Abstract
Is an attenuated physiological response to family conflict, seen in some youth exposed to early adversity, protective or problematic? A longitudinal study including 54 youth (average age 15.2) found that those with higher cumulative family aggression exposure showed lower cortisol output during a laboratory-based conflict discussion with their parents, and were less likely to show the normative pattern of increased cortisol reactivity to a discussion they rated as more conflictual. Family aggression interacted with cortisol reactivity in predicting youth adjustment: adolescents from more aggressive homes who were also more reactive to the discussion reported more post-traumatic stress symptoms and more antisocial behavior. These results suggest that attenuated reactivity may protect youth from the negative consequences associated with aggressive family environments.
Keywords: cortisol, HPA axis, family conflict, childhood adversity, antisocial behavior
How does a chronically aggressive family environment shape the development of children’s stress response systems? Children reared in harsh family environments have been found to show both heightened and dampened profiles of cortisol, a stress hormone secreted by the hypothalamic-pituitary-adrenal (HPA) axis that has been implicated in physical and mental health. For example, among adults with clinical disorders like depression and anxiety, past childhood adversity has been tied to elevated baseline cortisol and exaggerated stress reactivity (e.g. De Bellis et al., 1999; Elzinga, Spinhoven, Berretty, Jong, & Roelofs, 2010; Harkness, Stewart, & Wynne-Edwards, 2010). However, in studies of both children and adults without significant psychological symptoms, early life stress has been more often associated with low baseline cortisol and attenuated reactivity (e.g., Carpenter et al., 2007; Elzinga, et al., 2008; Granger, Serbin, Lehoux, Cooperman, & Ikeda, 1998; Luecken, Kraft, & Hagan, 2009; Roisman et al., 2009). Low cortisol, in turn, has been linked with externalizing problems (e.g. McBurnett, Lahey, Rathouz, & Loeber, 2000; Susman, 2006). A study comparing children exposed to severe versus moderate degrees of early life stress found that children with more severe stress exposure showed heightened, and those with moderate stress exposure diminished, reactivity to a laboratory stressor (Gunnar, Frenn, Wewerka, & Van Ryzin, 2009). The present study explores how a history of aggressive family behavior affects adolescents’ physiological responses to a lab-based family conflict discussion.
This variability in regulation of the HPA axis suggests that different pathways of risk and resilience may exist for children in adverse family environments (Gunnar, et al., 2009). For example, an attenuated physiological response to family conflict might protect children in chronically aggressive families. Researchers have suggested that children’s emotional and physiological reactivity to conflict might moderate associations between adverse early environments and later problems (Davies & Cummings, 1994; Steinberg & Avenovoli, 2000). In one study, children better able to regulate their vagal response to a simulated argument were less likely to develop adjustment problems in the context of chronic marital discord than children whose vagal response was less regulated (El-Sheikh & Whitson, 2006). Other studies have found interactions between physiological reactivity and family environments in predicting youth outcomes. For example, a study of kindergarteners found family adversity to be linked with poor social functioning, but only among children who were particularly physiologically reactive (as indexed by vagal and cortisol measures; Obradović, Bush, Stamperdahl, Adler, & Boyce, 2010). These studies are consistent with the theory of “biological sensitivity to context” (Boyce & Ellis, 2005): youth who are physiologically sensitive to their environments may be more vulnerable to compromised environments (and, conversely, might benefit more from salutary environments) than their less reactive peers. The current study uses a longitudinal design to explore how aggressive family environments affect HPA axis responses to laboratory-based family conflict, and whether family history interacts with HPA functioning in predicting youths’ well-being.
Family discord is a commonly occurring stressor in many children’s lives, one that has well-documented, deleterious effects on children’s well-being (Repetti, Taylor, & Seeman, 2002). Although many studies of aversive family behavior focus on either marital or parent-child conflict, studying family aggression across multiple domains may reveal additive effects. We use a cumulative aggression exposure index created by summing across multiple reporters, waves of data collection, and domains (father-to-mother, mother-to-father, father-to-child, and mother-to-child). An earlier investigation using the same sample of youth (Margolin, Vickerman, Oliver, & Gordis, 2010) found that a similarly constructed index had a dose-response relation with outcomes including academic failure, depression, and somatic complaints. Similarly, while many laboratory conflict paradigms have focused either on youth witnessing a marital argument (e.g. Davies, Sturge-Apple, Cicchetti & Cummings, 2007) or on conflict with a single parent (e.g. Granger et al., 1998), we engaged youth in a triadic conflict task that included both parents and the child.
This study employs a diverse sample of youth in early-to-mid adolescence, a key time for social development. Our longitudinal design allows us to examine whether family aggression over three waves of data collection predicts cortisol responding and youth outcomes in a subsequent wave.
Hypothesis 1
Given that we focus on a sample without significant psychopathology, we expect a history of family aggression to be associated with attenuated, rather than exaggerated, cortisol responding to a triadic conflict discussion. We will examine whether youths’ ratings of conflict during the discussion task interact with family aggression to predict cortisol responses.
Hypothesis 2
We expect that greater family aggression will predict more adverse youth outcomes. We focus on two outcomes: post-traumatic stress (PTS) symptoms, a potential indicator of the psychological cost of family aggression and violence, and antisocial behavior, which may reflect youths’ externalizing of aggressive family behavior. We will examine the possible interaction of cortisol reactivity with family aggression, with the expectation that attenuated cortisol may be risk-protective in more aggression-exposed youth.
Methods
Participants
We focus on 54 adolescents (29 boys, 25 girls) who participated in a laboratory-based conflict discussion with both parents as part of Wave 4 of a multi-wave study examining family aggression and child development. Families, recruited through advertisements and referrals, met the following criteria: parents had lived together for the past 3 years, had a co-resident child aged 9–10 years, and were able to complete measures in English. Study waves were spaced 1–3 years apart; Wave 4 data collection took place an average of six years after Wave 1. (See Margolin et al., 2010 for further details.) In Wave 1, 119 families participated. In Wave 2 and Wave 3, 102 and 101 families participated respectively. In Wave 4, 84 families participated in the discussion task, but only 73 families provided saliva samples. Of these, 14 had only one parent participating, two were dropped from analyses due to possible blood contamination of the youth’s saliva, and three youth were dropped for cortisol levels that were consistently out of range (each level >3 SDs above the mean value for each timepoint), a common approach with extreme cortisol values (e.g. Dettling, Gunnar, & Donzella, 1999).
Participant characteristics and measure descriptives are presented in Table 1. Youth identified as Hispanic/Latino (35.2% of participating youth), Caucasian (27.8%), African-American (18.5%), Asian (11.1%), and Multi-ethnic/Other (7.4%). Most of the participating youth (96%) came into the lab with biological parents. Compared to the full sample of youth eligible in Wave 4, families included in this study did not differ from the larger sample in age, gender, ethnicity, parents’ age or education, or family income, except that parents were more likely to be biologically related to the youth, t(116) = 2.56, p = .01).
Table 1.
Sample Characteristics and Descriptives
| Variable | M | SD | Range |
|---|---|---|---|
| Age | |||
| Youth | 15.2 | 0.8 | 13.8–18.6 |
| Mother | 45.1 | 5.2 | 33.8–54.5 |
| Father | 47.0 | 6.2 | 36.8–61.8 |
| Years of education (both parents averaged) | 14.4 | 2.4 | 9–20 |
| Family income, Wave 1 | $72,784 | $39,580 | $0–$165,000 |
| Pubertal status (sum of mothers’ 0–4 rating of growth spurt, skin changes, body hair) | 8.9 | 1.3 | 6–12 |
| Youth rating of conflict discussion | |||
| Conflict with mother | 1.7 | 1.1 | 0–4 |
| Conflict with father | 1.6 | 0.9 | 0–3 |
| Conflict with both parents (sum) | 3.3 | 1.7 | 0–6 |
| Youth PTS symptoms | 9.4 | 7.1 | 0–28 |
| Youth antisocial behavior | 6.8 | 7.2 | 0–38 |
| Youth cortisol indices | |||
| AUCg | 368.96 | 149.32 | 44.34–802.32 |
| AUCi | −23.56 | 121.86 | −268.29−241.92 |
| Baseline sample | .083 | .045 | .013–.27 |
| Peak cortisol | .100 | .055 | .009–.35 |
| Peak minus baseline | .017 | .053 | −.06–.27 |
Procedures
At Wave 4, families visited the lab for a 3–4 hour visit. All visits took place between 11:15 am and 7:15 pm, with a mean start time of 1:55 pm (SD = 2 hours). Families were instructed not to eat or smoke for an hour before their visit and not to consume alcohol or caffeine for 24 hours. Compliance was assessed both through verbal questioning and a questionnaire asking about medications, food, and beverages consumed within the previous 24 hours. One family had eaten within an hour of their visit and saliva collection was postponed to allow an hour interval after eating. Five saliva samples were collected: at baseline (0 minutes), immediately post-discussion (+ 40 min), and at three post-discussion timepoints (+ 50 min, + 60 min, and + 80 min). Since cortisol levels peak approximately 20–30 minutes after onset of an acute stressor (Kirschbaum & Hellhammer, 1989), these intervals were chosen to capture the full cortisol response including the beginning of recovery. Experimenters set timers to ensure consistency.
After consent procedures and background questionnaires (e.g., saliva compliance information), families were shown a 10-minute nature video with calming music and images. The baseline saliva sample was collected after this relaxation induction. Next followed the conflict discussion task. Families filled out a questionnaire to identify the most heated areas of conflict within the family and participated in 5-minute individual priming interviews designed to hone in on each person’s unique perspective of the hot topics and help that person formulate her or his side of the issue. These interviews took place concurrently, with each family member in a separate room with an experimenter. Families were then seated together and given 15 minutes to discuss at least one of the three topics, starting with the most conflictual. Families were instructed to discuss the topic as they would at home, and to “make sure that each of you gets your points across.” Immediately after the discussion, family members provided the second saliva sample and completed the Post-Discussion Questionnaire.
Measures
Post-Discussion Questionnaire
Youth responded to items “How much conflict did you experience with your mom?” and “How much conflict did you experience with your dad?” on a scale from 0 (“None”) to 4 (“A Lot”). In order to approximate the overall amount of conflict experienced by the youth during the task, these ratings, which were modestly correlated, r(53) = .35, p = .01, were averaged (the results reported in this paper were unchanged when we examined conflict with each parent separately in a follow-up analysis).
Cumulative family aggression exposure
We created a cumulative family aggression variable that combined spousal aggression ratings (from the Domestic Conflict Index; Margolin, John, & Foo, 1998) and parent-child conflict ratings (from the Conflict Tactics Scale – Parent/Child; Straus, Hamby, & Finkelhor, 1998), over the first three waves of the study. For each measure, we focused on a subset of items (36 in total) assessing particularly aversive, physically aggressive behavior within the past year, such as shaking or slapping the child, twisting the spouse’s arm, and shoving the spouse (see Margolin et al., 2010, for more details). Three reporters (mother, father, and youth) reported on these behaviors at each wave. If any of the aversive conflict behaviors were endorsed by any reporter, we assigned a score of ‘1’; if not, we assigned a ‘0’. Maximizing across reporters helps adjust for the under-reporting biases often seen in family conflict studies. Agreement between reporters ranged from 60%–84% depending on the domain (father-to-mother and mother-to-father spousal aggression; mother-to-child and father-to-child parental aggression).
Family aggression was positively correlated across waves (correlations ranging from .44 to .66, p values all < .01) and across domains (ranging from .23–.66, p values ranging from .00 to .09). After dichotomously coding each wave (1, 2, or 3) and each domain, we summed all of the waves and domains together to create a composite score. The composite family aggression variable had a possible range of 0 (no aggression reported by any reporter in any wave) to 12 (aggression reported by at least one family member in every domain and at every wave). The variable was normally distributed, with both a median and mode of 5 and a mean score of 5.20 (SD = 2.98). Three families reported zero aggression across all waves; 1 family reported aggression within each domain over all three waves.
Post-traumatic stress (PTS) symptoms
At Wave 4, youth completed the 17-item Youth Symptom Severity Checklist (Margolin, 1999), which assesses PTS symptoms among adolescents, including questions pertaining to each of the DSM-IV symptom clusters (re-experiencing, numbness, and hyperarousal). Youth rated whether they had, for example, felt “irritable,” “more upset than you wanted to be, when you were reminded of something bad that happened,” or “worried about being safe” from 0 (“Not At All”) to 3 (“Almost Always”). Cronbach’s alpha in this sample = .88.
Antisocial behavior
At Wave 4, youth completed the 40-item Self-Reported Antisocial Behavior Scale (SRA; Loeber, Farrington, Stouthamer-Loeber, & Van Kämmen, 1998), adapted for younger adolescents from the Self-Reported Delinquency Scale (SRD; Elliot, Huizinga, & Ageton, 1985). Both scales demonstrate good test-retest reliability and have been validated against crime and gang participation statistics (e.g. Gordon et al., 2004). Youth rated the frequency of behaviors such as cheating on a test, stealing or damaging property, physical fighting, and skipping school from 0 (“Never”) to 2 (“3 or More Times”). Cronbach’s alpha in this sample = .89.
Measurement of cortisol
Saliva samples were analyzed by Salimetrics, LLC, using an enzyme immunoassay with a lower limit of sensitivity of 0.003 μg/dl, and intra-assay and inter-assay coefficients of variation of 3.5% and 5.1%, respectively. Each saliva sample was assayed twice, and analyses were repeated if any pair of results differed by > 7%.
Four indices of cortisol output related to the conflict task were calculated: peak cortisol (each individual’s highest cortisol level, post-baseline); peak minus baseline; area under the curve with respect to ground (AUCg), a measure of total cortisol secretion that includes baseline value; and area under the curve with respect to increase (AUCi), a measure of reactivity to the task including cortisol output minus the baseline value to approximate task-related change in cortisol. Peak cortisol was correlated with AUCg, r(53) = .71, p = .00, and peak cortisol minus baseline was associated with AUCi, r(53) = .83, p = .00. Both AUCg and AUCi were calculated using the trapezoidal formulae described by Pruessner, Kirschbaum, Meinlschmid, and Hellhammer (2003), and both appeared normally distributed and did not require log transformation. Although acute stress tasks are typically expected to produce positive AUCi values, or cortisol increases over baseline, mean negative AUCi values have been reported in other family conflict studies (e.g., Davies et al., 2007; Granger et al., 1998). Because cortisol declines over the course of the day, negative AUCi scores are consistent with the normal functioning of the HPA axis.
Results
Table 1 presents descriptives for youth cortisol values (AUCi, AUCg, baseline and peak cortisol) and Table 2 presents zero-order correlations between all measures used in the final analyses.
Table 2.
Intercorrelations Between Study Variables
| Measure | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. Youth AUCg | _ | ||||
| 2. Youth AUCi | −.04 | _ | |||
| 3. Youth discussion rating (conflict) | .04 | .12 | _ | ||
| 4. Family aggression, Waves 1–3 | −.18 | .01 | .08 | _ | |
| 5. Antisocial behavior, Wave 4 | −.13 | .00 | −.04 | .40** | _ |
| 6. Post-traumatic stress symptoms, Wave 4 | −.26 | −.18 | .10 | .12 | .52** |
p <.01;
p <.05
Hypothesis 1
Table 3 shows regression analyses using the two cortisol indices, AUCg and AUCi, as outcome variables (we also examined peak cortisol and peak minus baseline, but, since the results closely paralleled our results for AUCg and AUCi, respectively, we only report the AUC results). Each model included the following (centered) predictors: the starting time of the laboratory visit, cumulative family aggression over waves 1–3; youths’ rating of conflict during the discussion; and the interaction of the conflict discussion rating with family aggression. Exploratory analyses also included age, gender, pubertal status, youth medications, and biological parent status; these variables were not associated with cortisol indices and did not alter results so were dropped from the final models.
Table 3.
Cortisol (Wave 4) Predicted by Cumulative Family Aggression (Waves 1–3), and Youths’ Parent-Child Conflict Rating of Discussion
| Cortisol area under the curve with respect to ground (AUCg)
| ||
|---|---|---|
| Beta | t | |
| (Constant) | 7.16*** | |
| Starting time of lab visit | −.52 | −4.20*** |
| Family aggression, waves 1–3 | −.29 | −2.34* |
| Youth discussion rating | .06 | .52 |
| Interaction of family aggression & discussion rating | −.02 | −.20 |
| Cortisol area under the curve with respect to increase (AUCi) | ||
| Beta | t | |
| (Constant) | .35 | |
| Starting time of lab visit | −.05 | −.40 |
| Family aggression, waves 1–3 | −.02 | −.13 |
| Youth discussion rating | .11 | .82 |
| Interaction of family aggression & discussion rating | −.43 | −3.33** |
Note. R (53, 4) = .54, F = 5.10, p = .00 predicting AUCg; R (53, 4) = .45, F = 3.04, p = .03 predicting AUCi
p<.001;
p<.01;
p<.05
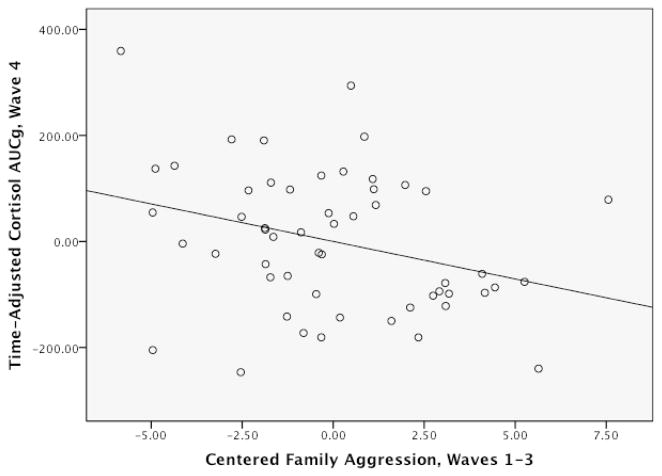
Consistent with expectations, family aggression and starting time were both negatively associated with AUCg (see Figure 1). Youths’ discussion rating and its interaction with family aggression were not associated with AUCg, and their inclusion or exclusion from the model did not affect results.
Figure 1.
Partial Regression Plot Showing Wave 1–3 Family Aggression Predicting Wave 4 Cortisol AUCg
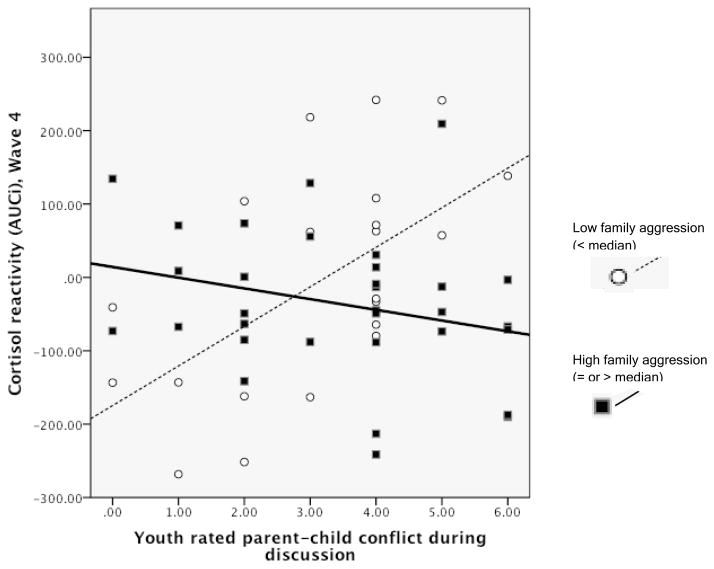
Family aggression interacted with youth’s conflict discussion rating in predicting AUCi. Figure 2 illustrates the association between AUCi and the conflict rating for youth from high (at or above the sample median) or low (below the sample median) family aggression. Youth with low family aggression show increased cortisol reactivity when the discussion is more conflictual. However, for the youth from aggressive families, the opposite pattern appeared, such that a higher-conflict discussion was linked with lower cortisol reactivity than a lower-conflict discussion. AUCi was not directly associated with family aggression or the conflict discussion rating.
Figure 2.
Ratings of parent-child conflict in the triadic discussions and cortisol reactivity (AUCi) for youth with a history of high versus low family aggression (median split). Youth with AUCi above ‘0’ are “responders” and those with AUCi below ‘0’ are “non-responders.”
Hypothesis 2
Table 4 presents regression models with family aggression and AUCi as predictors of youths’ Wave 4 PTS symptoms and antisocial behaviors. Exploratory analyses also included gender, pubertal status, biological parent status, and youth ratings of conflict during the discussion; these predictors did not affect results and were therefore dropped. Cortisol AUCg was not associated with either outcome variable so these analyses focus only on AUCi.
Table 4.
Youth Outcomes Associated With Cumulative Family Aggression and Cortisol Reactivity
| PTS symptoms
| ||
|---|---|---|
| Beta | t | |
| (Constant) | 10.43*** | |
| Family aggression, waves 1–3 | .13 | 1.01 |
| Cortisol reactivity (AUCi) | −.01 | −.10 |
| Interaction of family aggression & AUCi | .38 | 2.70** |
| Antisocial behavior | ||
| Beta | t | |
| (Constant) | 7.68*** | |
| Family aggression, waves 1–3 | .38 | 3.06** |
| Cortisol reactivity (AUCi) | .15 | 1.04 |
| Interaction of family aggression & AUCi | .34 | 2.41* |
Note. R (51, 3) = .41, F = 3.40, p = .03, associated with PTS symptoms; R (51, 3) = .48, F = 4.96, p = .01, associated with antisocial behavior
p<.001;
p<.01;
p<.05
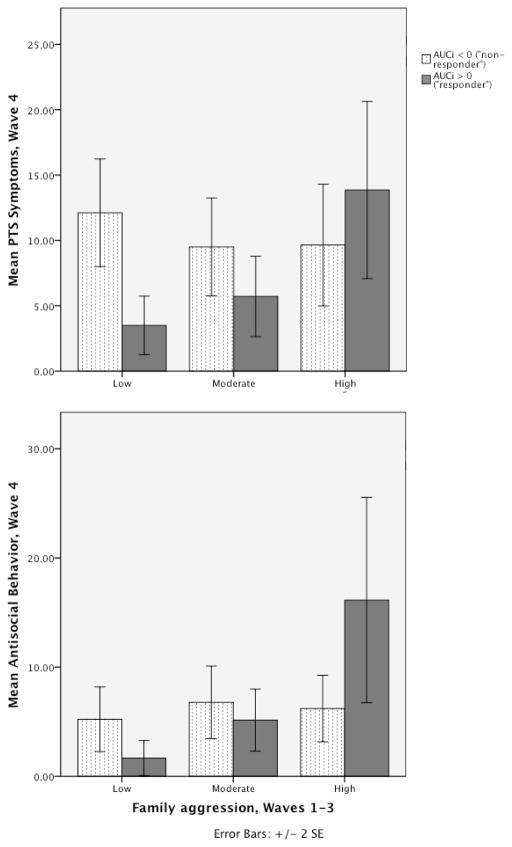
We found significant interactions between cortisol reactivity (AUCi) and family aggression for both PTS symptoms and antisocial behaviors. Family aggression also had a direct positive association with antisocial behavior. To examine these interactions, we divided the sample into youth with a positive value for AUCi (suggesting reactivity above baseline throughout the discussion task & recovery period, n = 20; “responders”) and youth whose AUCi value was negative or flat (showing little sustained reactivity, n = 34; “non-responders”). Among “responders,” family aggression was positively correlated with PTS symptoms, r(19) = .57, p = .01, and with antisocial behavior, r(19) = .59, p = .01. However, among the “non-responders,” family aggression and PTS symptoms were not associated, r(33) = −.14, p = .42, nor was family aggression and antisocial behavior, r(33) = .20, p = .26. We repeated the analysis after redefining “responders” as youth whose cortisol increased over baseline in the first two timepoints following the discussion task, and found similar results: the “responders” showed positive correlations between family history and outcomes that the “non-responders” did not show. Figure 3 illustrates associations between family aggression and youth outcomes among both “responders” and “non-responders,” displaying family aggression grouped by a tercile split (Low (0–3); Moderate (4–7); and High (>7). As shown, “responders” from the most aggressive families appeared most likely to report both PTS symptoms and antisocial behavior.
Figure 3.
Associations Between Family Aggression, Cortisol Reactivity (AUCi), and Youth Outcomes
Discussion
We found that youth exposed to higher cumulative family aggression showed lower total cortisol output (AUCg) during and after a family conflict discussion task. Family aggression history interacted with youths’ ratings of conflict with their parents during the discussion in predicting task-related cortisol reactivity (AUCi): youth from less aggressive families who reported greater parent-child conflict showed greater reactivity, whereas youth from more aggressive families showed the opposite pattern. These results are consistent with prior research establishing the “attenuation hypothesis” that links moderate childhood adversity with an attenuated HPA axis response to stress (e.g. Carpenter et al., 2007, Elzinga, et al., 2008, Granger et al., 1998, and Roisman et al., 2009). Also supporting our hypotheses, family aggression interacted with cortisol reactivity in predicting youths’ self-reported outcomes: youth from more aggressive families who reacted more strongly to the discussion task reported more psychological and behavioral problems. When we separated youth by AUCi indices, those whose task-related cortisol showed sustained increases over baseline (positive AUCi) had strong positive correlations between family aggression and negative outcomes that “non-responders” (negative AUCi) did not show. Thus, the generally attenuated response to family conflict we found among aggression-exposed youth may be adaptive, protecting youth from the deleterious effects of recurring family conflict. Our results identify a potentially high-risk group of youth: those who have been exposed to high levels of family aggression yet continue to show physiological arousal in response to conflict. These youth may be sensitized, rather than habituated, to family aggression, and have difficulty modulating their conflict-related arousal.
Although these findings initially appear contradictory with evidence linking attenuated cortisol with youths’ PTSD, antisocial behavior and externalizing symptoms (e.g., Susman, 2006; Shirtcliff, Granger, Booth & Johnson, 2005; Davies et al. 2007), our results suggest that youth show different pathways of risk and resilience depending on the family environment. Among youth from low-aggression families, a “normative” response to the conflict discussion may be a short-term increase in cortisol, as seen in our data. However, for youth exposed to aversive, aggressive behavior at home, the ability to down-regulate in the face of conflict may be more adaptive. Our results are consistent with the theory of biological sensitivity to context (Boyce & Ellis, 2005) and the closely related differential susceptibility hypothesis (e.g. Belsky & Pluess, 2009): “biologically sensitive” youth raised in supportive environments may not exhibit problems (and, in fact, may actually fare better than less reactive youth), but physiologically reactive youth are especially vulnerable within adverse family environments.
We also found different patterns of results for AUCg and AUCi. AUCg, an index of total cortisol output that includes the baseline (starting) value, was negatively associated with family aggression once we took time of day into account. In contrast, AUCi reflects task-specific change in cortisol, and was predicted by an interaction of family aggression history with conflict discussion ratings. The intensity of the conflict discussion appeared linked to higher AUCi values, but only for youth from less aggressive families. Family aggression interacted with AUCi but not AUCg in predicting youth adjustment, suggesting that young people’s reactivity to short-term, interpersonal stressors may be a more meaningful marker of their adaptation to aggressive family environments than other cortisol parameters.
Further research can help uncover the specific pathways by which family environments shape cortisol regulation. Sustained hyperarousal of the HPA axis may translate into eventual hypoarousal as the organism down-regulates to protect itself (Fries, Hesse, Hellhammer, & Hellhammer, 2005); alternatively, attenuated cortisol in aggression-exposed youth could result from stress inoculation, e.g. moderate adversity strengthening the body’s regulatory systems (e.g. Gunnar et al., 2009). Additionally, youths’ behavioral coping styles, such as withdrawal, may undergird different patterns of physiological responding to stress. Youth with aggressive parents may learn to “tune out” or detach from heated family arguments. This style of responding to family conflict may be maladaptive in low-aggression families, but protective for youth in high-aggression families.
Importantly, the outcomes used in this paper – youths’ PTS symptoms and antisocial behavior – were assessed with youth self-report, which limits the conclusions we can draw from our findings. It is possible, for example, that youths’ reports of their own functioning might be biased in some systematic way that is linked to the constructs of family risk and physiological reactivity. For example, low-reactive youth from more aggressive families might downplay, or underreport, problems, while high-reactive youth from more aggressive families might over-report problems. It is also possible that low-reactive youth from aggressive families evidence problems in other domains besides the ones we sampled in this paper. For example, if these youth cope with conflictual interactions by “tuning out,” they may be at risk for peer and romantic relationship problems in the future. Therefore, while we believe our findings point to an adaptive function for the down-regulation of cortisol in high-conflict families, we cannot definitively conclude that low reactivity is protective in all situations.
This paper was also limited by a small sample size that restricted the number of predictors we could examine. Though we did not find effects or interactions by gender or pubertal timing, a larger sample might have given us more power to detect such results. Our triadic conflict design, although a methodological strength, confines our findings to two-parent families. Finally, cortisol and youth outcome measures were sampled during the same wave, making it impossible to specify the direction of effects: it is possible that symptomatic youth develop greater cortisol reactivity over time, for example. However, this study also had several strengths, including its longitudinal design, a family aggression measure combining data from multiple reporters and across multiple waves, and a conflict discussion paradigm that included both parents and the youth.
In conclusion, we found that consistently aggressive family environments generally predicted low, attenuated profiles of cortisol release in adolescents engaged in a triadic family conflict task. Adolescents from aggressive families who did not show this pattern, and instead exhibited more exaggerated reactivity to the conflict discussion, were the most likely to report psychological and behavioral problems. Physiological reactivity, which could also be termed biological sensitivity to context, may be an important moderator of the link between family conflict and youth adjustment. Further research examining stress responding in a family context can help to clarify pathways of risk and resilience among youth in adverse environments.
Acknowledgments
This research was supported by NIH-NRSA Grant F32HD063255 awarded to Saxbe and by NIH-NICHD Grant R01 HD046807 and the David and Lucile Packard Foundation Grant 00-12802 awarded to Margolin. We thank the families who participated in the study and other members of the USC Family Studies Project.
References
- Belsky J, Pluess M. Beyond diathesis-stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/S0954579405050145. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AM, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PT, Cummings ME. Marital conflict and child adjustment: An emotional security hypothesis. Psychological Bulletin. 1994;116:387–411. doi: 10.1037/0033-2909.116.3.387. [DOI] [PubMed] [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, Cummings EM. The role of child adrenocortical functioning in pathways between interparental conflict and child maladjustment. Developmental Psychology. 2007;43:918–930. doi: 10.1037/0012-1649.43.4.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis M, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Ryan ND. Developmental traumatology: Biological stress systems. Biological Psychiatry. 1999;9:1259–1270. doi: 10.1016/S0006-3223(99)00044-X. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Gunnar MR, Donzella B. Cortisol levels of young children in full-day child care centers: Relations with age and temperament. Psychoneuroendocrinology. 1999;24:519–536. doi: 10.1016/S0306-4530(99)00009-8. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Whitson SA. Longitudinal relations between marital conflict and child adjustment: vagal regulation as a protective factor. Journal of Family Psychology. 2006;20:30–39. doi: 10.1037/0893-3200.20.1.30. [DOI] [PubMed] [Google Scholar]
- Elliott D, Huizinga D, Ageton S. Explaining Delinquency and Drug Use. Beverly Hills, CA: Sage; 1985. [Google Scholar]
- Elzinga BM, Spinhoven P, Berretty EW, Jong P, Roelofs K. The role of childhood abuse in HPA-axis reactivity in Social Anxiety Disorder: A pilot study. Biological Psychology. 2010;83:1–6. doi: 10.1016/j.biopsycho.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events. Psychoneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer D. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gordon RA, Lahey BB, Kawai E, Loeber R, Stouthamer-Loeber M, Farrington DP. Antisocial behavior and youth gang membership: Selection and socialization. Criminology. 2004;42:55–87. doi: 10.1111/j.1745-9125.2004.tb00513.x. [DOI] [Google Scholar]
- Granger DA, Serbin LA, Schwartzman A, Lehoux P, Cooperman J, Ikeda S. Children’s salivary cortisol, internalising behaviour problems, and family environment: Results from the Concordia longitudinal risk project. International Journal of Behavioral Development. 1998;22:707–728. doi: 10.1080/016502598384135. [DOI] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KL, Stewart JG, Wynne-Edwards KE. Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2010.07.006. in press. [DOI] [PubMed] [Google Scholar]
- Loeber R, Farrington D, Stouthamer-Loeber M, Van Kämmen W. Antisocial behavior and mental health problems: Explanatory factors in childhood and adolescence. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 1998. [Google Scholar]
- Luecken L, Kraft A, Hagan MJ. Negative relationships in the family-of-origin predict attenuated cortisol in emerging adults. Hormones and Behavior. 2009;55:412–417. doi: 10.1016/j.yhbeh.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin G. Unpublished scale. University of Southern California; Los Angeles: 1999. Youth Symptom Severity Checklist. [Google Scholar]
- Margolin G, John RS, Foo L. Interactive and unique risk factors for husbands’ emotional and physical abuse of their wives. Journal of Family Violence. 1998;13:315–344. [Google Scholar]
- Margolin G, Vickerman KA, Oliver PH, Gordis EB. Violence exposure in multiple interpersonal domains: Cumulative and differential effects. Journal of Adolescent Health. 2010;47:198–205. doi: 10.1016/j.jadohealth.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low Salivary Cortisol and Persistent Aggression in Boys Referred for Disruptive Behavior. Archives of General Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- McEwen B. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Obradović J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development. 2010;81:270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner J, Kirschbaum C, Meinlschmid G, Hellhammer D. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Repetti R, Taylor S, Seeman T. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. doi: 10.1037//0033-2909.128.2.230. [DOI] [PubMed] [Google Scholar]
- Roisman GI, Susman E, Barnett-Walker K, Booth-LaForce C, Owen MT, Belsky J, Bradley RH, Houts R, Steinberg L. Early family and child-care antecedents of awakening cortisol levels in adolescence. Child Development. 2009;80:907–20. doi: 10.1111/j.1467-8624.2009.01305.x. [DOI] [PubMed] [Google Scholar]
- Shirtcliff E, Granger D, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17:167–184. doi: 10.1017/S0954579405050091. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Avenevoli S. The role of context in the development of psychopathology: A conceptual framework and some speculative propositions. Child Development. 2000;71:66–74. doi: 10.1111/1467-8624.00119. [DOI] [PubMed] [Google Scholar]
- Straus MA, Hamby SL, Finkelhor DW. Identification of child maltreatment with the Parent Child Conflict Tactics Scales: Development and psychometric data for a national sample of American parents. Child Abuse & Neglect. 1998;22:249–270. doi: 10.1016/S0145-2134(97)00174-9. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities, and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]