Abstract
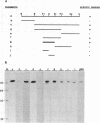
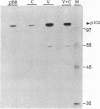
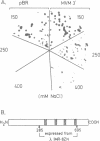
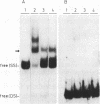
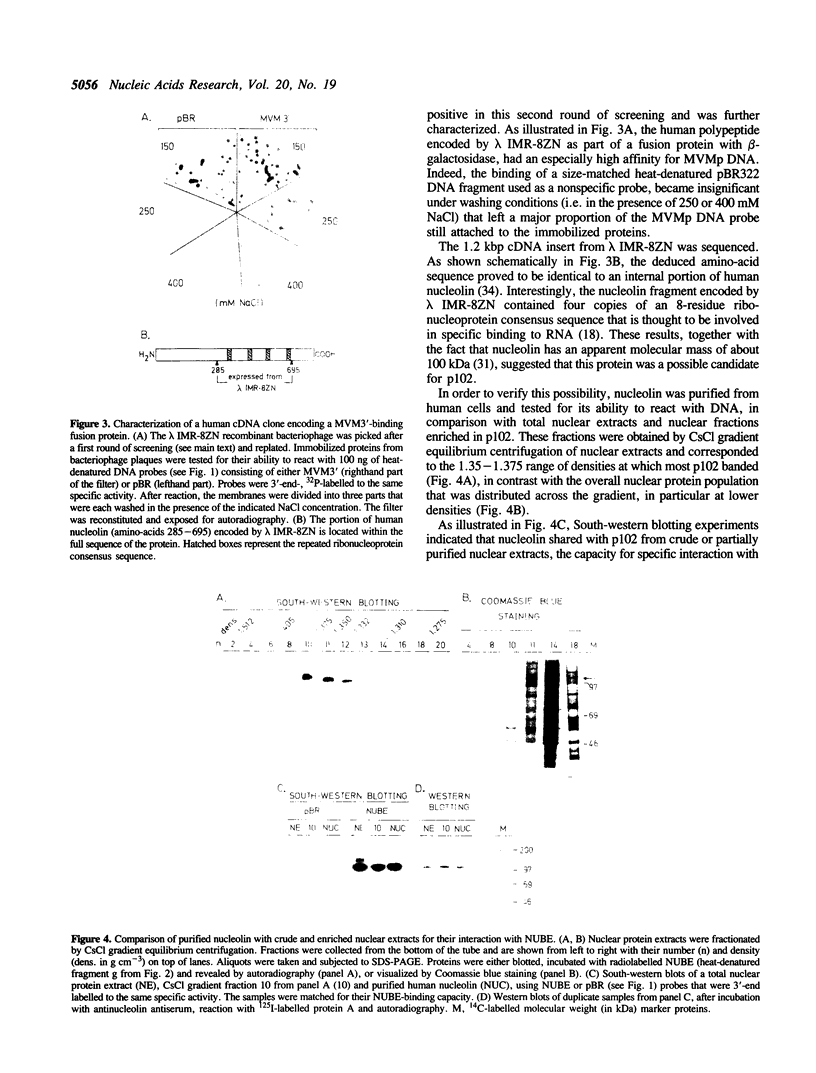
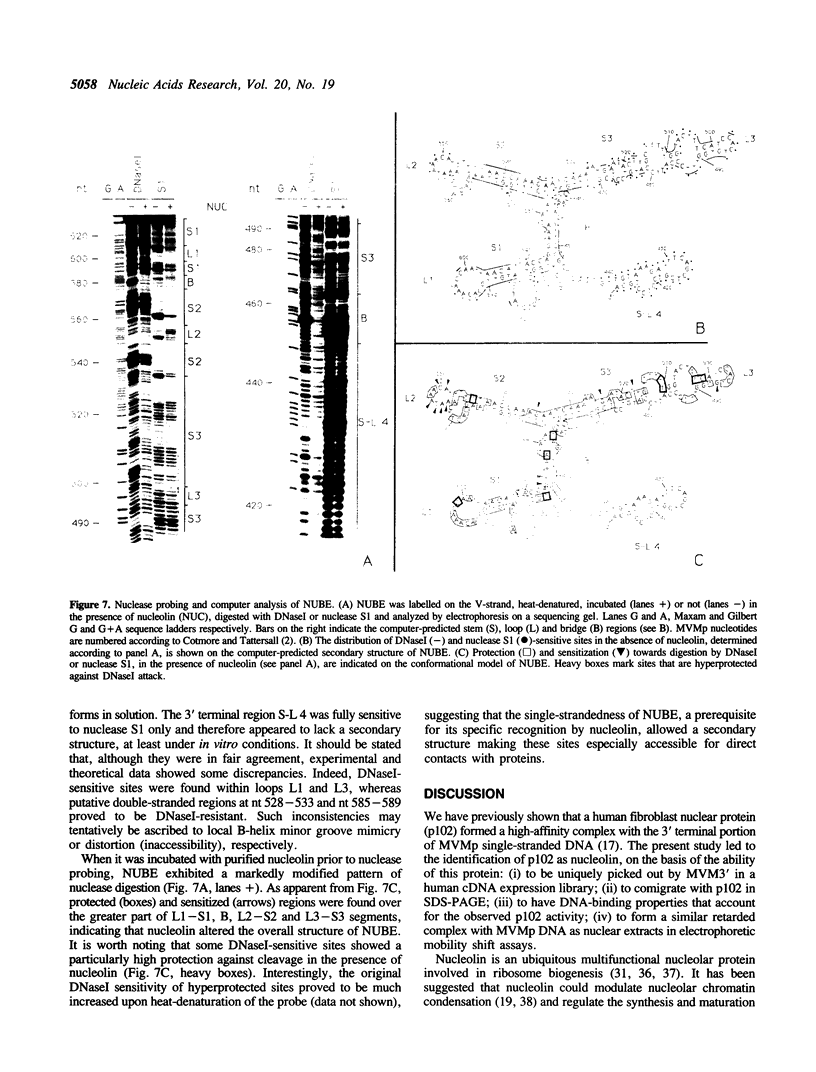
Nucleolin, a major nucleolar protein, forms a specific complex with the genome (a single-stranded DNA molecule of minus polarity) of parvovirus MVMp in vitro. By means of South-western blotting experiments, we mapped the binding site to a 222-nucleotide motif within the non-structural transcription unit, referred to as NUBE (nucleolin-binding element). The specificity of the interaction was confirmed by competitive gel retardation assays. DNaseI and nuclease S1 probing showed that NUBE folds into a secondary structure, in agreement with a computer-assisted conformational prediction. The whole NUBE may be necessary for the interaction with nucleolin, as suggested by the failure of NUBE subfragments to bind the protein and by the nuclease footprinting experiments. The present work extends the previously reported ability of nucleolin to form a specific complex with ribosomal RNA, to a defined DNA substrate. Considering the tropism of MVMp DNA replication for host cell nucleoli, these data raise the possibility that nucleolin may contribute to the regulation of the parvoviral life-cycle.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn J. K., Gavin B. J., Kumar G., Ward D. C. Transcriptional analysis of minute virus of mice P4 promoter mutants. J Virol. 1989 Dec;63(12):5425–5439. doi: 10.1128/jvi.63.12.5425-5439.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashktorab H., Srivastava A. Identification of nuclear proteins that specifically interact with adeno-associated virus type 2 inverted terminal repeat hairpin DNA. J Virol. 1989 Jul;63(7):3034–3039. doi: 10.1128/jvi.63.7.3034-3039.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalosse B. L., Barrijal S., Chen Y. Q., Cassiman J. J., Rommelaere J. Identification of a transformation-sensitive nuclear protein from normal human fibroblasts that specifically interacts with minute virus of mice DNA and correlates with cell resistance to the parvovirus. Mol Carcinog. 1989;2(5):245–251. doi: 10.1002/mc.2940020504. [DOI] [PubMed] [Google Scholar]
- Belenguer P., Baldin V., Mathieu C., Prats H., Bensaid M., Bouche G., Amalric F. Protein kinase NII and the regulation of rDNA transcription in mammalian cells. Nucleic Acids Res. 1989 Aug 25;17(16):6625–6636. doi: 10.1093/nar/17.16.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenguer P., Caizergues-Ferrer M., Labbé J. C., Dorée M., Amalric F. Mitosis-specific phosphorylation of nucleolin by p34cdc2 protein kinase. Mol Cell Biol. 1990 Jul;10(7):3607–3618. doi: 10.1128/mcb.10.7.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I. Parvovirus replication. Microbiol Rev. 1990 Sep;54(3):316–329. doi: 10.1128/mr.54.3.316-329.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell M. C., Astell C. R. A GC-box motif upstream of the B19 parvovirus unique promoter is important for in vitro transcription. J Virol. 1989 Nov;63(11):4814–4823. doi: 10.1128/jvi.63.11.4814-4823.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche G., Caizergues-Ferrer M., Bugler B., Amalric F. Interrelations between the maturation of a 100 kDa nucleolar protein and pre rRNA synthesis in CHO cells. Nucleic Acids Res. 1984 Apr 11;12(7):3025–3035. doi: 10.1093/nar/12.7.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche G., Gas N., Prats H., Baldin V., Tauber J. P., Teissié J., Amalric F. Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0----G1 transition. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6770–6774. doi: 10.1073/pnas.84.19.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbon H., Bugler B., Caizergues-Ferrer M., Amalric F. Role of phosphorylation on the maturation pathways of a 100 kDa nucleolar protein. FEBS Lett. 1983 May 8;155(2):218–222. doi: 10.1016/0014-5793(82)80606-6. [DOI] [PubMed] [Google Scholar]
- Bugler B., Bourbon H., Lapeyre B., Wallace M. O., Chang J. H., Amalric F., Olson M. O. RNA binding fragments from nucleolin contain the ribonucleoprotein consensus sequence. J Biol Chem. 1987 Aug 15;262(23):10922–10925. [PubMed] [Google Scholar]
- Bugler B., Caizergues-Ferrer M., Bouche G., Bourbon H., Amalric F. Detection and localization of a class of proteins immunologically related to a 100-kDa nucleolar protein. Eur J Biochem. 1982 Nov 15;128(2-3):475–480. doi: 10.1111/j.1432-1033.1982.tb06989.x. [DOI] [PubMed] [Google Scholar]
- Chang L. S., Shi Y., Shenk T. Adeno-associated virus P5 promoter contains an adenovirus E1A-inducible element and a binding site for the major late transcription factor. J Virol. 1989 Aug;63(8):3479–3488. doi: 10.1128/jvi.63.8.3479-3488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow M., Bodnar J. W., Polvino-Bodnar M., Ward D. C. Identification and characterization of a protein covalently bound to DNA of minute virus of mice. J Virol. 1986 Mar;57(3):1094–1104. doi: 10.1128/jvi.57.3.1094-1104.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Detera S. D., Becerra S. P., Swack J. A., Wilson S. H. Studies on the mechanism of DNA polymerase alpha. Nascent chain elongation, steady state kinetics, and the initiation phase of DNA synthesis. J Biol Chem. 1981 Jul 10;256(13):6933–6943. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erard M. S., Belenguer P., Caizergues-Ferrer M., Pantaloni A., Amalric F. A major nucleolar protein, nucleolin, induces chromatin decondensation by binding to histone H1. Eur J Biochem. 1988 Aug 15;175(3):525–530. doi: 10.1111/j.1432-1033.1988.tb14224.x. [DOI] [PubMed] [Google Scholar]
- FRESCO J. R., ALBERTS B. M., DOTY P. Some molecular details of the secondary structure of ribonucleic acid. Nature. 1960 Oct 8;188:98–101. doi: 10.1038/188098a0. [DOI] [PubMed] [Google Scholar]
- Forgac M. D. Characterization of a Mg2+-stabilized state of the (Na+ and K+)--stimulated adenosine triphosphatase using a fluorescent reporter group. J Biol Chem. 1980 Feb 25;255(4):1547–1553. [PubMed] [Google Scholar]
- Gaillard C., Strauss F. Sequence-specific single-strand-binding protein for the simian virus 40 early promoter stimulates transcription in vitro. J Mol Biol. 1990 Sep 20;215(2):245–255. doi: 10.1016/S0022-2836(05)80343-2. [DOI] [PubMed] [Google Scholar]
- Gas N., Escande M. L., Stevens B. J. Immunolocalization of the 100 kDa nucleolar protein during the mitotic cycle in CHO cells. Biol Cell. 1985;53(3):209–218. doi: 10.1111/j.1768-322x.1985.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Gu M. L., Rhode S. L. Autonomous parvovirus DNA replication requires topoisomerase I and its activity is increased during infection. J Virol. 1991 Mar;65(3):1662–1665. doi: 10.1128/jvi.65.3.1662-1665.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A. H., Olson M. O. Association of protein C23 with rapidly labeled nucleolar RNA. Biochemistry. 1986 Oct 7;25(20):6258–6264. doi: 10.1021/bi00368a063. [DOI] [PubMed] [Google Scholar]
- Herrick G., Alberts B. Purification and physical characterization of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2124–2132. [PubMed] [Google Scholar]
- Im D. S., Muzyczka N. Factors that bind to adeno-associated virus terminal repeats. J Virol. 1989 Jul;63(7):3095–3104. doi: 10.1128/jvi.63.7.3095-3104.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan G. At the heart of the nucleolus. Nature. 1987 Oct 8;329(6139):489–490. doi: 10.1038/329489a0. [DOI] [PubMed] [Google Scholar]
- Kharrat A., Derancourt J., Dorée M., Amalric F., Erard M. Synergistic effect of histone H1 and nucleolin on chromatin condensation in mitosis: role of a phosphorylated heteromer. Biochemistry. 1991 Oct 22;30(42):10329–10336. doi: 10.1021/bi00106a034. [DOI] [PubMed] [Google Scholar]
- Krauskopf A., Resnekov O., Aloni Y. A cis downstream element participates in regulation of in vitro transcription initiation from the P38 promoter of minute virus of mice. J Virol. 1990 Jan;64(1):354–360. doi: 10.1128/jvi.64.1.354-360.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merchlinsky M. J., Tattersall P. J., Leary J. J., Cotmore S. F., Gardiner E. M., Ward D. C. Construction of an infectious molecular clone of the autonomous parvovirus minute virus of mice. J Virol. 1983 Jul;47(1):227–232. doi: 10.1128/jvi.47.1.227-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf J. B., Bates R. C., Lederman M. Interaction of virally coded protein and a cell cycle-regulated cellular protein with the bovine parvovirus left terminus ori. J Virol. 1990 Nov;64(11):5485–5490. doi: 10.1128/jvi.64.11.5485-5490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. O., Rivers Z. M., Thompson B. A., Kao W. Y., Case S. T. Interaction of nucleolar phosphoprotein C23 with cloned segments of rat ribosomal deoxyribonucleic acid. Biochemistry. 1983 Jul 5;22(14):3345–3351. doi: 10.1021/bi00283a007. [DOI] [PubMed] [Google Scholar]
- Orrick L. R., Olson M. O., Busch H. Comparison of nucleolar proteins of normal rat liver and Novikoff hepatoma ascites cells by two-dimensional polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1973 May;70(5):1316–1320. doi: 10.1073/pnas.70.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Cornelis J. J. Antineoplastic activity of parvoviruses. J Virol Methods. 1991 Aug;33(3):233–251. doi: 10.1016/0166-0934(91)90024-t. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp M., Knippers R., Richter A. DNA binding properties of a 110 kDa nucleolar protein. Nucleic Acids Res. 1986 Sep 11;14(17):6803–6820. doi: 10.1093/nar/14.17.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp M., Richter A., Weisshart K., Caizergues-Ferrer M., Amalric F., Wallace M. O., Kirstein M. N., Olson M. O. Characterization of a 48-kDa nucleic-acid-binding fragment of nucleolin. Eur J Biochem. 1989 Feb 15;179(3):541–548. doi: 10.1111/j.1432-1033.1989.tb14581.x. [DOI] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Srivastava M., Fleming P. J., Pollard H. B., Burns A. L. Cloning and sequencing of the human nucleolin cDNA. FEBS Lett. 1989 Jun 19;250(1):99–105. doi: 10.1016/0014-5793(89)80692-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton T. H., Moen P. T., Jr, Fox E., Bodnar J. W. Interactions of minute virus of mice and adenovirus with host nucleoli. J Virol. 1989 Sep;63(9):3651–3660. doi: 10.1128/jvi.63.9.3651-3660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalkinoglu A. O., Zentgraf H., Hübscher U. Origin of adeno-associated virus DNA replication is a target of carcinogen-inducible DNA amplification. J Virol. 1991 Jun;65(6):3175–3184. doi: 10.1128/jvi.65.6.3175-3184.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]