Abstract
Antioxidant enzymes (AOEs) catalase and superoxide dismutase (SOD) detoxify harmful reactive oxygen species, but the therapeutic utility of AOEs is hindered by inadequate delivery. AOE modification by polyethylene glycol (PEG) and encapsulation in PEG-coated liposomes increases the AOE bioavailability and enhances protective effects in animal models. Pluronic-based micelles formed with AOEs show even more potent protective effects. Furthermore, polymeric nanocarriers (PNCs) based on PEG-copolymers protect encapsulated AOEs from proteolysis and improve delivery to the target cells, such as the endothelium lining the vascular lumen. Antibodies to endothelial determinants conjugated to AOEs or AOE carriers provide targeting and intracellular delivery. Targeted liposomes, protein conjugates and magnetic nanoparticles deliver AOEs to sites of vascular oxidative stress in the cardiovascular, pulmonary and nervous systems. Further advances in nanodevices for AOE delivery will provide a basis for the translation of this approach in the clinical domain.
Keywords: antioxidant enzymes, catalase, liposomes, magnetic nanoparticles, oxidative stress, polymer nanocarrier, SOD, vascular immunotargeting
Oxidants, including reactive oxygen species (ROS) superoxide anion and H2O2 cause oxidative stress in many pathological conditions. Megadoses of nonenzymatic antioxidants may alleviate subtle and modest chronic oxidative stress, but are only marginally effective in acute severe conditions including ischemia-reperfusion, inflammation and radiation injury [1]. Antioxidant enzymes (AOEs) including superoxide dismutase (SOD) and catalase, decomposing superoxide and H2O2, respectively, are more potent antioxidants that are not consumed in reaction with ROS, but are eliminated from blood within minutes [2]. Conjugation with polyethylene glycol (PEG), PEG-based pluronics and encapsulation in PEG-liposomes prolongs circulation of SOD and catalase, thus enhancing their bioavailability and efficacy in some forms of inflammation and conditions associated with elevated ROS level in plasma and tissue parenchyma [3].
A significant hurdle in the translation of antioxidant therapies into the clinical domain lies in the inadequate delivery of these agents to their intended site of action. In particular, the vascular endothelium represents a key therapeutic target in ischemia and inflammation [4]. Mixed results from several decades of antioxidant research, including large scale clinical trials [5–7], have shown that for antioxidant therapy to work, it must effectively detoxify the selected ROS, target the cells suffering oxidative stress and offer a suitable therapeutic time window. For example, antioxidant interventions in acute pathological conditions (e.g., radiation injury, ischemia-reperfusion and acute respiratory distress syndrome) should cover a time interval from a few hours to a few days, whereas treatment of chronic conditions such as atherosclerosis should last many weeks and months [8–10]. While the exact requirements vary in specific disease conditions, nanoscale drug carriers are a promising avenue for achieving these goals.
For example, conjugation of AOEs with antibodies to cell surface determinants including platelet-endothelial cell adhesion molecule-1 (PECAM) and intercellular adhesion molecule (ICAM)-1 enables their specific delivery to endothelial cells lining vascular lumen, the key target of oxidants [11–13]. Anti-PECAM/catalase and anti-PECAM/SOD conjugates detoxify endothelial ROS [14–16] and protect animals against acute vascular oxidative stress [15,17,18]. Rapid endothelial internalization of PECAM-targeted conjugates enables detoxification of intracellular ROS [19,20]. However, protein conjugates are degraded in the lysosomes within a few hours [21]. Recycling of the target molecules to the plasmalemma helps to prolong the delivery and effect of conjugates [22], yet more sustainable means for targeted antioxidant interventions would be highly desirable. In theory, this can be achieved by encapsulating AOEs into a polymeric nanocarrier protecting AOEs from proteases. In addition, this approach offers a new level of controlled modulation of the affinity, geometry and degradation of the drug-delivery system, even further improving precision of vascular and subcellular addressing of the cargoes and their effects. This article reviews the design and application of nanocarriers for delivery of antioxidants, with a focus on recently published studies.
Antioxidants & their properties
As a group of molecules inhibiting oxidative stress, antioxidants are numerous and diverse. Semantically, any compound that reduces toxicity caused by oxidation is an antioxidant. Antioxidants decompose ROS, block lipid peroxidation or scavenge oxidants, among other functions. Although the sheer number of antioxidants makes them prohibitive to list here, they can be broken into two general types: nonenzymatic antioxidants and antioxidant enzymes (Table 1). Antioxidant enzymes are highly potent and specific, with high affinities and rates of reaction that detoxify ROS with a high efficacy. AOEs are not consumed in reaction with ROS, whereas nonenzymatic antioxidants are. As examples, SOD accelerates superoxide anion conversion into H2O2, while catalase reduces the latter and into oxygen and water [2]. Catalase has a turnover of 40 million molecules of H2O2 degraded per second [23]. Therefore, AOE interventions offer efficacy for ROS quenching not attainable by small molecule antioxidants.
Table 1.
Antioxidant therapeutics, therapeutic action and delivery methods.
| Antioxidant type | Therapeutic action | Delivery vehicle |
|---|---|---|
| Antioxidant enzymes | ||
| Superoxide dismutase | Converts superoxide (O2•−) to H2O2 | Conjugates [3,14–16,57], liposomes [33,46,48,135], enzymosomes [49,50], magnetic nanocarriers [30] |
| Catalase | Converts H2O2 to H2O | Conjugates [11,17,18,136], polymeric nanocarriers [71,75,99,100,105] and microspheres [72], macrophage-nanozymes [31,51] |
| Glutathione peroxidase (GPx) | Reduces lipid hydroperoxides and converts H2O2 to H2O | GSH-PEGDA oligomer nanoparticle [44] |
| Nonenzymatic antioxidants | ||
| Vitamins Ascorbic acid (vitamin C) Tocopherol (vitamin E) (α-tocopherol, β-tocopherol) Retinoids (vitamin A) |
Scavenges ROS and upregulates antioxidant enzyme activities. Ascorbic acid scavenges O2•− Tocopherol scavenges lipid peroxyl radicals [137] Retiniods regulate epithelial cell growth. Essential vitamin for vision. Regulates NADPH-dependent lipid peroxidation |
Liposomes, emulsions, micelles, solid lipid nanoparticles, microspheres [35] Liposomes [138,139], niosomes, microemulsions, solid lipid nanoparticles [139] |
| Caroteniods Lycopene β-carotene |
Quenches singlet oxygen, free radicals Pro-vitamin A carotenoid | Solid nanoparticles [140] Liposomes, niosomes [35,141] |
| Polyphenols Flavonoids-catechins, Quercetin, fisetin Phenolic acids- curcumin Resveratrol | Scavenge free radicals, inhibit proinflammatory kinases, antitumor activity Affect inflammatory enzyme activity, protects LDL from oxidation Antitumor interferes with carcinogenesis during initiation, promotion and progression, topoisomerases inhibitor, upregulates MnSOD [142] |
Liposomes [143,144], quercetin nanosuspension [145] Iron-containing solid nanoparticles [146], liposomes, nanoparticles, nanoemulsions [40] Liposomes [147], solid lipid nanoparticles [148], lipid core nanocapsules [149] |
| Thiols N-acetylcysteine | Thiol-disulfide redox, metal chelators, radical quenchers, substrate for GSH redox, reductants of disulfate bonds [137] | Liposomes [36,37] |
| Antioxidant cofactors- coenzyme Q10 (ubiquinone) | Functions in the electron transport chain. Electron carrier from complex I and II to III. Circulating coenzyme Q10 in LDL prevents oxidation of LDL; cardioprotective | Liposomes [150,151], microspheres, nanoparticles, nanoemulsions [151] |
PEG: Polyethylene glycol; ROS: Reactive oxygen species; SOD: Superoxide dismutase. Adapted with permission from [1]
The focus of this article is on the delivery of antioxidant enzymes by nanocarriers; however, the delivery of nonenzymatic antioxidants has been the focus of considerable research interest also. The following paragraphs contain a brief, and by no means exhaustive, examination of some of the nonenzymatic antioxidants that have been studied for nanocarrier delivery.
The principle source of many small-molecule antioxidants is diet. In general, small molecule antioxidants nonspecifically quench a variety of ROS and reactive nitrogen species. For the most part, they are nontoxic, stable for storage, resistant to damage during complex and/or aggressive formulation processing methods and relatively inexpensive. Ascorbic acid (vitamin C) is a major water-soluble antioxidant obtained through dietary sources. Oil-soluble tocopherol (vitamin E), retiniods (vitamin A) and carotinoids (e.g., lycopene and β-carotene), as well as coenzyme Q10 (CoQ10), carotenoids and polyphenols function as antioxidants in myriad processes in the cells and tissues to reduce oxidative stress throughout the body.
Multifunctional glutathione, particularly in reduced form (GSH), is an intracellular thiol found in all tissues that has an important role protecting the lower airspaces of the lung and the their epithelial layer [24]. GSH also aids in xenobiotics detoxification, protein and nucleic acid synthesis and pulmonary protection from oxidative damage by endogenous and exogenous toxins [1]. The sulfydryl groups in N-acetylcysteine (NAC) interact with free radicals resulting in the nonoxidizing end-product NAC disulfide. NAC scavenges hydroxyl radical (OH•), H2O2 and hypochlorous acid (HOCl). Upon deacetylation, NAC reverts to cysteine, a precursor of GHS synthesis, thereby replenishing the glutathione system.
Polyphenol antioxidants are numerous, with over 4000 species [25]. Generally water-soluble, polyphenols include tannins, flavonoids and phenolic acids, are characterized by the presence of multiple aromatic ring groups and, as a group, have many and varied antioxidant functions. The antioxidant and pro-apoptotic properties [26] of the polyphenol curcumin has been examined for treatment of hepatocellular carcinoma [27]. Resveratrol, a natural polyphenol with strong antioxidant and free-radical scavenging properties, found in the skins of grapes and other fruits, has been studied extensively for antioxidant protective and anticancer properties [28].
Nonpolymeric carriers
Antioxidants have been formulated into nonpolymeric nanocarriers including traditional self-assembly phospholipid carriers, liposomes [29], as well as magnetic nanocarriers [30] or diverse polyplex complexes [31]. Coupling of enzymes to the carrier surface provides an alternative to encapsulation and has been shown to improve their stability when exposed to changes in temperature and pH [32]. For example, SOD associated with the surface of liposomes was shown to be more resistant than free SOD to inactivation by high concentrations of H2O2 [33]. Delivery methods of small antioxidants have been studied using numerous delivery vehicle platforms including liposomes, micelles and solid lipid nanoparticles, among others (Table 1). This section focuses on the nonpolymeric antioxidant formulation using liposomes and nanocarriers based on different formulation mechanisms.
Antioxidant liposomes
In the early 1980s, liposomes were proposed as a means to protect antioxidants from clearance and deactivation in vivo, and to facilitate access to desired cells and tissues. The amphiphilic nature of liposome’s bilayer structure allows the delivery of both hydrophilic and hydrophobic agents including lipid soluble antioxidants incorporated within the hydrophobic bilayer including vitamin E (TOHs and tocotrienols), ubiquinones, retinoids, caratenoids, flavonoids, soy isoflavones and synthetic butylated hydroxytoluene (BHT) among many others [34]. Likewise, water soluble antioxidants such as ascorbate, urate and glutathione have been studied as liposomal cargoes, enclosed in the hydrophilic core of the liposome. Inclusion of tocopherol and other oil-soluble antioxidant vitamins into the lipophilic inner membrane of liposomes has been shown to lend greater antioxidant efficacy, while also serving to protect the light and oxidation-sensitive molecules from damage by environmental exposure [35].
N-acetylcysteine (NAC) has a low bioavailability, likely due to acetylation, which necessitates use of vehicular drug-delivery systems. Suntres et al. found that liposomal NAC protected against liver injury in rats in a sepsis model better than free NAC [36,37]. A liposomal formulation of NAC showed a higher protective potency than the free drug when delivered intratracheally in a rat model of acute respiratory-distress syndrome [38]. A combination of tocopherols and NAC in liposomes were administered to guinea pigs after exposure to a mustard gas and provided a protective effect in the lungs [39].
Delivery and controlled release of curcu-min by various methods has been studied and reviewed [40]. Among the numerous techniques employed to deliver the lipophilic antioxidant, inclusion within dimyristoyl phophatidylcho-line (DMPC) liposomes that were targeted to using prostate membrane antigen specific antibodies showed enhanced anticancer properties in vitro and in vivo as compared with free curcuman [41]. Research into delivery of resveratrol by liposomes is extensive. Liposomal resveratrol delivery reduced vascular intimal thickening after endothelial injury in normal rats [42]. A combination of resveratrol and curcumin was shown to reduce prostate cancer in a mouse model by liposomal delivery [43]. GSH and polyethylene diacrylate (PEGDA) oligomers created liposome-like spherical self-assembly nanoparticles which have been shown to protect cells from oxidative stress in vitro [44].
Antioxidant enzyme liposomes
In early work by Freeman et al., liposomal delivery of SOD to cultured endothelial cells demonstrated a 6–12-fold increase in SOD activity compared with the control cells treated with free SOD. In addition, cells that received liposomal SOD were more resistant to oxidative damage by hyperoxia [29]. The same group reported a protective effect against exposure to high respiratory oxygen in newborn rats treated with daily injections of liposomes containing SOD and catalase [45]. Furthermore, they have reported enhanced delivery of SOD encapsulated in pH sensitive liposomes containing surfactant protein A (SP-A) to primary fetal pulmonary epithelial cells via SP-A receptors [46]. Hypertension caused through angiotensin II (Ang II) activation of membrane-bound NADPH oxidase generating superoxide was reduced in rats treated with liposomal SOD [47]. These earlier studies showed advantages of nanocarrier-formulated AOEs versus free AOEs and provided the basis for the subsequent improvements involving PEG-stealth liposomes, vascular immunotargeting and advanced nanocarrier design.
Testing of the anti-inflammatory effect of liposomal SOD formulations in a rat arthritic model showed that SOD liposomes formed by the dehydration–rehydration method without extrusion yielded the greatest loading efficiency of active SOD and inhibited edema more effectively than naked SOD [48]. The incorporation of an acetylated hydrophobic derivative of SOD, Ac-SOD, improved loading efficiency of the enzyme compared with unmodified SOD [49]. Since the Ac-SOD localized to the bilayer and 50% of the enzyme was exposed to the exterior, the site of activity was focused at the surface of the liposome or ‘enzymosome’, instead of within the aqueous interior. The change in conformation reduced the effect of release rate on the activity of the liposome and increased bioavailability of the enzyme [49]. This effect of SOD localization within the liposome structure was tested in a rat adjuvant arthritis model comparing PEG-coated liposomes with either Ac-SOD or plain SOD. The circulation time of the PEGylated liposomes increased regardless of the SOD type included and a faster anti-inflammatory effect was observed with the As-SOD PEG liposomes versus the plain SOD PEG liposomes [50].
Antioxidant enzyme nanoparticle complexes
Batrakova et al. developed a macrophage-driven system for the delivery of AOEs to the brain [31]. Catalase was electrostatically complexed with a cationic block copolymer, polyethyleneimine-poly(ethylene glycol) (PEI-PEG). These ‘nanozymes’ in which the PEI-PEG shielding protected the enzyme within the structure were phagocytized by macrophages, targeted by the subsequent migration of the cell to the inflamed brain in a mouse model of Parkinson’s disease [31]. Testing morphological and biochemical parameters of the brain injury showed promising therapeutic efficacy of this delivery system [51]. Similarly formed SOD nanozymes alleviated neuronal oxidative stress after local administration in the CNS in rodents [52].
Complexation with PEG-containing pluronics has been reported to increase cellular permeability of diverse drugs in cultured cells [53] and in vivo [53,54]. Although mechanism of intracellular transport remains to be defined, no deleterious effects have been noted [55]. For example, SOD-pluronic conjugates were reported to deliver enzymatically active SOD to neuronal cells more effectively than naked SOD or PEG-SOD without neuronal toxicity [3].
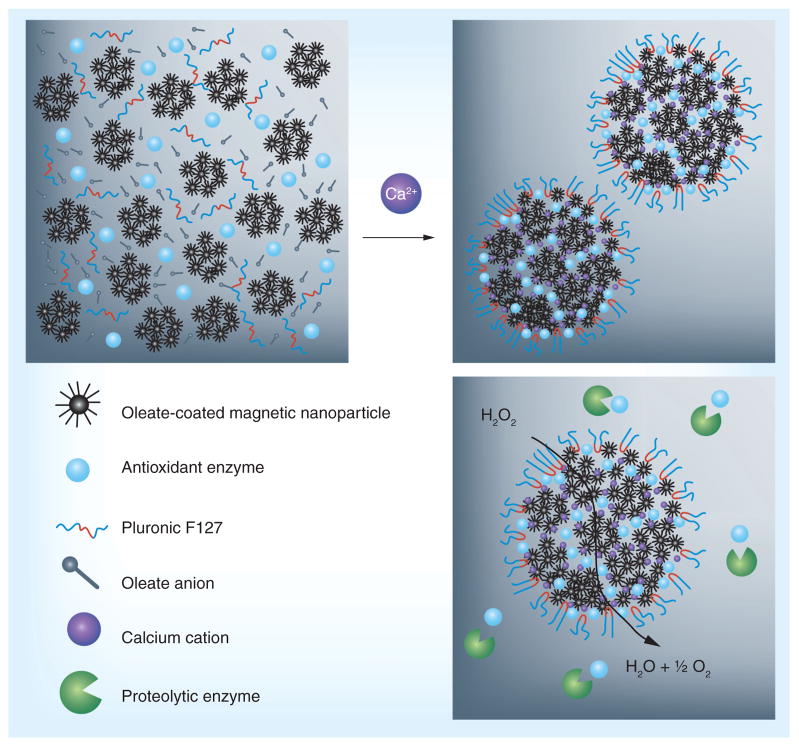
Recently, magnetic nanoparticles (MNPs) have been proposed for delivery of biotherapeutics. Chorny et al. reported delivery of plasmid DNA loaded in magnetically driven biocompatible polymeric MNPs composed of oleate-coated magnetite and surface modified with PEI oleate ion-pair complexes enabling DNA complexation [56]. They also reported that paclitaxel loaded MNPs could be delivered to arterial stents in rats using magnetic field: target accumulation was an order of magnitude higher in magnetically treated animals versus non magnetic controls [56]. Capitalizing on this, similarly formulated MNPs have been used to load either active SOD or catalase through a controlled precipitation method of two aqueous phases. The complexation of AOEs into MNP, as shown in Figure 1, is advantageous over other more traditional carriers since the synthesis does not require the use of either the strong sheer force required for emulsion techniques, or organic solvents, both of which are damaging to large AOEs. The formulation loaded active enzyme cargo efficiently and protected it from proteolytic degradation in vitro. MNPs demonstrated a protracted release of their cargo protein in plasma. Additionally, magnetically delivered, catalase-containing MNPs protected endothelial cells in vitro, with 62 ± 12% cells rescued from H2O2 induced cell death versus 10 ± 4% under nonmagnetic conditions [30].
Figure 1. Formation of antioxidant enzyme nanocarriers.
Mixing aqueous solutions of antioxidant enzymes, oleate-coated magnetite particles and Pluronic® F127 with an aqueous solution of CaCl2leads to fast assembly of composite nano-sized aggregates (300–400 nm) that protect antioxidant enzymes from proteolysis.
Reproduced with permission from [30].
Polymer nanocarriers for delivery of antioxidants
As described above, antioxidants include small molecules such as ROS scavengers (e.g., trolox), reducing agents (e.g., glutathione) and antioxidant inducers (e.g., curcumin) and antioxidant enzymes (e.g., SOD and catalase), each with a unique set of delivery challenges described below. However, both nonenzymatic and enzymatic antioxidant interventions may greatly benefit from the use of appropriate nanocarriers. The functions of nanocarriers include protection of antioxidant cargoes from inactivation, improved vascular targeting and intracellular delivery. Characteristics of a drug nanocarrier can be divided into several categories: surface chemistry, size, shape, mechanical properties, responsiveness and mechanisms for elimination, all directly related to the outcome of pharmacotherapy (Table 2). For vascular delivery, spherical nanocarriers should be smaller than 500 nm in diameter to minimize reticular endothelial system (RES) uptake and retention in microvasculature [57]. More detailed design considerations are provided in the following sections.
Table 2.
Simplified summary of nanocarrier properties†.
| Design features | Pharmacological outcomes | |||||
|---|---|---|---|---|---|---|
| Localization | Duration of effect | Delivered dose | ||||
| Circulation | Tissue/cellular | Subcellular | ||||
| Surface chemistry (PEG, affinity molecules, surface charge) | PEG coating prolongs circulation [152] | Affinity molecule provide cellular binding [153] | Positive charge facilitates endosomal escape [154,155] | - | Surface loading | |
| Size and shape (spherical, cylindrical, elliptical, complex) | Spheres: ~50–500 nm, maximal circulation, FMs: very long circulation [156–158] | Small, spherical particles extravasate into fenestrated tissue [156–158] | Regular geometry permits internalization [154,156–159] | Fickian release Mt/M∞ = ktnn = 0.43 (s), 0.45(c) [160] | Available volume 4/3πr3(s) πr2z(c) | |
| Responsiveness (pH, T, EM field) | Responsive size change modulates circulation and RES uptake [158,161] | Responsive size change permits local tissue retention [158,161] | Responsive swelling facilitates endosomal escape [158,161] | On/off release or case II transport Mt/M∞ = kt0.5–1.0 [160] | - | |
| Mechanical properties | Glassy | Rigidity shortens, [98,162] | - | - | Low diffusivity | - |
| Rubbery | Plasticity prolongs [98,162] | - | - | High diffusivity | - | |
| Mechanism for metabolism (hydrolysis, dissolution) | Release of coating/size change, inducing early clearance | Potential accumulation of degradation products | Potential accumulation of degradation products | Case II transport Mt/M∞ = kt0.5–1.0 [160] | Drug conjugate increased loading [65] | |
Examples of the complex relationship between the tunable nanocarrier design parameters and the pharmacological features that can be affected by these modulations are presented. A power law model of release is presented for simplicity of comparison. Case II transport describes non-Fickian release, which is controlled by solvent front swelling or surface erosion.
C: Cylinder; EM: Electromagnetic; FM: Filomicille; PEG: Polyethylene glycol; r: Radius; S: Sphere; T: Temperature; Z: Length.
Polymeric carriers for small molecule antioxidant delivery
Nonenzymatic antioxidants typically reduce oxidizing species in stoichiometric ratios, being consumed in the process [58]. Thus, large sustained doses are required to achieve the effect. Cellular metabolic pathways can help recycle a radical scavenger [59], usually at the expense of the cell’s own glutathione reducing capacity. Furthermore, exceeding optimal dose of some antioxidants may lead to pro-oxidant effects [26,60,61]. Therefore, nanocarriers for such antioxidants should be designed for delivery of therapeutically significant amounts at a gradual, controlled rate while avoiding burst release, which could result in transient pro-oxidant localized concentrations.
This makes delivery of antioxidants challenging as most of the carriers can achieve a maximum loading of 35–45 wt% for small molecule drugs [62–64]. The larger carriers needed to achieve sufficient dosing may cause side effects and exceed size limits for adequate circulation. Furthermore, the passive loading of drugs into nanocarriers can result in limited control of the drug release profile [65,66]. Coupling antioxidants covalently to a biodegradable polymer backbone can overcome these limitations, by enhancing the total mass of drug in the nanocarrier and providing specific chemical cleavage mechanism to control the release rate. Many antioxidants have phenol and thiol reactive groups that are easily functionalized and incorporated into polymers using polyester, polyanhydride or poly(β-amino ester) chemistry [67]. This approach also helps to protect the labile groups of antioxidants from premature oxidation.
Several approaches have been pursued to conjugate antioxidants to polymers and proteins. For instance, vitamin E was conjugated to poly(acrylic acid) to synthesize a water-soluble carrier for vitamin E that suppressed oxidative stress in sperm cells in vitro [68]. Antioxidant polymers with glutathione, ascorbic acid, gallic acid and catechin conjugated to PEG, poly(methyl methacrylate) and gelatin have also been synthesized and shown to have antioxidant properties in vitro [44,69,70]. However, therapeutic capacity of these antioxidant polymers is limited by their low relative mass of antioxidant compared with the bulk material (~0.1–35 wt%).
As an alternative to pendant conjugation strategies, trolox, a water-soluble analogue of vitamin E, was recently polymerized through a polycondensation reaction mediated by Stagelich esterification [67]. As the condensation pairs used result in a zero length conjugation bond, the resulting biodegradable poly(trolox ester) possesses 100% antioxidant mass. The heterocyclic polymer, poly(trolox ester) has a high glass transition temperature (Tg; 130–155°C), that results in a rigid polymer at physiological temperatures. By using a single-step solvent extraction method, the poly(trolox ester) can be formulated into nano-particles of sizes 100–250 nm, that are able to quench ROS and suppress oxidative stress in cell culture of mouse pulmonary microvascular endothelial cells exposed to pro-oxidant challenge [67]. Interestingly, poly(trolox ester) nanoparticles, but not free trolox, were reported to suppress protein oxidation [61], emphasizing the importance of the nanocarrier in antioxidant delivery and effect. As the emerging approaches using antioxidant polymers have shown promising in vitro results, testing the efficacy of these methods in animal models becomes increasingly critical.
Polymeric nanocarriers for antioxidant enzyme delivery
Although AOEs are highly potent and specific, unfortunately, they are labile and easily inactivated during nanocarrier formulation conditions. Furthermore, they are prone to proteolytic inactivation, which limits their therapeutic duration in the lysosomes [21,71]. In view of these vulnerabilities, biodegradable polymeric nano-carriers (PNCs) seem a logical choice for delivery of AOEs (Figure 2). Encapsulation of AOEs in a polymer shell can provide protection from proteolytic inactivation, thereby extending therapeutic duration. For instance, using a standard water/oil/water emulsification method ~7wt% of SOD or catalase was loaded into poly(lactide-co-glycolide) (PLGA) polymer microspheres. as a stabilizer, these particles By using PEG400 (sized ~10–15 μm) released active enzyme for over 50 days [72]. However, their size exceeded the circulation limit of less than 500 nm.
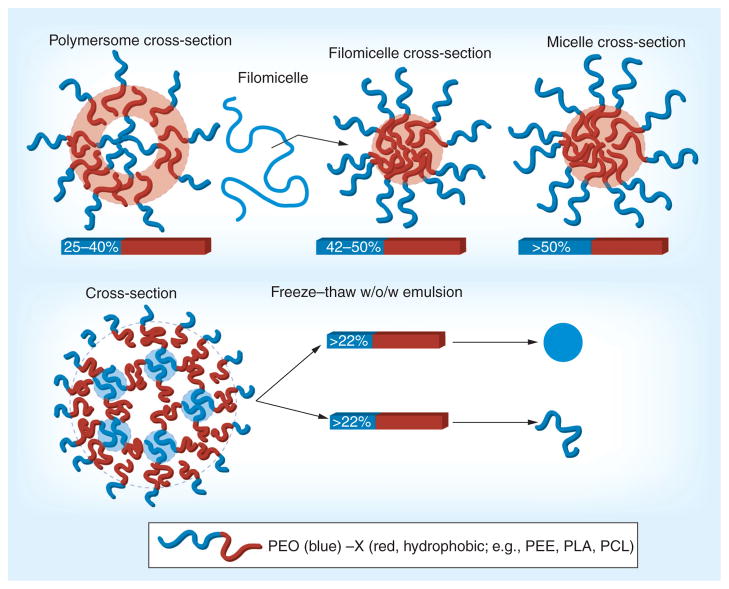
Figure 2. Formulation approach and polymer control of polymeric nanocarrier morphology.
Hydrophilic drugs can be loaded in the internal blue regions (external PEG brushes are freely water soluble and thus do not retain hydrophilic drugs) and hydrophobic drugs in the red regions.
PCL: Polycaprolactone; PEG: Polyethylene glycol; PEO: Polyethylene oxide; PLA: Polylactic acid.
Another potential mechanism of enzyme deactivation is through a sharp drop in pH resulting from delivery to the lysosomes or other areas of acidosis, including carrier-induced acidosis (i.e., the release of lactic acid during the degradation of PLGA). To avoid the latter problem, nanocarriers can be synthesized from polyketal based polymers which do not produce acidic groups during degradation. For example, SOD loaded polyketal microspheres injected in the cardiac muscle have been reported to alleviate myocardial ischemia-reperfusion injury [73]. Furthermore, SOD-loaded polyketal particles introduced intratracheally alleviated bleomycin induced pulmonary injury [74]. However, these carriers are too big for intravascular delivery. In order to decrease the size of nanocarriers made through these standard methods, increased shear rates are needed during the last emulsification step, inactivating the labile AOEs. Furthermore, significant burst release (~50–60%) within the first few hours suggests that the majority of protein loading was limited to surface absorption, not incorporated within the particle interior [72].
Introducing a freezing step during the primary emulsification helps to minimize enzyme inactivation and enhance internal loading in PNCs [71,75]. When using an amphiphilic encapsulating polymer (e.g, poly(ethylene glycol)-b-poly(lactic-co-glycolic acid) [PEG-PLGA]), PNCs produced through this method were 200–300 nm in size and contained ten-times the mass of catalase compared with synthesis without the cryogenic step. Also, these carriers maintained approximately 25% of the original catalase activity after 24 h incubation with a proteolytic enzyme, while free enzyme lost more than 90% activity within the first hour [71]. This loading mechanism and protection was independent of the enzyme used, yet substrate diffusivity through the polymer shell greatly determined the ability of internally loaded enzyme to express its function [75]. For instance, horseradish peroxidase, catalase and xanthine oxidase possessed 25% of the original mass of loaded protein after 4 h incubation with protease. In contrast, PNC-encapsulated xanthine oxidase had no measurable activity, since its substrate (hypoxanthine) has diffusivity two orders of magnitude slower than the other two enzymes’ substrates. Therefore, through design of the encapsulating material, it is possible to selectively impact the substrate accessibility of the PNC-loaded enzyme [75].
As an alternative approach, one can enhance enzyme stability through the addition of a protecting molecule. For instance, since inactivation occurs in part due to the enzyme denaturation in the hydrophobic/hydrophilic interface, addition of a decoy protein (e.g., serum albumin) can prolong the activity of the loaded enzyme, at the expense of total loading capacity. The hydrophobic interface provides an absorptive surface, which can encourage the unfolding of proteins. As this surface is not attracted to the antioxidant enzyme specifically, by allowing a nonactive protein to absorb to the surface instead, the active protein is inhibited from absorbing and thereby able to maintain its activity. For example, the activity of SOD released from PLGA nanoparticles along with protective albumin retained activity after 7 days, versus 4 days in the case of omitting albumin [76]. Local injection of these SOD-loaded carriers via the cerebral artery upon reperfusion after transient ischemia alleviated the brain injury in a rat model [77].
Local infusion similar to that described above facilitates the retention and uptake in the downstream vasculature in pathological conditions with a known location and accessible conduit artery. However, this mechanism of local delivery is not specific and provides no targeting to selected vascular cell types suffering oxidative stress. In this context, targeted delivery of anti-oxidants to vascular endothelial cells is a primary therapeutic target and combating oxidative stress of the systemic, pulmonary, cerebral and cardiac endothelium represents an important and challenging goal [78]. As discussed above, conjugation with antibodies to ICAM and PECAM provides intracellular endothelial delivery of multivalent conjugates and nanocarriers. Besides providing potential ‘stealth’ properties, amphiphilic di-block copolymers (e.g., polyethylene glycol-poly-lactic acid [PEG-PLA] and PEG-PLGA) can be used to couple targeting agents to the nanocarriers post formulation. For instance, PECAM-1 antibody (anti-PECAM) was coupled to biotinylated PEG-PLGA PNCs loaded with catalase, providing delivery of 12% of the injected dose in the lungs (a preferred vascular target), as compared with 2% for nontargeted control nanocarriers. As a result, anti-PECAM/coated PNC loaded with catalase, but not nontargeted counterparts, inhibited the ROS levels in the lungs [75]. Furthermore, endothelial cells pretreated with catalase-loaded, anti-PECAM/nanocarriers were protected against oxidative stress for at least 20 h after drug delivery, as compared with less than 3 h in the case of protein anti-PECAM/catalase conjugate [75].
Recently, several proteins including SOD were covalently functionalized with vinyl groups followed by free radical polymerization in the presence of other diacrylate monomers, which resulted in encapsulation of protein molecule in a nanometer thick (~5 nm) polymer shell [79]. Surface properties and degradation rate of polymer shell was controlled by monomer selection. SOD nanocapsules prevented cell death in an in vitro paraquat injury model, suggesting that their polymer shell is permeable to the substrate molecules (e.g., O2·−) [79]. Furthermore, polymer nanocapsules having large aqueous cores synthesized from (allyloxy)12-cucurbit(6)uril, a rigid disk-shaped molecule, is another potential vehicle for protein delivery [80]. Even though their use for delivery of AOEs has not been studied, polymer nanocapsules incorporating disulfide bridges that can degrade in a reducing environment could be applicable for release of the cargo triggered by reduced environment in the host cell [81].
Modulation of geometry of polymeric carriers for antioxidants
Spherical shape is the most common among the PNC formulations that range from relatively small (50–300 nm diameter, including polymersomes [82], dendrimers and polyplexes [83]) to large spheroid PNCs (300–1,000 nm diameters [84]). Some of these carriers are self-assembled, while others are formulated by other methods. For example, similar to amphiphilic phospholipid-based liposomes, self-assembled spherical polymersomes typically consist of amphiphilic diblock copolymers [85]. However, the same chemistry, as well as other techniques mentioned below, also yields nonspherical nanocarriers. It has been recognized in the last decade that carrier geometry modulates its drug-delivery functions. This section will discuss geometry aspects of polymer-based carriers for AOEs. For a more general outline of nonspherical liposomes and carbon nanotubes, see reviews [86] and [87], respectively.
PNC geometries
One class of nonspherical model PNCs includes flat elliptical disks [88,89]. PNC formulation techniques include micro-fluidics to form emulsions [90] and/or microscope projection photolithography to form micron-scale particles [91]. Another nanofabrication technique (Particle Replication In Nonwetting Templates [PRINT]), nanoimprinting, affords formulation of diverse-geometry nanocarriers [92–94]. Furthermore, depending on the molar ratio of the hydrophilic PEG or polyethylene oxide (PEO) block to the hydrophobic (e.g., polycaprolactone [PCL]) block, such amphiphilic copolymers self-assemble into either spherical polymersomes (~50 nm to several μm aqueous core vesicles, ~25–40% PEG) [95], filomicelles (width ~10–40 nm, length ≤ 50μm, ~42–50% PEG) [96] or spherical micelles (solid core, >50% PEG) [97]. Spherical polymer-somes are analogous in structure to liposomes with an amphiphilic bilayer enclosing an aqueous core. Filomicelles are long tubular structures with micellular cross-sections that contain hydrophobic interiors and hydrophilic surfaces. The persistence length lp describes stiffness and for filomicelles varies from 0.5 μm (fluid) to 5 μm (stiff), dependent on the total polymer molecular weight [98].
Alternatively, a freeze–thaw emulsion technique provided PEO-PLA diblock copolymer-based filamentous nanocarriers (width ~50 nm, length 5–20 μm) protective encapsulation of active catalase [99]. The absolute ratios of hydrophobic to hydrophilic blocks in the polymer were different than those used to form self-assembled poly-mersomes or filomicelles; nevertheless, this ratio dictates the resulting spherical or filamentous geometry (figure 2). These filaments have lp from approximately 600 nm to several microns [100]. Stiffness, thickness and absolute length of these filaments can be controlled by the molecular weight of the polymer used to make them, while the ratio of hydrophobic to hydrophilic domains in the diblock copolymer is kept constant for the formation of filaments.
Drug loading & carrier degradation
Loading of hydrophilic or amphiphilic antioxidant drugs is possible in emulsion-based PNCs, as well as in the polymersome aqueous core, while hydrophobic antioxidant drugs can intercalate within the lipophilic polymer domains of the carriers discussed. Diverse PRINT particles have encapsulated both hydrophobic [101] and hydrophilic (e.g., siRNA) cargoes [102]. Hydrophilic compounds can be coupled to the surface of polymersomes [103,104] and filomicelles. Emulsion-based PEG-PLA nanofilaments can encapsulate large hydrophilic enzymes including catalase [99]. Interestingly, PEGylation of the cargo itself takes advantage of the unique phase-alignment that occurs during formulation and further enhances loading and resistance of the cargo to external proteases through more efficient encapsulation [105].
Biodegradability is one of the key requirements for biocompatibility of a drug delivery system if its size does not permit excretion via physiological pathways. Two typical materials at opposite ends of the degradation kinetics spectrum are fast-degrading polyanhydrides and slow-degrading polyesters (degradation time of the latter ranges from weeks to years) [106–108]. Polyanhydride microparticles have tunable degradation times from hours to weeks, achievable by alteration of the ratio of constituent co-polymers [109–111]. Examples of these copolymers are poly(carboxy-phenoxy propane or hexane) (CPP or CPH, relatively hydrophobic) and poly(sebacic acid) (SA, relatively hydrophilic). Higher CPP:SA ratios result in slower degradation and vice versa. Spherical polyanhydride carriers have been synthesized on the nanoscale [112–114] yet shape control has not been documented and degradation kinetics often are too rapid for practical applications.
Polymer backbone structure regulates hydrolysis kinetics of polymers and their macromolecular assemblies. For example, PCL’s longer alkane segments, which are free of functional groups between their hydrolysable bonds (vs PLA/PLGA), mediate tighter polymer chain packing, enhance crystallinity and decrease water penetration into PCL structures. Similarly, polymers composed of the same-handed enantiomer pack tighter, are more crystalline and resilient to degradation vs. polymers composed of racemic mixtures of both-handed enantiomers (e.g., D-PLA degrades slower than D,L-PLA) [115,116]. PLGA has random glycolic acid segments inserted into the backbone of PLA, reducing crystallinity and increasing degradability. Glycolic acid is also devoid of the side methyl groups found in lactic acid, further enhancing water accessibility. The PLGA degradation products are primarily lactic acid and/or glycolic acid (<100 Da), which are hydrophilic, diffusible and rapidly metabolized.
Geometry impacts degradation of carriers made of relatively slow degrading polymers, such as polyesters through diffusion. The kinetics of water penetration into, and therefore degradation of, a polymer matrix is inversely proportional to the polymer structure’s minimum dimension. Millimeter-scale polyester spheres exhibit heterogeneous surface erosion in water; the surface degrades and erodes faster than water can penetrate into and degrade the interior. In contrast, nanometer scale polyester spheres experience uniform water penetration throughout the polymer matrix leading to homogeneous degradation and erosion. The internal degradation can actually exceed exterior degradation as acidic degradation products accumulate inside the carrier [117]. PEO-PLA polymersomes and PEO-PCL filomicelles degrade primarily by PLA and PCL hydrolysis [107,108], yielding pore-preferring copolymers [85] and filomicelle fragmentation [118]. The degradation can be delayed by blending in nondegradable diblocks such as PEO-PEE/poly(ethyl ethylene), which are renally excreted. PEO-PCL filomicelles degrade within days despite the fact that PCL is typically thought of as slow-degrading (e.g., in months) [118]. It appears that the small water diffusion distances through filomicelles (cross sectional radii <20 nm) enables rapid PCL degradation. Interestingly, since PEO does not degrade in water, the degradation occurs at the PCL end, which results in an increased ratio of PEO:PCL and thus a transition to micelle-preferring polymers. Filamentous PEO-PLA catalase-carriers experienced similar degradation, although the overall kinetics were longer as compared with filomicelles (weeks vs. days) likely due to a two- to three-fold enhancement in cross sectional diameter, different formulations and resultant macromolecular structure [99].
Environmental pH and hydrolytic enzymes also play a role in degradation. Degradable polymers typically contain hydrolysable bonds and undergo faster acid-catalyzed hydrolysis at low pH [119]. For example, the rate of polymersome hydrolysis and release of loaded drugs is further accelerated at acidic lysosomal pH (~5.0) [120]. The AOE-loaded PEO-PLA nanofilament preparation mentioned earlier also demonstrated significant acid-catalyzed degradation [99]. Interestingly, polyanhydrides degrade more slowly at acidic pH with far more rapid degradation in basic solutions [113,121]. Enzymatic (e.g., proteases) degradation of polymers has been extensively studied [122–124].
Circulation
Covalent coupling of PEG to carriers or proteins (PEG-ylation) is known to enhance circulation (e.g., liposomes [125]) and reduce immune system interactions. For example, the PEG brush of polymersomes and filomicelles enhances compatibility with blood [82,97,126], as they: remain suspended in plasma, avoid RBC and leukocyte adhesion, do not fix complement and other defensive plasma proteins [82,97,126], and do not cause hemolysis [98]. Polymersomes circulate longer than liposomes with t1/2 approximately 24 h vs 1–3 h [127], potentially due to their denser PEG brush (and perhaps higher structural durability). By design, polymersomes are made of individual polymers that possess a 100 mol% degree of PEGylation, whereas liposomes with more than 10% PEGylation of their constituent phospholipids are unstable [128].
Carrier geometry also regulates nanocarrier circulation. Small spheres (<20 nm) extravasate via vascular pores, enhancing clearance. Large spheres (>1 μm) clear rapidly due to retention in the microvasculature, whereas spherical carriers of intermediate size may circulate for many hours and even days depending on their stealth features and geometry. Comparison of polymer-somes and filomicelles illustrates the latter aspect. Highly flexible filomicelles have circulation t1/2 approaching 1 week in mice [127]. This is thought to be due to filomicelles’ ability to flow-align and thereby avoid vascular collisions, extravasation and phagocytosis. Indeed, longer filomicelles circulate longer and are less readily internalized by macrophages under flow. Their length makes filomicelles a difficult substrate for macrophages to engulf and the carrier’s flow-aligning structure experiences extensive drag forces from directional flow that oppose phagocytosis [88].
Cellular uptake & subcellular trafficking
Carrier geometry also modulates cellular uptake and subsequent lysosomal delivery in a cell- specific way. Macrophages internalize and deliver more than 1000 nm IgG-opsonized particles to lysosomes faster than particles less than 500 nm in size [129]. Parenchymal cells internalize particles less than 100 nm in size faster than particles more than 500 nm in size [130], unless they have an elastic membrane [131]. Phagocytosis of rigid disks is dependent on the degree of curvature of the disk at the point of contact [88]. Similarly, stiff filaments of the same composition are only internalized if the point of contact is at the carrier’s point of maximum curvature (the ends) [132]. Carrier geometry also modulates receptor-mediated PNC endocytosis: antibody targeted PNC spheres are internalized and trafficked by endothelial cells to lysosomes faster than disks [89]. Studies suggest that cylindrical PRINT particles are internalized via clathrin-mediated endocytosis and macropinocytosis in multiple cell types [92,94]. In epithelial cells, filomicelles (PEGylated by definition) undergo fragmentation upon macropinocytosis [127]. It should be noted, not surprisingly, that the very same PEGylation that enhances circulation (see ‘Circulation’ section) can impact internalization and subcellular trafficking to varying extents (sometimes negatively) [133,134]. Therefore, while several studies have discussed the complex interplay of surface chemistry (e.g., PEGylation) and nanocarrier surface binding moiety affinity (e.g., antibodies), nanocarrier geometry is an emerging approach that also modulates uptake and intracellular addressing of carrier cargo, which may be used for prolongation of effects of antioxidant enzymes.
Conclusion & future perspective
Based on the current knowledge of cardiovascular pathology, it is safe to postulate that endothelial cells lining vascular lumen represent the key therapeutic target for management of several forms of vascular oxidative stress (ischemia, hyperoxia, radiation injury, inflammation and acute lung injury). Current pharmacotherapy for severe acute forms of these conditions is either grossly suboptimal or nonexistent. At least in theory, targeting antioxidants to endothelium (and other cardiovascular targets such as cardiomyocytes) may help to improve management of these grave conditions.
One of the authors of this article has worked in the area of targeted vascular delivery of anti-oxidant enzymes for a quarter of century, which makes our analysis of the current state of affairs in the field and predictions for its development fairly conservative. It has been a long journey from the early 1980s (development of PEG-enzymes and PEG-liposomes), through the 1990s (design of targeted antioxidant conjugates) and the first decade of this century (design of protective nanocarriers with controlled affinity, geometry and durability) to the main challenges of this decade: preclinical validation of efficacy and safety, and industrial development of this approach. There are good reasons to expect that early in this decade some of the nanodevices described in this review (or newer iterations) will establish superiority in treatment of acute vascular oxidative stress over nontargeted and unprotected antioxidants in animal studies.
This outcome will motivate industrial investment in translation of this approach into preclinical and clinical domains, encompassing scaling up and quality control of the production and replacement of current affinity moieties by analogues accessible for human use (i.e., binding to human tissue targets, humanized and minimized to scFv format). Toxicological studies in large animals and primates will complement fine-tuning of optimally effective doses and administration regimens in the same species, providing a template for the subsequent human studies.
The safety and benefit/risk ratio for interventions intended to manage vascular oxidative stress are higher than in many other areas of nanomedicine, such as cancer. Rigorous testing of adverse effects associated with unintended features of carrier nanodevices is necessary. Nanocarriers (including stealthy PEG-coated ones) must be carefully tested in terms of activation of defense systems (e.g., complement and RES), inflammation or thrombosis, as well as aggregation in circulation and embolism of the microvasculature. Nanocarrier surface properties (that control many of the biocompatibility phenomena mentioned above), rate of absorption of components of plasma, carrier degradation and drug release (that may be fast due to huge surface/mass ratio) and effects of anchoring on and uptake by target and bystander cells must be fully characterized for each formulation. In addition, evaluation of potential side effects of quenching ROS signaling and other physiological functions in the vasculature is critically important.
In this context, we believe that management of acute vascular ischemia-reperfusion in organ transplantation represents arguably the most attractive application of antioxidant nanodevices targeted to endothelium. First, time for antioxidant intervention is well defined and relatively short in this setting. Second, the drugs infused into a donor or locally into an organ graft during procurement will quickly bind to endothelium and nonbound material will be removed from the graft by perfusion just prior to the grafting; hence the recipient body actually will not be exposed to the nanodevices. These two factors greatly boost the efficacy and safety of the approach. Taken together with a great need for improving both the quality of organs for transplantation and the outcome, this may generate sufficient industrial and financial incentives for the clinical development and testing. Results of animal studies of organ transplantation show superb effects of targeted antioxidants in several animal species.
It is tempting to speculate that the positive outcome of this intervention in clinical trials will open up other applications: for treatment of acute myocardial infarction, acute lung injury and stroke, among others. Of course, the complexity and cost of these interventions are challenging, yet they promise unparalleled efficacy in treatment of many life-threatening conditions including those lacking effective pharmacotherapy. If this is the case, the trade-off is well worth the investment. However, “it is very difficult to make predictions, especially when they concern the future”.
Executive summary.
Reactive oxygen species are implicated in the inflammatory pathogenesis of numerous diseases.
Antioxidant enzymes, catalase and superoxide dismutase, are highly effective at quenching antioxidants, but have poor bioavailability and therapeutic index.
Varied size, shape and composition nanocarriers have been developed to overcome the challenge of enzyme delivery.
Polymer nanocarrier design features modulate pharmacological outcomes such as tissue localization, dose, circulation and therapeutic efficacy.
Nonpolymeric nanoparticles, such as liposomes, polyplex nanoparticles and magnetic nanocarriers improve stability, impart protection and target delivery of enzymes.
Developments in the field of antioxidant enzyme delivery bring the field closer to clinical translation.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organizati on or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Christofidou-Solomidou M, Muzykantov VR. Antioxidant strategies in respiratory medicine. Treat Respir Med. 2006;5(1):47–78. doi: 10.2165/00151829-200605010-00004. [DOI] [PubMed] [Google Scholar]
- 2.Muzykantov VR. Targeting of superoxide dismutase and catalase to vascular endothelium. J Control Release. 2001;71(1):1–21. doi: 10.1016/s0168-3659(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 3.Yi X, Zimmerman MC, Yang R, Tong J, Vinogradov S, Kabanov AV. Pluronic-modified superoxide dismutase 1 attenuates angiotensin II-induced increase in intracellular superoxide in neurons. Free Radic Biol Med. 2010;49(4):548–558. doi: 10.1016/j.freeradbiomed.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muzykantov VR. Delivery of antioxidant enzyme proteins to the lung. Antioxid Redox Signal. 2001;3(1):39–62. doi: 10.1089/152308601750100489. [DOI] [PubMed] [Google Scholar]
- 5.Suarna C, Wu BJ, Choy K, et al. Protective effect of vitamin E supplements on experimental atherosclerosis is modest and depends on preexisting vitamin E deficiency. Free Radic Biol Med. 2006;41(5):722–730. doi: 10.1016/j.freeradbiomed.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Siekmeier R, Steffen C, Marz W. Role of oxidants and antioxidants in atherosclerosis: results of in vitro and in vivo investigations. J Cardiovasc Pharmacol Ther. 2007;12(4):265–282. doi: 10.1177/1074248407299519. [DOI] [PubMed] [Google Scholar]
- 7.Thomson MJ, Puntmann V, Kaski JC. Atherosclerosis and oxidant stress: the end of the road for antioxidant vitamin treatment? Cardiovasc Drugs Ther. 2007;21(3):195–210. doi: 10.1007/s10557-007-6027-1. [DOI] [PubMed] [Google Scholar]
- 8.Dziubla TD, Muro S, Muzykantov VR, Koval M. Nanoscale antioxidant therapeutics. In: Singh KK, editor. Oxidative Stress, Disease and Cancer. Imperial College Press; London, UK: 2006. [Google Scholar]
- 9.Delles C, Miller WH, Dominiczak AF. Targeting reactive oxygen species in hypertension. Antioxid Redox Signal. 2008;10(6):1061–1077. doi: 10.1089/ars.2007.2008. [DOI] [PubMed] [Google Scholar]
- 10.Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Kumar MN. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J Control Release. 2006;113(3):189–207. doi: 10.1016/j.jconrel.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Muzykantov VR, Atochina EN, Ischiropoulos H, Danilov SM, Fisher AB. Immunotargeting of antioxidant enzyme to the pulmonary endothelium. Proc Natl Acad Sci USA. 1996;93(11):5213–5218. doi: 10.1073/pnas.93.11.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, et al. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci USA. 1999;96(5):2379–2384. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nowak K, Weih S, Metzger R, et al. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia-reperfusion injury of the lung in vivo. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L162–L169. doi: 10.1152/ajplung.00001.2007. [DOI] [PubMed] [Google Scholar]
- 14.Shuvaev VV, Tliba S, Nakada M, Albelda SM, Muzykantov VR. Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J Pharmacol Exp Ther. 2007;323(2):450–457. doi: 10.1124/jpet.107.127126. [DOI] [PubMed] [Google Scholar]
- 15.Shuvaev VV, Christofidou-Solomidou M, Bhora F, et al. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J Pharmacol Exp Ther. 2009;331(2):404–411. doi: 10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shuvaev VV, Han J, Yu KJ, et al. PECAM-targeted delivery of SOD inhibits endothelial inflammatory response. FASEB J. 2010;25(1):348–357. doi: 10.1096/fj.10-169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, et al. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol. 2003;21(4):392–398. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 18.Christofidou-Solomidou M, Scherpereel A, Wiewrodt R, et al. PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2003;285(2):L283–292. doi: 10.1152/ajplung.00021.2003. [DOI] [PubMed] [Google Scholar]
- 19.Muro S, Wiewrodt R, Thomas A, et al. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116(Pt 8):1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 20.Wiewrodt R, Thomas AP, Cipelletti L, et al. Size-dependent intracellular immunotargeting of therapeutic cargoes into endothelial cells. Blood. 2002;99(3):912–922. doi: 10.1182/blood.v99.3.912. [DOI] [PubMed] [Google Scholar]
- 21.Muro S, Cui X, Gajewski C, Murciano JC, Muzykantov VR, Koval M. Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress. Am J Physiol Cell Physiol. 2003;285(5):C1339–C1347. doi: 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- 22.Muro S, Gajewski C, Koval M, Muzykantov VR. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105(2):650–658. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]
- 23.Stenesh J. Biochemistry. Plenum; NY, USA: 1998. [Google Scholar]
- 24.Papi A, Chicca M, Pandit A, Caramori G, Geoffrey JL, Steven DS. Encyclopedia of Respiratory Medicine. Academic Press; Oxford, UK: 2006. Oxidants and antioxidants/Antioxidants, Nonenzymatic; pp. 266–271. [Google Scholar]
- 25.Quideau S, Deffieux D, Douat-Casassus C, Pouysegu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl. 2011;50(3):586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 26.Sandur SK, Ichikawa H, Pandey MK, et al. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane) Free Radic Biol Med. 2007;43(4):568–580. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol. 2011 doi: 10.2174/138920112798868791. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 28.Pezzuto JM. The phenomenon of resveratrol: redefining the virtues of promiscuity. Ann NY Acad Sci. 2011;1215(1):123–130. doi: 10.1111/j.1749-6632.2010.05849.x. [DOI] [PubMed] [Google Scholar]
- 29.Freeman BA, Young SL, Crapo JD. Liposome-mediated augmentation of superoxide dismutase in endothelial cells prevents oxygen injury. J Biol Chem. 1983;258(20):12534–12542. [PubMed] [Google Scholar]
- 30▪.Chorny M, Hood E, Levy RJ, Muzykantov VR. Endothelial delivery of antioxidant enzymes loaded into non-polymeric magnetic nanoparticles. J Control Release. 2010;146(1):144–151. doi: 10.1016/j.jconrel.2010.05.003. Nonsolvent, nonshear preparation of magnetically active nanocarriers efficiently load active catalase or SOD, provide proteins with protection from proteolysis and protect cells from oxidative damage from hydrogen peroxide in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Batrakova EV, Li S, Reynolds AD, et al. A macrophage-nanozyme delivery system for Parkinson’s disease. Bioconjug Chem. 2007;18(5):1498–1506. doi: 10.1021/bc700184b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vertegel AA, Reukov V, Maximov V. Enzyme-nanoparticle conjugates for biomedical applications. Methods Mol Biol. 2011;679:165–182. doi: 10.1007/978-1-60761-895-9_14. [DOI] [PubMed] [Google Scholar]
- 33.Nagami H, Yoshimoto N, Umakoshi H, Shimanouchi T, Kuboi R. Liposome-assisted activity of superoxide dismutase under oxidative stress. J Biosci Bioeng. 2005;99(4):423–428. doi: 10.1263/jbb.99.423. [DOI] [PubMed] [Google Scholar]
- 34.Stone WL, Smith M. Therapeutic uses of antioxidant liposomes. Mol Biotechnol. 2004;27(3):217–230. doi: 10.1385/MB:27:3:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonnet M, Lethuaut L, Boury F. New trends in encapsulation of liposoluble vitamins. J Control Release. 2010;146(3):276–290. doi: 10.1016/j.jconrel.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 36.Alipour M, Omri A, Smith MG, Suntres ZE. Prophylactic effect of liposomal N-acetylcysteine against LPS-induced liver injuries. J Endo Res. 2007;13(5):297–304. doi: 10.1177/0968051907085062. [DOI] [PubMed] [Google Scholar]
- 37▪▪.Mitsopoulos P, Omri A, Alipour M, Vermeulen N, Smith MG, Suntres ZE. Effectiveness of liposomal-N-acetylcysteine against LPS-induced lung injuries in rodents. Int J Pharma. 2008;363(1–2):106–111. doi: 10.1016/j.ijpharm.2008.07.015. Liposomal delivery of antioxidants provided protection against LPS in multiple lung injury measures. [DOI] [PubMed] [Google Scholar]
- 38.Fan J, Shek PN, Suntres ZE, Li YH, Oreopoulos GD, Rotstein OD. Liposomal antioxidants provide prolonged protection against acute respiratory distress syndrome. Surgery. 2000;128(2):332–338. doi: 10.1067/msy.2000.108060. [DOI] [PubMed] [Google Scholar]
- 39.Mukhopadhyay S, Mukherjee S, Stone WL, Smith M, Das SK. Role of MAPK/AP-1 signaling pathway in the protection of CEES-induced lung injury by antioxidant liposome. Toxicology. 2009;261(3):143–151. doi: 10.1016/j.tox.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug-delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res (Phila) 2011;4(8):1158–1171. doi: 10.1158/1940-6207.CAPR-10-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thangapazham RL, Puri A, Tele S, Blumenthal R, Maheshwari RK. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int J Oncol. 2008;32(5):1119–1123. [PMC free article] [PubMed] [Google Scholar]
- 42.Hung CF, Chen JK, Liao MH, Lo HM, Fang JY. Development and evaluation of emulsion-liposome blends for resveratrol delivery. J Nanosci Nanotechnol. 2006;6(9–10):2950–2958. doi: 10.1166/jnn.2006.420. [DOI] [PubMed] [Google Scholar]
- 43.Narayanan NK, Nargi D, Randolph C, Narayanan BA. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125(1):1–8. doi: 10.1002/ijc.24336. [DOI] [PubMed] [Google Scholar]
- 44.Williams SR, Lepene BS, Thatcher CD, Long TE. Synthesis and characterization of poly(ethylene glycol)-glutathione conjugate self-assembled nanoparticles for antioxidant delivery. Biomacromolecules. 2009;10(1):155–161. doi: 10.1021/bm801058j. [DOI] [PubMed] [Google Scholar]
- 45.Tanswell AK, Freeman BA. Liposome-entrapped antioxidant enzymes prevent lethal O2 toxicity in the newborn rat. J Appl Physiol. 1987;63(1):347–352. doi: 10.1152/jappl.1987.63.1.347. [DOI] [PubMed] [Google Scholar]
- 46.Briscoe P, Caniggia I, Graves A, et al. Delivery of superoxide dismutase to pulmonary epithelium via pH-sensitive liposomes. Am J Physiol. 1995;268(3 Pt 1):L374–380. doi: 10.1152/ajplung.1995.268.3.L374. [DOI] [PubMed] [Google Scholar]
- 47.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95(3):588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 48.Corvo LM, Jorge JCS, van’t Hof R, Cruz MEM, Crommelin DJA, Storm G. Superoxide dismutase entrapped in long-circulating liposomes: formulation design and therapeutic activity in rat adjuvant arthritis. Biochim Biophys Acta. 2002;1564(1):227–236. doi: 10.1016/s0005-2736(02)00457-1. [DOI] [PubMed] [Google Scholar]
- 49.Gaspar MM, Martins MB, Corvo ML, Cruz ME. Design and characterization of enzymosomes with surface-exposed superoxide dismutase. Biochim Biophys Acta. 2003;1609(2):211–217. doi: 10.1016/s0005-2736(02)00702-2. [DOI] [PubMed] [Google Scholar]
- 50.Gaspar MM, Boerman OC, Laverman P, Corvo ML, Storm G, Cruz ME. Enzymosomes with surface-exposed superoxide dismutase: in vivo behaviour and therapeutic activity in a model of adjuvant arthritis. J Control Release. 2007;117(2):186–195. doi: 10.1016/j.jconrel.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Brynskikh AM, Zhao Y, Mosley RL, et al. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson’s disease. Nanomedicine. 2010;5(3):379–396. doi: 10.2217/nnm.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenbaugh EG, Roat JW, Gao L, et al. The attenuation of central angiotensin II-dependent pressor response and intra-neuronal signaling by intracarotid injection of nanoformulated copper/zinc superoxide dismutase. Biomaterials. 2010;31(19):5218–5226. doi: 10.1016/j.biomaterials.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Batrakova EV, Li S, Miller DW, Kabanov AV. Pluronic P85 increases permeability of a broad spectrum of drugs in polarized BBMEC and Caco-2 cell monolayers. Pharm Res. 1999;16(9):1366–1372. doi: 10.1023/a:1018990706838. [DOI] [PubMed] [Google Scholar]
- 54.Batrakova EV, Miller DW, Li S, Alakhov VY, Kabanov AV, Elmquist WF. Pluronic P85 enhances the delivery of digoxin to the brain: in vitro and in vivo studies. J Pharmacol Exp Ther. 2001;296(2):551–557. [PubMed] [Google Scholar]
- 55.Batrakova EV, Zhang Y, Li Y, et al. Effects of pluronic P85 on GLUT1 and MCT1 transporters in the blood-brain barrier. Pharm Res. 2004;21(11):1993–2000. doi: 10.1023/b:pham.0000048189.79606.6e. [DOI] [PubMed] [Google Scholar]
- 56.Chorny M, Fishbein I, Alferiev I, Levy RJ. Magnetically responsive biodegradable nanoparticles enhance adenoviral gene transfer in cultured smooth muscle and endothelial cells. Mol Pharm. 2009;6(5):1380–1387. doi: 10.1021/mp900017m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shuvaev VV, Tliba S, Pick J, et al. Modulation of endothelial targeting by size of antibody-antioxidant enzyme conjugates. J Control Release. 2011;149(3):236–241. doi: 10.1016/j.jconrel.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villano D, Fernandez-Pachon MS, Moya ML, Troncoso AM, Garcia-Parrilla MC. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta. 2007;71(1):230–235. doi: 10.1016/j.talanta.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 59.Rock CL, Jacob RA, Bowen PE. Update on the biological characteristics of the antioxidant micronutrients: vitamin C, vitamin E, and the carotenoids. J Am Diet Assoc. 1996;96(7):693–702. doi: 10.1016/S0002-8223(96)00190-3. quiz 703–694. [DOI] [PubMed] [Google Scholar]
- 60.de la Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans. 2007;35(Pt 5):1156–1160. doi: 10.1042/BST0351156. [DOI] [PubMed] [Google Scholar]
- 61.Wattamwar PP, Hardas SS, Butterfield DA, Anderson KW, Dziubla TD. Tuning of the pro-oxidant and antioxidant activity of trolox through the controlled release from biodegradable poly(trolox ester) polymers. Acta Biomaterialia. 2011 doi: 10.1002/jbm.a.33174. (In Press) [DOI] [PubMed] [Google Scholar]
- 62.Kumar V, Prud’Homme RK. Thermodynamic limits on drug loading in nanoparticle cores. J Pharm Sci. 2008;97(11):4904–4914. doi: 10.1002/jps.21342. [DOI] [PubMed] [Google Scholar]
- 63.De Villiers MM, Aramwit P, Kwon GS. Nanotechnology in Drug Delivery. Springer, AAPS Press; NY and VA, USA: 2009. [Google Scholar]
- 64.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 65.Leonarduzzi G, Testa G, Sottero B, Gamba P, Poli G. Design and development of nanovehicle-based delivery systems for preventive or therapeutic supplementation with flavonoids. Curr Med Chem. 17(1):74–95. doi: 10.2174/092986710789957760. [DOI] [PubMed] [Google Scholar]
- 66.Kumar V, Hong SY, Maciag AE, et al. Stabilization of the nitric oxide (NO) prodrugs and anticancer leads, PABA/NO and double JS-K, through incorporation into PEG-protected nanoparticles. Mol Pharm. 2010;7(1):291–298. doi: 10.1021/mp900245h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wattamwar PP, Mo YQ, Wan R, Palli R, Zhang QW, Dziubla TD. Antioxidant activity of degradable polymer poly(trolox ester) to suppress oxidative stress injury in the cells. Adv Func Mater. 2010;20(1):147–154. [Google Scholar]
- 68.Fleming C, Maldjian A, Da Costa D, et al. A carbohydrate-antioxidant hybrid polymer reduces oxidative damage in spermatozoa and enhances fertility. Nat Chem Biol. 2005;1(5):270–274. doi: 10.1038/nchembio730. [DOI] [PubMed] [Google Scholar]
- 69.Spizzirri UG, Iemma F, Puoci F, et al. Synthesis of antioxidant polymers by grafting of gallic acid and catechin on gelatin. Biomacromolecules. 2009;10(7):1923–1930. doi: 10.1021/bm900325t. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Singh A, Xu P, Pindrus MA, Blasioli DJ, Kaplan DL. Expansion and osteogenic differentiation of bone marrow-derived mesenchymal stem cells on a vitamin C functionalized polymer. Biomaterials. 2006;27(17):3265–3273. doi: 10.1016/j.biomaterials.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 71.Dziubla TD, Karim A, Muzykantov VR. Polymer nanocarriers protecting active enzyme cargo against proteolysis. J Control Release. 2005;102(2):427–439. doi: 10.1016/j.jconrel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 72.Giovagnoli S, Luca G, Casaburi I, et al. Long-term delivery of superoxide dismutase and catalase entrapped in poly(lactide-co-glycolide) microspheres: In vitro effects on isolated neonatal porcine pancreatic cell clusters. J Control Release. 2005;107(1):65–77. doi: 10.1016/j.jconrel.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 73.Seshadri G, Sy JC, Brown M, et al. The delivery of superoxide dismutase encapsulated in polyketal microparticles to rat myocardium and protection from myocardial ischemia-reperfusion injury. Biomaterials. 2010;31(6):1372–1379. doi: 10.1016/j.biomaterials.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fiore VF, Lofton MC, Roser-Page S, et al. Polyketal microparticles for therapeutic delivery to the lung. Biomaterials. 2010;31(5):810–817. doi: 10.1016/j.biomaterials.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75▪▪.Dziubla TD, Shuvaev VV, Hong NK, et al. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials. 2008;29(2):215–227. doi: 10.1016/j.biomaterials.2007.09.023. Degradable polymer nanocarriers modified to accommodate endothelial targeting antibodies successfully load active catalase, bind to endothelial cells and impart antioxidant protection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reddy MK, Wu L, Kou W, Ghorpade A, Labhasetwar V. Superoxide dismutase-loaded PLGA nanoparticles protect cultured human neurons under oxidative stress. Appl Biochem Biotechnol. 2008;151(2–3):565–577. doi: 10.1007/s12010-008-8232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reddy MK, Labhasetwar V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia-reperfusion injury. FASEB J. 2009;23(5):1384–1395. doi: 10.1096/fj.08-116947. [DOI] [PubMed] [Google Scholar]
- 78.Muro S, Dziubla T, Qiu W, et al. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule 1. J Pharmacol Exp Ther. 2006;317(3):1161–1169. doi: 10.1124/jpet.105.098970. [DOI] [PubMed] [Google Scholar]
- 79▪.Yan M, Du JJ, Gu Z, et al. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nat Nanotechnol. 2010;5(1):48–53. doi: 10.1038/nnano.2009.341. Novel biodegradable protein delivery nanocapsules. [DOI] [PubMed] [Google Scholar]
- 80.Kim D, Kim E, Kim J, et al. Direct synthesis of polymer nanocapsules with a noncovalently tailorable surface. Angew Chem Int Ed. 2007;46(19):3471–3474. doi: 10.1002/anie.200604526. [DOI] [PubMed] [Google Scholar]
- 81.Kim E, Kim D, Jung H, et al. Facile, Template-Free Synthesis of Stimuli-Responsive Polymer Nanocapsules for Targeted Drug Delivery. Angew Chem Int Ed. 2010;49(26):4405–4408. doi: 10.1002/anie.201000818. [DOI] [PubMed] [Google Scholar]
- 82.Photos PJ, Bacakova L, Discher B, Bates FS, Discher DE. Polymer vesicles in vivo: correlations with PEG molecular weight. J Control Release. 2003;90(3):323–334. doi: 10.1016/s0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- 83.Saad M, Garbuzenko OB, Ber E, et al. Receptor targeted polymers, dendrimers, liposomes: which nanocarrier is the most efficient for tumor-specific treatment and imaging? J Control Release. 2008;130(2):107–114. doi: 10.1016/j.jconrel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dziubla TD, Karim A, Muzykantov VR. Polymer nanocarriers protecting active enzyme cargo against proteolysis. J Control Release. 2005;102(2):427–439. doi: 10.1016/j.jconrel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 85.Ahmed F, Discher DE. Self-porating polymersomes of PEG-PLA and PEG-PCL: hydrolysis-triggered controlled release vesicles. J Control Release. 2004;96(1):37–53. doi: 10.1016/j.jconrel.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 86.Zarif L. Elongated supramolecular assemblies in drug delivery. J Control Release. 2002;81(1–2):7–23. doi: 10.1016/s0168-3659(02)00010-x. [DOI] [PubMed] [Google Scholar]
- 87.Bianco A, Kostarelos K, Prato M. Opportunities and challenges of carbon-based nanomaterials for cancer therapy. Expert Opin Drug Deliv. 2008;5(3):331–342. doi: 10.1517/17425247.5.3.331. [DOI] [PubMed] [Google Scholar]
- 88▪▪.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103(13):4930–4934. doi: 10.1073/pnas.0600997103. Demonstrates that the effect of high aspect ratio and shape on inhibition of phagocytosis of drug-delivery particles is possible by minimizing the size-normalized curvature of particles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Muro S, Garnacho C, Champion JA, et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol Ther. 2008;16(8):1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu S, Nie Z, Seo M, et al. Generation of monodisperse particles by using microfluidics: control over size, shape, and composition. Angew Chem Int Ed Engl. 2005;44(5):724–728. doi: 10.1002/anie.200462226. [DOI] [PubMed] [Google Scholar]
- 91.Dendukuri D, Pregibon DC, Collins J, Hatton TA, Doyle PS. Continuous-flow lithography for high-throughput microparticle synthesis. Nat Mater. 2006;5(5):365–369. doi: 10.1038/nmat1617. [DOI] [PubMed] [Google Scholar]
- 92▪.Gratton SE, Pohlhaus PD, Lee J, Guo J, Cho MJ, Desimone JM. Nanofabricated particles for engineered drug therapies: a preliminary biodistribution study of PRINT nanoparticles. J Control Release. 2007;121(1–2):10–18. doi: 10.1016/j.jconrel.2007.05.027. Investigates cellular internalization pathways of varied size, shape and surface charge of nanofabricated particles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc. 2005;127(28):10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 94.Gratton SE, Napier ME, Ropp PA, Tian S, Desimone JM. Microfabricated particles for engineered drug therapies: elucidation into the mechanisms of cellular internalization of PRINT particles. Pharm Res. 2008;25(12):2845–2852. doi: 10.1007/s11095-008-9654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee JC, Bermudez H, Discher BM, et al. Preparation, stability, and in vitro performance of vesicles made with diblock copolymers. Biotechnol Bioeng. 2001;73(2):135–145. doi: 10.1002/bit.1045. [DOI] [PubMed] [Google Scholar]
- 96.Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297(5583):967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 97.Lee JC, Wong DT, Discher DE. Direct measures of large, anisotropic strains in deformation of the erythrocyte cytoskeleton. Biophys J. 1999;77(2):853–864. doi: 10.1016/S0006-3495(99)76937-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dalhaimer P, Bates FS, Discher DE. Single molecule visualization of stable, stiffness-tunable, flow-conforming worm micelles. Macromolecules. 2003;36(18):6873–6877. [Google Scholar]
- 99.Simone EA, Dziubla TD, Colon-Gonzalez F, Discher DE, Muzykantov VR. Effect of polymer amphiphilicity on loading of a therapeutic enzyme into protective filamentous and spherical polymer nanocarriers. Biomacromolecules. 2007;8(12):3914–3921. doi: 10.1021/bm700888h. [DOI] [PubMed] [Google Scholar]
- 100.Simone EA, Dziubla TD, Discher DE, Muzykantov VR. Filamentous polymer nanocarriers of tunable stiffness that encapsulate the therapeutic enzyme catalase. Biomacromolecules. 2009;10(6):1324–1330. doi: 10.1021/bm900189x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Petros RA, Ropp PA, DeSimone JM. Reductively labile PRINT particles for the delivery of doxorubicin to HeLa cells. J Am Chem Soc. 2008;130(15):5008–5009. doi: 10.1021/ja801436j. [DOI] [PubMed] [Google Scholar]
- 102.Kelly JY, DeSimone JM. Shape-specific, monodisperse nano-molding of protein particles. J Am Chem Soc. 2008;130(16):5438–5439. doi: 10.1021/ja8014428. [DOI] [PubMed] [Google Scholar]
- 103.Castillo J, Curley J, Hotz J, et al. Glucocorticoids prolong rat sciatic nerve blockade in vivo from bupivacaine microspheres. Anesthesiology. 1996;85(5):1157–1166. doi: 10.1097/00000542-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 104.Hickey T, Kreutzer D, Burgess DJ, Moussy F. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials. 2002;23(7):1649–1656. doi: 10.1016/s0142-9612(01)00291-5. [DOI] [PubMed] [Google Scholar]
- 105.Simone EA, Dziubla TD, Arguiri E, et al. Loading PEG-catalase into filamentous and spherical polymer nanocarriers. Pharm Res. 2009;26(1):250–260. doi: 10.1007/s11095-008-9744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumar N, Ravikumar MN, Domb AJ. Biodegradable block copolymers. Adv Drug Deliv Rev. 2001;53(1):23–44. doi: 10.1016/s0169-409x(01)00219-8. [DOI] [PubMed] [Google Scholar]
- 107.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28(1):5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 108.Siepmann J, Gopferich A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv Drug Deliv Rev. 2001;48(2–3):229–247. doi: 10.1016/s0169-409x(01)00116-8. [DOI] [PubMed] [Google Scholar]
- 109.Hanes J, Chiba M, Langer R. Synthesis and characterization of degradable anhydride-co-imide terpolymers containing trimellitylimido-l-tyrosine: novel polymers for drug delivery. Macromolecules. 1996;29(16):5279–5287. [Google Scholar]
- 110.Leong KW, Brott BC, Langer R. Bioerodible polyanhydrides as drug-carrier matrices. I: Characterization, degradation, and release characteristics. J Biomed Mater Res. 1985;19(8):941–955. doi: 10.1002/jbm.820190806. [DOI] [PubMed] [Google Scholar]
- 111.Tabata Y, Gutta S, Langer R. Controlled delivery systems for proteins using polyanhydride microspheres. Pharm Res. 1993;10(4):487–496. doi: 10.1023/a:1018929531410. [DOI] [PubMed] [Google Scholar]
- 112.Carino GP, Jacob JS, Mathiowitz E. Nanosphere based oral insulin delivery. J Control Release. 2000;65(1–2):261–269. doi: 10.1016/s0168-3659(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 113.Fu J, Wu C. Laser light scattering of the degradation of poly(sebacic anhydride) nanoparticles. J Polymer Sci B Polymer Phys. 2001;39(6):703–708. [Google Scholar]
- 114.Pfeifer BA, Burdick JA, Langer R. Formulation and surface modification of poly(ester-anhydride) micro- and nanospheres. Biomaterials. 2005;26(2):117–124. doi: 10.1016/j.biomaterials.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 115.Li S, Garreau H, Vert M. Structure-property relationships in the case of the degradation of solid aliphatic poly-(α-hydroxy acids) in aqueous media part 3 influence of the morphology of poly(L-lactic acid) J Mat Sci. 1990;1(4):198–206. [Google Scholar]
- 116.Li S, Garreau H, Vert M. Structure-property relationships in the case of the degradation of solid aliphatic poly-(α-hydroxy acids) in aqueous media. Part 1 Poly(DL-lactic acid) J Mat Sci. 1990;1(3):123. [Google Scholar]
- 117.Vert M, Li SM, Garreau H. Attempts to map the structure and degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. J Biomater Sci Polym Ed. 1994;6(7):639–649. doi: 10.1163/156856294x00581. [DOI] [PubMed] [Google Scholar]
- 118.Geng Y, Discher DE. Hydrolytic degradation of poly(ethylene oxide)-block-polycaprolactone worm micelles. J Am Chem Soc. 2005;127(37):12780–12781. doi: 10.1021/ja053902e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shih C. A graphical method for the determination of the mode of hydrolysis of biodegradable polymers. Pharm Res. 1995;12(12):2036–2060. doi: 10.1023/a:1016276830464. [DOI] [PubMed] [Google Scholar]
- 120.Ahmed F, Pakunlu RI, Srinivas G, et al. Shrinkage of a rapidly growing tumor by drug-loaded polymersomes: pH-triggered release through copolymer degradation. Mol Pharm. 2006;3(3):340–350. doi: 10.1021/mp050103u. [DOI] [PubMed] [Google Scholar]
- 121.Gopferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 1996;17(2):103–114. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- 122.Li S, Molina I, Martinez MB, Vert M. Hydrolytic and enzymatic degradations of physically crosslinked hydrogels prepared from PLA/PEO/PLA triblock copolymers. J Mater Sci Mater Med. 2002;13(1):81–86. doi: 10.1023/a:1013651022431. [DOI] [PubMed] [Google Scholar]
- 123.MacDonald RT, McCarthy SP, Gross RA. Enzymatic degradability of poly(lactide): effects of chain stereochemistry and material crystallinity. Macromolecules. 1996;29(23):7356–7361. [Google Scholar]
- 124.Tokiwa Y, Jarerat A. Biodegradation of poly(L-lactide) Biotechnol Lett. 2004;26(10):771–777. doi: 10.1023/b:bile.0000025927.31028.e3. [DOI] [PubMed] [Google Scholar]
- 125.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268(1):235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 126.Bermudez H, Brannan AK, Hammer DA, Bates FS, Discher DE. Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules. 2002;35(21):8203–8208. [Google Scholar]
- 127.Geng Y, Dalhaimer P, Cai S, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nano. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bedu-Addo FK, Tang P, Xu Y, Huang L. Interaction of polyethyleneglycol-phospholipid conjugates with cholesterol-phosphatidylcholine mixtures: sterically stabilized liposome formulations. Pharm Res. 1996;13(5):718–724. doi: 10.1023/a:1016043431778. [DOI] [PubMed] [Google Scholar]
- 129.Koval M, Preiter K, Adles C, Stahl PD, Steinberg TH. Size of IgG-opsonized particles determines macrophage response during internalization. Exp Cell Res. 1998;242(1):265–273. doi: 10.1006/excr.1998.4110. [DOI] [PubMed] [Google Scholar]
- 130.Brewer JM, Tetley L, Richmond J, Liew FY, Alexander J. Lipid vesicle size determines the Th1 or Th2 response to entrapped antigen. J Immunol. 1998;161(8):4000–4007. [PubMed] [Google Scholar]
- 131.Romero EL, Morilla MJ, Regts J, Koning GA, Scherphof GL. On the mechanism of hepatic transendothelial passage of large liposomes. FEBS Lett. 1999;448(1):193–196. doi: 10.1016/s0014-5793(99)00364-6. [DOI] [PubMed] [Google Scholar]
- 132.Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2008;26(1):244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Izumisawa T, Hattori Y, Date M, Toma K, Maitani Y. Cell line-dependent internalization pathways determine DNA transfection efficiency of decaarginine-PEG-lipid. Int J Pharm. 2010;404(1–2):264–270. doi: 10.1016/j.ijpharm.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 134.Shi F, Wasungu L, Nomden A, et al. Interference of poly(ethylene glycol)-lipid analogues with cationic-lipid-mediated delivery of oligonucleotides; role of lipid exchangeability and non-lamellar transitions. Biochem J. 2002;366(Pt 1):333–341. doi: 10.1042/BJ20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aoki H, Fujita M, Sun CQ, Fuji K, Miyajima K. High-efficiency entrapment of superoxide dismutase into cationic liposomes containing synthetic aminoglycolipid. Chem Pharm Bull. 1997;45(8):1327–1331. doi: 10.1248/cpb.45.1327. [DOI] [PubMed] [Google Scholar]
- 136.Atochina EN, Balyasnikova IV, Danilov SM, Granger DN, Fisher AB, Muzykantov VR. Immunotargeting of catalase to ACE or ICAM-1 protects perfused rat lungs against oxidative stress. Am J Physiol. 1998;275(4 Pt 1):L806–817. doi: 10.1152/ajplung.1998.275.4.L806. [DOI] [PubMed] [Google Scholar]
- 137.Deneke SM. Thiol-based antioxidants. Curr Top Cell Regul. 2000;36:151–180. doi: 10.1016/s0070-2137(01)80007-8. [DOI] [PubMed] [Google Scholar]
- 138.Pedersen PJ, Adolph SK, Subramanian AK, et al. Liposomal formulation of retinoids designed for enzyme triggered release. J Med Chem. 2010;53(9):3782–3792. doi: 10.1021/jm100190c. [DOI] [PubMed] [Google Scholar]
- 139.Trapasso E, Cosco D, Celia C, Fresta M, Paolino D. Retinoids: new use by innovative drug-delivery systems. Expert Opin Drug Deliv. 2009;6(5):465–483. doi: 10.1517/17425240902832827. [DOI] [PubMed] [Google Scholar]
- 140.Wegmann, Krucker M, Bachmann S, et al. Characterization of lycopene nanoparticles combining solid-state and suspended-state NMR spectroscopy. J Agricult Food Chem. 2002;50(26):7510–7514. doi: 10.1021/jf020715g. [DOI] [PubMed] [Google Scholar]
- 141.Palozza P, Muzzalupo R, Trombino S, Valdannini A, Picci N. Solubilization and stabilization of [beta]-carotene in niosomes: delivery to cultured cells. Chem Phys Lipids. 2006;139(1):32–42. doi: 10.1016/j.chemphyslip.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 142.Robb EL, Page MM, Wiens BE, Stuart JA. Molecular mechanisms of oxidative stress resistance induced by resveratrol: specific and progressive induction of MnSOD. Biochem Biophys Res Comm. 2008;367(2):406–412. doi: 10.1016/j.bbrc.2007.12.138. [DOI] [PubMed] [Google Scholar]
- 143.Mignet N, Seguin J, Romano MR, et al. Development of a liposomal formulation of the natural flavonoid fisetin. Int J Pharm. 2011 doi: 10.1016/j.ijpharm.2011.04.066. (In Press) [DOI] [PubMed] [Google Scholar]
- 144.Yuan Z-p, Chen L-j, Fan L-y, et al. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin Cancer Res. 2006;12(10):3193–3199. doi: 10.1158/1078-0432.CCR-05-2365. [DOI] [PubMed] [Google Scholar]
- 145.Gao L, Liu G, Wang X, Liu F, Xu Y, Ma J. Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int J Pharm. 2010;404(1–2):231–237. doi: 10.1016/j.ijpharm.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 146.Nilsson L, LoÌ̂f D, BergenstÃ¥hl Br. Phenolic acid nanoparticle formation in iron-containing aqueous solutions. J Agricult Food Chem. 2008;56(23):11453–11457. doi: 10.1021/jf8025925. [DOI] [PubMed] [Google Scholar]
- 147.Coimbra M, Isacchi B, van Bloois L, et al. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int J Pharm. 2011 doi: 10.1016/j.ijpharm.2011.01.056. (In Press) [DOI] [PubMed] [Google Scholar]
- 148.Teskac K, Kristl J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int J Pharm. 2009;390(1):61–69. doi: 10.1016/j.ijpharm.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 149.Frozza RL, Bernardi A, Paese K, et al. Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. J Biomed Nanotechnol. 2011;6(6):694–703. doi: 10.1166/jbn.2010.1161. [DOI] [PubMed] [Google Scholar]
- 150.Lee W-C, Tsai T-H. Preparation and characterization of liposomal coenzyme Q10 for in vivo topical application. Int J Pharm. 2010;395(1–2):78–83. doi: 10.1016/j.ijpharm.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 151.Beg S, Javed S, Kohli K. Bioavailability enhancement of coenzyme Q10: an extensive review of patents. Recent Pat Drug Deliv Formul. 2010;4(3):245–255. doi: 10.2174/187221110793237565. [DOI] [PubMed] [Google Scholar]
- 152.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61(6):428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Carnemolla R, Shuvaev VV, Muzykantov VR. Targeting antioxidant and antithrombotic biotherapeutics to endothelium. Semin Thromb Hemost. 2010;36(3):332–342. doi: 10.1055/s-0030-1253455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Harush-Frenkel O, Altschuler Y, Benita S. Nanoparticle-cell interactions: drug delivery implications. Crit Rev Ther Drug Carrier Syst. 2008;25(6):485–544. doi: 10.1615/critrevtherdrugcarriersyst.v25.i6.10. [DOI] [PubMed] [Google Scholar]
- 155.Sun X, Zhang N. Cationic polymer optimization for efficient gene delivery. Mini Rev Med Chem. 2010;10(2):108–125. doi: 10.2174/138955710791185109. [DOI] [PubMed] [Google Scholar]
- 156▪.Simone EA, Dziubla TD, Muzykantov VR. Polymeric carriers: role of geometry in drug delivery. Expert Opin Drug Deliv. 2008;5(12):1283–1300. doi: 10.1517/17425240802567846. Recent review on the role that the size, shape and surface modifications play on polymer carriers in vitro and in vivo localization and drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tao L, Hu W, Liu Y, Huang G, Sumer BD, Gao J. Shape-specific polymeric nanomedicine: emerging opportunities and challenges. Exp Biol Med. 2011;236(1):20–29. doi: 10.1258/ebm.2010.010243. [DOI] [PubMed] [Google Scholar]
- 158.Caldorera-Moore M, Guimard N, Shi L, Roy K. Designer nanoparticles: incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opin Drug Deliv. 2010;7(4):479–495. doi: 10.1517/17425240903579971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: relevance to drug delivery. Cell Mol Life Sci. 2009;66(17):2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC) Adv Drug Deliv Rev. 2001;48(2–3):139–157. doi: 10.1016/s0169-409x(01)00112-0. [DOI] [PubMed] [Google Scholar]
- 161.Gao W, Chan JM, Farokhzad OC. pH-Responsive nanoparticles for drug delivery. Mol Pharm. 2010;7(6):1913–1920. doi: 10.1021/mp100253e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yoo JW, Chambers E, Mitragotri S. Factors that control the circulation time of nanoparticles in blood: challenges, solutions and future prospects. Curr Pharm Des. 2010;16(21):2298–2307. doi: 10.2174/138161210791920496. [DOI] [PubMed] [Google Scholar]