Abstract
Background and Aims
The importance of chemokines in alcoholic liver injury has been implicated. The role of chemokine, monocyte chemoattractant protein-1 (MCP-1) elevated in patients with alcoholic liver disease is not yet understood. Here we evaluate the pathophysiological significance of MCP-1 and its receptor CCR2 in alcoholic liver injury.
Methods
Leiber-DeCarli diet containing alcohol or isocaloric control diets were fed to wild-type (WT) and MCP-1 deficient (KO) mice for 5 weeks. In vivo and in vitro assays were performed to study the role of MCP-1 in alcoholic liver injury.
Results
MCP-1 was increased in Kupffer cells as well as hepatocytes of alcohol-fed mice. Alcohol feeding increased serum ALT, in WT and CCR2KO but not MCP-1KO mice. Alcohol-induced liver steatosis and triglyceride was attenuated in alcohol-fed MCP-1KO but high in CCR2KO compared to WT, whereas serum endotoxin was high in alcohol-fed WT and MCP-1KO mice. Expression of liver pro-inflammatory cytokines TNFα, IL-1β, IL-6, KC/IL-8, ICAM-1 and CD68 was induced in alcohol-fed WT mice but inhibited in MCP-1KO, independent of NFκB activation in Kupffer cells. Oxidative stress, but not CYP2E1, was prevented in chronic alcohol-fed MCP-1KO mice compared to WT. Increased expression of PPARα and PPARγ, was accompanied by nuclear translocation, DNA binding and induction of fatty acid metabolism genes, ACOX and CPT-1, in livers of alcohol-fed MCP-1KO mice compared to WT controls. In vitro assays uncovered an inhibitory effect of recombinant MCP-1 on PPARα mRNA and PPRE binding in hepatocytes, independent of CCR2.
Conclusion
Deficiency of MCP-1 protects mice against alcoholic liver injury, independent of CCR2, by inhibition of pro-inflammatory cytokines and induction of genes related to fatty acid oxidation, linking chemokines to hepatic lipid metabolism.
Keywords: chemokines, alcohol, CCL2, PPAR, hepatic lipid metabolism
INTRODUCTION
Alcoholic liver disease (ALD) is a major health concern and about ninety percent of heavy drinkers develop fatty liver disease or steatosis. Fatty liver is occasionally accompanied by or progresses to inflammation in human alcoholic liver disease. The essential role of innate immune cell activation and circulating endotoxin/lipopolysaccharide in ALD has been proposed (1, 2). Circulating endotoxin activates liver macrophages and leads to induction of cytokines, chemokines and reactive oxygen species (3). While pro-inflammatory cytokine production in alcoholic liver is extensively investigated, the importance of chemokines is still unknown. Elevation of chemokines such as IL-8, MCP-1 and MIP-1 in alcoholic hepatitis and cirrhotic patients and correlation with recruitment of polymorphonuclear leukocytes is reported (4, 5). However, the pathophysiological mechanisms affected by chemokines in alcoholic liver disease are yet to be determined. CC-chemokines induce recruitment and activation of mononuclear cells such as monocytes/macrophages, T cells and NKT cells (6, 7), and these cells play an important role in development and propagation of alcoholic liver injury (8).
MCP-1 or CCL2, an important CC-chemokine recruits and activates monocytes/macrophages to the site of tissue injury, and regulates adhesion molecules and pro-inflammatory cytokines TNFα, IL-1β and IL-6 (9, 10). The pivotal role of MCP-1 in alcoholic liver injury was first recognized by studies showing higher amounts of MCP-1 as compared to other CC-chemokines, MIP-1α and MIP-1β, in the liver and mononuclear cells of patients with alcoholic hepatitis (4, 5). Subsequently, the pathogenic role of MCP-1 expressed by liver macrophages and endothelial cells was demonstrated in rodent models of alcoholic hepatitis (11). Besides macrophage activation, MCP-1 appears to play a significant role in hepatic steatosis or early liver injury. Recently, transgenic mice overexpressing MCP-1 in adipose tissue exhibited insulin resistance and increased hepatic triglyceride content (12). These studies were based on the observations that mice fed a high-fat diet led to MCP-1 induction in adipose tissue but not liver (12). In vitro studies also demonstrated that MCP-1 can induce lipid accumulation in hepatocyte cultures (13). In general, MCP-1 seems to play an important role in hepatic inflammatory responses and steatosis during tissue injury.
Previous studies from our laboratory and others have shown the pathophysiological importance of pro-inflammatory cytokines in ALD (1, 2, 14). However, the pathophysiological role of chemokines such as MCP-1 in alcoholic liver injury is still uncertain. Based on preferential elevation of MCP-1 amongst other CC-chemokines, in alcoholic hepatitis patients (4, 5) and its importance in modulation of pro-inflammatory cytokines (9, 10), we hypothesized that MCP-1 contributes to chronic alcoholic liver injury and steatosis via modulation of inflammatory cytokines. Using MCP-1 deficient mice we sought to investigate whether MCP-1 and its receptor CCR2, plays a causative role in alcoholic liver injury.
MATERIALS AND METHODS
Additional Methods are available as Supplementary Methods
Animal Studies
All animals received proper care in agreement with animal protocols approved by the Institutional Animal Use and Care Committee of the University of Massachusetts, Medical School. Six- to eight-week-old, female wild type (C57BL/6), and MCP-1-deficient and CCR2-deficient mice (all generated on a C57BL/6 background; Jackson Labs) received Lieber-DeCarli diet (Bio-Serv, Frenchtown, NJ) with 5% (v/v) ethanol (36% ethanol-derived calories) for 6 weeks; pair-fed control mice received an equal amount of calories as their alcohol-fed counterparts with the alcohol-derived calories substituted with dextrinmaltose. All strains of mice consumed comparable daily calories. In some cases, the mice from both the alcohol-fed and pair-fed groups were administered an intraperitoneal (i.p.) injection of either 0.2ml 0.9% saline (phosphate-buffered, pH 7.4) alone as a vehicle control or 0.2ml 0.9% saline containing 0.5mg/kg non-purified lipopolysaccharide (LPS, from Escherichia coli 0111:B4, Sigma, St. Louis, MO) and sacrificed 2 hours later. There were 9–12 mice in each experimental group. Serum was separated from whole blood and frozen at −80°C. Liver tissue was rapidly excised and a portion was snap-frozen in liquid nitrogen and stored at −80°C. Additional portions of the livers were stored in RNA stabilization reagent, RNA later (Qiagen GmbH, Hilden, Germany), for RNA extraction or fixed in 10% neutral-buffered formalin for histopathological analysis.
Statistical analysis
Statistical significance was determined by analysis of variance (in vivo) and t test (in vitro) using the GraphPad Prism 5.01 (GraphPad software, Inc., La Jolla, CA) software. Data are shown as mean ± standard error of the mean (SEM) and were considered statistically significant at P < 0.05.
RESULTS
Chronic alcohol consumption induces MCP-1 in Kupffer cells and hepatocytes
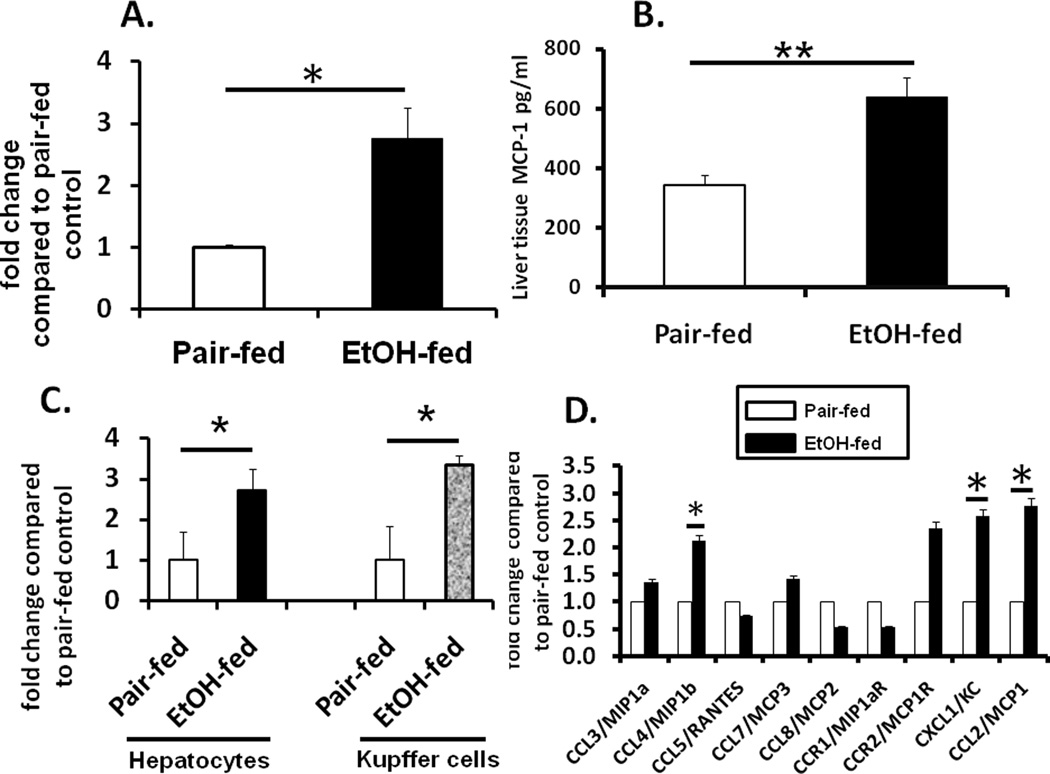
MCP-1 is increased during ALD however its cellular source in the liver is not yet identified. Here, C57Bl/6 mice were fed the Leiber-Decarli alcohol diet or its isocaloric control (pair-fed) diet to determine expression of MCP-1 in the liver. Chronic alcohol feeding for 6 weeks induced MCP-1 mRNA (Fig 1A) and protein (Fig 1B) in whole livers compared to pair-fed controls. Next to identify the cell types expressing MCP-1, we isolated hepatocytes and Kupffer cells and estimated MCP-1 mRNA. Fig 1C shows that isolated hepatocytes as well as Kupffer cells express high amounts of MCP-1 mRNA in chronic alcohol-fed mice compared to isocaloric pair-fed controls with similar expression levels of baseline MCP-1 in hepatocytes relative to Kupffer cells (Fig S1). Expression analysis of the CC-chemokine gene family revealed significant increase in CCL4/MIP-1β and KC/IL-8/CXCL1 with a maximal elevation in MCP-1 in livers of chronic alcohol fed mice compared to pair-fed controls (Fig 1D).
Fig 1. MCP-1 is induced in hepatocytes and Kupffer cells during chronic alcohol exposure.
C57Bl/6 mice were fed 5% alcohol-containing Leiber-DeCarli and isocaloric pair-fed diet or for 6 weeks. (A) Total RNA from liver tissue was subjected to real-time qPCR for determination of MCP-1 mRNA *p<0.04 compared to pair-fed control; n=12; (B) Total liver MCP-1 protein levels were analyzed in tissue extracts by ELISA of alcohol-fed and pair-fed mice **p<0.045 compared to pair-fed control; n=12; (C) Hepatocytes and Kupffer cells isolated from alcohol-fed and pair-fed mice were subjected to real-time qPCR for determination of MCP-1 mRNA *p<0.05 compared to corresponding pair-fed control, n=9. (D) Total RNA from liver tissue was subjected to real-time qPCR for determination of chemokine mRNA *p<0.05 compaired to pair-fed control; n=9. Values depicted in the graph are mean fold change ± SEM.
MCP-1 deficiency protects against alcoholic liver injury
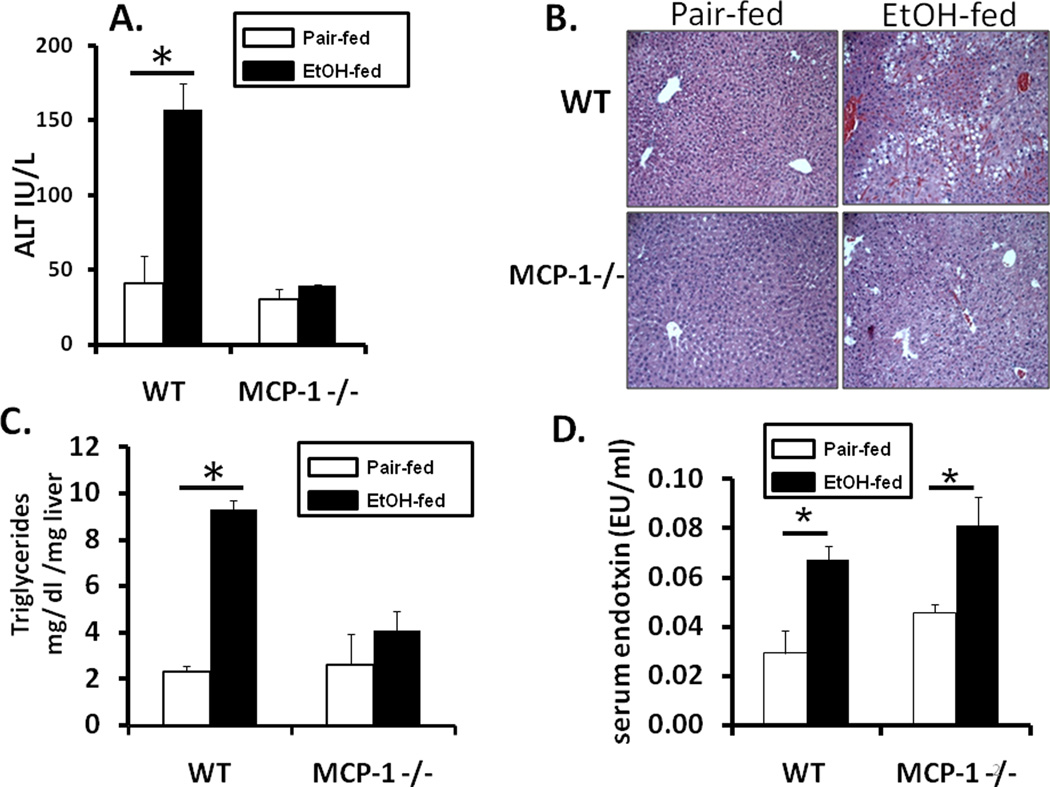
To investigate the role of MCP-1 in alcoholic liver disease, wild-type (WT) and MCP-1 deficient (MCP-1KO) mice were fed Leiber-DeCarli diet with 5% ethanol or isocaloric control diet for 6 weeks to induce alcoholic liver damage. Prolonged alcohol feeding resulted in liver injury as assessed by significantly increased serum ALT levels (Fig 2A) and higher liver/body weight ratio (Suppl Fig S2A) in alcohol-fed WT mice compared to pair-fed controls and MCP-1KO mice. Despite no liver damage, serum alcohol levels in MCP-1KO were comparable to alcohol-fed wild-type mice (Suppl Fig 2B). Histological analysis showed micro and macro-steatosis in chronic alcohol-fed WT mice whereas fat deposition was not detectable in pair-fed controls and MCP-1KO mice (Fig 2B). In agreement with the histological data, liver triglyceride levels were significantly higher in alcohol-fed WT mice compared to pair-fed controls and MCP-1KO mice (Fig 2C). Further, chronic alcohol-fed wild-type and MCP-1KO mice show significantly increased serum endotoxin compared to pair-fed controls (Fig 2D). Collectively these data show that chronic alcohol feeding induces liver damage in wild-type mice and regardless of high blood alcohol levels and elevated endotoxin MCP-1KO mice are protected from alcoholic liver injury.
Fig 2. MCP-1 deficiency protects against alcohol induced liver injury.
MCP-1 deficient (MCP-1 −/−) or wild-type (WT) mice were fed an isocaloric pair-fed diet or 5% alcohol-containing Leiber-DeCarli diet for 6 weeks and (A) serum ALT analyzed *p<0.001, n=12 compared to pair-fed controls, (B) liver sections were fixed in formalin and stained with hematoxylin-eosin; magnification 100X and (C) Liver triglycerides (mg/g liver tissue) measured * p<0.001 compared to pair-fed control. (D) serum endotoxin analyzed *p<0.046, n=9 compared to pair-fed control. Values shown in the graph are mean ± SEM (9–12 mice per group).
Chronic alcohol induced up-regulation of pro-inflammatory cytokines is prevented by MCP-1 deficiency independent of NFκB activation
MCP-1 plays an important role in induction of pro-inflammatory cytokines at the site of tissue injury (10). Here we investigated the impact of MCP-1 deficiency on alcohol-induced expression of cytokines in the liver. We elucidated expression of circulating endotoxin (baseline) mediated induction of pro-inflammatory cytokines TNFα, IL-1β and IL-6 as well as CC-chemokine mRNA levels in liver of alcohol-fed WT and MCP-1KO mice. Here in Fig 3A we show that TNFα, IL-1β and IL-6 mRNA was increased significantly in alcohol-fed WT mice compared to pair-fed WT controls, whereas alcohol-fed MCP-1KO mice were unable to induce pro-inflammatory cytokine mRNA in the liver. Figure 3B shows that MCP-1 deficiency also prevented chronic alcohol induced liver tissue TNFα as compared to WT mice. Interestingly amongst CC-chemokine genes, KC/IL-8 mRNA was significantly decreased but CCL4/MIP-1β and CCL5/RANTES mRNA was high in alcohol-fed MCP-1KO mice compared to pair-fed controls (Fig 3C). Furthermore investigation of MCP-1 mediated adhesion molecules and macrophage markers demonstrate significant induction of ICAM-1 and CD68 but unchanged VCAM-1 and F4/80 in livers of alcohol-fed WT but not MCP-1KO mice (Fig 3D). Since NFκB is important in chronic alcohol mediated pro-inflammatory cytokine production and macrophage activation (15), we next determined whether inhibition of inflammatory cytokines was regulated by lack of NFκB activation in MCP-1 deficient mice. Interestingly, our results show that NFκB binding activity in whole livers was significantly increased in alcohol-fed MCP-1 deficient mice (Fig 3E) compared to alcohol-fed WT and pair-fed MCP-1KO mice. Furthermore increased NFκB activation was observed in isolated Kupffer cells of alcohol-fed MCP-1KO and WT mice compared to pair-fed controls (Fig 3F). Immunohistochemical analysis revealed NFκB p65 staining in non-parenchymal cells of alcohol fed WT and MCP-1KO mice (Suppl Fig S3). On the other hand isolated hepatocytes show decreased NFκB activation in alcohol-fed WT mice compared to pair-fed controls and this inhibition was prevented in alcohol-fed MCP-1KO mice (Fig 3F), likely contributing to NFκB mediated hepatocyte survival in alcohol-fed MCP-1KO mice. These results indicate that liver pro-inflammatory cytokine mRNA, ICAM-1 and CD68 are significantly decreased in chronic alcohol-fed MCP-1KO mice compared to their WT counterparts, in an NFκB independent manner.
Fig 3. Chronic alcohol induced inflammatory cytokine production is prevented in MCP-1 −/− mice.

Chronic alcohol fed WT and MCP-1 −/− mice were subjected to analysis of (A) TNFα mRNA, *p<0.05 compared to pair-fed control (n=9); IL-1β mRNA, *p<0.05 compared to pair-fed control (n=9); IL-6 mRNA, *p<0.05 compared to pair-fed control, n=9, and (B) Tissue TNFα estimated by ELISA, *p<0.05 compared to pair-fed control (n=9) and (C) Total RNA from liver tissue was subjected to real-time qPCR for determination of chemokine mRNA, * p<0.05, **p<0.001, #p<0.01 compaired to pair-fed controls, n=9, (D) Total RNA from liver tissue was subjected to real-time qPCR for determination of adhesion molecules ICAM-1 and VCAM-1 and macrophage markers, F4/80 and CD68 mRNA, *p<0.04, n=7, #p<0.03, n=9, (E) NFκB DNA binding activity in whole livers *p<0.05; **p<0.01, #p<0.001 compared to corresponding pair-fed control (n=8) (F) Kupffer cells (left panel) *p<0.05, n=6 compared to pair-fed control ; primary hepatocytes (right panel) *p<0.03 compared to pair-fed control n=6, was analyzed by EMSA shown representative gel (upper panels). To confirm specificity, a 20-fold excess of unlabeled oligonucleotide was included as cold competitor (Comp). Density units of the upper and lower bands together are shown in bar graph (lower panel). Values shown in graphs are mean ± SEM. ns, non-significant.
Oxidative stress and sensitization to LPS induced pro-inflammatory cytokine production is inhibited by MCP-1 deficiency
The classical feature of alcoholic liver injury is alcohol-mediated oxidative stress and increased sensitization to lipopolysaccharide (LPS) resulting in enhanced pro-inflammatory cytokine expression in the liver (1, 16). To further test the effect of sensitization to LPS in chronic alcohol-fed MCP-1 deficient mice, an in vivo LPS challenge (0.5mg/kg body weight; i.p.) was administered at the end of the chronic alcohol feeding. In figure 4A our results show that in vivo LPS challenge increased pro-inflammatory cytokine, TNFα, IL-1β and IL-6 mRNA in liver of WT mice as compared to pair-fed controls and this induction was prevented in chronic alcohol-fed MCP-1KO mice. Interestingly no changes were observed in TLR4 expression, a receptor for TLR4 (Suppl Fig S4). We next determined whether MCP-1 deficiency affects alcohol induced oxidative stress and alcohol metabolizing enzyme CYP2E1 in the liver. Fig 4B shows that chronic alcohol induced oxidative stress as illustrated by increased TBARS in WT mice was significantly blunted in alcohol-fed MCP-1KO mice. However, CYP2E1 levels estimated in liver microsomal preparations from alcohol-fed WT and MCP-1KO mice remained similar (Fig 4C). These results suggest that MCP-1 contributes to chronic alcohol induced oxidative stress, in a CYP2E1 independent fashion and sensitizes the liver to LPS, resulting in enhanced pro-inflammatory cytokine production.
Fig 4. MCP-1 deficiency prevents oxidative stress and inhibits sensitization to LPS in chronic alcohol fed mice.
Chronic alcohol fed WT and MCP-1 −/− mice were injected with lipopolysaccharide (LPS) and livers subjected to analysis of mRNA by real-time PCR after 2 hours for (A) TNFα mRNA *p<0.01 compared to pair-fed control n=9; IL-6 mRNA *p<0.05, n=9; IL-1β mRNA *p<0.02, n=9 (B) Livers from WT and MCP-1 −/− mice were subjected to analysis of thiobarbituric acid substance (TBARS), marker of oxidative stress **p<0.01 compared to pair-fed. (C) CYP2E1 was detected in liver microsomal fractions by western blotting. Anti-calnexin antibody was used as an internal loading control. Values shown in the graphs are mean ± SEM.
Chronic alcohol feeding induces liver injury in CCR2-deficient mice
MCP-1 is known to mediate inflammatory cell activation in the liver via its receptor CCR2 (17). The importance of CCR2, predominantly expressed in monocyte/macrophage cells, is shown in liver diseases such as fibrosis (18). To investigate the role of CCR2 in alcoholic liver injury we fed CCR2 deficient mice (CCR2KO) with Leiber-DeCarli diet containing 5% ethanol for 6 weeks. Figure 5A shows that similar to WT mice, alcohol feeding increases serum ALT in CCR2KO mice indicating liver damage in the absence of CCR2. Further, histological examination showed that micro- and macro-steatosis was observed in alcohol-fed WT and CCR2KO mice compared to pair-fed controls (Fig 5B). Quantitation of liver triglycerides exhibited significantly high levels in alcohol-fed WT and CCR2KO mice compared to pair-fed mice (Fig 5C), supporting histological findings. Thus, it is evident that chronic alcohol feeding induces liver injury irrespective of the absence of CCR2 and suggests that MCP-1 mediated protection from alcoholic liver injury is independent of CCR2.
Fig 5. CCR2 deficiency does not prevent against alcoholic liver injury.
CCR2 deficient (CCR2 −/−) or wild-type (WT) mice were fed an isocaloric pair-fed diet or 5% alcohol-containing Leiber-DeCarli diet for 6 weeks and (A) serum ALT analyzed *p<0.002, n=12 compared to pair-fed controls, (B) liver sections were fixed in formalin and stained with hematoxylin-eosin; magnification 100X and (C) Liver triglycerides (mg/g liver tissue) measured * p<0.02 compared to pair-fed control n=12. Values shown in the graph are mean ± SEM.
PPARα and PPARγ mRNA expression, DNA binding and target genes is increased in chronic-alcohol fed MCP-1 knock-out mice
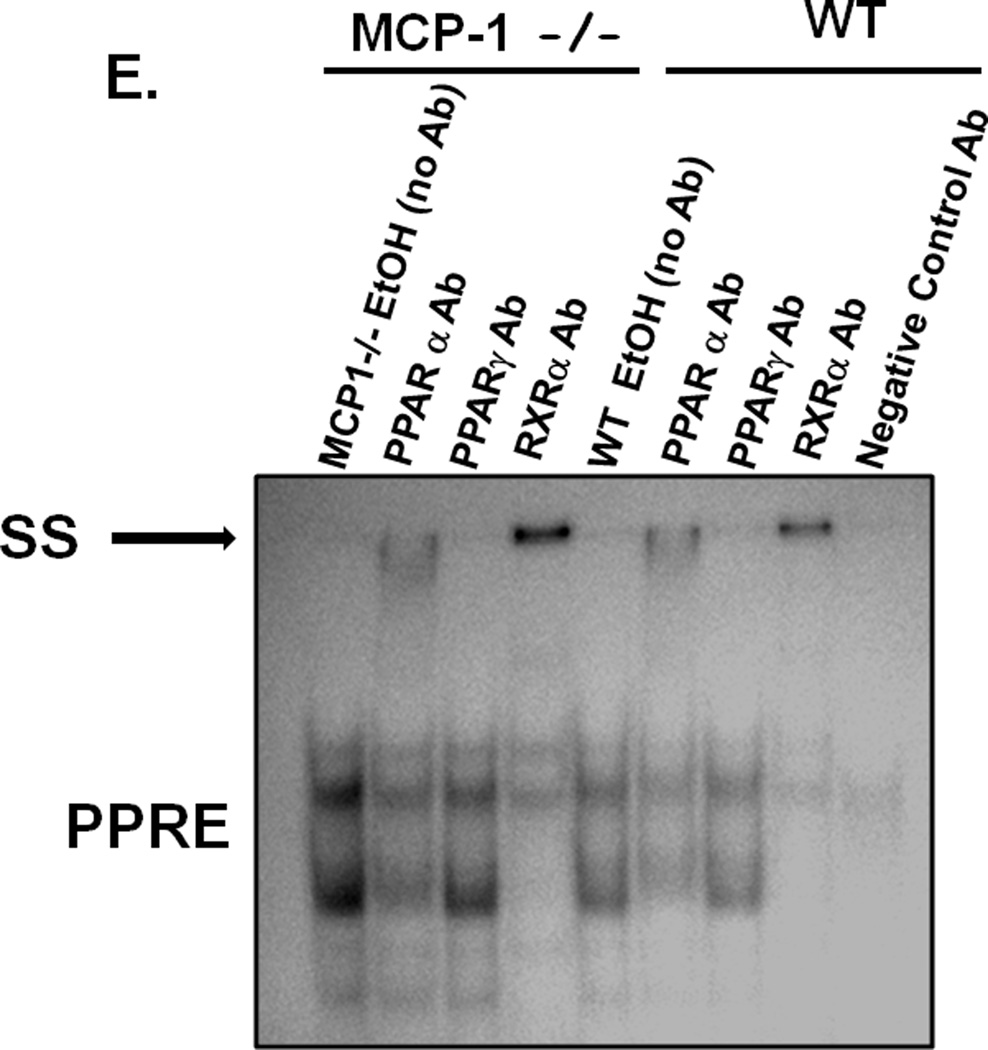
Having observed inhibitory effects on inflammatory responses in the liver we next wanted to determine whether the decrease in hepatic steatosis in alcohol fed MCP-1KO mice (in Figure 2D & E) was related to regulation of fatty acid metabolism genes. We analyzed PPARα and PPARγ, important transcription factors in metabolism as well as inflammatory responses (19). Fig 6A shows that while chronic alcohol feeding decreases PPARα mRNA in WT, alcohol-fed MCP-1KO mice show comparable levels to pair-fed controls, indicating prevention of PPARα down-regulation by alcohol. On the other hand PPARγ mRNA was not affected in alcohol-fed WT mice but was significantly increased in alcohol-fed MCP-1KO mice as compared to controls (Fig 6A). PPARα and PPARγ mRNA was not altered between genotypes, but showed a significant up-regulation in alcohol-fed MCP-1KO compared to WT counterparts (Fig 6A). Upon activation PPARs translocate to the nucleus and bind to promoter elements of target gene involved in fatty acid metabolism (20, 21). Fig 6B shows that nuclear PPARα and PPARγ levels are increased in alcohol-fed MCP-1KO mice compared to pair-fed controls. Using EMSA we next analyzed the DNAbinding activity of PPARs in livers of alcohol-fed WT and MCP-1KO mice. Our results show that PPRE binding activity was significantly reduced in alcohol-fed WT mice compared to pair-fed controls whereas down-regulation of PPRE binding activity was prevented in alcohol-fed livers of MCP-1KO mice (Fig 6C). Similar to PPRE activation in whole livers, figure 6D shows that PPRE binding activity in isolated hepatocytes is significantly reduced in alcohol-fed WT mice whereas this down-regulation was prevented in alcohol-fed MCP-1KO mice compared to pair-fed controls. It is worthy to note that PPRE binding is significantly higher in alcohol-exposed hepatocytes (Fig 6D) and whole livers (Fig 6C) of MCP-1KO compared to alcohol-fed WT mice. Supershift analysis in whole livers of alcohol-fed MCP-1KO and WT mice revealed presence of PPARα and RXRα in the PPAR binding complex (Fig 6E). Next, to further evaluate whether increased PPRE binding activity in MCP-1KO mice results in target gene induction related to fatty acid metabolism (20) we estimated mRNA levels of acyl-CoA oxidase (ACOX), carnitine palmitolyl transferase (CPT-1), long chain acyl-CoA dehydrogenase (LCAD) and medium chain acyl-CoA dehydrogenase (MCAD). Our results show that ACOX (Fig 7A) and CPT-1 (Fig 7B) mRNA levels are significantly decreased in alcohol-fed WT mice and this down-regulation was prevented in MCP-1KO mice. Further, LCAD (Fig 6C) and MCAD (Fig 6D) mRNA did not show significant changes in alcohol-fed MCP-1KO and WT mice. These results indicate that MCP-1 regulates PPAR mRNA expression, nuclear translocation, DNA binding and down-stream target gene expression related to fatty acid metabolism in alcoholic liver injury.
Fig 6. Deficiency of MCP-1 restores chronic alcohol induced PPAR expression and DNA binding activity.
Livers from chronic alcohol fed WT and MCP-1 −/− mice were subjected to analysis of (A) PPARα mRNA *p<0.05, **p<0.03 compared to pair-fed control, n=12; PPARγ mRNA *p<0.05, **p<0.04 compared to pair-fed control n=12, by real time PCR, (B) Nuclear PPARα (*p<0.01 compared to pair-fed control, n=6) and PPARγ (*p<0.05 compared to pair-fed control, n=6) levels by western blotting are shown in representative gels (upper panel) and graphs of density units (lower panel) (C) PPAR binding activity to PPAR-response element (PPRE) in whole livers (*p<0.05 compared to pair-fed control; #p<0.05 compared to WT pair-fed mice) and (D) in isolated hepaocytes (*p<0.01 compaired to pair-fed control, n=6, #p<0.04 compared to WT EtOH-fed mice) by EMSA shown as a representative in gel (upper panel). To confirm specificity, a 20-fold excess of unlabeled oligonucleotide was included as cold competitor (Comp). Density unit of the band is shown in bar graph (lower panel). (E) Supershift analysis was carried out using anti-PPARα, anti-PPARγ, anti-RXRα and negative control (non-specific) antibody (Ab) added 30 min prior to the PPRE oligo in EMSA. Supershifted band (SS) is indicated by bold arrow and indicates presence of that protein in the binding complex. ns, non-significant.
Fig 7. Down-regulation of PPARα target genes is prevented in chronic alcohol fed MCP-1 deficient mice.
Chronic alcohol fed WT and MCP-1 −/− mice were subjected to analysis of PPARα target gene expression by real time PCR for (A) ACOX mRNA *p<0.02 compared to pair-fed control, n=9 (B) CPT-1 mRNA *p<0.01 compared to pair-fed control,n=9 (C) LCAD mRNA, (D) MCAD mRNA. Values shown in the graphs are mean ± SEM. ns, non-significant.
MCP-1 negatively interferes with PPARα mRNA and PPRE binding in cultured hepatocytes
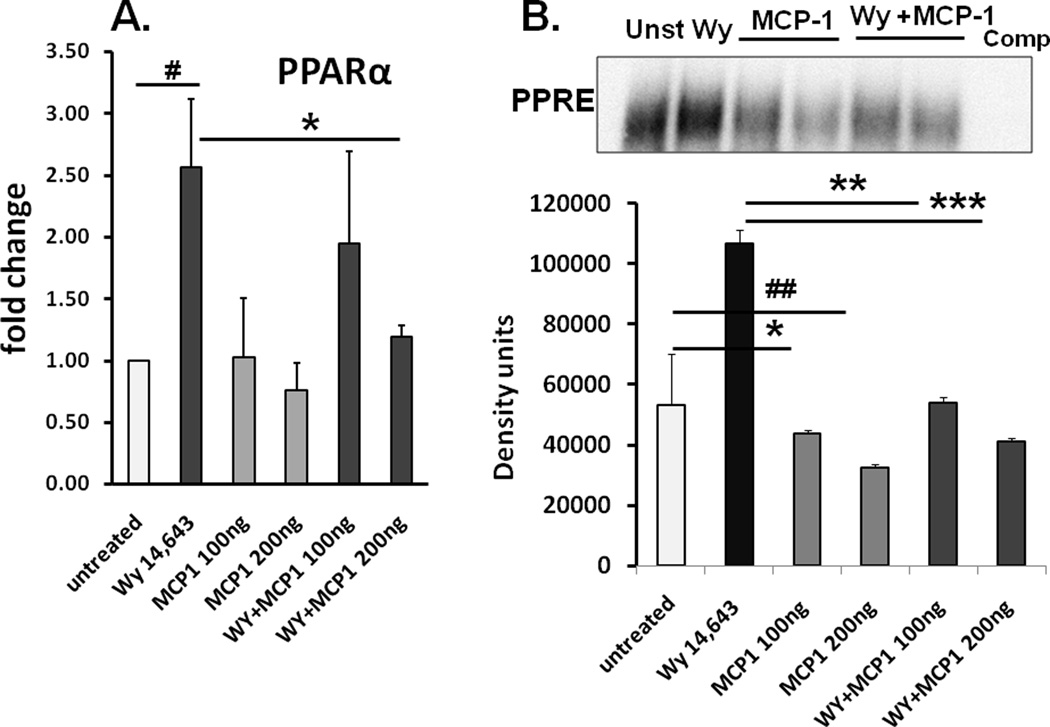
Since lack of MCP-1 correlates with PPRE binding and expression of fatty acid oxidation genes, we wanted to next evaluate whether MCP-1 directly affects PPARα expression and DNA binding activity in hepatocytes. To this end, we performed in vitro experiments using recombinant MCP-1 and determined its effect on PPAR agonist, WY-14,643 induced PPARα mRNA and PPRE binding activity in human hepatocyte Huh7 cells. In accordance with previous studies showing lack of CCR2 expression in hepatocytes (18) and Huh7 cells (13), our results show absence of CCR2 expression in hepatocytes and Huh7 cells compared to high expression in monocyte/macrophages (Suppl Fig S5A). Figure 8A shows that recombinant MCP-1 significantly decreases baseline and WY-induced PPARα mRNA compared to WY treatment alone in Huh7 hepatocytes. Further MCP-1 treatment of Huh7 cells significantly reduced baseline and WY-induced PPRE activation (Fig 8B), shown in triplicates in Suppl Fig S5B. Collectively our results suggest that MCP-1 can directly inhibit PPARα induction and activation impeding fatty acid oxidation in hepatocytes.
Fig 8. MCP-1 interferes with PPARα expression and DNA binding activity.
Huh7 cells treated with 100, 200 ng/ml MCP-1 in the presence or absence of PPARα agonist WY 14,643 (100µM) (WY) was subjected to (A) PPARα mRNA #p<0.03 compared to untreated cells; *p<0.05 compared to WY14,643, n=9, and (values on bar graphs are mean fold change ± SEM (B) PPRE DNA binding activity by EMSA is shown in a representative gel (upper panel) and average density of the bands ± SEM from a total of 9 experiments is shown, *p<0.05 MCP-1 (100ng) vs untreated cells; #p<0.04 MCP-1 (200ng) vs untreated; **p<0.002 MCP-1 (100ng) vs WY14,643; ***p<0.001 MCP-1 (200ng) vs WY14,643.
DISCUSSION
The significance of MCP-1 as a master regulator of monocyte/macrophage function has been proposed in various chronic inflammatory diseases (22). MCP-1 was previously identified to direct trafficking of immune cells to the site of tissue injury (6, 7). However, recent studies have suggested a role for MCP-1 in metabolic diseases such as diabetes and obesity-related insulin resistance and hepatic steatosis (12, 23). Here we report novel data that MCP-1 contributes to alcohol induced fatty liver likely via down-regulation of PPARα and its target fatty acid metabolism genes, independent of its receptor CCR2. These results for the first time indicate a link between inflammatory chemokines and lipid metabolism in alcoholic liver injury. We show that chronic alcohol consumption increases MCP-1 in Kupffer cells and hepatocytes in the liver. Deficiency of MCP-1 protects against chronic alcohol induced liver injury by reducing expression of pro-inflammatory cytokines and macrophage activation markers, ICAM-1 and CD68, and increasing PPARα expression and DNA binding leading to induction of fatty acid metabolism genes.
Chronic alcohol induced liver injury is characterized by steatosis, inflammatory cell activation and hepatocyte damage (1, 2, 24). Inflammatory cell mediators produced in the liver during chronic alcohol exposure contribute to liver injury. For instance, TNFα induces hepatocyte apoptosis in the liver (25), whereas IL-6 can generate hepatoprotective or damaging effects based on the target cells (26). Human studies and animal models of alcoholic hepatitis show that MCP-1 is upregulated in Kupffer cells (11). Our novel observations show that chronic alcohol feeding induces MCP-1 in Kupffer cells and hepatocytes, suggesting a functional role in inflammatory responses and steatosis. Previous studies in genetically obese and high fat diet fed mice showed that MCP-1 significantly increased in adipose tissue and plasma, but not in the liver, contributing to insulin resistance and hepatic steatosis (12). Recent studies by Obstfeld et. al. (23) demonstrate that leptin deficient ob/ob mice exhibit increased MCP-1 in hepatocytes only. Contrary to obesity and diabetes, chronic alcohol induces MCP-1 in Kupffer cells as well as hepatocytes ascribing a pathogenic role for MCP-1 in the alcoholic liver. Activating signals including LPS/TLR4 and pro-inflammatory cytokines are potent inducers of MCP-1 (27, 28). Interestingly recent studies show that homocysteine, increased in alcoholic liver injury (29) also induces MCP-1 expression in hepatocytes (30). The investigation of cell specific regulation of MCP-1 during alcoholic liver injury will provide further insights into its functional significance.
The contribution of MCP-1 in various models of liver injury has been under investigation. While in some cases of liver injury such as hepatic granuloma formation and obesity induced fatty liver, lack of MCP-1 is protective (12, 23, 31), in other instances such as Con-A induced liver injury and lethal endotoxemia absence of MCP-1 worsens disease (32, 33). Here we show that MCP-1 deficiency is protective against chronic alcohol induced liver injury as indicated by decreased serum ALT and reduced steatosis. Patients with severe alcoholic hepatitis and cirrhosis displayed the highest elevation of MCP-1 in liver and plasma compared to other CC-chemokines (4, 5). Previous studies indicated that CC-chemokines including MCP-1 played a major role in late stage alcoholic hepatitis directing migration of inflammatory cells and leading to fibrosis and cirrhosis (8). Studies from Seki and colleagues (18) indicated the significance of the MCP-1/CCR2 axis in liver fibrosis. Our studies provide novel direct evidence for the importance of MCP-1 in pathogenesis of early alcoholic liver injury.
Chronic alcohol feeding induces gut permeability and increases serum endotoxin levels which in turn upregulate pro-inflammatory cytokine production in the liver (2, 3). Our results show that similar to alcohol-fed wild-type, MCP-1 KO animals also demonstrate an elevation in serum endotoxin, suggesting that chronic alcohol does not affect mechanisms related to gut permeability in MCP-1 deficient mice. MCP-1 regulates production of pro-inflammatory cytokines and adhesion molecules in monocytes/macrophages (9, 10). Despite increased endotoxin we observed a significant reduction in mRNA expression of pro-inflammatory cytokines TNFα, IL-1β, IL-6 and KC/IL-8 in the liver of alcohol-fed MCP-1 KO mice compared to wild-type controls. In addition we also observed a significant decrease in adhesion moelcule, ICAM-1 and macrophage activation marker, CD68 in alcohol-fed MCP-1KO mice. Further our data indicate that down-regulation of pro-inflammatory cytokines, adhesion molecule and macrophage activation marker is independent of NFκB activation in Kupffer cells in alcohol-fed MCP-1KO mice. Noteworthy is the lack of reduction in NFκB DNA binding activity in isolated hepatocytes from alcohol-fed MCP-1KO compared to inhibition of NFκB activation in hepatocytes of alcohol-fed WT mice indicates a role for NFκB in hepatocyte survival. Future studies will delineate the mechanism of reduction in pro-inflammatory responses in alcohol-fed MCP-1 deficient mice.
Oxidative stress and sensitization to LPS are hallmarks of molecular mechanisms of alcoholic liver injury (1, 2, 16). Interestingly our results show that MCP-1 deficiency prevents induction of chronic alcohol-induced oxidative stress compared to wild-type mice. A similar correlation between MCP-1 and induction of oxidative stress in a toxic model of acute liver injury using carbon tetrachloride was previously observed (34). How MCP-1 modulates oxidative stress pathways or reduces anti-oxidants will be investigated in the future. Similar up-regulation of microsomal CYP2E1 (35) in alcohol-fed WT and MCP-1KO mice indicate induction of oxidative stress independent of alcohol metabolizing CYP2E1. Besides cellular mechanisms such as toll-like receptor expression, oxidative stress contributes to LPS sensitization in ALD and enhancement of pro- inflammatory cytokine gene expression (36, 37). An in vivo LPS challenge given at the end of chronic alcohol feeding led to augmentation of pro-inflammatory cytokines TNFα, IL-1β and IL-6 in wild-type mice and this was prevented in MCP-1 KO mice. Our results suggest that MCP-1 deficiency inhibits oxidative stress and also impedes sensitization to LPS, independent of TLR4 expression, during alcoholic liver injury. Studies to unravel the mechanisms of LPS sensitization affected by MCP-1 during chronic alcohol exposure will be examined in the future.
Amongst the various mechanistic studies for alcoholic steatosis, alterations in transcription factors such as PPARα and PPARγ, controlling lipid metabolism have been recognized (24). Previous studies showed that alcohol feeding in rats decreased PPARα activation and down-stream target genes important in fatty acid oxidation (19). Here we show that alcohol fed MCP-1KO mice exhibit increased PPARα and PPARγ mRNA expression, enhanced nuclear PPARα and PPARγ, PPRE activation in whole liver and isolated hepatocytes, presence of PPARα/RXRα in the DNA binding complex and induction of target genes such as carnitine palmitoyltransferase (CPT-1) and acyl CoA oxidase (ACOX), both enzymes critical in fatty acid oxidation. Previous studies have shown that secretory product of adipose tissue, likely MCP-1, can induce lipid accumulation in hepatoyctes (13). Our in vitro findings demonstrate that recombinant MCP-1 down-regulates PPARα mRNA expression and DNA binding activity in hepatocytes, likely contributing to increased triglyceride accumulation in ALD. These results suggest a direct effect of MCP-1 on PPARα and its target genes and thus steatosis.
MCP-1 mediates its action via receptor CCR2 (38) or independent of CCR2 (39). Our results show that CCR2KO mice induce alcoholic liver injury similar to alcohol-fed WT mice indicating CCR2 independent effects of MCP-1. Furthermore, since hepatocytes do not express CCR2 (18) as reported here (Suppl Fig 5A), we predict that MCP-1 mediates its effects in the liver independent of CCR2. Another lipid-modulating transcription factor with anti-inflammatory properties PPARγ, was up-regulated in alcohol-fed MCP-1KO livers. It is likely that PPARγ inhibits pro-inflammatory cytokine production in chronic alcohol exposed MCP-1 KO mice. Our results here show that MCP-1 expression is directly upregulated in hepatocytes during chronic alcohol exposure and likely regulates fatty acid oxidation resulting in steatosis. LPS can modulate fatty acid oxidation genes via TLR4/IRAK-1 and PPARα reduction (40). We predict that alcohol mediated increase in circulating endotoxin (LPS) induces MCP-1 in hepatocytes and macrophages to regulate fatty acid oxidation pathways in an autocrine or paracrine fashion in the liver. Future studies using MCP-1 targeting strategies will provide mechanistic insights into the pathophysiological mechanisms affected by MCP-1 in alcoholic liver injury.
Overall our studies show for the first time that MCP-1 in the liver regulates macrophage activation, pro-inflammatory responses and hepatic steatosis in alcoholic liver disease. These studies provide a link between inflammatory cell activation and pathways of fatty acid metabolism during alcoholic liver injury likely involved in amplification and progression of disease. Therefore it appears plausible that pharmacological approaches to block MCP-1 in the alcoholic liver may be beneficial to early alcoholic fatty liver injury and also abrogate inflammatory pathways contributing to propagation in ALD.
Supplementary Material
Table I.
Real time PCR primers
| Target genes | Forward Primer (5’ → 3’) | Reverse Primer (5’ → 3’) |
|---|---|---|
| TNFα | cac cac cat caa gga ctc aa | agg caa cct gac cac tct cc |
| IL-1β | ct ttg aag ttg acg gac cc | tga gtg ata ctg cct gcc tg |
| IL-6 | aca acc acg gcc ttc cct act t | cac gat ttc cca gag aac atg tg |
| MCP-1 | cag gtc cct gtc atg ctt ct | cag gtc cct gtc atg ctt ct |
| KC/IL-8 | gga agt gtg atg act cag gtt tgc | gat ggt tcc ttc cgg tgg ttt ctt c |
| CCL3/MIP-1α | tct cag cgc cat atg gag ct | ttc cgg ctg tag gag aag ca |
| CCL4/MIP-1β | ccg agc aac acc atg aag c | cca ttg gtg ctg aga acc ct |
| CCL5/RANTES | gct gct ttg cct acc tct cc | tcg agt gac aaa cac gac tgc |
| CCL8/MCP2 | cca gat aag gct cca gtc acc t | ggc act gga tat tgt tga ttc tct c |
| CCL7/MCP3 | gca gag aag caa ggc cag cac a | agc agg cac aga agc gtg gc |
| CCR1 | tag tga act tgg acc cca gg | tca agg ttc aag gtc cca ac |
| CCR2 | gtg tac ata gca aca agc ctc aaa g | ccc cca cat agg gat cat ga |
| ICAM | SA Biosciences Cat # PPM03196A | |
| VCAM | SA Biosciences Cat # PPM03208B | |
| F4/80 | tgc atc tag caa tgg aca gc | gcc ttc tgg atc cat ttg aa |
| CD68 | ccc aca ggc agc aca gtg gac | tcc aca gca gaa gct ttg gcc c |
| TLR4 | gcc ttt cag gga att aag ctc c | aga tca acc gat gga cgt gta a |
| PPARα | aac atc gag tgt cga ata tgt gg | agc cga ata gtt cgc cga aag |
| PPARγ | gga aga cca ctc gca ttc ctt | tcg cac ttt ggt att ctt gga g |
Acknowledgement
We thank Karen Kodys for labeling the oligonucleotides for the EMSA analysis.
Financial Support: This work was supported by PHS grant # AA017545 (to PM) and AA017986 (to PM) from the National Institute of Alcohol Abuse and Alcoholism and by the resources of University of Massachusetts Medical School CFAR (grant 5P30 AI42845) and its contents are solely the responsibility of the authors and do not necessarily represent the views of the NIAAA.
Abbreviations
- MCP-1
Monocyte chemoattractant protein-1
- CCL2
Chemokine (C-C motif) ligand 2 (CCL2)
- CCR2
chemokine (C-C motif) receptor 2
- ALT
Alanine Amino Transferase
- PPARα
peroxisome proliferator-activated receptor α
- PPARγ
peroxisome proliferator-activated receptor γ
- PPRE
peroxisome proliferator response element
- ACOX
Acyl CoA oxidase
- CPT-1
Carnitine palmitoyl transferase 1A
- LCAD
Long chain acyl-CoA dehydrogenase
- MCAD
Medium chain acyl-CoA dehydrogenase
- MIP-1
Macrophage inflammatory protein 1
Contributor Information
Pranoti Mandrekar, Email: Pranoti.Mandrekar@umassmed.edu.
Aditya Ambade, Email: Aditya.Ambade@umassmed.edu.
Arlene Lim, Email: Arlene.Lim@umassmed.edu.
Gyongyi Szabo, Email: Gyongyi.Szabo@umassmed.edu.
Donna Catalano, Email: Donna.Catalano@umassmed.edu.
REFERENCES
- 1.Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med (Maywood) 2003;228(8):882–890. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- 2.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50(6):1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, Goto M, Yamashina S, et al. Role of kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15(Suppl):D20–D25. doi: 10.1046/j.1440-1746.2000.02179.x. [DOI] [PubMed] [Google Scholar]
- 4.Afford SC, Fisher NC, Neil DA, Fear J, Brun P, Hubscher SG, Adams DH. Distinct patterns of chemokine expression are associated with leukocyte recruitment in alcoholic hepatitis and alcoholic cirrhosis. J Pathol. 1998;186(1):82–89. doi: 10.1002/(SICI)1096-9896(199809)186:1<82::AID-PATH151>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 5.Fisher NC, Neil DA, Williams A, Adams DH. Serum concentrations and peripheral secretion of the beta chemokines monocyte chemoattractant protein 1 and macrophage inflammatory protein 1alpha in alcoholic liver disease. Gut. 1999;45(3):416–420. doi: 10.1136/gut.45.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 7.Adams DH, Lloyd AR. Chemokines: Leucocyte recruitment and activation cytokines. Lancet. 1997;349(9050):490–495. doi: 10.1016/s0140-6736(96)07524-1. [DOI] [PubMed] [Google Scholar]
- 8.Bautista AP. Impact of alcohol on the ability of kupffer cells to produce chemokines and its role in alcoholic liver disease. J Gastroenterol Hepatol. 2000;15(4):349–356. doi: 10.1046/j.1440-1746.2000.02174.x. [DOI] [PubMed] [Google Scholar]
- 9.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, et al. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187(4):601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Y, Beller DI, Frendl G, Graves DT. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992;148(8):2423–2428. [PubMed] [Google Scholar]
- 11.Bautista AP. Chronic alcohol intoxication primes kupffer cells and endothelial cells for enhanced CC-chemokine production and concomitantly suppresses phagocytosis and chemotaxis. Front Biosci. 2002;7:a117–a125. doi: 10.2741/a746. [DOI] [PubMed] [Google Scholar]
- 12.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clement S, Juge-Aubry C, Sgroi A, Conzelmann S, Pazienza V, Pittet-Cuenod B, Meier CA, et al. Monocyte chemoattractant protein-1 secreted by adipose tissue induces direct lipid accumulation in hepatocytes. Hepatology. 2008;48(3):799–807. doi: 10.1002/hep.22404. [DOI] [PubMed] [Google Scholar]
- 14.Mandrekar P. Signaling mechanisms in alcoholic liver injury: Role of transcription factors, kinases and heat shock proteins. World J Gastroenterol. 2007;13(37):4979–4985. doi: 10.3748/wjg.v13.i37.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183(2):1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29(2):141–154. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
- 17.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100(10):2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, et al. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50(1):185–197. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454(7203):470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 20.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 21.Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358(6389):771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): An overview. J Interferon Cytokine Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, Ables EV, et al. C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes. 2010;59(4):916–925. doi: 10.2337/db09-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295(1):E10–E16. doi: 10.1152/ajpendo.00011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pastorino JG, Hoek JB. Ethanol potentiates tumor necrosis factor-alpha cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology. 2000;31(5):1141–1152. doi: 10.1053/he.2000.7013. [DOI] [PubMed] [Google Scholar]
- 26.Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, Lafdil F, Osei-Hyiaman D, et al. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134(4):1148–1158. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines--CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 28.Taub DD, Oppenheim JJ. Chemokines, inflammation and the immune system. Ther Immunol. 1994;1(4):229–246. [PubMed] [Google Scholar]
- 29.Stickel F, Choi SW, Kim YI, Bagley PJ, Seitz HK, Russell RM, Selhub J, et al. Effect of chronic alcohol consumption on total plasma homocysteine level in rats. Alcohol Clin Exp Res. 2000;24(3):259–264. [PubMed] [Google Scholar]
- 30.Woo CW, Siow YL, O K. Homocysteine induces monocyte chemoattractant protein-1 expression in hepatocytes mediated via activator protein-1 activation. J Biol Chem. 2008;283(3):1282–1292. doi: 10.1074/jbc.M707886200. [DOI] [PubMed] [Google Scholar]
- 31.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci U S A. 1997;94(22):12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zisman DA, Kunkel SL, Strieter RM, Tsai WC, Bucknell K, Wilkowski J, Standiford TJ. MCP-1 protects mice in lethal endotoxemia. J Clin Invest. 1997;99(12):2832–2836. doi: 10.1172/JCI119475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ajuebor MN, Hogaboam CM, Le T, Swain MG. C-C chemokine ligand 2/monocyte chemoattractant protein-1 directly inhibits NKT cell IL-4 production and is hepatoprotective in T cell-mediated hepatitis in the mouse. J Immunol. 2003;170(10):5252–5259. doi: 10.4049/jimmunol.170.10.5252. [DOI] [PubMed] [Google Scholar]
- 34.Zamara E, Galastri S, Aleffi S, Petrai I, Aragno M, Mastrocola R, Novo E, et al. Prevention of severe toxic liver injury and oxidative stress in MCP-1-deficient mice. J Hepatol. 2007;46(2):230–238. doi: 10.1016/j.jhep.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Cederbaum AI. Role of CYP2E1 in ethanol-induced oxidant stress, fatty liver and hepatotoxicity. Dig Dis. 2010;28(6):802–811. doi: 10.1159/000324289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat kupffer cells: Role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79(6):1348–1356. doi: 10.1189/jlb.1005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, et al. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43(5):989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- 38.New DC, Wong YH. CC chemokine receptor-coupled signalling pathways. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2003;35(9):779–788. [PubMed] [Google Scholar]
- 39.Viedt C, Orth SR. Monocyte chemoattractant protein-1 (MCP-1) in the kidney: Does it more than simply attract monocytes? Nephrol Dial Transplant. 2002;17(12):2043–2047. doi: 10.1093/ndt/17.12.2043. [DOI] [PubMed] [Google Scholar]
- 40.Maitra U, Chang S, Singh N, Li L. Molecular mechanism underlying the suppression of lipid oxidation during endotoxemia. Mol Immunol. 2009;47(2–3):420–425. doi: 10.1016/j.molimm.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.