) | 40 (36) | 26 (65) | 32 ± 2.7 (25) | 22 ± 1.8 (74) |
| AC/AC | 96 | 32 (33) | 9 (9) | 55 (57) | 30 (55) | 62 ± 4.4 (30) | 39 ± 4.7 (63) |
Oviposition rates were significantly affected by blood meal source for the first but not the second blood meal (Table 1) (first blood meal: χ2= 39.19, df = 4, P < 0.0001, second blood meal: χ2= 3.70, df = 4, P = 0.449). For the first blood meal, the highest oviposition rates were observed in the AC/AC group; however, both fecundity and fertility were greatest in the LC/LC and LC/DB groups (Table 1).
Hatch rates for both the first and second blood meals were significantly different between treatments (Table 1) (first blood meal: χ2= 196.18, df = 4, P < 0.0001, second blood meal: χ2= 200.60, df = 4, P < 0.0001). The highest hatch rates for the first blood meal were observed in the LC/LC and AC/AC groups, while the second blood meal showed the highest hatch rate in the LC/DB group (Table 1). Both the DB/DB and CB/CB groups showed consistently low oviposition and hatch rates, as well as fecundity and fertility rates during both blood feeding trials (Table 1).
Effects of blood feeding trial on rates of feeding, oviposition, and hatch
Results for these analyses are shown in Tables 1 and 2. Both treatment groups using LC (i.e., LC/LC and LC/DB) and also the CB/CB group showed significantly higher numbers of fully engorged mosquitoes in the first, compared to the second, blood feeding trial. All other groups did not show any differences in engorgement rate between trials. Only the groups using bovine blood for both feeding trials (i.e., DB/DB and CB/CB) showed significant differences in rates of partial feeding in the first, compared to the second, blood feeding trial and higher rates occurred during the second feeding trial.
Table 2.
Results from χ2 analyses for differences in rates of feeding (fully engorged and partially fed), oviposition, and hatch between the first and second blood feeding trials. Significant P-values in bold.
| Rate | χ2 | df | P |
|---|---|---|---|
| LC/LC
|
|||
| Fully engorged | 10.17 | 1 | 0.001 |
| Partially fed | 1.19 | 1 | 0.276 |
| Oviposition | 3.51 | 1 | 0.061 |
| Hatch | 11.94 | 1 | < 0.0001 |
| LC/DB
|
|||
| Fully engorged | 73.60 | 1 | < 0.0001 |
| Partially fed | 3.80 | 1 | 0.051 |
| Oviposition | 2.19 | 1 | 0.139 |
| Hatch | 2.15 | 1 | 0.143 |
| DB/DB
|
|||
| Fully engorged | 0.36 | 1 | 0.546 |
| Partially fed | 13.79 | 1 | 0.0002 |
| Oviposition | 2.85 | 1 | 0.092 |
| Hatch | 5.26 | 1 | 0.022 |
| CB/CB
|
|||
| Fully engorged | 3.97 | 1 | 0.046 |
| Partially fed | 8.94 | 1 | 0.003 |
| Oviposition | 0.03 | 1 | 0.874 |
| Hatch | 65.62 | 1 | < 0.0001 |
| AC/AC
|
|||
| Fully engorged | 0.25 | 1 | 0.617 |
| Partially fed | 0.63 | 1 | 0.429 |
| Oviposition | 7.32 | 1 | 0.007 |
| Hatch | 270.56 | 1 | < 0.0001 |
Except for the DB/DB group, a greater percentage of fully engorged mosquitoes oviposited after the first, compared to the second, blood feeding trial. Oviposition rate was significantly different and higher for the first, compared to the second, blood feeding trial in the AC/AC group. Hatch rates were significantly higher for the first, compared to the second, blood feeding trial in all groups except LC/DB.
Effects of blood feeding trial and blood meal source on fecundity and fertility in subsets
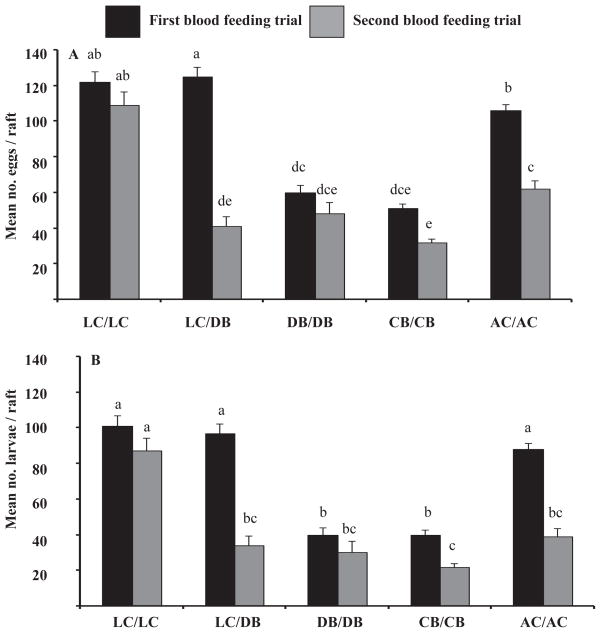
Both fecundity (mean number of eggs/raft) and fertility (mean number of larvae hatched/raft) in subsets were significantly affected by blood feeding trial, blood meal source, and the blood feeding trial × blood meal source interaction (Figure 1, Table 1) (fecundity, blood feeding trial: F = 75.54, df = 1, 257, P <0.0001; blood meal source: F = 60.50, df = 4, 257, P <0.0001; blood feeding trial × blood meal source: F = 9.13, df = 4, 257, P <0.0001) (fertility, blood feeding trial: F = 69.45, df = 1, 257, P <0.0001; blood meal source: F = 51.80, df = 4, 257, P <0.0001; blood feeding trial × blood meal source: F = 7.53, df = 4, 257, P <0.0001). Significantly more eggs were present in rafts oviposited during the first (range = 20–208 eggs/raft), compared to the second, (range = 9–260 eggs/raft) gonotrophic cycle and this varied by treatment group. The LC/DB treatment group during the first blood feeding trial showed significantly greater fecundity, while the CB/CB group during the second blood feeding trial showed significantly lower fecundity than all other groups (Figure 1). Significantly more larvae hatched from egg rafts during the first (range = 1–168), compared to the second (range = 5–156), gonotrophic cycle. The LC/LC (first trial), LC/DB (first trial), AC/AC (first trial), and LC/LC (second trial) groups showed significantly greater fertility, while the CB/CB group during the second trial showed significantly lower fertility than other groups (Figure 1).
Figure 1.
The mean number of eggs/raft (i.e., fecundity) (± standard error) (A) and the mean number of larvae/raft (i.e., fertility) (± standard error) (B) in subsets for the first and second blood feeding trial in each treatment group. Groups with different lowercase letters are significantly different in each figure.
DISCUSSION
Our results show that the blood meal source affects feeding rates and reproduction in colonized Cx. p. quinquefasciatus under the conditions of this test. Mosquitoes fed on a live chicken or chicken blood in Alsever’s solution delivered artificially exhibited higher feeding, fecundity, and fertility rates than those fed on either type of treated bovine blood tested. For both blood feeding trials, mosquitoes fed defibrinated bovine blood exhibited the lowest rates of fecundity and fertility. The current study shows that mosquitoes laid more than twice as many eggs/raft during the first gonotrophic cycle when fed on chicken blood (live or in Alsever’s) (~118 eggs/raft) compared to bovine blood (defibrinated or in citrate) (~54 eggs/raft). Chicken blood contains nucleated blood cells, while mammalian blood contains anucleated cells, which has been hypothesized to influence fecundity if nucleated cells contain more nutrition (Bennett 1970, Downe and Archer 1975). Furthermore, the live chicken blood used here was untreated and this could have also influenced reproductive differences observed between blood sources.
Fecundity may depend, in part, on incubation temperature and host species. A previous study found lower fecundity rates when Cx. p. quinquefasciatus was fed on mice or pigeons at 25° C (~105 or 93 eggs/raft, respectively) (Suleman and Shirin 1981). In a separate study, higher fecundity rates were observed when Cx. p. quinquefasciatus was fed on mice at 25° C or 30° C (~283 or 240 eggs/raft, respectively) (Oda et al. 1980). In addition, Cx. tarsalis Coquillet shows higher fecundity when fed on chickens compared to guinea pigs or snakes, possibly due to different rates of digestion and/or nutrition for different blood sources (Downe and Archer 1975). The differences in fecundity and fertility between these studies may be attributed to genetic differences in mosquito populations or species.
Of the blood meal sources used here, mosquitoes showed the greatest fecundity and fertility rates during the first blood feeding trial using LC. The rates of oviposition and fertility (ranges = 40–75% and 70–83%) we observed for the first blood feeding trial varied by treatment. We observed a reduction in rates of oviposition and fertility (ranges = 40–65% and 56–82%) after the second blood feeding trial, likely due to increased chronological and/or gonotrophic age of the mosquito. We also observed a significant decrease in fecundity from the first to the second blood feeding. A previous study showed that increased chronological age at the time of the first blood meal on a chicken reduced fecundity in Cx. p. quinquefasciatus (McCann et al. 2009). In the same study, mosquitoes that were 5 d old at the time of the first blood meal produced (laid + retained) 115–230 eggs, while mosquitoes that were 13 d old at the first blood meal produced only 25–200 eggs (McCann et al. 2009). Others have also reported a reduction in fecundity with increasing age in Cx. p. quinquefasciatus fed repeatedly on a mouse (Suleman 1979, Walter and Hacker 1974) or chicken (Akoh et al. 1992) and in Cx. p. pipiens fed repeatedly on a chicken (Awahmukalah and Brooks 1985). In addition to the reproductive effects of different blood sources, virus infection rates may be influenced by blood source. It is widely accepted that higher virus doses are required to infect mosquitoes using artificial blood sources, compared to infectious hosts (e.g., Jupp 1976, Meyer et al. 1983, Turell 1988). One reason for this may be that artificially added anticoagulants in the blood source affect mosquito infection rates. Mourya (2002) showed an increase in DENV infection rates in Ae. aegypti attributed to heparinized blood that may increase blood clotting time in the mosquito midgut. Others have hypothesized that the use of defibrinated blood in infection studies inhibits the attachment of virions in the mosquito midgut, due to variation in the attractive forces of hemoglobin (Dodge et al. 1963, Mitchell 1983). The results of our study demonstrate that the blood meal source may impact physiological processes related to fecundity and fertility in Cx. p. quinquefasciatus. However, further studies are needed to address the extent to which these effects alter interactions between mosquitoes and virions contained in the blood meal.
Artificial blood meal delivery systems are an important tool for laboratory studies where the use of animals is not possible. However, these studies should consider that the blood meal source, whether delivered via animal or artificial method, may affect physiological processes leading to reproduction in Cx. p. quinquefasciatus and could influence results. Colonized mosquitoes are laboratory animals that are commonly used to investigate pathogen-host interactions, as well as in basic biological research. Hence, observations of mosquito colonies are beneficial to improve future experiments. We have no doubt that other mosquito colonies would also show variation in the processes observed here and it would be beneficial to test additional populations to determine the extent of this variation. These findings contribute to our understanding of the factors that influence the reproduction of Cx. p. quinquefasciatus.
Acknowledgments
We thank Sara Lynn, Hilda Lynn, and Timothy Hope for laboratory assistance and we are grateful to Gregg Ross, Chelsea Smartt, and two anonymous reviewers for critically reviewing earlier drafts of the manuscript. This research was supported by the National Institutes of Health grant AI-42164. A University of Florida Graduate Alumni Award was used to support Sheri Anderson.
REFERENCES CITED
- Akoh JI, Aigbodion FI, Kumbak D. Studies on the effect of larval diet, adult body weight, size of blood meal, and age on the fecundity of Culex quinquefasciatus (Diptera: Culicidae) Insect Sci Appl. 1992;13:177–181. [Google Scholar]
- Awahmukalah DST, Brooks MA. Viability of Culex pipiens pipiens eggs affected by nutrition and aposymbiosis. J Invert Path. 1985;45:225–230. doi: 10.1016/0022-2011(85)90012-6. [DOI] [PubMed] [Google Scholar]
- Begum MN, Ahmed TU, Khoda ME. Gonotrophic cycle of Culex quinquefasciatus Say (Diptera: Culicidae) in Dhaka. Bangl J Zool. 1985;14:111–115. [Google Scholar]
- Bennett GF. The influence of the blood meal type on the fecundity of Aedes (Stegomyia) aegypti L. (Diptera: Culicidae) Canad J Zool. 1970;48:539–543. doi: 10.1139/z70-090. [DOI] [PubMed] [Google Scholar]
- Chamberlain RW, Sudia WD, Gillett JD. St. Louis encephalitis virus in mosquitoes. Am J Hyg. 1959;70:221–236. doi: 10.1093/oxfordjournals.aje.a120072. [DOI] [PubMed] [Google Scholar]
- Clements AN. Development, Nutrition, and Reproduction. Vol. 1. Chapman and Hall Publishers; London: 1992. The Biology of Mosquitoes. [Google Scholar]
- Dodge JT, Mitchell G, Hanahan DJ. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963;100:110–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Downe AER, Archer JA. The effects of different blood-meal sources on digestion and egg production in Culex tarsalis Coquillet (Diptera: Culicidae) J Med Entomol. 1975;12:431–437. doi: 10.1093/jmedent/12.4.431. [DOI] [PubMed] [Google Scholar]
- Jansen CC, Webb CE, Graham GC, Craig SB, Zborowski P, Ritchie SA, Russell RC, van den Hurk AF. Blood sources of mosquitoes collected from urban and peri-urban environments in eastern Australia with species-specific molecular analysis of avian blood meals. Am J Trop Med Hyg. 2009;81:849–857. doi: 10.4269/ajtmh.2009.09-0008. [DOI] [PubMed] [Google Scholar]
- Jupp P. The susceptibility of four South African species of Culex to West Nile and Sindbis viruses by two different infecting methods. Mosq News. 1976;36:166–173. [Google Scholar]
- Kasili S, Oyieke F, Wamae C, Mbogo C. Seasonal changes of infectivity rates of Bancroftian filariasis vectors in Coast Province, Kenya. J Vector Borne Dis. 2009;46:219–224. [PubMed] [Google Scholar]
- Labarthe N, Serrão ML, Melo Y, José-Oliveira S, Lourenço-de-Oliveira R. Potential vectors of Dirofilaria immitis in Itacoatiara, Oceanic Region of Niterói Municipality, state of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 1998;93:425–432. doi: 10.1590/s0074-02761998000400001. [DOI] [PubMed] [Google Scholar]
- Lai CH, Tung KC, Ooi HK, Wang JS. Susceptibility of mosquitoes in central Taiwan to natural infections of Dirofilaria immitis. Med Vet Entomol. 2001;15:64–67. doi: 10.1046/j.1365-2915.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- MacKay AJ, Kramer WL, Meece JK, Brumfield RT, Foil LD. Host feeding patterns of Culex mosquitoes (Diptera: Culicidae) in east Baton Rouge Parish, Louisiana. J Med Entomol. 2010;47:238–248. doi: 10.1603/me09168. [DOI] [PubMed] [Google Scholar]
- McCann S, Day JF, Allan S, Lord CC. Age modifies the effect of body size on fecundity in Culex quinquefasciatus Say (Diptera: Culicidae) J Vector Ecol. 2009;34:174–181. doi: 10.1111/j.1948-7134.2009.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RP, Hardy JL, Presser SB. Comparative vector competence of Culex tarsalis and Culex quinquefasciatus from the Coachella, Imperial, and San Joaquin Valleys of California for St. Louis encephalitis virus. Am J Trop Med Hyg. 1983;32:305–311. doi: 10.4269/ajtmh.1983.32.305. [DOI] [PubMed] [Google Scholar]
- Mitchell CJ. Mosquito vector competence and arboviruses. In: Harris KF, editor. Current Topics in Vector Research. New York: Praeger Publishers; 1983. p. 31. [Google Scholar]
- Mourya DT. Effect of anticoagulants on the susceptibility of Aedes aegypti mosquitoes to dengue virus infection. Acta Virol. 2002;46:51–53. [PubMed] [Google Scholar]
- Oda T, Mori A, Ueda M, Kurokawa K. Effects of temperatures on the oviposition and hatching of eggs in Culex pipiens molestus and Culex pipiens quinquefasciatus. Trop Med. 1980;22:167–172. [Google Scholar]
- Richards SL, Lord CC, Pesko KA, Tabachnick WJ. Environmental and biological factors influencing Culex pipiens quinquefasciatus Say (Diptera: Culicidae) vector competence for St. Louis encephalitis virus. Am J Trop Med Hyg. 2009;81:264–272. [PMC free article] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Dohm DJ, O’Guinn ML. Vector competence of selected North American Culex and Coquilletidia mosquitoes for West Nile virus. Emerg Infect Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT User’s guide for personal computers. computer program, version 8.0. SAS; Cary, North Carolina: 2002. [Google Scholar]
- Suleman M. Culex quinquefasciatus Say: Life table characteristics of adults reared from wild-caught pupae from North West Frontier Province, Pakistan. Mosq News. 1979;39:756–762. [Google Scholar]
- Suleman M, Shirin M. A comparison of the reproductive capacity of Culex quinquefasciatus Say fed on cold- and warm-blooded vertebrates. Pakistan J Zool. 1981;13:221–228. [Google Scholar]
- Turell MJ. Reduced Rift Valley fever virus infection rates in mosquitoes associated with pledget feedings. Am J Trop Med Hyg. 1988;39:597–602. doi: 10.4269/ajtmh.1988.39.597. [DOI] [PubMed] [Google Scholar]
- Walter NM, Hacker CS. Variation in life table characteristics among three geographic strains of Culex pipiens quinquefasciatus. J Med Entomol. 1974;11:541–550. [PubMed] [Google Scholar]