Abstract
The endothelium lies in a strategic anatomical position between the circulating blood and vascular smooth-muscle cells as a source of vasodilators such as nitric oxide, prostacyclin, and hyperpolarizing factor as well as heparin-like substances as well as other molecules with anti-proliferative properties. These effects of endothelial cells may explain why platelets and monocytes usually do not adhere at the blood vessel wall. However, under pathological conditions, endothelial dysfunction occurs and significantly contributes to the increase of platelet-vessel wall interaction, vasoconstriction, pro-inflammation, and proliferation. Under these conditions, endothelium-dependent vasodilation is reduced and endothelium-dependent constrictor responses are augmented. Upon vessel wall injury platelets rapidly adhere to the exposed sub-endothelial matrix which is mediated by several cellular receptors present on platelets or endothelial cells and various adhesive proteins. Subsequent platelet activation results in the recruitment of additional platelets and the generation of platelet aggregates forming a stable platelet plug. Therapeutic strategies aimed at improving or preserving endothelial function therefore may be promising in the prevention and treatment of coronary artery disease. Diagnostic modalities for assessment of endothelial function should allow for the early detection of vascular endothelial dysfunction before the manifestation of serious adverse vascular disorders.
Keywords: vascular, endothelial cell, cardiovascular disorders, coronary syndrome, vasoconstriction, vasodilation, inflammation, anti-inflammation, anti-platelet, pro-platelet, anticoagulant, pro-coagulant, Diagnosis of endothelial function
Introduction
Cardiovascular Disease (CVD) resultant from atherosclerosis has been a longstanding, significant contributor to morbidity and mortality worldwide with a prevalence of 80 million people age 20 and over in the United States in 2006. In the United States in 2006, approximately 7,095,000 inpatient cardiovascular operations and procedures were performed, and the estimated cost (direct and indirect) in the United States for CVD in 2009 was $475.3 billion. Furthermore, increased risk is associated with advancing age; in 2005 more than 150,000 Americans who died from CVD were younger than 65.[1] Considering an average life expectancy of about 77 years in the United States, these deaths are deemed premature, and necessitate advancements in the treatment and prevention of CVD.
Among the 80 million people over age 20 in the United States affected by CVD from atherosclerosis, Peripheral Arterial Disease (PAD) affects about 8 million Americans and has risk factors similar to those of Coronary Artery Disease [1]. These similar risk factors are likely due to homologous origins of disease, further underscoring the need to address the notion of endothelial cell dysfunction – the apparent source of CVD. Major advances have been made in understanding the early mechanisms of endothelial dysfunction. Given the complexity of the genetics, molecular mechanics and physiology of this disease, it is important to continue to pursue a deeper understanding of its intricacies. Such research will likely lead to prevention strategies as early in the disease process as possible, a far more cost-effective alternative to treatment of established CVD. Certainly, any advances in treatment and prevention strategies against CVD will greatly benefit our healthcare system.
Endothelial cell dysfunction is a hallmark of the early development of atherosclerosis. While many medications that reduce the risk of CVD exist, including statins, purified fish oils, ACEIs/ARBS, and thiazide diuretics, “all the major risk factors for atherosclerosis have been associated with impaired nitric oxide activity,”[2] which is a primary marker for endothelium dysfunction[3–4]. This, combined with the rise of flow-mediated dilation (FMD) as an indicator of a patient’s NO bioactivity, can open the door for identifying patients with early endothelium dysfunction[4]. These patients would likely be more susceptible to various forms of CVD. Early identification of these patients can be very beneficial. This practice can pave the way to testing a rising array of medications aimed at correcting early endothelial cell dysfunction. It is all but certain that such correction will lead to longer term risk reduction and improved clinical outcomes.
Reduced bioactivity of nitric oxide (NO) is the current benchmark for recognizing endothelial cell dysfunction[3]. However, despite NO’s predictive value, many other biological molecules are responsible for endothelial cell homeostasis, and provide a wide variety of potential drug targets. Identification and understanding of key biological markers/mediators involved in the pathophysiology behind endothelium dysfunction should ultimately lead to methods for treating or preventing the early onset of atherogenesis. Literature shows that endothelial cell dysfunction and plaque formation is characterized by a balance shift. The shift is characterized by decreased vasodilatory, thrombolytic, anti-inflammatory, and anti-coagulant molecules (of which NO is arguably the most significant) relative to their pro-coagulant, vasoconstrictive, and thrombotic counterparts [3].
This review is a brief summary of some potential drug targets geared toward stimulating the anti-atherogenic properties of the endothelium, centered on some of the key biological functions of the endothelial cell. Table 1, adapted from Blann et al.[2], identifies key components of the endothelium, some of which will be discussed in this review. In addition, there are other molecules that are pro-atherogenic and are not directly related to the endothelium such as Angiotensin II, Thrombin, Plasmin, and Fibrin that are not listed in Table 1. The anti-atherogenic column in Table 1 lists molecules which are of anti-coagulant, anti-thrombotic, anti-inflammatory, anti-adhesive, or vasodilatory nature. The opposite is true of the pro-atherogenic column. The molecules in either column should not be considered healthy, or unhealthy, because pathological conditions tend to arise only from irregular expression of these molecules. It is important to note that factors in the pro-atherogenic” category are of great physiologic importance for normal health, and that complete inhibition of any of these factors will likely have adverse results. However, these factors, in certain cascades, can play atherogenic roles in dysfunctional endothelium. Furthermore, some anti-atherogenic molecules, which lyses clots, may play a role in triggering thrombosis in advanced lesions.
Table 1.
Types of anti-pro-atherothrombogenic molecules in endothelium
| Anti-atherothrombogenic Mediators | Pro-atherothrombogenic Mediators | |
|---|---|---|
|
Cell Surface Molecules |
Thrombomodulin Protein C Heparin and Heparan sulfate Ectonucleotidases Tissue Factor Pathway Inhibitor Endothelial Estrogen Receptor-alpha (ERα) |
Tissue Factor Binding Sites for coagulation factors |
| Released Molecules | Nitric Oxide Prostacyclin Tissue Plasminogen Activator (t-PA) t-PA binding sites |
Thromboxane A2 Platelet Activating Factor Von Willebrand Factor Endothelin |
ERα plays key role in the E2-induced prevention of endothelial dysfunction after ischemia/reperfusion (60)
Organic nitrates and innovative NO donors for the endothelium
It is generally known that organic nitrates such as isosorbide dinitrate (ISDM) or isosorbide mononitrate (ISMN) are commonly used in the treatment of stable angina and improvement of exercise tolerance in the clinical setting. However, little attention has been paid to the great potential that may be derived from recent advances in the understanding of nitrate function. For example, nitrates that can increase NO production and perhaps slow or prevent the progression of atherosclerosis, unlike traditional nitrates used for stable angina, have been identified. Such molecules may be important to address the fact that routine administration of organic nitrates is typically associated with tachyphylaxis, decreased NO bioavailability, and development of endothelial cell dysfunction [5]. This is typically caused by the generation of reactive oxygen species (ROS), generally produced by the resultant superoxide anion from the nitrate metabolic pathway [6–7]. ROS inhibit mitochondrial aldehyde dehydrogenase (ALDH-2), which is a primary enzyme for metabolism of certain nitrates. Consequently, long term repetitive treatment with nitroglycerin, versus ISMN and ISDN, is more associated with worsened endothelial cell dependent vasodilation and a decrease in NO bioavailability [5, 7]. However, among all organic nitrates, repeated long term administration is still linked to nitrate tolerance.
While nitrate free-intervals generally decrease or prevent long term nitrate tolerance [7], control of ROS is still of significant pharmacologic importance, and has been a focus of recent research regarding organic nitrate function. Compounds possessing 3 nitrate groups go through a mitochondrial biotransformation which is not undergone by compounds with 2 or less nitrate groups. This biotransformation is associated with the increased generation of ROS [6]. This led to the preference of ISMN and ISDN in clinical practice for long-term treatment of stable angina since they do not seem to significantly stimulate the production of ROS [6]. This is likely due to their metabolism being through the non-mitochondrial “low affinity pathway” in tissues[8]. However, Dragoni et al. have recently shown that pentaerithrityl tetranitrate (PETN), typically avoided due to its mitochondrial metabolism, exhibits an additional antioxidant effect and an ability to stimulate protective genes that code for the generation of heme-oxygenase-1 and ferritin. The authors hypothesize that these genes play a role in the establishment of endothelial cell pre-conditioning [6]. The phenomenon of pre-conditioning is that tissues, such as the myocardium, undergoing transient periods of ischemia, can become resistant to periods of severe ischemia. This phenomenon was shown to be consistent in the endothelium by Zahler et al., and is another area of great interest and therapeutic potential [9].
On a similar front, dietary nitrite is making a return appearance. Despite the history of nitrites being associated with negative consequences, a recent extensive report has shown the presence of many beneficial effects on the endothelium by nitrites.[10] In rats with micro-vascular inflammation induced by a high cholesterol diet, nitrite supplementation in water proved protective against endothelial dysfunction. Nitrite ingestion is involved in the reduction of C - reactive protein levels back to normal in these mice and the preservation of BH4, a redox enzyme which is involved in NO formation. Nitrite is also known to be depleted in hyper-cholesterolemic states. Given these data, the abundance of nitrite in vegetables (which are associated with “heart health”), and with the hypothesis that N-nitrosamine formation is the true carcinogenic worry, re-evaluation of nitrite supplementation is defensible[10]. Perhaps nitrite given with compounds that inhibit N-nitrosamine formation as a cocktail could provide long-term protection of the endothelium without adverse consequences.
Thrombomodulin
Thrombomodulin (TM) is a 100 kDa membrane protein that is expressed on the surface of endothelial cells. It is involved in maintaining the balance between anti-coagulant and pro-coagulant properties of the endothelium [11–12] Each endothelial cell typically contains about 50,000–100,000 TM molecules [13]. TM possesses a great deal of anti-atherogenic functions. Among these are its activation of anti-coagulant Protein C via formation of the thrombin-TM complex, and its facilitation of thrombin-anti-thrombin complex formation via its chondroitin sulfate moiety [14–15]. TM is also responsible for activation of Thrombin Activatable Fibrinolysis Factor (TAFI) which is also being examined for its anti-inflammatory properties [15]. TM is a multi-domain peptide which can be recombinantly expressed in various forms with a large variety of functional activities [16–17]. Noting TM’s wide array of anti-coagulant functions, it is not surprising that local TM deficiencies are linked to thrombosis and atherogenesis in animal models[14]. The functions of TM and the physiologic consequences of localized TM deficiency clearly underscore its role in prevention of plaque formation.[14]. In addition to this, TM is sub-normally expressed in endothelial cells lining atherosclerotic plaques [14, 18]. And, loss of TM activity in vein grafts is linked to early thrombosis [19].
Given this information underscoring the physiological consequences of lacking TM, a natural question to follow is: what would be the results of hyper-expression of TM along cells in the endothelium? Furthermore, since TM is less prevalent in endothelium lining atherosclerotic plaques, the next question is: what is TM’s role in stabilization of plaques? While treatment of atherosclerotic plaques would ideally involve reversal and removal of the fatty streak, perhaps stabilizing a slightly occluded vessel could be a less invasive approach to preventing plaque growth and minimizing risk of occlusive thrombosis.
Many genes have been identified as associated with changes in TM function and therefore clinical manifestations of disease. These genetic polymorphisms tend to be associated with an increased risk of myocardial infarction or atherosclerosis, and are listed in Weiler et al.[14]. These clinical correlations match up with the notion that loss of TM or inactivation of endothelial TM can lead to vascular complications. Loss of TM expression on the endothelial cell membrane can occur via multiple mechanisms. These include oxidation of functional domains of TM, any trigger of large amounts of cytokines—generally from sufficient endothelial cell injury—which in turn suppress its gene transcription, or neutrophil-triggered release of soluble TM [14–15]. These facts further refine the previous questions: would increased cellular TM expression make the endothelium more resistant to these inflammatory response cascades? Would such hyper-expression of TM lead to a more non-reactive endothelium that is more resistant to the development of atherosclerotic lesions? Or, would this lead to the endothelium having a decreased ability to repair vascular injury? Furthermore, this information underscores the need to identify the genetic targets which control TM expression, so that they can be modified in order to design experiments to answer these questions.
In 2004, a new gene transfer method using a “ligand-facilitated transfer of the cationic liposome: TM gene complex” was used to induce the over-expression of TM in rat inferior vena cavas [19]. This over-expression was induced prior to excision and vein graft operation. Amazingly, under reduced-flow conditions of venous endothelium, over-expression of thrombomodulin was achieved by this high-efficiency method. This translated to a greater thrombo-resistance (less clotting induced by thrombin) in the grafts with over-expressed TM versus control groups, thus preventing acute graft failure [19]. These results combined with full length TM’s ability to inhibit smooth muscle cell (SMC) proliferation make it a good candidate for in vivo studies related to operative occlusion prophylaxis[20]. TM over-expression can perhaps protect against stent failure, and a variety of graft failures. While experimentation is currently focused on venular endothelium TM, the advent of even higher efficiency gene transfer techniques (potentially used under normal flow conditions) could open a world of clinical opportunities with regard to arterial endothelium TM.
Soluble Thrombomodulin
Soluble TM is another interesting factor with potential for controlling atherogenesis/thrombosis. Soluble TM appears to be a cleaved fragment of the membrane TM that is released into the bloodstream via “neutrophil-elastase-dependent proteolytic release” [13–14]. Soluble TM is known to be elevated in a variety of disease states associated with endothelial cell injury [21]. This form of TM still retains significant amounts of cofactor/biologic activity relative to cellular TM. Soluble TM still has the ability to inhibit fibrinolysis, and some fragments maintain the ability to activate protein C at 30–50% of the affinity of cellular TM. Variations in the types and sizes of soluble TM fragments also seem to produce drastic differences in functional activity [21]. An example of this variability is likely due to an array of expressions of its chondroitin sulfate (CS) moiety among fragments[16]. The CS moiety is responsible for increasing TM’s ability to inhibit thrombin, and furthers the binding of anti-thrombin III to thrombin. TM possessing the chondroitin sulfate moiety (at full length) is more prevalent in arterial endothelial cells vs. venous endothelial cells[16]. Another example of different functional fragment activity involves the lectin domain. The lectin-like domain is associated with anti-inflammatory properties, while showing no anti-coagulant or fibrinolytic actions. It has been shown to protect endothelial cells in certain destructive conditions, and disrupts monocyte adhesion to activated endothelium [14, 22].
There are a variety of different soluble TM fragments identified, found in different concentrations among different cardiac risk disease states [21]. Isolating and understanding the different structure-function relationships among soluble TM fragments can certainly lead to identification of the most therapeutically beneficial components for correlating disease states. For instance, it is important to identify the beneficial or adverse effects of routine administration of rTM consisting of only the lectin-like domain. Thus far, it has been shown to reduce inflammation in mouse models, and seems to hold promise in various inflammatory disorders including arthritis [22]. Perhaps it is possible that these anti-inflammatory properties can provide long-term risk reduction by reducing micro-vascular inflammation in Cardiovascular Diseases.
Other manipulations may provide a safer and more specific alternative for anti-coagulant therapies in the setting of acute coronary syndromes. While the current paradigm is centered on unfractionated and low molecular weight heparins, bleeding complications are still of significant concern. However, manipulated soluble TM fragments can be explored as a potentially safer and comparably efficacious alternative. For example, the soluble-TM-thrombin complex still exhibits the anti-coagulant property of protein C activation. In addition, some soluble and recombinant TM does not have the chondroitin sulfate moiety, which directly interferes with its abilities to activate protein C, and facilitate the formation of the thrombin-anti-thrombin complex [14, 16]. Perhaps these fragments can provide a therapeutically beneficial balance of activity. Different concentrations of these fragments may exhibit safer degrees of anti-coagulation while maintaining efficacy. In short, purifying and experimenting with various TM domain fragments may unlock the keys to an entirely new class of acute care blood thinners or anti-inflammatory agents.
Tissue Factor and Tissue Factor Pathway Inhibitor
Tissue factor is a transmembrane protein that plays a large role in the extrinsic pathway of the coagulation cascade. It is expressed on various immune cells and smooth muscle cells, and certain conditions cause it to be expressed in endothelial cells. Tissue factor is abundant in atherosclerotic lesions, and is associated with an increased risk of thrombus formation within those lesions. It is regulated by tissue factor pathway inhibitor (TFPI) which exerts its effects via factor Xa inhibition, and is predominantly seen in the endothelial cells of the microvasculature[23].
There are a variety of TFPIs, and one of the most clinically promising versions is the active-site-inactivated recombinant factor VIIa. Its mechanism of action is competitive inhibition of factor VIIa to prevent binding to tissue factor. Inhibition of thrombosis without prolonged bleeding time has been observed in animal models using this inhibitor[23]. Additionally, monoclonal antibodies against tissue factor provide a variety of beneficial effects, including reduction in thrombin-mediated inflammation[23].
A recent trial has demonstrated that thrombin is a key molecule for initiating tissue factor in human umbilical vein endothelial cells. This mechanism is further enhanced by plasmin treatment. It was concluded that plasmin’s proteolytic activity was needed to create this increased expression of tissue factor. While other explanations are possible, it is likely that plasmin increases local tissue factor by cleaving TFPI[24]. Should this be true, the next question is whether or not an elevated level of TFPI would be completely degraded by the same level of enzyme activity, or if plasmin’s activity is of limited capacity.
It appears that plasmin activity can be overridden, since recombinant tissue factor pathway inhibitor inhibits thrombus formation and fibrin deposition in injured arterial segments (two events dependent on thrombin activating TF)[23]. Increased TF activity in atherosclerotic plaques is associated with conditions more conducive to thrombosis[23]. Perhaps increased TFPI levels locally can provide protection against thrombus formation when performing stent operations. This opens a door to therapeutic options similar to those of thrombomodulin. Perhaps high-efficiency gene transfer over-expression of TFPI in atheroprone segments of endothelium can correlate with better clinical outcomes during stenting or angioplasty.
With regard to tissue factor itself, some tissue factor polymorphisms are associated with poor clinical outcomes in CVD. The +5466A>G polymorphism has been shown to influence not only risk of cardiac death, but the effect of statins on thrombin formation [25]. Undas et al. have identified a certain polymorphism that may be linked to variable pleiotropic effects in statins, aside from cholesterol lowering [25]. This study is the first on a path toward potentially identifying optimum drug regimens based on patient genotype, and may be important in the setting of personalized medicine approaches in the future by aiding with the risk stratification of patients. Further identifying and characterizing high risk polymorphisms with regard to factors related to the endothelium will be crucial in the design of new patient specific therapies. Tissue factor, and its pathway inhibitor, is certainly molecules of interest for future Pharmacogenomic approaches.
Endothelial Lipase and HDL Modulation by Tissue Factor Pathway Inhibitor
Endothelial lipase (EL) is an intriguing lipid hydrolyzing enzyme that robustly influences high density lipoprotein (HDL) metabolism[26] but the health implications of this modulating activity in humans are unknown. It is the most recently discovered member of the lipase gene family that includes lipoprotein lipase and hepatic lipase. In contrast to these other two lipases, endothelial lipase is produced by endothelial cells. EL activity is increased in the midst of inflammatory states as reflected by its upregulation by proinflammatory cytokines. Of great interest is the fact that EL has been found to hydrolyze HDL and that EL activity may explain the inverse relationship that exists between inflammatory states and levels of HDL [27]. Although the possibility exist that downregulating EL will lead to increased HDL and reduced risk for CVD, the health implications of modulating EL are unknown. However, if a therapeutic agent reduces EL and inflammatory cytokines while increasing HDL, this would suggest that the agent possesses strong therapeutic potential. The benefits of such metabolic regulation by this novel agent await investigation of the cardiovascular effects on humans in states of health and disease.
Prostacyclin
Prostacyclin is another molecule endogenous to the endothelium which is responsible for vasodilatory and protective actions on the endothelium. Inhibitors of prostacyclin formation or activity will likely inhibit normal endothelial function and consequently open the door to atherosclerotic plaque formation [28]. Increased oxidative stress via superoxide anion formation leads to peroxynitrate formation [8]. Peroxynitrate is noted to inhibit prostacyclin synthase (PGI-S) [28]. Given that prostacyclin is vital for controlling vasodilation and inhibition of thrombus formation [3, 29], this suggests a mechanism for how oxidative stress can make the endothelium more prone to developing atherosclerotic lesions. In addition, the notion that various lipid peroxides inhibit PGI-S further supports the role of decreased prostacyclin protection in atherosclerosis[28], because oxidant injury is linked to both lipid peroxide formation and atherosclerosis[30].
The role of prostacyclin has been established as vital to a healthy endothelium, and so the development of prostacyclin analogues followed. Prostacyclin analogues have already shown degrees of efficacy in an array of disease states including pulmonary arterial hypertension (PAH), Buerger’s Disease, and critical limb ischemia[28, 31]. More recently, it has been shown that iloprost, a prostacyclin analogue, has the ability to increase circulating endothelial progenitor cells (EPCs) in patients with critical limb ischemia [31]. Following up on data showing improvement in ulcer healing, level of pain, and delayed amputation in previous studies, the authors decided to uncover the potential mechanism behind these clinical benefits. Based on the evidence that iloprost can activate many pro-angiogenic genes, and that clinical benefits in critical limb ischemia come from more than anti-platelet and vasodilatory actions, the authors set out to determine iloprost’s effect on EPCs. The dosing rate for the IV infusion was a median of 1.2 ng/kg/min for 6 hours to prevent hypotension and tachycardia. Of the 23 enrolled patients, 14 had reduced pain and the other 9 had stabilized pain. EPC levels were increased from a median of 13,812/ml pre-treatment versus 23,739/ml post treatment. These results demonstrate that iloprost does seem to increase EPCs, an event to which the authors attributed the positive clinical response as reflected by pain reduction and delayed time to amputation consistent with previous studies. The authors acknowledged that the data are short term, that the data may not correlate with long-term outcomes, and that the population sample was very small. However, given the data supporting the ability of EPCs to promote angiogenesis, longer term trials are certainly warranted to clarify iloprost’s benefit in a variety of vascular conditions [31]. Iloprost is also associated with good tolerability in recent trials involving limb ischemia [31–32]. Concordantly, it seems appropriate to study tolerable doses of iloprost for angiogenic benefits in other patient populations at high risk for vascular disease. Perhaps, future research can focus on the development of an oral or SQ formulation, suitable for outpatient therapy, and investigations regarding that dosage form’s effect on overall atherosclerotic plaque formations in high risk patients. With the mechanistic potential for endothelium preservation and these newfound efficacy data in peripheral ischemic disease, perhaps the next stage is to identify the most tolerable analogs and test them in clinical trials. Circulating endothelial progenitor cells can be indicative of the severity of vascular disease. Increased EPCs of normal functional capacity correlate to less risk for developing atherosclerotic lesions, and EPCs are linked to the regeneration of new blood vessels [5, 31].
Moreover, work by He. et al. [33] has demonstrated that the angiogenic properties of EPCs are dependent on the biosynthesis of prostacyclin. Characterizations of the functions of iloprost on EPC function have helped elucidate some of the mechanisms and pathways by which EPCs function to proliferate and promote angiogenesis [33]. Continuing research along the lines of prostacyclin analogues with regard to EPC function will likely lead to classification of more specific therapeutic targets in EPCs; this should ultimately lead to new drug classes for the prevention and treatment of atherosclerotic vascular diseases.
Ligustrazine derivatives
Recent publications discuss various forms of ligustrazine derivatives as novel compounds for use in the protection of endothelial cells. While these are not endogenous compounds in the body, they deserve attention for anti-oxidant protection of the endothelium. The studies examined the effects of various derivatives of ligustrazine on ECV-304 and HUVEC cells. While ligustrazine possesses a wide variety of pharmacological uses, this compound has been more recently noted to have protective effects on injured vascular endothelial cells [34–35].
The first study evaluated a variety of 2-acyloxymethyl-3, 5, 6-trimethylpyrazine derivatives. Some compounds exhibited the ability to protect HUVEC cells from hydrogen peroxide (oxidative stress) damage, while others stimulated the proliferation of normal cells while being unable to protect the cells from damage.[35]
In the second study, ECV-304 cells were used instead of HUVECs. It was found that one particular derivative produced a greater protective effect against hydrogen peroxide damage of endothelial cells vs. the other derivatives or original ligustrazine. The derivative, which included the bisphenylmethyl group on the methyl position of ligustrazine, provided the lowest EC50 for ECV-304 protection against hydrogen peroxide damage.
Part 3 of these experiments included acylpiperazinyl derivatives. 2-(4-salicyloyl-1-piperazinmethyl)-3,5,6-trimethylpyrazine (compound E33) was determined to have the most beneficial effect, again on ECV-304 cells exposed to hydrogen peroxide[36]. Structure-activity relationships are discussed in each of the respective articles, and biological assays are in progress to further understand the effects of these compounds. These are an interesting class of molecules derived from a traditional Chinese medicine herb, and perhaps may pave the way toward new agents in preserving the integrity and balance of the endothelium. Considering that compounds in each of the 3 studies were found to protect against oxidative stress, coupled with the physiological role of oxidative stress on atherogenesis and NO dysfunction, it is easy to see why these are attractive compounds.
Adiponectin
A recent review has been written by Shibata et al. regarding adiponectin and cardiovascular disease. In summary, adiponectin is a plasma protein derived from adipose tissue. It is typically found in human plasma with concentrations between 3 and 30 mcg/ml. When this protein is inadequate in patients with existing cardiovascular conditions, the result is increased incidence of adverse events [37]. For example, low plasma adiponectin concentration is found in patients with acute coronary syndrome, while higher levels are associated with decreased MI in healthy males. Another example is that higher serum adiponectin levels in patients with PAD correlate with better maximum walking distances and other positive signs. Furthermore, weak association with better cardiovascular outcomes in end-stage-renal patients was shown in a recent Meta analysis. Admittedly, the sum total of clinical evidence still remains to show clear cut benefit of higher adiponectin levels in general cardiovascular diseases[37].
Despite its semi-controversial clinical benefits, adiponectin has shown some evidence in animal models with regard to endothelium preservation and reduced levels of atherosclerosis. Adiponectin has been shown to play roles in angiogenesis, EPC levels, nitric oxide production by endothelial cells, and in the prevention of endothelial apoptosis in an in vitro models[37]. Adiponectin also plays a stabilizing role in the endothelium by decreasing the expression of certain cell adhesion molecules via NFkB suppression. Lastly, its role in endothelium health is further established by its ability to reduce reactive oxygen and reactive nitrogen species (thus preserving NO function) in endothelial cells.
Adiponectin exerts its effects apparently via AMP-activated protein kinase (AMPK). There are various forms of adiponectin as well translating to different physiological effects. Primarily the high molecular weight (HMW) form is responsible for endothelial cell functions while the other trimer form exerts effects more on myocytes [38].
Shear stress and its mechanical stimulation of genetic and cellular targets
There is much argument in favor of the benefits of exercise to reduce atherosclerotic risk. The mechanism behind this is likely related to the concept of shear stress on the endothelium, and how it regulates the function of molecules related to endothelial cell homeostasis. Shear stress is essentially the drag force of blood rushing parallel along the vessel wall. This force is crucial in maintaining the anti-atherogenic state of the endothelium. Shear stress also helps explain why only certain parts of the human vasculature are susceptible to atherosclerotic plaque formation via endothelial cell dysfunction. This susceptibility is from areas of low or oscillatory shear stress which shifts the balance of the endothelium more toward a pro-atherogenic and pro-inflammatory state. Shear stress is associated with the regulation of genes that control endothelial nitric oxide synthetase (eNOS) and thrombomodulin (TM) [39–41]. These two molecules are key players associated with maintaining an anti-coagulant and anti-inflammatory state in the endothelium [15]. Additionally, areas of low shear stress are shown to have lower expression of eNOS and are vulnerable to the development of atherosclerosis [41–42]. Two key transcription factors, Nrf2 and KLF2 have been identified as responsible for about 70% of the expression of endothelial cell genes induced by shear stress [40]. It has been suggested that developing inducers of these molecules may provide the ability to stimulate an anti-inflammatory/anti-coagulant state in areas of low or oscillatory shear stress which are normally prone to atherosclerosis [40–41]. While the physiologic consequences of such inducers are still unknown, development of these compounds can certainly lead to a greater understanding of the pathway and perhaps lead to the development of clinically important compounds.
Sirt-1 genetic modulator
Other methods for regulating the expression of eNOS, and thereby increasing NO output include examination of the Silent information regulator class of proteins (Sirt). Sirt-1 is a deactylating protein covered extensively in a recent review by Potente and Dimmeler [43], as a novel target for maintaining endothelial cell homeostasis. To summarize, this protein is responsible for the regulation of many transcriptional factors including Foxo1, p53, and nuclear factor LXR in hepatocyte. Most notably for the topic at hand, is its regulation of eNOS. Sirt-1 is thought to regulate eNOS expression based on some key pieces of evidence. The administration of resveratrol, which activates Sirt-1, has been shown to also increase the expression of eNOS [43]. In addition, Sirt-1 inhibition in the arterial endothelium inhibits endothelium dependent vasodilation. It has been shown that Sirt-1 promotes the output of NO via deacetylation of eNOS [44]. Furthermore, caloric restriction, which is shown to lower blood pressure in healthy individuals, has been shown to promote the deacetylation of eNOS in mice. Putting this all together, Sirt-1 is a deactylating regulator of the expression of eNOS [43–44]. Sirt-1 may also play roles in vascular growth, removal of cholesterol from peripheral tissues, improvement of insulin sensitivity, and endothelial senescence[43].
Essentially, Sirt-1 has the ability to control risk factors across the board with respect to diabetes, high cholesterol, and aging effects in the body. On the downside (and potential upside), Sirt-1 activity is enhanced in certain tumors, which may be a pitfall to chronic administration of a Sirt-1 activator (yet paves the way for new anti-tumor agents). Consequently, it seems simultaneously attractive yet potentially hazardous as a drug target for disease[43]. While Sirt-1 activity suppresses cancer in breast and colon cells, it appears that Sirt-1 also is responsible for the maintenance of prostate cancer and certain epithelial cell cancers[45]. As noted by Kim and Um, further understanding Sirt-1’s different mechanisms in these cancer cells vs. its anti-aging properties can pave the way to a whole new avenue for targeting atherosclerosis and related chronic diseases[45].
Resolvins and Lipoxins in Endothelial Dysfunction
Endothelial-leukocyte interactions trigger a novel and interesting set of inflammation-resolving, potent lipid mediators known as lipoxins and resolvins. Lipoxins are metabolites of arachidonic acid and resolvins are metabolites of the omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Through a yet not fully understood mechanism, they are known to confer an end to inflammatory processes occurring within the endothelium. Aspirin seems to play a key role in the intercellular interactions leading to the formation of these compounds[46]. For example, the formation of some lipoxins is mediated through aspirin acetylated PGHS-2 in endothelial cells and 5-lipoxygenase in leukocytes [47]. The role of lipoxins, in particular, appears to be centered upon the resolution phase of the inflammatory process, playing an inhibitory role on polymorphonuclear cell adhesion and chemotaxis. Lipoxins tend to inhibit formation of Leukotriene B4(LTB4), a primary mediator of inflammatory cellular processes centered on recruitment of polymorphonuclear cells from post-capillary venules and their adhesion to endothelial cells [46, 48]. Furthermore, lipoxin mediated enhancement of TGF-β serves as a cue to further promote the resolution of fibrosis[46].
During the later stages of the inflammatory process, signals from prostaglandins E2 and D2 trigger the resolution mechanism necessary for the recall of blood granulocytes. The mechanism consists of enhancing the formation of the omega-3 fatty acid derived resolvins through independent EPA and DHA pathways[49]. After an insult, these additional mediators allow for endothelium homeostasis return. In a similar fashion to lipoxin production, resolvin formation is heavily influenced by the role of aspirin mediated acetylation of the COX-2 enzyme [46, 50].
Aspirin-triggered lipoxins when used in an in vivo animal models promote inflammation resolution seemingly by shortening the maximal neutrophil number present during the inflammatory response. Resolvin E1 reduces the maximal neutrophil number and the time when this effect occurs [51]. Both lipoxins and resolvins seem to act through concomitant increase in the tissue repair and wound healing molecule TGF-β. Tissue repair and leukocyte infiltration is of key importance at the endothelial level since macrophage incorporation and subsequent foam cell transformation plays a central role in the pathogenesis of atherosclerotic plaque formation[52]. Given this information, it is speculated that potent lipid mediators, such as lipoxins and resolvins, reduce the process of leukocyte rolling and transmigration and thus potentially attenuate formation of the atherosclerotic plaque on the vascular endothelium.
The role of lipoxins and resolvins has already been evaluated in apoprotein E-deficient mice with global leukocyte 12/15-lipoxygenase deficiency [53]. Expression of the 12/15-lipoxygenase was shown to be a protective mechanism against atherosclerosis, suggesting its lipoxins A4 and resolvin D1 products may be the mediators by which this protection is conferred. In the same study, treatment of human aortic endothelial cells with resolvin D1 led to a significant decrease in MCP-1 and IL-8, and a significant up regulation of the anti-inflammatory compound PDGF-β. Lipoxin A4 in turn was found to down regulate p-selectin expression. In another study[54], diminution of leukocyte transmigration was also found in choroid-retinal endothelial cell and leukocyte co-cultures, where addition of resolvins E1 and resolvin D1 not only decreased the number of leukocyte transmigrating past the endothelial layer, but also diminished IL-8, protein 1β and TNF-α.
Lastly, thrombus formation after endothelial plaque rupture could potentially be attenuated by these compounds. Using intravital microscopy and a murine animal model, Dona et al [55] showed that in vivo administration of resolvin effectively reduces leukocyte rolling by 40%. It was also reported that resolvin E1 inhibits ADP and U46619 activated platelet aggregation in a concentration dependent manner. These effects are indicative of this resolvin’s ability to reduce platelet aggregation and leukocyte specific biochemical and structural processes that allow for homing, rolling, and migration across the endothelium.
Research suggests that resolvin E1 binds with high affinity to the G-protein coupled chemerin receptor (ChemR23) [56–57] present in monocytes, macrophages, dendritic and endothelial cells. Despite recent evidence indicating the presence of ChemR23 receptors on endothelial cells [58], to date no studies have been published directly investigating the relationship between resolvins and Chem23 receptors and effects on the endothelium. In other cells, RvE1 binding counter-regulates TNF-α-stimulated NF-κB activation and enhances macrophage phagocytosis of polymorphonuclear cells via Akt and ribosomal subunit rS6 downstream phosphorylation [59]. Lower binding affinity has also been reported between resolvins and the BLT-1 (leukotriene B4 receptor 1) present on neutrophils. It is possible however that these potent lipid mediators act through a series of receptors and pathways, and that additional targets remain to be identified on the endothelial cell surface.
Taken together, these findings suggest that atherosclerotic plaque formation is affected by a failure of the inflammation resolution mechanism through which potent lipid mediator’s act on vascular endothelial cells to achieve homeostasis. Potent lipid mediators, such as resolvins and lipoxins, may serve as potential novel therapeutic agents to attenuate ongoing inflammatory processes within the vascular endothelium. Even more intriguing, exogenous administration of these and similar potent lipid mediators may even work as alternatives for individuals unable to obtain biochemical benefit from aspirin’s anti-platelet effects. It may also be that the ingestion of EPA and DHA will lead to a sufficient quantity of these potent lipid mediators required for the activation of these mechanisms, at least in most individuals at high risk for cardiovascular disease.
Diagnostic modalities for assessment of endothelial function might include local vasodilation by venous occlusion plethysmography, flow-mediated dilatation, arterial pulse wave analysis, pulse amplitude tonometry, microvascular blood flow by laser Doppler flowmetry, biochemical markers, measurement of endothelial-derived microparticles progenitor cells, and glycocalyx measurements should advance the early detection of vascular endothelial dysfunction before the manifestation of serious adverse vascular or cardiovascular disorders.
Conclusions
The stage is set for the rise of novel, long term risk reduction CVD therapies with regard to the endothelium. Nitrates with supplementary anti-oxidant effects such as PETN are likely ready for human safety trials, and dietary nitrite may also be deserving of such research. These are basic ways of restoring some NO activity, and this logically would have a good chance of reducing the occurrence of long term CVD development.
With regard to thrombomodulin, devising a method for localized expression would open the door to a much greater understanding of its ability as a potential therapy. Again, these attempts may include higher efficiency gene transfer methods, or even less invasive forms of creating reduced flow conditions, i.e. a meshwork (to slow flow) angioplasty device which at the peak of inflation releases a TM gene transfer vehicle. In addition, functional assays and subsequent clinical testing of various soluble thrombomodulin fragments just may open the door to a superior breed of anti-coagulant molecules. TFPI also presents itself as a possible player in this respect. Given the significant risks of thrombosis and re-occlusion in current angioplasty techniques, TFPI can have a role in localized reduction in risk of intimal thickening[23]. Perhaps stents secreting TFPI can prevent the initial TF cascade from inducing SMC proliferation and therefore reduce long-term re-occlusion risk.
The idea of exploring the clinical potential of long term low dose prostacyclin analogues seems plausible as well. Just as ACE inhibitors and ARBs are used to protect the vasculature and renal system in hypertensive states, perhaps prostacyclin can be a well-tolerated protector of those with impaired endothelial function. These analogs along with ligustrazine derivatives may turn out to be molecules of great tolerability that can be used as a long-term hedge against accelerated endothelial cell dysfunction in high risk patients. Furthermore, evidence is growing for direct clinical utility of iloprost in the settings of critical limb ischemia, warranting larger and more controlled trials to assess its efficacy and tolerability.
The elucidation of further drug targets such as Nrf2, KLF2, and Sirt-1 open the door for new compounds to be created as agonists or antagonists of these factors. Animal experimentation involving chemical modulation of these entities may shed further light on the favorability of these pathways for human clinical applications.
This review is an outline of some emerging therapies centered mainly upon preservation of endothelial cell function. With vigilant scientific efforts continually plowing the way forward, perhaps CVD therapy will soon be revolutionized. In time, CVD may no longer be looked at as a treatable condition that is identified after much of its damage is done, but instead as a syndrome preventable through stabilization of the endothelium.
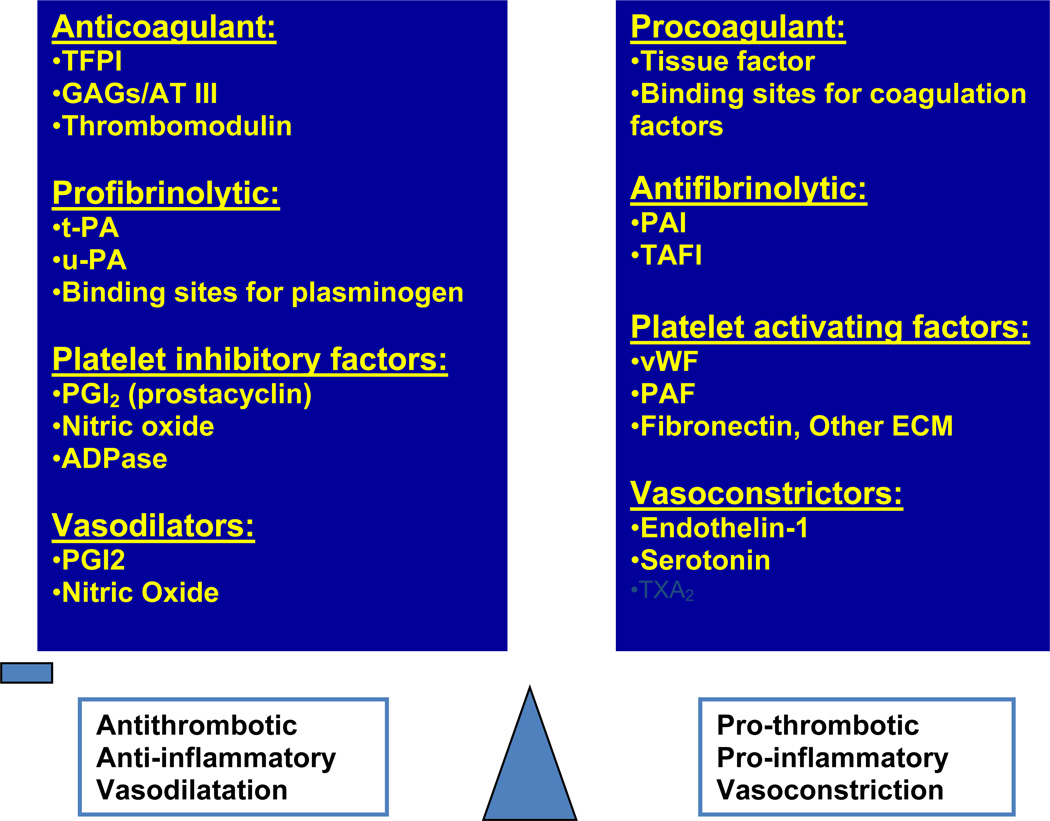
Figure 1.
Hemostasis of vascular endothelium: Endothelium as an organ represents 1–6 × 1013 cells monolayer, weighs 1 kg, covers 6 tennis courts, and it maintains hemostasis of various physiological functions. Modulator of vascular tone via nitric oxide (NO), prostaglandin I2 (PGI2, prostacyclin), endothelin and various receptors, regulator of hemostasis via antithrombotic, pro-thrombotic mediators and modulator of inflammation via pro- and anti-inflammatory mediators maintain endothelial hemostasis.
Acknowledgements
This publication was made possible by Grant Number KL2 RR 024136 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/.
Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
References
- 1.Lloyd-Jones D, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Blann AD. How a damaged blood vessel wall contibutes to thrombosis and hypertenasion. Pathophysiol Haemost Thromb. 2003;33(5–6):445–448. doi: 10.1159/000083843. [DOI] [PubMed] [Google Scholar]
- 3.Esper RJ, et al. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol. 2006;5:4. doi: 10.1186/1475-2840-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steffel J, Luscher TF. Predicting the development of atherosclerosis. Circulation. 2009;119(7):919–921. doi: 10.1161/CIRCULATIONAHA.108.846725. [DOI] [PubMed] [Google Scholar]
- 5.Thum T, et al. Differential effects of organic nitrates on endothelial progenitor cells are determined by oxidative stress. Arterioscler Thromb Vasc Biol. 2007;27(4):748–754. doi: 10.1161/01.ATV.0000258787.18982.73. [DOI] [PubMed] [Google Scholar]
- 6.Dragoni S, et al. Pentaerythrityl tetranitrate and nitroglycerin, but not isosorbide mononitrate, prevent endothelial dysfunction induced by ischemia and reperfusion. Arterioscler Thromb Vasc Biol. 2007;27(9):1955–1959. doi: 10.1161/ATVBAHA.107.149278. [DOI] [PubMed] [Google Scholar]
- 7.Fayers KE, et al. Nitrate tolerance and the links with endothelial dysfunction and oxidative stress. Br J Clin Pharmacol. 2003;56(6):620–628. doi: 10.1046/j.1365-2125.2003.01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daiber A, et al. New insights into bioactivation of organic nitrates, nitrate tolerance and cross-tolerance. Clin Res Cardiol. 2008;97(1):12–20. doi: 10.1007/s00392-007-0588-7. [DOI] [PubMed] [Google Scholar]
- 9.Zahler S, Kupatt C, Becker BF. Endothelial preconditioning by transient oxidative stress reduces inflammatory responses of cultured endothelial cells to TNF-alpha. FASEB J. 2000;14(3):555–564. doi: 10.1096/fasebj.14.3.555. [DOI] [PubMed] [Google Scholar]
- 10.Stokes KY, et al. Dietary Nitrite Prevents Hypercholesterolemic Microvascular Inflammation and Reverses Endothelial Dysfunction. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.01291.2008. [DOI] [PubMed] [Google Scholar]
- 11.Adams TE, Huntington JA. Thrombin-cofactor interactions: structural insights into regulatory mechanisms. Arterioscler Thromb Vasc Biol. 2006;26(8):1738–1745. doi: 10.1161/01.ATV.0000228844.65168.d1. [DOI] [PubMed] [Google Scholar]
- 12.Kapur NK, et al. Hemodynamic modulation of endocardial thromboresistance. Circulation. 2007;115(1):67–75. doi: 10.1161/CIRCULATIONAHA.106.640698. [DOI] [PubMed] [Google Scholar]
- 13.Dittman WA, Majerus PW. Structure and function of thrombomodulin: a natural anticoagulant. Blood. 1990;75(2):329–36. [PubMed] [Google Scholar]
- 14.Weiler H, Isermann BH. Thrombomodulin. J Thromb Haemost. 2003;1(7):1515–1524. doi: 10.1046/j.1538-7836.2003.00306.x. [DOI] [PubMed] [Google Scholar]
- 15.Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein CEPCR system: integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol. 2004;24(8):1374–1383. doi: 10.1161/01.ATV.0000134298.25489.92. [DOI] [PubMed] [Google Scholar]
- 16.Lin JH, et al. Modulation of glycosaminoglycan addition in naturally expressed and recombinant human thrombomodulin. J Biol Chem. 1994;269(40):25021–25030. [PubMed] [Google Scholar]
- 17.Tsiang M, Lentz SR, Sadler JE. Functional domains of membrane-bound human thrombomodulin. EGF-like domains four to six and the serine/threoninerich domain are required for cofactor activity. J Biol Chem. 1992;267(9):6164–6170. [PubMed] [Google Scholar]
- 18.Mangan S, Clancy P, Golledge J. Modulation of endothelial cell thrombomodulin by PPAR ligands--variation according to environment. Thromb Res. 2008;121(6):827–834. doi: 10.1016/j.thromres.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabuchi N, et al. Non-viral in vivo thrombomodulin gene transfer prevents early loss of thromboresistance of grafted veins. Eur J Cardiothorac Surg. 2004;26(5):995–1001. doi: 10.1016/j.ejcts.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Li J, et al. Recombinant thrombomodulin inhibits arterial smooth muscle cell proliferation induced by thrombin. J Vasc Surg. 2000;32(4):804–813. doi: 10.1067/mva.2000.107992. [DOI] [PubMed] [Google Scholar]
- 21.Ohlin AK, Larsson K, Hansson M. Soluble thrombomodulin activity and soluble thrombomodulin antigen in plasma. J Thromb Haemost. 2005;3(5):976–982. doi: 10.1111/j.1538-7836.2005.01267.x. [DOI] [PubMed] [Google Scholar]
- 22.Van de Wouwer M, et al. The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. J Thromb Haemost. 2006;4(8):1813–1824. doi: 10.1111/j.1538-7836.2006.02033.x. [DOI] [PubMed] [Google Scholar]
- 23.Sajadi S, et al. Tissue factor pathway inhibitors as a novel approach to antithrombotic therapy. Drug News Perspect. 2003;16(6):363–369. doi: 10.1358/dnp.2003.16.6.829308. [DOI] [PubMed] [Google Scholar]
- 24.Kothari H, et al. Plasmin enhances cell surface tissue factor activity in mesothelial and endothelial cells. J Thromb Haemost. 2009;7(1):121–131. doi: 10.1111/j.1538-7836.2008.03218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Undas A, et al. Tissue factor +5466A>G polymorphism determines thrombin formation following vascular injury and thrombin-lowering effects of simvastatin in patients with ischemic heart disease. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Paradis ME, et al. Endothelial lipase is associated with inflammation in humans. J Lipid Res. 2006;47(12):2808–2813. doi: 10.1194/jlr.P600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed W, et al. High-density lipoprotein hydrolysis by endothelial lipase activates PPARalpha: a candidate mechanism for high-density lipoprotein-mediated repression of leukocyte adhesion. Circ Res. 2006;98(4):490–498. doi: 10.1161/01.RES.0000205846.46812.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gryglewski RJ. Prostacyclin among prostanoids. Pharmacol Rep. 2008;60(1):3–11. [PubMed] [Google Scholar]
- 29.Britten MB, Zeiher AM, Schachinger V. Clinical importance of coronary endothelial vasodilator dysfunction and therapeutic options. J Intern Med. 1999;245(4):315–327. doi: 10.1046/j.1365-2796.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- 30.Morrow JD, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332(18):1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 31.Di Stefano R, et al. The prostacyclin analogue iloprost increases circulating endothelial progenitor cells in patients with critical limb ischemia. Thromb Haemost. 2008;100(5):871–877. [PubMed] [Google Scholar]
- 32.de Donato G, et al. The ILAILL study: iloprost as adjuvant to surgery for acute ischemia of lower limbs: a randomized, placebo-controlled, double-blind study by the italian society for vascular and endovascular surgery. Ann Surg. 2006;244(2):185–193. doi: 10.1097/01.sla.0000217555.49001.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He T, et al. Angiogenic function of prostacyclin biosynthesis in human endothelial progenitor cells. Circ Res. 2008;103(1):80–88. doi: 10.1161/CIRCRESAHA.108.176057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng XC, et al. Design, synthesis, and biological activities of novel Ligustrazine derivatives. Bioorg Med Chem. 2007;15(10):3315–3320. doi: 10.1016/j.bmc.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, et al. Synthesis of the novel liqustrazine derivatives and Their protective effect on injured vascular endothelial cell damaged by hydrogen peroxide. Bioorg Med Chem Lett. 2003;13(13):2123–2126. doi: 10.1016/s0960-894x(03)00359-7. [DOI] [PubMed] [Google Scholar]
- 36.Cheng XC, et al. Ligustrazine derivatives. Part 3: Design, synthesis and evaluation of novel acylpiperazinyl derivatives as potential cerebrocardiac vascular agents. Bioorg Med Chem. 2009 doi: 10.1016/j.bmc.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Shibata R, Ouchi N, Murohara T. Adiponectin and cardiovascular disease. Circ J. 2009;73(4):608–614. doi: 10.1253/circj.cj-09-0057. [DOI] [PubMed] [Google Scholar]
- 38.Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol. 2007;27(5):996–1003. doi: 10.1161/ATVBAHA.106.131755. [DOI] [PubMed] [Google Scholar]
- 39.van Thienen JV, et al. Shear stress sustains atheroprotective endothelial KLF2 expression more potently than statins through mRNA stabilization. Cardiovasc Res. 2006;72(2):231–240. doi: 10.1016/j.cardiores.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Boon RA, Horrevoets AJ. Key transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie. 2009;29(1):39–40. 41–43. [PubMed] [Google Scholar]
- 41.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85(1):9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 42.Zarins CK, et al. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53(4):502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 43.Potente M, Dimmeler S. Emerging roles of SIRT1 in vascular endothelial homeostasis. Cell Cycle. 2008;7(14):2117–2122. doi: 10.4161/cc.7.14.6267. [DOI] [PubMed] [Google Scholar]
- 44.Mattagajasingh I, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104(37):14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim EJ, Um SJ. SIRT1: roles in aging and cancer. BMB Rep. 2008;41(11):751–756. doi: 10.5483/bmbrep.2008.41.11.751. [DOI] [PubMed] [Google Scholar]
- 46.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 47.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92(21):9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yokomizo T, Izumi T, Shimizu T. Leukotriene B4: metabolism and signal transduction. Arch Biochem Biophys. 2001;385(2):231–241. doi: 10.1006/abbi.2000.2168. [DOI] [PubMed] [Google Scholar]
- 49.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 50.Serhan CN, et al. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39(11):1125–1132. doi: 10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- 51.Bannenberg GL, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174(7):4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 52.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 53.Merched AJ, et al. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22(10):3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian H, et al. Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: biosynthesis and mechanisms of anti-inflammatory actions. Invest Ophthalmol Vis Sci. 2009;50:3613–3620. doi: 10.1167/iovs.08-3146. [DOI] [PubMed] [Google Scholar]
- 55.Dona M, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112(3):848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arita M, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201(5):713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arita M, et al. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178(6):3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 58.Kaur J, et al. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun. 2010;391(4):1762–1768. doi: 10.1016/j.bbrc.2009.12.150. [DOI] [PubMed] [Google Scholar]
- 59.Ohira T, et al. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2010;285(5):3451–3461. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Favre J, Gao J, Henry JP, Remy-Jouet I, Fourquaux I, Billon-Gales A, Thuillez C, Arnal JF, Lenfant F, Richard V. Endothelial estrogen receptor {alpha} plays an essential role in the coronary and myocardial protective effects of estradiol in ischemia/reperfusion. Arterioscler Thromb Vasc Biol. 2010 Dec;30(12):2562–2567. doi: 10.1161/ATVBAHA.110.213637. [DOI] [PubMed] [Google Scholar]