Abstract
Background
Endotoxin mediated pro-inflammatory cytokines play a significant role in pathogenesis of acute and chronic liver diseases. Hsp90 functions as an important chaperone of LPS signaling and is required for production of pro-inflammatory cytokines. We hypothesized that inhibition of hsp90 prevents LPS induced liver injury by decreasing pro-inflammatory cytokines.
Methods
C57BL/6 mice were injected i.p. with an hsp90 inhibitor, 17-DMAG, and LPS. Parameters of liver injury, pro-inflammatory cytokines, and mechanisms associated were studied by in vivo and in vitro experiments.
Results
Inhibition of hsp90 by 17-DMAG prevented LPS induced serum ALT and significantly reduced serum TNFα and IL-6 protein as well as mRNA in liver. Enhanced DNA binding activity of heat shock factor 1 (HSF1) and induction of target gene hsp70 confirmed hsp90 inhibition in liver. 17-DMAG treatment decreased CD14 mRNA and LPS induced NFκB DNA binding without affecting TLR4 mRNA in liver. Mechanistic studies revealed that 17-DMAG mediated inhibition of TNFα showed no effect on LPS induced NFκB promoter driven reporter activity but significantly decreased TNFα promoter driven reporter activity. Chromatin immunoprecipitation assays showed that 17-DMAG enhanced HSF1 binding to TNFα promoter, but not IL-6 promoter, suggesting HSF1 mediated direct inhibition of TNFα but not IL-6. We show that HSF1 indirectly regulates IL-6 via induction of another transcription factor, ATF3. Inhibition of HSF1 using siRNA prevented 17-DMAG mediated down-regulation of NFκB binding activity, TNFα and IL-6 induction supporting a repressive role for HSF1 on pro-inflammatory cytokine genes during hsp90 inhibition.
Conclusion
Hsp90 inhibition in vivo reduces pro-inflammatory cytokines and prevents LPS induced liver injury likely via repressive action of HSF1. Our results suggest a novel application for 17-DMAG in alleviating LPS induced liver injury.
Keywords: pro-inflammatory cytokines, 17-DMAG, Endotoxin, HSF1, hsp70
Introduction
The importance of macrophage activation and endotoxin mediated pro-inflammatory cytokine production in liver injury is evident from numerous models of acute and chronic liver disease (1). For instance in non-alcoholic steatohepatitis (NASH), endotoxin or lipopolysaccharide (LPS) triggered TNFα and other pro-inflammatory cytokines (2). Exposure of genetically obese mice to LPS exhibit hepatotoxicity and develop steatohepatitis (3). In alcoholic liver disease (ALD), gut derived endotoxin (LPS) activates liver macrophages and production of pro-inflammatory cytokines TNFα, IL-6 and IL-1β that contribute to the pathogenesis of liver injury (4–6). Acetaminophen mediated liver injury (7), ischemia-reperfusion injury (8) and liver cancer (9), are all linked to LPS, macrophage activation and pro-inflammatory cytokines. It is thus evident from literature that exposure to LPS induces pro-inflammatory cytokines and reactive oxygen species (10) leading to development and progression of liver injury (1, 11, 12).
The significance of stress induced heat shock proteins as molecular chaperones of the LPS signalling pathway in macrophage activation is reported (13–16). Hsp70 and hsp90 bind to LPS signaling molecules culminating in activation of NFκB and expression of pro-inflammatory cytokines, TNFα, IL-1β and IL-6 in macrophages (17–20). Hsp90, an important molecular chaperone, is responsible for tertiary folding of client proteins such as IKK (21), IRAK-1 (22) and MAP kinases (23) and inhibition of hsp90 diminishes innate immune responses via TLR signaling (14). Targeting hsp90 as an attractive therapeutic strategy was evaluated in treatment of cancers and is currently in clinical trials (24–27). Preclinical data also suggest that hsp90 inhibition is an effective treatment approach for alleviating chronic inflammatory diseases such uveitis (18) and rheumatoid arthritis (28).
The role of hsp90 in liver diseases remains elusive. Our earlier studies reported that chronic alcohol induced macrophage activation and liver disease is associated with increased hsp90 (17). Based on the requirement of hsp90 in LPS pathway, we hypothesized that inhibition of hsp90 prevents LPS induced liver injury via decreased pro-inflammatory cytokine production. To this end, we tested the effect of hsp90 inhibition in vivo using 17-DMAG, a water-soluble derivative of benzoquinone ansamycin antibiotic geldanamycin, on endotoxin mediated liver injury and pro-inflammatory cytokine production in mice. In order to dissect the molecular mechanisms underlying the inhibition of pro-inflammatory cytokines by 17-DMAG, we performed in vitro studies in RAW 264.7 macrophages. Here we show that hsp90 inhibition prevents LPS induced liver injury by down-regulation of pro-inflammatory cytokine, TNFα and IL-6 likely via HSF1 activation in the liver.
Materials and Methods
Animals and Experimental Protocol
All animals received proper care in agreement with animal protocols approved by the Institutional Animal Care and Use Committee at the University of Massachusetts Medical School. Six-week old C57BL/6 female and male mice were purchased from Jackson Labs (Bar Harbor, ME). Prior to LPS injection, mice were injected intraperitoneally (i.p.) with either 0.1mL saline or 0.5 mg/kg body weight lipopolysaccharide in 0.1ml (LPS, from E. coli 0111:B4, Sigma, St. Louis, MO). Mice were intraperitoneally administered a single dose of hsp90 inhibitor 17-DMAG [17-Dimethylamino-ethylamino-17-demethoxygeldanamycin] (NCI, NSC 707545) 2.5 mg/kg, 5 mg/kg or 30 mg/kg BW. Mice were sacrificed at 2 hrs or 18hrs after 17-DMAG and LPS administration. Serum was separated from whole blood and frozen at −80°C. Liver tissue was rapidly excised and a portion was snap-frozen in liquid nitrogen and stored at −80°C. Additional portions of the livers were stored in RNA stabilization reagent, RNAlater (Qiagen GmbH, Hilden, Germany), for RNA extraction.
Other Methods
The following methods are described in Supplementary Information, including serum biochemical assay and cytokines, EMSA, RNA extraction and real-time PCR, western blot analysis, cell culture reagents and stimulations, transfections and luciferase reporter assay, chromatin immunoprecipitation.
HSF1 siRNA transfection
RAW macrophages were transiently transfected with 20pM of HSF1 siRNA (Invitrogen, Carlsbad, CA) in Opti-MEM for 6 hrs (sequence listed in supplementary information Table 1,) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). RNA and nuclear protein extraction were done as reported in supplementary information.
Statistical Analysis
Statistical significance was determined using the T-test or nonparametric ANOVA followed by Kruskal-Wallis test. Data are presented as mean ± standard error, and were considered statistically significant at p< 0.05.
Results
Hsp90 inhibitor 17-DMAG reduces serum ALT levels
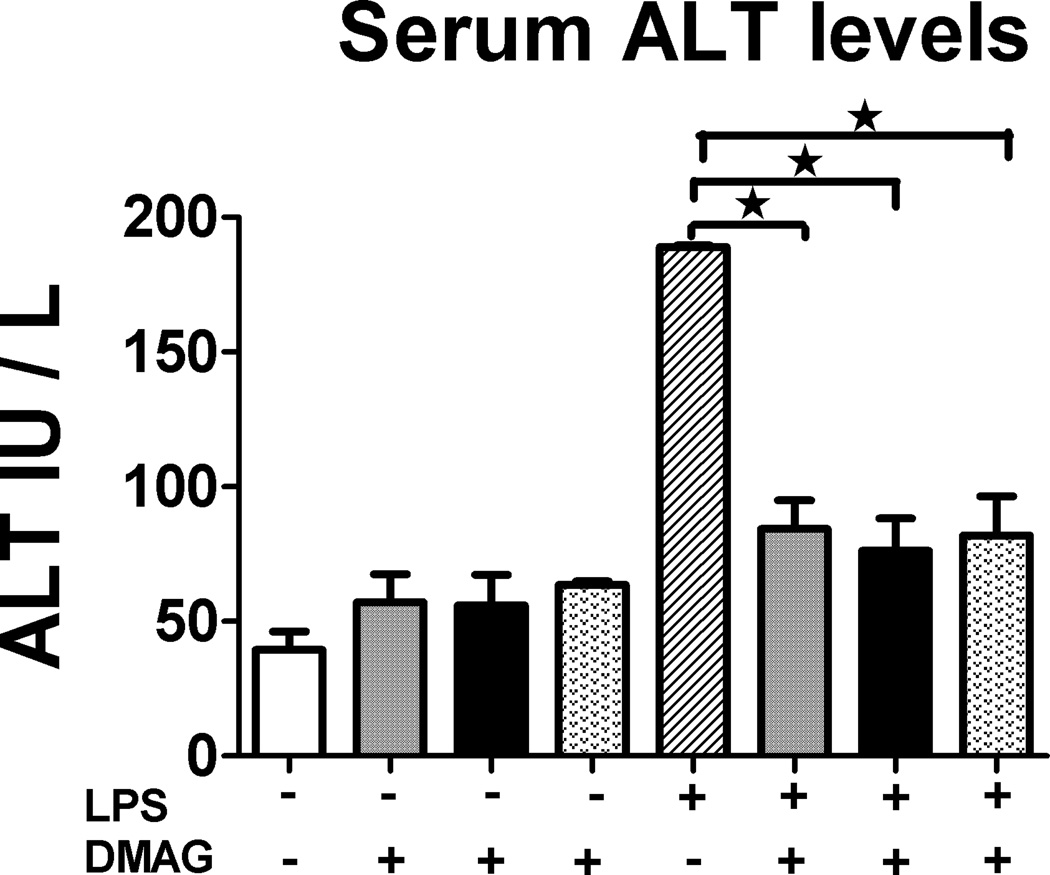
The significance of hsp90 in liver inflammatory responses is unknown. Here, we determined the effect of 17-DMAG, a water soluble hsp90 inhibitor in vivo on liver inflammatory responses and injury. The levels of serum alanine aminotransferase (ALT), a marker of liver injury were assessed after 18 hrs of 17-DMAG and LPS administration in vivo. Figure 1 shows that LPS injection in vivo at 0.5 mg/kg BW induces significantly high serum ALT levels as compared to saline injected controls after 18 hrs. Hsp90 inhibition by 17-DMAG administered at 2.5, 5 and 30 mg/kg BW exhibited significant reduction of serum ALT at all three doses (Fig. 1) independent of the dose used. These experiments suggest that hsp90 inhibition prevented LPS induced liver injury. Since all the doses showed similar effects, subsequent experiments were performed with lower doses 2.5 and 5 mg/kg BW 17-DMAG concentrations in vivo.
Fig. 1. Hsp90 inhibitor 17-DMAG reduces LPS induced serum ALT.
LPS ( 0.5 mg/kg BW) and 17-DMAG (
0.5 mg/kg BW) and 17-DMAG ( 2.5,
2.5,  5 and
5 and 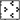 30 mg/kg BW) were injected intraperitoneally in C57BL/6 mice. All mice were sacrificed 18 hrs post injection and alanine aminotransferase [ALT] activity was determined in serum as described in supplementary information. Values are shown as mean ±SEM (5 mice per group). *p < 0.05 vs. LPS injected mice.
30 mg/kg BW) were injected intraperitoneally in C57BL/6 mice. All mice were sacrificed 18 hrs post injection and alanine aminotransferase [ALT] activity was determined in serum as described in supplementary information. Values are shown as mean ±SEM (5 mice per group). *p < 0.05 vs. LPS injected mice.
Hsp90 inhibition decreases pro-inflammatory cytokine production in the liver
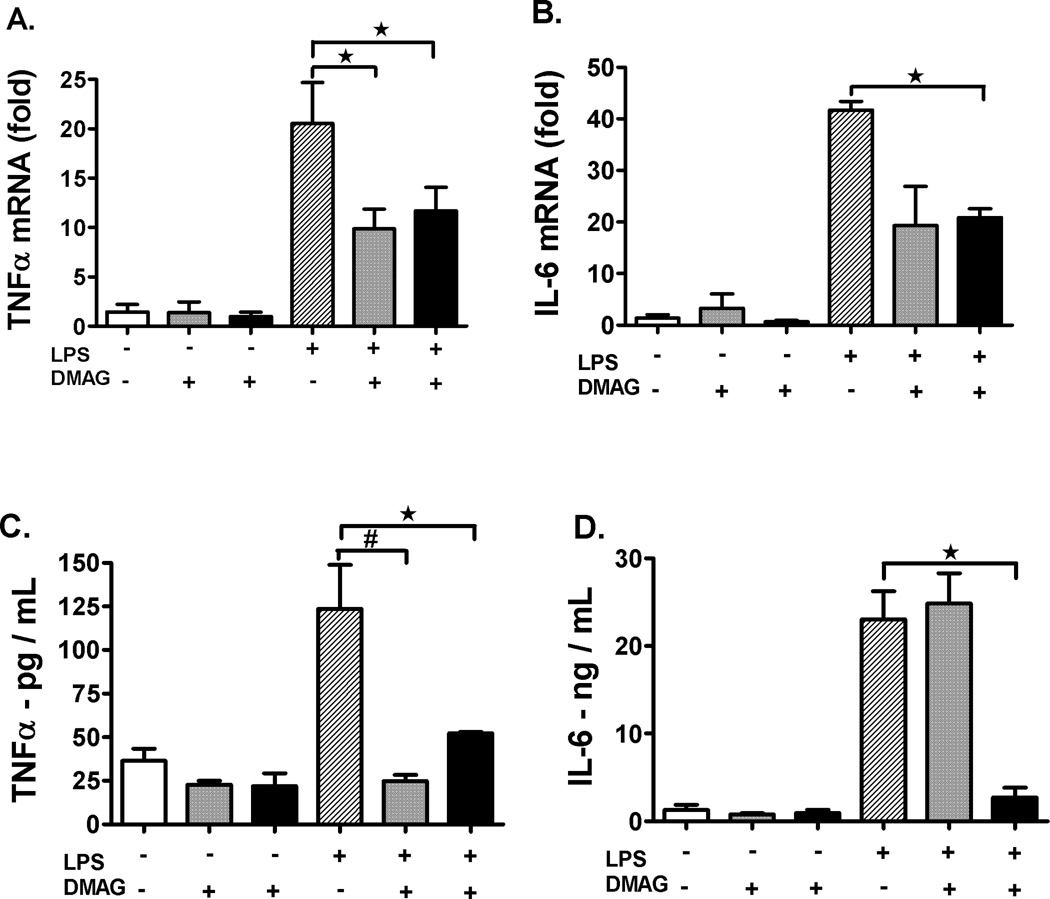
Since LPS induced liver injury is largely mediated by pro-inflammatory cytokines, we determined if 17-DMAG has any effect on pro-inflammatory cytokine production in the liver. First, we analyzed mRNA levels of pro- inflammatory cytokines by real-time PCR in whole livers after treatment with 17-DMAG in vivo. Pro-inflammatory cytokine TNFα mRNA (Fig. 2A) was significantly reduced at 2.5 and 5 mg/kg 17-DMAG treatment compared to LPS alone whereas IL-6 mRNA (Fig. 2B) was decreased at the higher dose of 5 mg/kg of 17-DMAG compared to LPS alone in liver. Second, we measured serum cytokine levels by ELISA and observed that TNFα (Fig. 2C) was significantly reduced at both doses of 17-DMAG whereas IL-6 (Fig. 2D) showed significant reduction only at the 5 mg/kg 17-DMAG compared to LPS alone. These results suggest that hsp90 inhibition by 17-DMAG prevented LPS induced pro-inflammatory cytokines, TNFα and IL-6 at both mRNA and protein levels in the liver.
Fig. 2. Hsp90 inhibition decreases pro-inflammatory cytokine production in the liver.
C57BL/6 mice were injected intraperitoneally with LPS ( 0.5 mg/kg BW) and 17-DMAG (
0.5 mg/kg BW) and 17-DMAG ( 2.5 and
2.5 and  5 mg/kg BW). All mice were sacrificed 2 hrs post injection and total RNA was extracted from liver. Messenger RNA levels of liver (A) TNFα, (B) IL-6, were analyzed by quantitative real-time PCR, and normalized to 18S rRNA. Results are expressed as mean fold change ± SEM over mice injected with saline (5 mice per group). Another set of C57BL/6 mice were injected intraperitoneally with LPS (
5 mg/kg BW). All mice were sacrificed 2 hrs post injection and total RNA was extracted from liver. Messenger RNA levels of liver (A) TNFα, (B) IL-6, were analyzed by quantitative real-time PCR, and normalized to 18S rRNA. Results are expressed as mean fold change ± SEM over mice injected with saline (5 mice per group). Another set of C57BL/6 mice were injected intraperitoneally with LPS ( 0.5 mg/kg BW) and 17-DMAG (
0.5 mg/kg BW) and 17-DMAG ( 2.5 and
2.5 and  5 mg/kg BW) for 18 hrs and serum TNFα (C), IL-6 (D) was analyzed by ELISA. #p< 0.001, *p < 0.05 vs. LPS injected mice.
5 mg/kg BW) for 18 hrs and serum TNFα (C), IL-6 (D) was analyzed by ELISA. #p< 0.001, *p < 0.05 vs. LPS injected mice.
Hsp90 inhibition induces HSF1 DNA binding activity and up-regulates hsp70 expression in liver
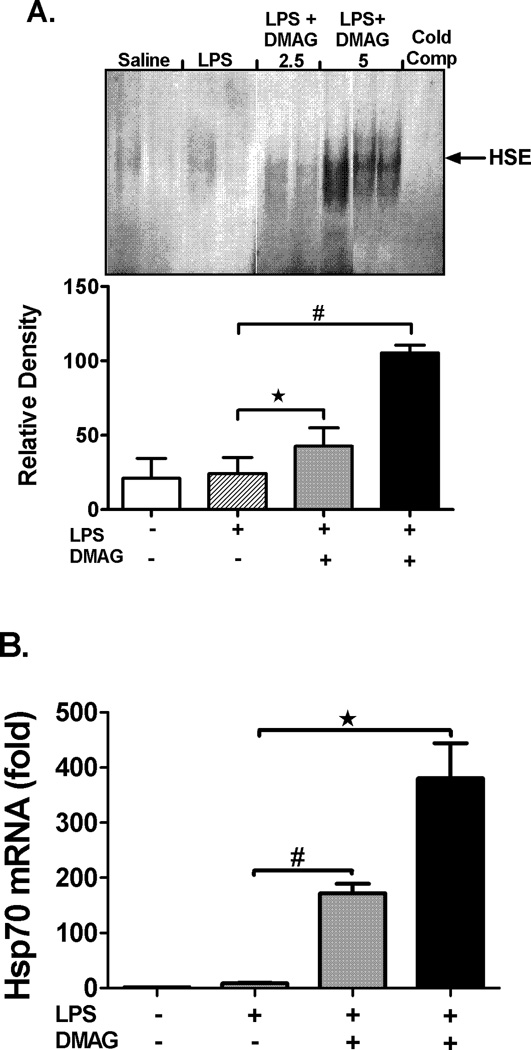
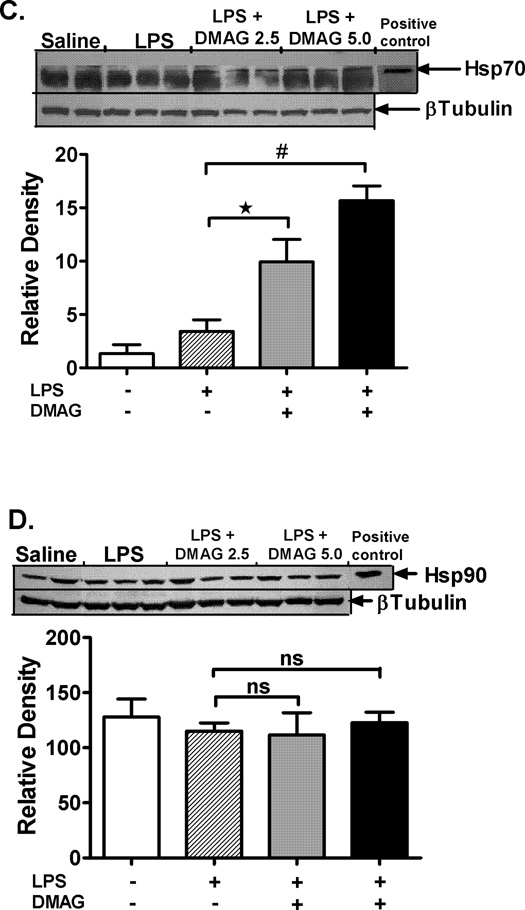
Hsp90 sequesters HSF1 in an inactive state (29) and inhibition of hsp90 dissociates this complex and releases HSF1 which translocates to the nucleus (30). To confirm inhibition of hsp90 activity in liver, we analyzed DNA binding activity of HSF1 by EMSA and expression of target gene, hsp70. Hsp90 inhibition by 17-DMAG significantly up-regulated HSF1 binding to DNA in a dose dependent manner (Fig. 3A) in liver. Complementary to HSF1 activation, hsp90 inhibition resulted in subsequent induction of hsp70 mRNA (Fig. 3B) and protein levels (Fig. 3C) in the liver. In accordance with the reported action of 17-DMAG on hsp90 chaperone function (31) no effect was observed on protein levels of hsp90 in liver (Fig. 3D). Our results suggest that 17-DMAG up-regulates HSF1 DNA binding activity and induces target gene hsp70, without affecting hsp90 levels, confirming inhibition of hsp90 function after 17-DMAG treatment in liver.
Fig. 3. Hsp90 inhibition induces HSF1 DNA binding activity and up-regulates hsp70 expression in the liver.
C57BL/6 mice were injected intraperitoneally with LPS ( 0.5 mg/kg BW) and 17-DMAG (
0.5 mg/kg BW) and 17-DMAG ( 2.5 and
2.5 and  5 mg/kg BW) and livers were collected at end of 2 hrs. DNA binding activity of HSF1 was detected in nuclear extracts of liver cells by EMSA using a 32P labeled, double stranded HSE oligonucleotide. (A) A representative EMSA picture is shown in upper panel and the bar graph in lower panel shows mean relative density ± SEM (5 mice per group). #p<0.01, *p < 0.05 compared to LPS injected mice. Hsp70 mRNA levels in the liver (B) were analyzed by quantitative real-time PCR and normalized to 18S rRNA. Results are expressed as mean fold change ± SEM over mice injected with saline (5 mice per group). #p<0.001, *p < 0.0001 vs. LPS injected mice. Hsp70 (C) and hsp90 (D) protein was detected in liver whole cell lysates by western blotting. β Tubulin is shown as internal loading control. A representative gel picture is shown with mean relative density ± SEM (3 mice per group). *p < 0.001 compared to LPS injected mice; ns-not significant.
5 mg/kg BW) and livers were collected at end of 2 hrs. DNA binding activity of HSF1 was detected in nuclear extracts of liver cells by EMSA using a 32P labeled, double stranded HSE oligonucleotide. (A) A representative EMSA picture is shown in upper panel and the bar graph in lower panel shows mean relative density ± SEM (5 mice per group). #p<0.01, *p < 0.05 compared to LPS injected mice. Hsp70 mRNA levels in the liver (B) were analyzed by quantitative real-time PCR and normalized to 18S rRNA. Results are expressed as mean fold change ± SEM over mice injected with saline (5 mice per group). #p<0.001, *p < 0.0001 vs. LPS injected mice. Hsp70 (C) and hsp90 (D) protein was detected in liver whole cell lysates by western blotting. β Tubulin is shown as internal loading control. A representative gel picture is shown with mean relative density ± SEM (3 mice per group). *p < 0.001 compared to LPS injected mice; ns-not significant.
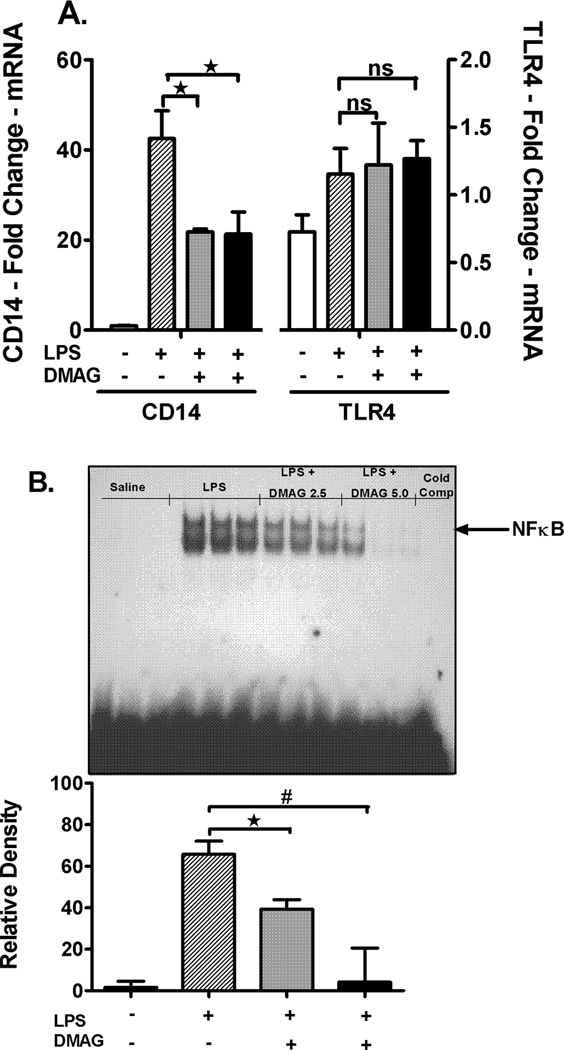
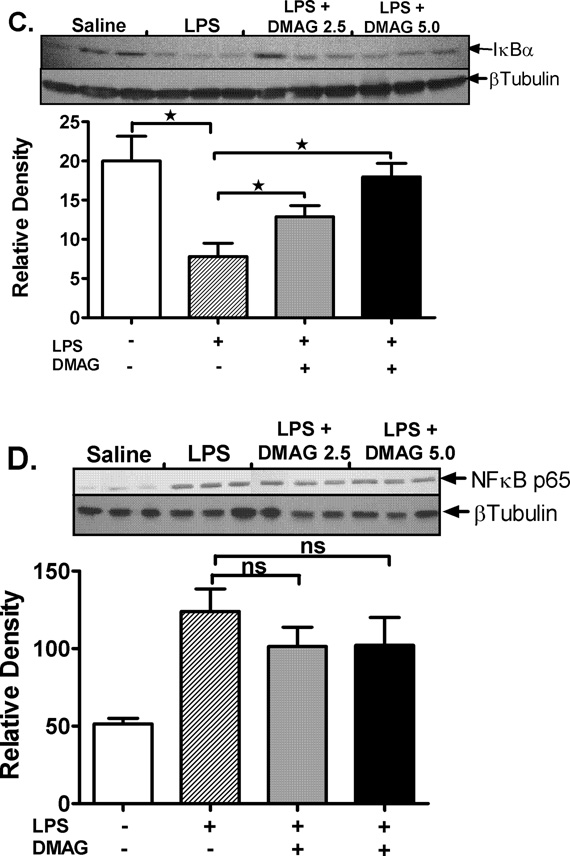
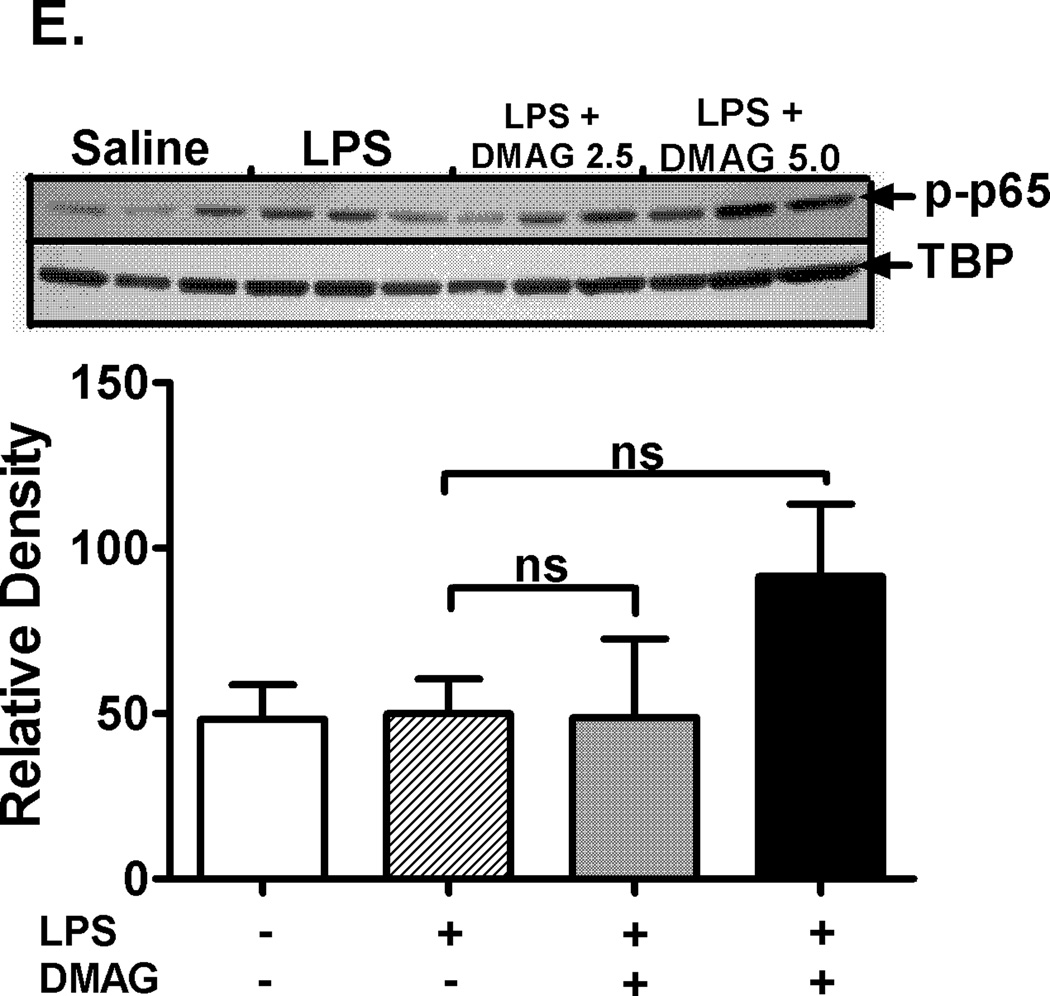
17-DMAG affects NFκB DNA binding activity and down-regulates CD14 mRNA in liver
Hsp90 chaperones the LPS receptors, CD14 and TLR4 resulting in activation of downstream signaling and pro-inflammatory cytokine production (14). We assessed CD14 and TLR4 mRNA levels, as a measure of the total cellular expression, in response to hsp90 inhibition. Liver CD14 mRNA was significantly down-regulated in response to hsp90 inhibition by 17-DMAG compared to LPS alone (Fig. 4A) while the TLR4 mRNA was unaffected (Fig. 4A). Subsequently, to determine the effect of 17-DMAG on downstream activation, we analyzed NFκB, a pivotal transcription factor in CD14/TLR4 signaling. Our results show that 17-DMAG treatment significantly decreased LPS induced NFκB DNA binding activity in a dose dependent manner (Fig. 4B). Next, we determined whether 17-DMAG mediated down-regulation of NFκB was IκBα dependent or due to alterations in NFκB p65 levels. We observed that 17-DMAG prevented the LPS-induced degradation of cytoplasmic IκBα (Fig. 4C) concomitant to reduced NFκB binding observed in liver (Fig. 4B), whereas total cellular NFκB p65 (Fig. 4D) was unchanged. Further, 17-DMAG did not alter nuclear phospho-p65 levels indicating a phosphorylation independent effect of NFκB inhibition (Fig. 4E). Together, these results suggest that hsp90 inhibition reduces CD14/TLR4 signaling and culminates in decreased NFκB DNA binding in an IκBα dependent manner.
Fig. 4. 17-DMAG affects NFκB DNA binding activity and down-regulates CD14 in the liver.
(A) Liver CD14 (left vertical axis), TLR4 (right vertical axis) mRNA levels were analyzed by quantitative real-time PCR and normalized to 18S rRNA. Results are expressed as mean fold change ± SEM over mice injected with saline (5 mice per group). *p < 0.05 vs. LPS injected mice. ns-not significant. (B) C57BL/6 mice were injected intraperitoneally with LPS ( 0.5 mg/kg BW) and 17-DMAG (
0.5 mg/kg BW) and 17-DMAG ( 2.5 and
2.5 and  5 mg/kg BW) and livers were collected at end of 2 hrs. NFκB DNA binding activity was detected in nuclear extracts of whole livers by EMSA using a 32P labeled, double stranded NFκB consensus oligonucleotide. A representative EMSA picture is shown in upper panel and the bar graph in lower panel shows mean relative density ± SEM (5 mice per group). #p<0.001, *p < 0.05 compared to LPS injected mice. Cytoplasmic IκBα (C), cellular NFκB p65 (D) and nuclear NFκB phospho p65 (E) were detected in livers collected at end of 2 hrs. A representative western blot picture is shown in upper panel and the bar graph in lower panel shows mean relative density ± SEM (5 mice per group). *p < 0.05 vs. LPS injected mice. ns-not significant.
5 mg/kg BW) and livers were collected at end of 2 hrs. NFκB DNA binding activity was detected in nuclear extracts of whole livers by EMSA using a 32P labeled, double stranded NFκB consensus oligonucleotide. A representative EMSA picture is shown in upper panel and the bar graph in lower panel shows mean relative density ± SEM (5 mice per group). #p<0.001, *p < 0.05 compared to LPS injected mice. Cytoplasmic IκBα (C), cellular NFκB p65 (D) and nuclear NFκB phospho p65 (E) were detected in livers collected at end of 2 hrs. A representative western blot picture is shown in upper panel and the bar graph in lower panel shows mean relative density ± SEM (5 mice per group). *p < 0.05 vs. LPS injected mice. ns-not significant.
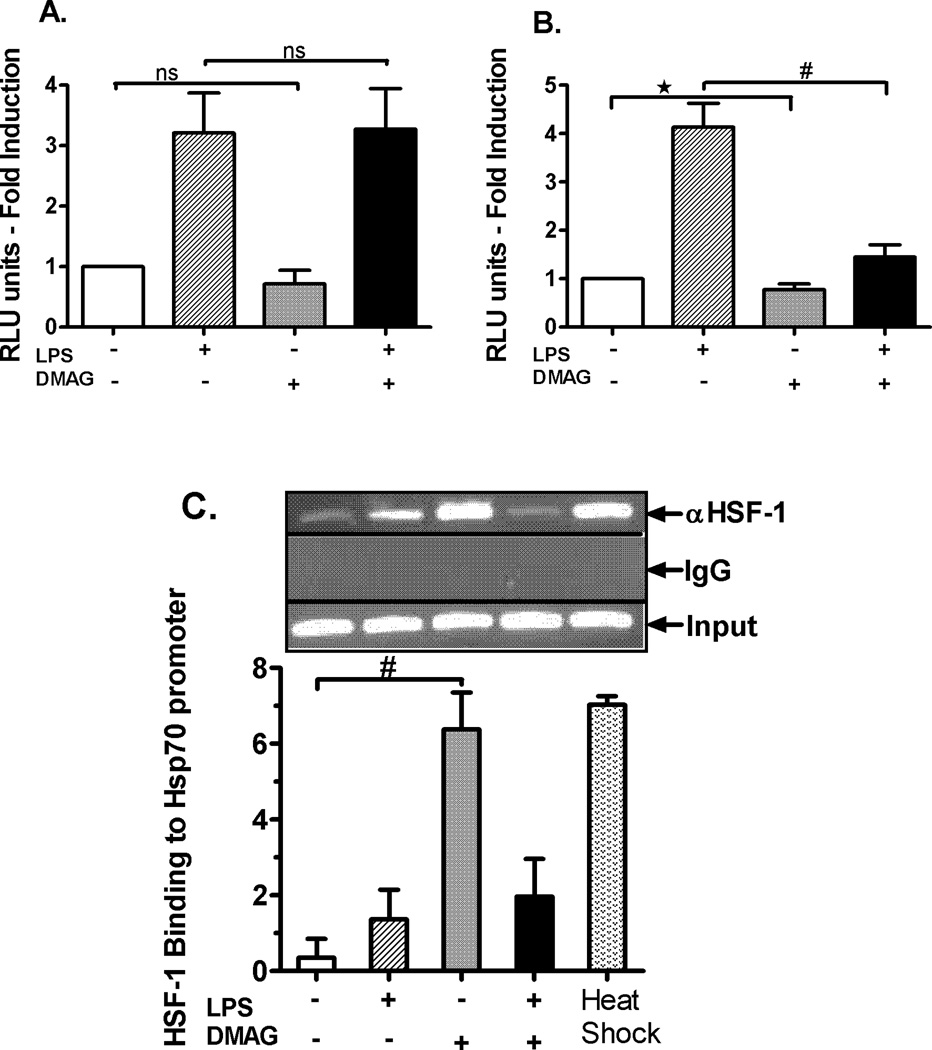
HSF1 regulates LPS induced TNFα expression in response to hsp90 inhibition
To further delineate if 17-DMAG mediated inhibition of pro-inflammatory cytokines is linked to reduced NFκB activity we determined the effect of 17-DMAG on NFκB promoter driven reporter gene activity in RAW 264.7 macrophages. RAW macrophages showed similar effect of 17-DMAG mediated inhibition of pro-inflammatory cytokines and NFκB activity as observed in the liver (data not shown) and were used as an in vitro model for subsequent mechanistic transfection experiments. LPS induced NFκB promoter driven luciferase reporter activity was significantly induced in RAW macrophages whereas treatment with 17-DMAG had no significant effect (Fig. 5A), indicating that inhibition of pro-inflammatory cytokines was not solely dependent on NFκB promoter. Next, we determined whether 17-DMAG treatment had any effect on TNFα promoter driven reporter activity. LPS stimulation induced TNFα promoter driven reporter activity which was significantly decreased by 17-DMAG treatment in RAW macrophages (Fig. 5B). These results suggest that 17-DMAG did not affect NFκB promoter driven reporter activity but reduced TNFα promoter driven reporter activity, suggesting that mechanisms other than NFκB binding may be involved in negatively regulating TNFα expression in response to hsp90 inhibition. Hsp70 induced during hsp90 inhibition (shown in Fig. 3C) interacts with NFκB proteins to suppress TNFα expression in heat shocked cells (32). Here we determined whether NFκB-p50 binds to hsp70 in macrophages after 17-DMAG treatment. There was no significant induction in the NFκB-p50-hsp70 complex formation after LPS and/or 17-DMAG treatment as compared to untreated samples (supplementary figure 1), ruling out the possibility of hsp70 mediated mechanism of inhibition of pro-inflammatory cytokines after treatment with 17-DMAG.
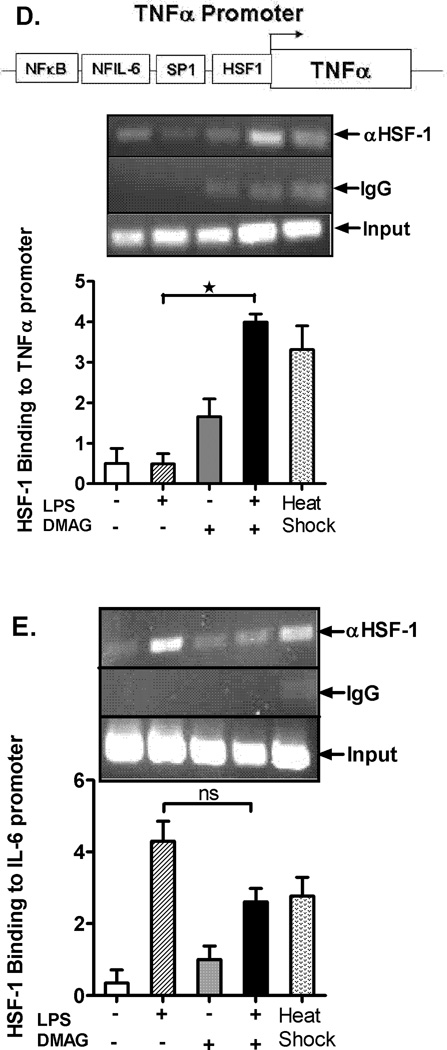
Fig. 5. HSF1 regulates TNFα and hsp70 expression in response to hsp90 inhibition.
RAW macrophages were transfected with either NFκB (A) or TNFα (B) promoter constructs separately. Next day the cells were stimulated with LPS ( 100 ng/ml), 17-DMAG (
100 ng/ml), 17-DMAG ( 0.5 µM) or both (
0.5 µM) or both ( ) for 6 hrs. Fold induction in NFκB (A) and TNFα (B) promoter activity over unstimulated cells is shown as bar graph. *p < 0.05 vs. unstimulated cells, #p < 0.001 vs. LPS stimulated cells. Data represents mean of 3 experiments ± SEM. Chromatin immunoprecipitation assay was performed using anti-HSF1 antibody and semi quantitative PCR was carried out using hsp70 (C), TNFα (D), and IL-6 (E) promoter specific primers. A representative gel picture for each gene is shown. The densitometry graph represents average of 3 independent experiments. *p < 0.0001 vs. LPS stimulated cells; #p < 0.001 vs. unstimulated cells.
) for 6 hrs. Fold induction in NFκB (A) and TNFα (B) promoter activity over unstimulated cells is shown as bar graph. *p < 0.05 vs. unstimulated cells, #p < 0.001 vs. LPS stimulated cells. Data represents mean of 3 experiments ± SEM. Chromatin immunoprecipitation assay was performed using anti-HSF1 antibody and semi quantitative PCR was carried out using hsp70 (C), TNFα (D), and IL-6 (E) promoter specific primers. A representative gel picture for each gene is shown. The densitometry graph represents average of 3 independent experiments. *p < 0.0001 vs. LPS stimulated cells; #p < 0.001 vs. unstimulated cells.
Next, we sought to determine whether another transcription factor was involved in modulation of 17-DMAG mediated reduction of pro-inflammatory cytokine production. Earlier studies have shown that HSF1 serves as a transcriptional repressor for pro-inflammatory cytokine expression during heat stress by NFκB inhibition (33). To determine whether HSF1 binds to the TNFα or IL-6 promoter, we performed chromatin immunoprecipitation of DNA-protein complexes using an anti-HSF1 antibody followed by semi-quantitative PCR using HSF1 binding site specific primers in TNFα (33), IL-6 promoter (34) and hsp70 promoter (56). Positive control heat shocked macrophages show a significant up-regulation in binding of HSF1 to the hsp70 promoter (7–8 fold) (Fig. 5C) and moderate binding to TNFα promoter (3 fold) (Fig. 5D) without changes in LPS-treated macrophages. HSF1 binding to TNFα promoter was up-regulated in response to hsp90 inhibition by 17-DMAG and LPS treatment (Fig. 5D). Interestingly, we observed that HSF1 binding to IL-6 promoter was not affected after hsp90 inhibition (Fig. 5E). Thus, our results here show that HSF1 binds to the TNFα but not IL-6 promoter and likely serves as a key transcriptional repressor down-regulating TNFα expression in response to hsp90 inhibition by 17-DMAG.
Knockdown of HSF1 restores the TNFα transcription
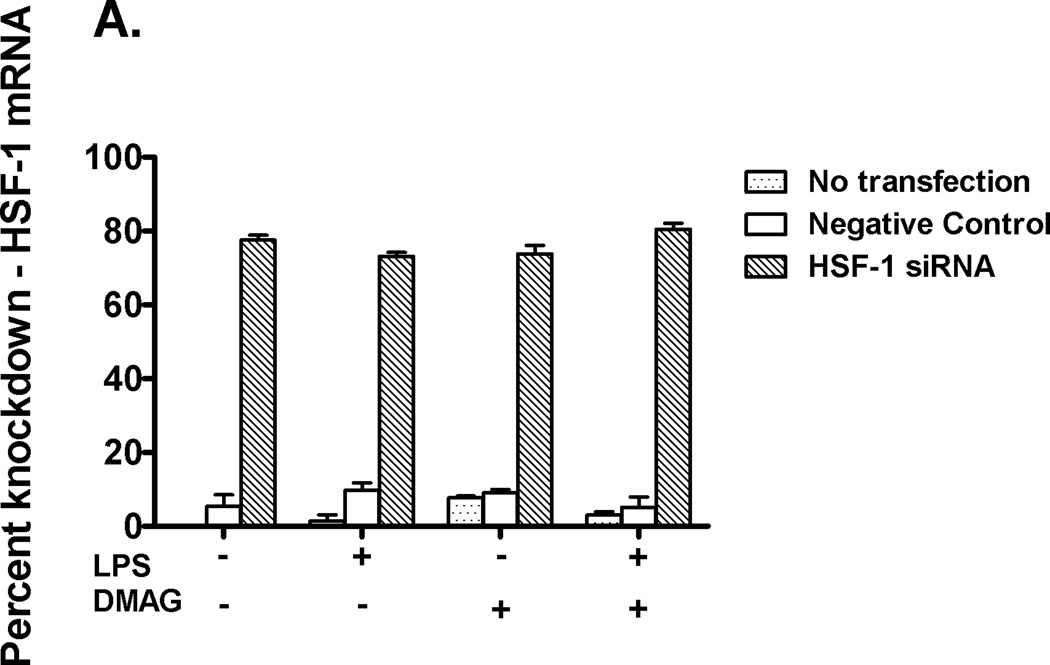
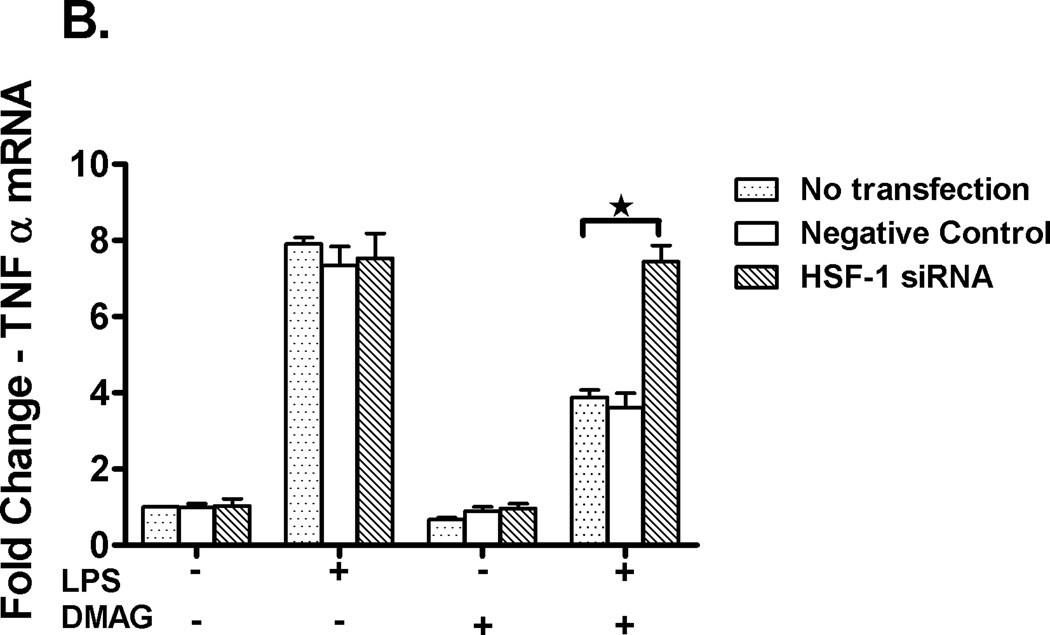
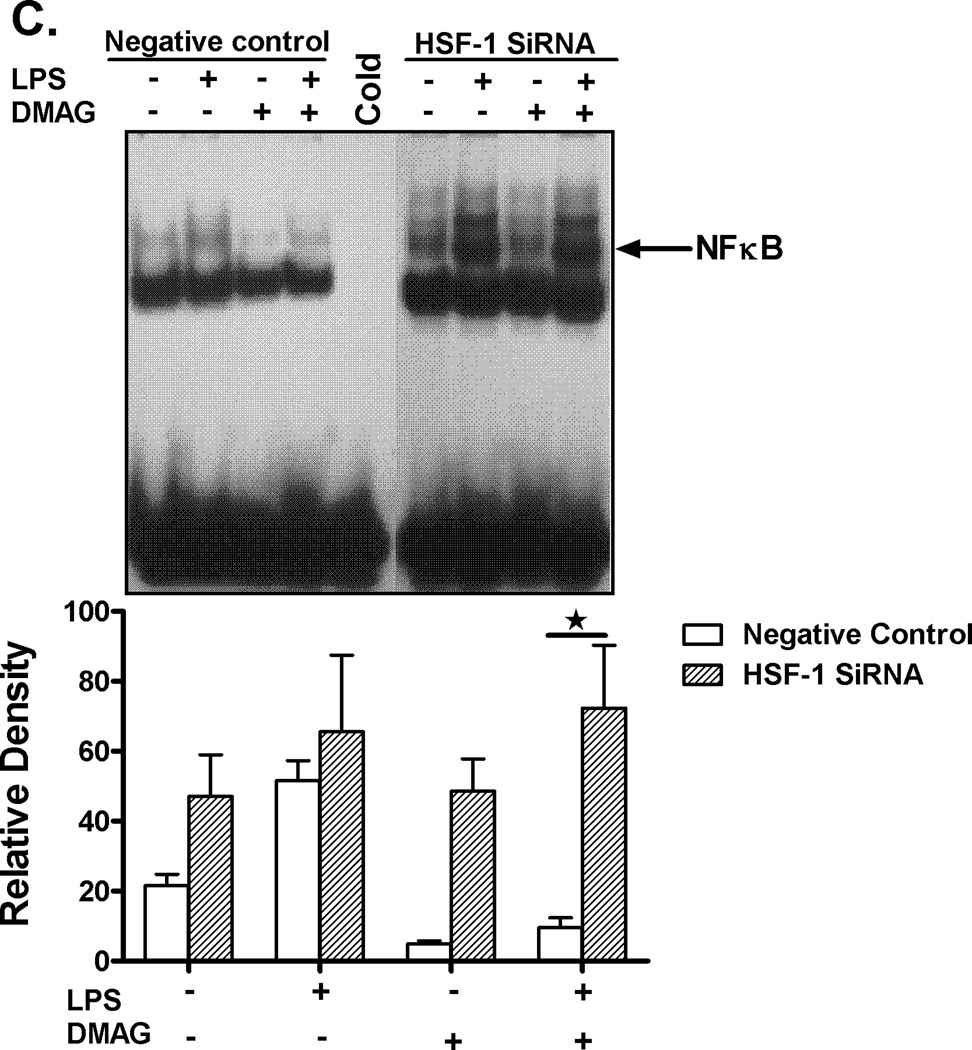
In order to confirm if HSF1 down-regulates and has a direct effect on TNFα expression during hsp90 inhibition, siRNA experiments targeting HSF1 were performed. Using specific HSF1 siRNA (35), transfection was performed in RAW macrophages followed by treatment with LPS ± 17-DMAG. As shown in fig 6A, ~ 80% knock down of HSF1 mRNA was achieved. RAW cells were then treated with LPS in the absence or presence of 17-DMAG, and TNFα mRNA was measured by real-time PCR. Knock-down of HSF1 prevented 17-DMAG mediated down regulation of LPS induced TNFα expression (Fig. 6B). Previous studies showed that HSF1 can bind to the 5’ end of the TNFα promoter (36) and likely reduce NFκB DNA binding due to inaccessible chromatin after HSF1 binding. We thus analyzed the effect of HSF1 knockdown on LPS-induced NFκB DNA binding activity in macrophages after hsp90 inhibition. Knockdown of HSF1 inhibited reduced LPS-induced NFκB DNA binding activity in 17-DMAG treated cells (Fig. 6C). These results indicate that HSF1 plays a significant role in down regulation of NFκB DNA binding and ultimately pro-inflammatory cytokine response after hsp90 inhibition by 17-DMAG in macrophages.
Fig. 6. HSF1 knockdown restores TNFα expression.
RAW macrophages were transfected with HSF1 siRNA and were stimulated next day with LPS, 17-DMAG or both for 2 hrs. HSF1 (A) and TNFα (B) mRNA were analyzed by real-time PCR. (A) Bar graph represents mean percent knockdown of HSF1 mRNA ± SEM of a total 3 experiments. (B) Data represents mean fold change in TNFα mRNA of 3 experiments ± SEM. *p < 0.0001 vs. non transfected, LPS+17-DMAG stimulated cells. NFκB DNA binding activity (C) was detected in nuclear extracts of RAW macrophages 24 hrs after HSF1 siRNA transfection and 2 hrs treatment with LPS ± 17-DMAG. A representative EMSA picture is shown in upper panel and the bar graph in lower panel shows mean relative density ± SEM. *p < 0.01 compared to negative control siRNA transfected, LPS+17-DMAG stimulated cells.
17-DMAG induced HSF-1 indirectly mediates IL-6 suppression through ATF3
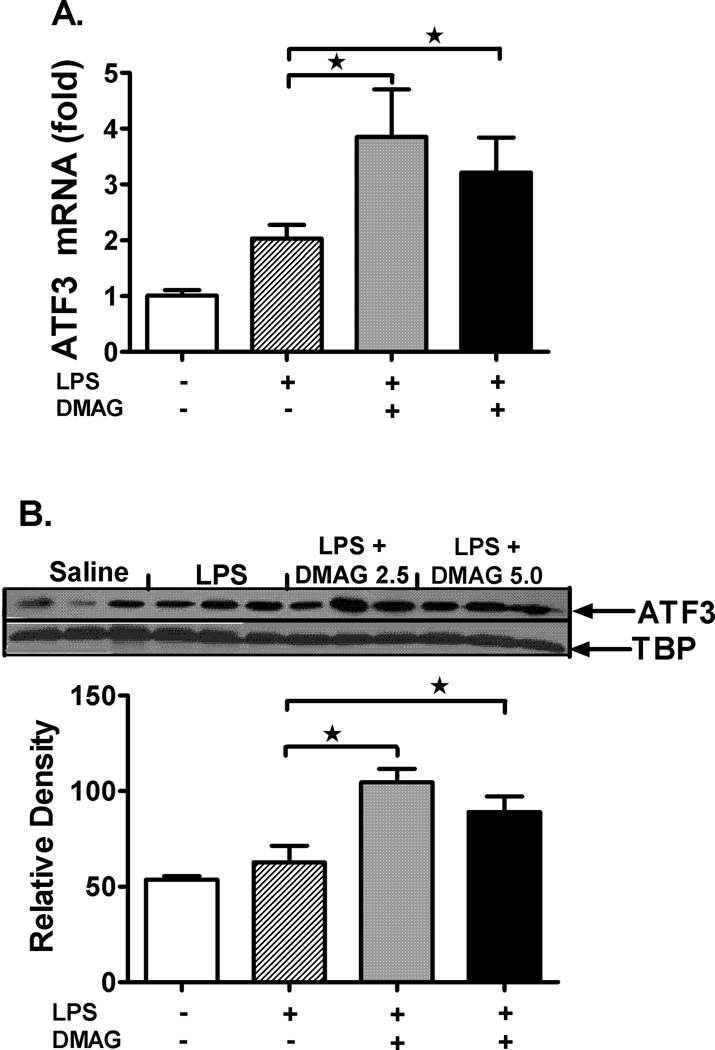
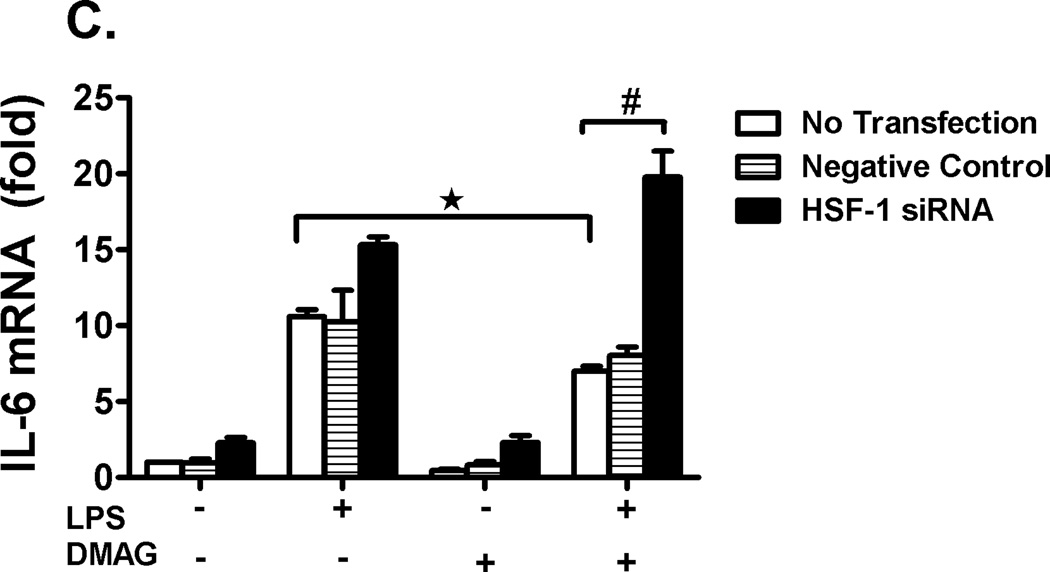
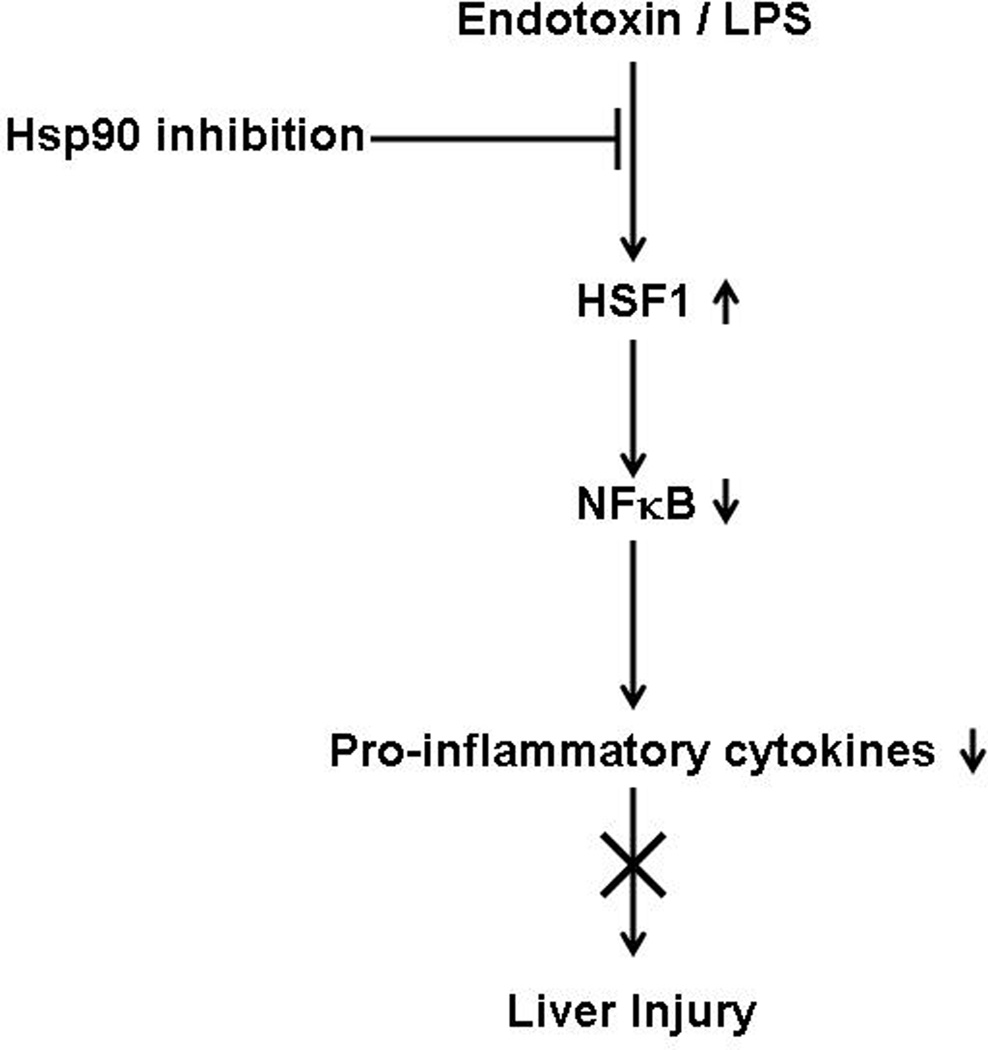
Recent studies show that heat shock induced HSF1 indirectly negatively regulates IL-6 promoter through induction of ATF3 (37). To check the possibility of this mechanism, we analyzed ATF3 mRNA (Fig. 7A) and protein levels (Fig. 7B) after 17-DMAG treatment in liver. We observed a significant induction of ATF3 mRNA and protein in 17-DMAG treated livers suggesting an ATF3 mediated IL-6 suppression. Further, we determined whether inhibition of HSF1 using siRNA affects IL-6 mRNA levels in RAW macrophages. Figure 7C shows that HSF-1 knockdown prevented the down-regulation of LPS-induced IL-6 mRNA during 17-DMAG treatment suggesting a role for HSF1 in regulation of IL-6 likely via ATF3.
Fig. 7. 17-DMAG induced HSF-1 indirectly mediates IL-6 suppression through ATF3.
C57BL/6 mice were injected intraperitoneally with LPS ( 0.5 mg/kg BW) and 17-DMAG (
0.5 mg/kg BW) and 17-DMAG ( 2.5 and
2.5 and  5 mg/kg BW) and livers were collected at end of 2 hrs. Messenger RNA levels of liver ATF3 (A) were analyzed by quantitative real-time PCR, and normalized to 18S rRNA. Results are expressed as mean fold change ± SEM over mice injected with saline (5 mice per group). Nuclear ATF3 protein (B) was detected in livers collected at end of 2 hrs. A representative western blot picture is shown in upper panel and the bar graph in lower panel shows mean relative density ± SEM (5 mice per group). *p < 0.05 vs. LPS injected mice. RAW macrophages were transfected with HSF1 siRNA and were stimulated next day with LPS, 17-DMAG or both for 2 hrs. IL-6 mRNA was analyzed by real-time PCR. (C) Data represents mean fold change in IL-6 mRNA of 3 experiments ± SEM. #p < 0.001 vs. non transfected, LPS+17-DMAG stimulated cells.
5 mg/kg BW) and livers were collected at end of 2 hrs. Messenger RNA levels of liver ATF3 (A) were analyzed by quantitative real-time PCR, and normalized to 18S rRNA. Results are expressed as mean fold change ± SEM over mice injected with saline (5 mice per group). Nuclear ATF3 protein (B) was detected in livers collected at end of 2 hrs. A representative western blot picture is shown in upper panel and the bar graph in lower panel shows mean relative density ± SEM (5 mice per group). *p < 0.05 vs. LPS injected mice. RAW macrophages were transfected with HSF1 siRNA and were stimulated next day with LPS, 17-DMAG or both for 2 hrs. IL-6 mRNA was analyzed by real-time PCR. (C) Data represents mean fold change in IL-6 mRNA of 3 experiments ± SEM. #p < 0.001 vs. non transfected, LPS+17-DMAG stimulated cells.
Discussion
Intracellular chaperones are necessary for the stability and function of signaling molecules down-stream to the LPS receptor (14, 15, 19). The role of hsp90, an important molecular chaperone in LPS signaling pathway, has been recognized (13, 19, 20). The significance of endotoxin (LPS) mediated macrophage activation and inflammatory responses in acute and chronic liver diseases is well known (1). In this study, we targeted hsp90 to inhibit LPS signaling in the liver and reduce pro-inflammatory cytokine production preventing liver injury. Experiments performed in vivo using water-soluble and less toxic, hsp90 specific inhibitor, 17-DMAG, we show that hsp90 inhibition decreases pro-inflammatory cytokine production and alleviates LPS induced liver injury. Previous limitations for in vivo use of geldanamycin and its derivatives has been their dose limiting toxicity leading to weight loss, hematologic, hepatic, and renal toxicity, and cell death (38, 39). Pharmacodynamic studies showed that medium tolerated dose of 17-DMAG in vivo is 75 mg/kg with minimal toxicity (40, 41). Here, we used a single dose of 17-DMAG ranging from 2.5 mg/kg to 30 mg/kg with less concern for non-specific or toxic effects of 17-DMAG. Our data here suggest hsp90 as an attractive therapeutic target in liver diseases.
While inhibitors of hsp90 were primarily identified of therapeutic importance in cancer, their role in inflammatory diseases (42) such as rheumatoid arthritis (28), endotoxin mediated uveitis (18), sepsis (43) and atherosclerosis (44, 45) is emerging. Since LPS mediated inflammatory responses are crucial to development of liver diseases, strategies that prevent this response in the liver could have a beneficial effect. The inhibitory function of hsp90 inhibitors on liver inflammatory responses could be a dual mechanism: either loss of client proteins due to loss of chaperone function (19, 21) or induction of anti-inflammatory transcription factor HSF1 and hsp70 expression (46). Here we report that inhibition of hsp90 in the liver induces HSF1 and inhibits LPS induced NFκB activation and pro-inflammatory cytokine production alleviating liver injury (Fig. 8). The chaperone function of hsp90 on LPS signaling intermediates explains its effect on expression of down-stream pro-inflammatory cytokines. In this context, 17-DMAG could affect transcription of cytokine genes and their production. We observed that pro-inflammatory cytokines, TNFα and IL-6 were significantly inhibited both at mRNA and protein level in whole livers treated with 17-DMAG and LPS. Concomitant reduction of serum TNFα and IL-6 paralleled the liver cytokine profile. We predict that hsp90 inhibition in liver alters LPS signaling events proximal to pro-inflammatory cytokine gene transcription.
Fig. 8. Mechanism of pro-inflammatory cytokine inhibition and alleviation of LPS induced live injury by 17-DMAG.
Inhibition of hsp90 by 17-DMAG induces heat shock transcription factor (HSF1) in liver which hinders binding of NFκB to the promoter region of pro-inflammatory cytokine target genes, resulting in reduction of LPS induced pro-inflammatory cytokines and prevent liver injury.
The transcription factor NFκB is a key down-stream signaling intermediate of LPS receptors, CD14 and TLR4. Earlier studies show that 17-DMAG reduces NFκB activity in respiratory epithelial cells (47) either directly or through inhibition of upstream LPS receptors, CD14 and TLR4 (14). Hsp90 associates with the LPS receptor complex (14) and its inhibition decreases CD14 expression (48). Our results show significant down-regulation in CD14 mRNA without changes in TLR4 mRNA in liver. Furthermore, 17-DMAG significantly inhibits NFκB DNA binding but does not affect NFκB driven reporter activity. Interestingly, 17-DMAG has a profound effect on TNFα promoter driven reporter activity pointing to the likely involvement of other repressive transcription factors in 17-DMAG-mediated pro-inflammatory cytokine reduction in the liver.
While inhibition of Hsp90 releases HSF1 from its inactive state to induce target gene expression (29, 30), HSF1 also negatively regulates induction of pro-inflammatory cytokine genes (49–51). Consistent with previous findings (31), no change in hsp90 protein levels was observed after 17-DMAG treatment in the liver. On the other hand, hsp90 inhibition resulted in a significant up-regulation of HSF1 DNA binding and induction of hsp70 mRNA and protein in the liver, confirming inhibition of hsp90 chaperone function. The repressive function of HSF1 on transcription of pro-inflammatory cytokine gene TNFα in macrophages during exposure to febrile temperatures has been shown (51, 52, 53). The TNFα promoter is reported to have a binding site for HSF1 (33). We postulated that activated HSF1 in liver may serve as a repressor of TNFα gene induction during treatment with 17-DMAG. Using chromatin immunoprecipitation assay we show binding of HSF1 to TNFα promoter in presence of 17-DMAG treatment in macrophages. This observation correlates with elevated DNA binding activity of HSF1 in response to hsp90 inhibition by 17-DMAG in liver. Further, while HSF1 bound to the hsp70 promoter, 17-DMAG treatment did not induce binding of HSF1 to IL-6 promoter indicating an HSF1 indirect or independent down-regulation of IL-6 during 17-DMAG treatment. Previous studies show that HSF1 indirectly negatively regulates IL-6 promoter through induction of ATF3 (37). Our studies exhibit an up-regulation of LPS-induced ATF3 mRNA and protein in liver during 17-DMAG treatment suggesting that HSF1 negatively regulates IL-6 likely through ATF3 induction. Future studies will determine the role of ATF3 in 17-DMAG treated macrophages and liver inflammatory responses. Finally, using HSF1 siRNA we also confirmed the direct repressive role for HSF1 in TNFα inhibition and an indirect regulation of IL-6 in 17-DMAG treated macrophages. Thus, HSF1 appears to play a significant role in down-regulation of pro-inflammatory cytokine responses in the liver on treatment with 17-DMAG, a specific hsp90 inhibitor.
The clinical significance of our study is related to the emerging function of hsp90 as a potential therapeutic target in different diseases (18, 28, 43, 44, 54). Compelling approaches using hsp90 inhibitors in hepatocellular carcinoma (41) and hepatitis C virus replication (54, 55) have been reported. Our results here for the first time suggest a novel application for hsp90 inhibitor 17-DMAG in alleviating LPS mediated liver injury providing a solid basis for clinical investigations using hsp90 inhibitors in acute and chronic liver diseases. A significant role for hsp90 in chronic alcohol mediated pro-inflammatory cytokine induction is shown earlier (17). Future studies in our laboratory are underway to target hsp90 in liver diseases regulated by pro-inflammatory responses such as alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD) and liver fibrosis.
Supplementary Material
Acknowledgement
The authors thank Karen Kodys for labeling oligonucleotides for EMSA analysis. This work was supported by the University of Massachusetts Center for AIDS Research (P30 AI042845).
Financial Support: This work was supported by the PHS grant # AA017986 (to PM) from the National Institute of Alcohol Abuse and Alcoholism and its contents are the sole responsibility of the authors and do not necessarily represent the views of the NIAAA.
Abbreviations
- 17-DMAG
17-Dimethylamino-ethylamino-17-demethoxygeldanamycin
- Hsp90
Heat Shock Protein mol wt. 90 kDa
- Hsp70
Heat Shock Protein mol wt. 70 kDa
- HSF1
Heat Shock Transcription Factor1
- HSE
Heat Shock binding Element
Contributor Information
Aditya Ambade, Email: Aditya.Ambade@umassmed.edu.
Donna Catalano, Email: Donna.Catalano@umassmed.edu.
Arlene Lim, Email: Arlene.Lim@umassmed.edu.
Pranoti Mandrekar, Email: Pranoti.Mandrekar@umassmed.edu.
References
- 1.Nolan JP. The role of intestinal endotoxin in liver injury: A long and evolving history. Hepatology. 2010;52(5):1829–1835. doi: 10.1002/hep.23917. [DOI] [PubMed] [Google Scholar]
- 2.Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, Sugiyama T. Lipopolysaccharide triggered TNF-alpha-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol. 2009;51(1):168–175. doi: 10.1016/j.jhep.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: Implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94(6):2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, Goto M, Yamashina S, et al. Role of kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15(Suppl):D20–D25. doi: 10.1046/j.1440-1746.2000.02179.x. [DOI] [PubMed] [Google Scholar]
- 5.Uesugi T, Froh M, Arteel GE, Bradford BU, Wheeler MD, Gabele E, Isayama F, et al. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol. 2002;168(6):2963–2969. doi: 10.4049/jimmunol.168.6.2963. [DOI] [PubMed] [Google Scholar]
- 6.Enomoto N, Schemmer P, Ikejima K, Takei Y, Sato N, Brenner DA, Thurman RG. Long-term alcohol exposure changes sensitivity of rat kupffer cells to lipopolysaccharide. Alcohol Clin Exp Res. 2001;25(9):1360–1367. [PubMed] [Google Scholar]
- 7.Su GL, Hoesel LM, Bayliss J, Hemmila MR, Wang SC. Lipopolysaccharide binding protein inhibitory peptide protects against acetaminophen-induced hepatotoxicity. Am J Physiol Gastrointest Liver Physiol. 2010;299(6):G1319–G1325. doi: 10.1152/ajpgi.00140.2010. [DOI] [PubMed] [Google Scholar]
- 8.Colletti LM, Green M. Lung and liver injury following hepatic ischemia/reperfusion in the rat is increased by exogenous lipopolysaccharide which also increases hepatic TNF production in vivo and in vitro. Shock. 2001;16(4):312–319. doi: 10.1097/00024382-200116040-00014. [DOI] [PubMed] [Google Scholar]
- 9.Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52(4):1322–1333. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 10.Uchikura K, Wada T, Hoshino S, Nagakawa Y, Aiko T, Bulkley GB, Klein AS, et al. Lipopolysaccharides induced increases in fas ligand expression by kupffer cells via mechanisms dependent on reactive oxygen species. Am J Physiol Gastrointest Liver Physiol. 2004;287(3):G620–G626. doi: 10.1152/ajpgi.00314.2003. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Cederbaum AI. CYP2E1 potentiation of LPS and TNFalpha-induced hepatotoxicity by mechanisms involving enhanced oxidative and nitrosative stress, activation of MAP kinases, and mitochondrial dysfunction. Genes Nutr. 2010;5(2):149–167. doi: 10.1007/s12263-009-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16(11):1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandrekar P. Signaling mechanisms in alcoholic liver injury: Role of transcription factors, kinases and heat shock proteins. World J Gastroenterol. 2007;13(37):4979–4985. doi: 10.3748/wjg.v13.i37.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triantafilou M, Triantafilou K. Heat-shock protein 70 and heat-shock protein 90 associate with toll-like receptor 4 in response to bacterial lipopolysaccharide. Biochem Soc Trans. 2004;32(Pt 4):636–639. doi: 10.1042/BST0320636. [DOI] [PubMed] [Google Scholar]
- 15.Hsu HY, Wu HL, Tan SK, Li VP, Wang WT, Hsu J, Cheng CH. Geldanamycin interferes with the 90-kDa heat shock protein, affecting lipopolysaccharide-mediated interleukin-1 expression and apoptosis within macrophages. Mol Pharmacol. 2007;71(1):344–356. doi: 10.1124/mol.106.024240. [DOI] [PubMed] [Google Scholar]
- 16.Triantafilou K, Triantafilou M, Ladha S, Mackie A, Dedrick RL, Fernandez N, Cherry R. Fluorescence recovery after photobleaching reveals that LPS rapidly transfers from CD14 to hsp70 and hsp90 on the cell membrane. J Cell Sci. 2001;114(Pt 13):2535–2545. doi: 10.1242/jcs.114.13.2535. [DOI] [PubMed] [Google Scholar]
- 17.Mandrekar P, Catalano D, Jeliazkova V, Kodys K. Alcohol exposure regulates heat shock transcription factor binding and heat shock proteins 70 and 90 in monocytes and macrophages: Implication for TNF-alpha regulation. J Leukoc Biol. 2008;84(5):1335–1345. doi: 10.1189/jlb.0407256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poulaki V, Iliaki E, Mitsiades N, Mitsiades CS, Paulus YN, Bula DV, Gragoudas ES, et al. Inhibition of Hsp90 attenuates inflammation in endotoxin-induced uveitis. FASEB J. 2007;21(9):2113–2123. doi: 10.1096/fj.06-7637com. [DOI] [PubMed] [Google Scholar]
- 19.Salminen A, Paimela T, Suuronen T, Kaarniranta K. Innate immunity meets with cellular stress at the IKK complex: Regulation of the IKK complex by HSP70 and HSP90. Immunol Lett. 2008;117(1):9–15. doi: 10.1016/j.imlet.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Joly AL, Wettstein G, Mignot G, Ghiringhelli F, Garrido C. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J Innate Immun. 2010;2(3):238–247. doi: 10.1159/000296508. [DOI] [PubMed] [Google Scholar]
- 21.Broemer M, Krappmann D, Scheidereit C. Requirement of Hsp90 activity for IkappaB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-kappaB activation. Oncogene. 2004;23(31):5378–5386. doi: 10.1038/sj.onc.1207705. [DOI] [PubMed] [Google Scholar]
- 22.De Nardo D, Masendycz P, Ho S, Cross M, Fleetwood AJ, Reynolds EC, Hamilton JA, et al. A central role for the Hsp90.Cdc37 molecular chaperone module in interleukin-1 receptor-associated-kinase-dependent signaling by toll-like receptors. J Biol Chem. 2005;280(11):9813–9822. doi: 10.1074/jbc.M409745200. [DOI] [PubMed] [Google Scholar]
- 23.Bandyopadhyay S, Chiang CY, Srivastava J, Gersten M, White S, Bell R, Kurschner C, et al. A human MAP kinase interactome. Nat Methods. 2010;710:801–805. doi: 10.1038/nmeth.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacey S, Wilson RH, Walton M, Eatock MM, Hardcastle A, Zetterlund A, Arkenau HT, et al. A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin Cancer Res. 2011;17(6):1561–1570. doi: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson PG, Mitsiades CS, Laubach JP, Lonial S, Chanan-Khan AA, Anderson KC. Inhibition of heat shock protein 90 (HSP90) as a therapeutic strategy for the treatment of myeloma and other cancers. Br J Haematol. 2011;152(4):367–379. doi: 10.1111/j.1365-2141.2010.08360.x. [DOI] [PubMed] [Google Scholar]
- 26.Sedlackova L, Spacek M, Holler E, Imryskova Z, Hromadnikova I. Heat-shock protein expression in leukemia. Tumour Biol. 2011;32(1):33–44. doi: 10.1007/s13277-010-0088-7. [DOI] [PubMed] [Google Scholar]
- 27.Allegra A, Sant'antonio E, Penna G, Alonci A, D'Angelo A, Russo S, Cannavo A, et al. Novel therapeutic strategies in multiple myeloma: Role of the heat shock protein inhibitors. Eur J Haematol. 2011;86(2):93–110. doi: 10.1111/j.1600-0609.2010.01558.x. [DOI] [PubMed] [Google Scholar]
- 28.Rice JW, Veal JM, Fadden RP, Barabasz AF, Partridge JM, Barta TE, Dubois LG, et al. Small molecule inhibitors of Hsp90 potently affect inflammatory disease pathways and exhibit activity in models of rheumatoid arthritis. Arthritis Rheum. 2008;58(12):3765–3775. doi: 10.1002/art.24047. [DOI] [PubMed] [Google Scholar]
- 29.Conde R, Belak ZR, Nair M, O'Carroll RF, Ovsenek N. Modulation of Hsf1 activity by novobiocin and geldanamycin. Biochem Cell Biol. 2009;87(6):845–851. doi: 10.1139/o09-049. [DOI] [PubMed] [Google Scholar]
- 30.Kim HR, Kang HS, Kim HD. Geldanamycin induces heat shock protein expression through activation of HSF1 in K562 erythroleukemic cells. IUBMB Life. 1999;48(4):429–433. doi: 10.1080/713803536. [DOI] [PubMed] [Google Scholar]
- 31.Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42(2):260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 32.Guzhova IV, Darieva ZA, Melo AR, Margulis BA. Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones. 1997;2(2):132–139. doi: 10.1379/1466-1268(1997)002<0132:msphiw>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh IS, He JR, Hester L, Fenton MJ, Hasday JD. Bacterial endotoxin modifies heat shock factor-1 activity in RAW 264.7 cells: Implications for TNF-alpha regulation during exposure to febrile range temperatures. J Endotoxin Res. 2004;10(3):175–184. doi: 10.1179/096805104225004851. [DOI] [PubMed] [Google Scholar]
- 34.Inouye S, Fujimoto M, Nakamura T, Takaki E, Hayashida N, Hai T, Nakai A. Heat shock transcription factor 1 opens chromatin structure of interleukin-6 promoter to facilitate binding of an activator or a repressor. J Biol Chem. 2007;282(45):33210–33217. doi: 10.1074/jbc.M704471200. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs AT, Marnett LJ. HSF1-mediated BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated colon cancer cells via stabilization of anti-apoptotic bcl-2 proteins. J Biol Chem. 2009;284(14):9176–9183. doi: 10.1074/jbc.M808656200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh IS, Viscardi RM, Kalvakolanu I, Calderwood S, Hasday JD. Inhibition of tumor necrosis factor-alpha transcription in macrophages exposed to febrile range temperature. A possible role for heat shock factor-1 as a negative transcriptional regulator. J Biol Chem. 2000;275(13):9841–9848. doi: 10.1074/jbc.275.13.9841. [DOI] [PubMed] [Google Scholar]
- 37.Takii R, Inouye S, Fujimoto M, Nakamura T, Shinkawa T, Prakasam R, Tan K, et al. Heat shock transcription factor 1 inhibits expression of IL-6 through activating transcription factor 3. J Immunol. 2010;184(2):1041–1048. doi: 10.4049/jimmunol.0902579. [DOI] [PubMed] [Google Scholar]
- 38.Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, Verbel D, Heller G, et al. 17-allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8(5):986–993. [PubMed] [Google Scholar]
- 39.Hollingshead M, Alley M, Burger AM, Borgel S, Pacula-Cox C, Fiebig HH, Sausville EA. In vivo antitumor efficacy of 17-DMAG (17-dimethylaminoethylamino-17-demethoxygeldanamycin hydrochloride), a water-soluble geldanamycin derivative. Cancer Chemother Pharmacol. 2005;56(2):115–125. doi: 10.1007/s00280-004-0939-2. [DOI] [PubMed] [Google Scholar]
- 40.Egorin MJ, Lagattuta TF, Hamburger DR, Covey JM, White KD, Musser SM, Eiseman JL. Pharmacokinetics, tissue distribution, and metabolism of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (NSC 707545) in CD2F1 mice and fischer 344 rats. Cancer Chemother Pharmacol. 2002;49(1):7–19. doi: 10.1007/s00280-001-0380-8. [DOI] [PubMed] [Google Scholar]
- 41.Breinig M, Caldas-Lopes E, Goeppert B, Malz M, Rieker R, Bergmann F, Schirmacher P, et al. Targeting heat shock protein 90 with non-quinone inhibitors: A novel chemotherapeutic approach in human hepatocellular carcinoma. Hepatology. 2009;50(1):102–112. doi: 10.1002/hep.22912. [DOI] [PubMed] [Google Scholar]
- 42.Yun TJ, Harning EK, Giza K, Rabah D, Li P, Arndt JW, Luchetti D, et al. EC144, a synthetic inhibitor of heat shock protein 90, blocks innate and adaptive immune responses in models of inflammation and autoimmunity. J Immunol. 2011;186(1):563–575. doi: 10.4049/jimmunol.1000222. [DOI] [PubMed] [Google Scholar]
- 43.Chatterjee A, Dimitropoulou C, Drakopanayiotakis F, Antonova G, Snead C, Cannon J, Venema RC, et al. Heat shock protein 90 inhibitors prolong survival, attenuate inflammation, and reduce lung injury in murine sepsis. Am J Respir Crit Care Med. 2007;176(7):667–675. doi: 10.1164/rccm.200702-291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madrigal-Matute J, Lopez-Franco O, Blanco-Colio LM, Munoz-Garcia B, Ramos-Mozo P, Ortega L, Egido J, et al. Heat shock protein 90 inhibitors attenuate inflammatory responses in atherosclerosis. Cardiovasc Res. 2010;86(2):330–337. doi: 10.1093/cvr/cvq046. [DOI] [PubMed] [Google Scholar]
- 45.Brouet A, Sonveaux P, Dessy C, Moniotte S, Balligand JL, Feron O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ Res. 2001;89(10):866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
- 46.Bagatell R, Paine-Murrieta GD, Taylor CW, Pulcini EJ, Akinaga S, Benjamin IJ, Whitesell L. Induction of a heat shock factor 1-dependent stress response alters the cytotoxic activity of hsp90-binding agents. Clin Cancer Res. 2000;6(8):3312–3318. [PubMed] [Google Scholar]
- 47.Malhotra V, Shanley TP, Pittet JF, Welch WJ, Wong HR. Geldanamycin inhibits NF-kappaB activation and interleukin-8 gene expression in cultured human respiratory epithelium. Am J Respir Cell Mol Biol. 2001;25(1):92–97. doi: 10.1165/ajrcmb.25.1.4384. [DOI] [PubMed] [Google Scholar]
- 48.Vega VL, De Maio A. Geldanamycin treatment ameliorates the response to LPS in murine macrophages by decreasing CD14 surface expression. Mol Biol Cell. 2003;14(2):764–773. doi: 10.1091/mbc.E02-08-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh IS, Gupta A, Nagarsekar A, Cooper Z, Manka C, Hester L, Benjamin IJ, et al. Heat shock co-activates interleukin-8 transcription. Am J Respir Cell Mol Biol. 2008;39(2):235–242. doi: 10.1165/rcmb.2007-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie Y, Chen C, Stevenson MA, Auron PE, Calderwood SK. Heat shock factor 1 represses transcription of the IL-1beta gene through physical interaction with the nuclear factor of interleukin 6. J Biol Chem. 2002;277(14):11802–11810. doi: 10.1074/jbc.M109296200. [DOI] [PubMed] [Google Scholar]
- 51.Cooper ZA, Singh IS, Hasday JD. Febrile range temperature represses TNF-alpha gene expression in LPS-stimulated macrophages by selectively blocking recruitment of Sp1 to the TNF-alpha promoter. Cell Stress Chaperones. 2010;15(5):665–673. doi: 10.1007/s12192-010-0179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18(21):5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooper ZA, Ghosh A, Gupta A, Maity T, Benjamin IJ, Vogel SN, Hasday JD, et al. Febrile-range temperature modifies cytokine gene expression in LPS-stimulated macrophages by differentially modifying NF-{kappa}B recruitment to cytokine gene promoters. Am J Physiol Cell Physiol. 2010;298(1):C171–C181. doi: 10.1152/ajpcell.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakagawa S, Umehara T, Matsuda C, Kuge S, Sudoh M, Kohara M. Hsp90 inhibitors suppress HCV replication in replicon cells and humanized liver mice. Biochem Biophys Res Commun. 2007;353(4):882–888. doi: 10.1016/j.bbrc.2006.12.117. [DOI] [PubMed] [Google Scholar]
- 55.Ujino S, Yamaguchi S, Shimotohno K, Takaku H. Heat-shock protein 90 is essential for stabilization of the hepatitis C virus nonstructural protein NS3. J Biol Chem. 2009;284(11):6841–6846. doi: 10.1074/jbc.M806452200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh IS, He JR, Calderwood S, Hasday JD. A high affinity HSF-1 binding site in the 5'-untranslated region of the murine tumor necrosis factor-alpha gene is a transcriptional repressor. J Biol Chem. 2002;277(7):4981–4988. doi: 10.1074/jbc.M108154200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.