Abstract
Objectives
Bipolar disorder and schizophrenia share common pathophysiological processes and may have similar perceptual abnormalities. Mismatch negativity (MMN) and P3a—event-related potentials associated with auditory preattentional processing—have been extensively studied in schizophrenia, but rarely in bipolar disorder. Furthermore, MMN and P3a have not been examined between diagnostic subgroups of patients with bipolar disorder. We evaluated MMN and P3a in patients with bipolar disorder compared to patients with schizophrenia and healthy controls.
Methods
MMN and P3a were assessed in 52 bipolar disorder patients, 30 schizophrenia patients, and 27 healthy control subjects during a duration-deviant auditory oddball paradigm.
Results
Significant MMN and P3a amplitude reductions were present in patients with bipolar disorder and schizophrenia relative to controls. The MMN reduction was more prominent in patients with schizophrenia than bipolar disorder, at a trend level. P3a did not differ significantly between patient groups. There were no MMN or P3a differences between patients with bipolar I (n = 34) and bipolar II (n = 18) disorder. Patients with bipolar I disorder failed to show lateralized MMN, in contrast to the other groups. No MMN or P3a differences were found between patients with bipolar disorder taking (n = 12) and not taking (n = 40) lithium, as well as between those taking (n = 30) and not taking (n = 22) antipsychotic medications.
Conclusions
Patients with bipolar disorder showed deficits in preattentive auditory processing, including MMN deficits that are less severe and P3a deficits that are slightly more pronounced, than those seen in schizophrenia.
Keywords: auditory processing, bipolar disorder, mismatch negativity, P3a, schizophrenia
Despite clear differences in clinical symptomatology between bipolar disorder and schizophrenia, the two disorders share many features, including high rates of functional disability and notable cognitive impairment that remains relatively stable. Recent genome-wide association studies (GWAS) have identified genetic factors that seem to confer comparable risk for the two disorders (e.g., 1–4). There is also the suggestion that N-methyl-D-aspartate (NMDA) abnormalities, which are well-documented in schizophrenia (5, 6), might also be present in bipolar disorder. For example, studies have found altered NMDA-receptor complexes in the brain tissue of patients with bipolar disorder (7–9), suggesting that disturbances in glutamatergic functioning are present in this disorder as well.
NMDA-receptor dysfunction has been linked to an auditory event-related potential (ERP), namely, mismatch negativity (MMN) (10, 11). NMDA antagonists can block MMN generation in primates (12), as well as selectively diminish MMN amplitude in healthy control subjects (13). MMN is elicited in response to infrequent, physically deviant tones interspersed in the repeated presentation of a standard tone. MMN is largest at central midline scalp sites and typically peaks between 160 and 220 msec after the onset of the deviant stimulus (14). MMN is thought to reflect automatic, preattentive information processing, as it can be elicited without directing attention to stimuli (15). It can even be elicited during sleep (16), detected in comatose states (17), and recorded in utero (18).
Patients with schizophrenia show robust deficits in MMN compared to healthy controls (e.g., 19–23). These deficits are thought to reflect impaired neurotransmission at NMDA-mediated glutamate receptors (24) and may reflect the early stages of a cascade of neurocognitive abnormalities resulting in impaired community outcome (25). In fact, smaller MMN responses have been associated with poorer psychosocial functioning in both patients with schizophrenia (e.g., 26, 27) and healthy subjects (28).
Given the established association between impaired NMDA function and reduced MMN, and the findings of NMDA abnormalities in bipolar disorder, it is possible that patients with bipolar disorder would exhibit abnormalities in MMN similar to those found in schizophrenia. To our knowledge, only five published studies have examined MMN in bipolar disorder (29–33). Only one of the five studies reported reduced MMN in patients with bipolar disorder compared with controls (29). However, four out of the five studies had very limited power with sample sizes ranging from 11–25. The only adequately-powered study (with 59 patients with bipolar disorder) failed to find significant patient-control differences (31). Yet, this study used a specialized ascertainment approach, in that it was based on two family studies in which patients with bipolar disorder were recruited along with relatives, but not alone. Furthermore, none of these studies included both patients with bipolar I and bipolar II disorder. Hence, it is not possible to infer MMN differences between these two subtypes. While some evidence suggests that MMN is unaffected by second-generation antipsychotic medications (e.g., 34–36), little is known about the effects of mood stabilizers, such as lithium, on MMN.
In addition to MMN, early auditory processing has also been assessed with the P3a ERP component. P3a is assumed to reflect the covert orienting or shift in attention (37). It is a positive-going wave that is frontally maximal and peaks between 250 and 300 msec post-stimulus. Both the NMDA- and gamma-aminobutyric acid (GABA)-receptor systems are thought to modulate P3a (38). Like MMN, P3a has been shown to be a strongly automatic process (39) and to be impaired in schizophrenia (e.g., 40–43). To our knowledge, only one study (29) examined P3a amplitude in a bipolar disorder sample and it failed to detect significant group differences.
In the current study, we examined MMN and P3a in a sample of 52 patients with bipolar disorder compared with healthy controls and patients with schizophrenia, using a duration-deviant auditory oddball paradigm. Our primary objective was to determine the extent of MMN and P3a deficits in the bipolar disorder group, using schizophrenia as a comparison group because of previous reports of MMN and P3a impairments associated with this disorder. An additional objective of the study was to examine subgroups of patients with bipolar I and bipolar II disorder and to assess possible effects of lithium and antipsychotics on MMN and P3a.
Methods
Participants
The sample consisted of 52 patients with bipolar disorder, 30 patients with schizophrenia, and 27 normal comparison subjects. All participants gave written informed consent after receiving a detailed explanation of study procedures in accordance with procedures approved by the Institutional Review Boards at the University of California, Los Angeles (UCLA) and the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS). Patients with schizophrenia were recruited from outpatient treatment clinics at the VAGLAHS and from board-and-care residences in the community through staff presentations and referral. Patients with bipolar disorder were recruited from outpatient clinics at UCLA and the VAGLAHS. Diagnosis was based on the Structured Clinical Interview for DSM-IV Axis-I disorders (SCID-I) (44). Of the patients with bipolar disorder, 34 were diagnosed with bipolar I disorder (7 of those with a history of psychosis) and 18 with bipolar II disorder. Patients were considered to be clinically stable, meaning that they had no medication changes in the past six weeks, no inpatient hospitalization in the past three months, and no changes in housing in the past two months. The mean age of onset of first symptoms was 18.12 [standard deviation (SD) = 7.05] for patients with bipolar disorder and 21.21 (SD = 5.25) for those with schizophrenia. The mean number of hospitalizations for patients with bipolar disorder was 2.75 (SD = 4.15) compared to 11.86 (SD = 16.83) for those with schizophrenia.
Healthy controls were recruited through newspaper and internet advertisements and were screened with the SCID-I and SCID-II (45). They were excluded if they met criteria for any lifetime psychotic disorder, bipolar mood disorder, recurrent depression, substance dependence, paranoid, schizotypal, or schizoid personality disorder, or if they reported a history of psychotic disorder among their first-degree relatives.
Additional exclusion criteria for all groups included being younger than 18 or older than 60 years; having a verbal IQ below 70, based on the reading subtest of the Wide Range Achievement Test-third edition (WRAT-3) (46); meeting diagnostic criteria for substance dependence in the past six months or substance abuse in the past month, based on interview; having an identifiable neurological disorder; seizures; history of head injury or loss of consciousness for more than one hour; or being insufficiently fluent in English, determined by testing the participant’s ability to understand the consent form.
Of the patients with schizophrenia, 22 were receiving second-generation antipsychotic medications, four were receiving first-generation antipsychotic medications, two were receiving both types of antipsychotics, and two were not taking an antipsychotic medication at the time of testing. Of the patients with bipolar disorder, most were receiving more than one psychoactive drug; 30 were taking second-generation antipsychotics, 26 were taking anticonvulsants, 12 were taking lithium, and 28 were taking antidepressants.
Clinical ratings
Psychiatric symptoms were evaluated using the expanded 24-item UCLA version of the Brief Psychiatric Rating Scale (BPRS) (47), the Hamilton Depression Rating Scale (HDRS) (48), as well as the Young Mania Rating Scale (YMRS) (49). Current level of functioning was assessed with the Role Functioning Scale (RFS) (50). All the clinical assessments were conducted by interviewers trained to reliability through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC) based on established procedures (51, 52).
ERP paradigm
Subjects were presented with standard and duration-deviant tones binaurally using foam ear inserts (1 kHz 85 dB sound pressure level, with 10 msec rise/fall) with a fixed stimulus onset asynchrony of 500 msec, using E-Prime 2.0. Standard (90% probability; 50 msec duration) and deviant (10% probability; 100 msec duration) tones were presented in a fixed, pseudorandom order (with the restriction that at least four standard tones were presented between deviant tones). A total of 2,000 trials were administered. During the 20-minute electrocephalogram (EEG) recording, subjects were instructed to watch a silent movie to divert attention from the stimuli.
EEG recordings were acquired with a 64-channel BioSemi ActiveTwo amplifier (Biosemi B.V., Amsterdam, The Netherlands). Data were sampled at 1024 Hz with a bandpass of 0.16 to 100 Hz. Additional electrodes were placed above and below the left eye and at the outer canthi of both eyes to monitor blinks and eye movements. Each active electrode was measured online with respect to a common mode sense electrode during data collection, forming a monopolar channel. An additional electrode was placed at the nose tip and all EEG data were re-referenced offline to this electrode.
Data processing was performed offline using BrainVision Analyzer 2 software (Brain Products, Gilching, Germany). Based on visual inspection, bad electrodes were removed from the recording and a spherical spline interpolation was used to recreate the electrode (53, 54). The mean interpolated electrodes was 1.5 (range: 1–4) for patients with bipolar disorder; 2.6 (range: 1–5) for patients with schizophrenia; and 1.4 (range: 1–2) for healthy controls. Ocular artifacts were removed from the data using a regression-based algorithm (51). Continuous data were then epoched at -100 to 500 msec relative to stimulus onset and were baseline corrected to the average of the prestimulus interval (−100 msec to 0). The MMN and P3a were derived from electrodes at frontocentral sites, based on previous studies (AF3, AF4, AF7, AF8, AFz, Cz, F1, F2, F3, F4, F5, F6, F7, F8, FC1, FC2, FC3, FC4, FC5, FC6, FCz, Fp1, Fp2, Fpz, Fz). Epochs that contained activity exceeding ± 75 μV at these electrode sites were automatically rejected. The mean number of acceptable deviant trials (out of 200) was 187 for patients with bipolar disorder, 190 for patients with schizophrenia, and 194 for healthy controls; the mean number of acceptable standard trials (out of 1,800) was 1,698 for patients with bipolar disorder, 1,709 for patients with schizophrenia, and 1,747 for healthy controls. There were no significant group differences in the number of acceptable deviant trials [F(2,106) = 1.43, p = 0.24].
MMN and P3a waveforms were generated by subtracting standard from deviant averaged waveforms. The waveforms were then low-pass filtered at 20 Hz (zerophase shift, 24 dB/octave rolloff) to remove any residual high-frequency artifact. MMN and P3a amplitudes were measured as the mean activity in the 135–205 msec and 250–350 msec latency range, respectively. These time windows were chosen based on prior studies in schizophrenia (27, 55, 56).
Data analysis
One-way ANOVAs and chi-square tests were used to assess group differences for continuous and categorical demographic variables, respectively. Electrodes were averaged together to examine regional activity: left anterior (Fp1, AF3, AF7, F1, F3, F5, F7, FC1, FC3, FC5), midline (Fpz, AFz, Fz, FCz, Cz), and right anterior (Fp2, AF4, AF8, F2, F4, F6, F8, FC2, FC4, FC6). One-way ANOVAs were conducted to investigate group differences in MMN and P3a amplitude separately at Fz and at the averaged midline sites. To assess laterality effects, a 2 × 3 repeated-measures ANOVA with region (left versus right) as the within-subject factor and group as the between-subject factor was performed for each component. Least significant difference (LSD) tests were conducted as post-hoc analyses to follow up on significant main effects. Significant group × region interactions were decomposed using paired t-tests to assess differences between regional clusters of electrodes within each group. We also conducted independent samples t-tests within the bipolar disorder group to examine MMN and P3a differences between (i) patients with bipolar I and bipolar II disorder, (ii) those taking lithium and those not taking lithium, and (iii) those taking second-generation antipsychotics and those not taking second generation antipsychotics. The laterality analyses were repeated for the bipolar I versus II comparison.
Results
Demographic and clinical characteristics
Demographic and symptom ratings can be seen in Table 1. There were significant group differences in age [F(2,106) = 3.80, p = 0.02]; healthy controls [mean (M) = 39.5] being significantly younger than patients with bipolar disorder (M = 45.2) (p = 0.04), but no significant differences in gender distribution or parental education were observed among the three groups. The groups differed on personal education [F(2,106) = 5.75, p = 0.004); however, our intent was to match the groups on parental, not personal, education. As expected, patients with schizophrenia had more severe overall symptoms than patients with bipolar disorder [BPRS: t(79) = 3.75, p < 0.001]. There were no significant differences in depression level [HDRS: t(79) = 0.30, p > 0.05] between the two patient groups. However, patients with schizophrenia had significantly higher scores on the YMRS [t(79) = 2.93, p = 0.004] compared to patients with bipolar disorder. This difference on the YMRS was due to the fact that patients with schizophrenia had higher scores on items related to psychosis, such as thought content (delusions, hallucinations), insight (denying illness), and appearance (being minimally unkempt).
Table 1.
Demographic and clinical characteristics
| Healthy controls (n = 27) | Bipolar disorder (n = 52) | Schizophrenia (n = 30) | |
|---|---|---|---|
| Age, mean (SD)a | 39.5 (9.9) | 45.2 (9.8) | 45.6 (8.7) |
| Gender, % male | 66.7 | 53.8 | 70.0 |
| Personal education, mean (SD)b | 14.7 (1.6) | 14.0 (2.2) | 12.9 (2.3) |
| Parental education, mean (SD) | 13.0 (3.2) | 11.9 (4.4) | 11.0 (5.0) |
| Race, % White | 40.7 | 75.0 | 53.3 |
| Handedness, % right | 92.6 | 86.5 | 83.3 |
| BPRS, mean (SD) | – | 33.8 (7.2) | 40.7 (9.1) |
| HDRS, mean (SD) | – | 9.8 (7.6) | 9.3 (6.4) |
| YMRS, mean (SD) | – | 3.2 (3.9) | 6.4 (5.9) |
SD = standard deviation; BPRS = Brief Psychiatric Rating Scale; HDRS = Hamilton Depression Rating Scale; YMRS = Young Mania Rating Scale.
Significant difference in age between healthy controls and patients with bipolar disorder, p < 0.05.
Significant difference in personal education between healthy controls and patients with schizophrenia, p < 0.05.
MMN and P3a analyses at Fz and midline electrodes
Because there was a group difference in age, we first examined the effect of age on MMN and P3a. Age was significantly correlated with P3a at electrode Fz only in the schizophrenia patients (r = −0.39, p = 0.03). However, the pattern of results was the same when using versus not using age as a covariate. We therefore report below only the analyses not including age as a covariate.
Examining MMN at electrode Fz, the ANOVA revealed a significant main effect of group [F(2,106) = 11.44, p < 0.001]. LSD-corrected pairwise comparisons showed that patients with bipolar disorder (M = −1.35, SD = 0.87) and schizophrenia (M = −0.94, SD = 0.78) had significantly reduced MMN amplitude at Fz (both p < 0.005) relative to healthy controls (M = −2.12, SD = 1.22); the difference in MMN amplitude between the two patient groups was at a trend level (p = 0.06) (see Table 2). Examining MMN over the midline region, the ANOVA revealed a significant main effect of group [F(2,106) = 10.63, p < 0.001]. The group contrasts with the midline cluster were similar to those with Fz alone; patients with bipolar disorder (M = −1.18, SD = 0.80) and schizophrenia (M = −0.85, SD = 0.81) had significantly reduced MMN amplitude (both < 0.005) relative to healthy controls (M = −1.82, SD = 0.81), the difference in MMN amplitude between the two patient groups was at a trend level (p = 0.08).
Table 2.
Mean and standard deviation (SD) for the mismatch negativity and P3a amplitudes for each group
| Healthy controls (n = 27) | Bipolar disorder (n = 52) | Schizophrenia (n = 30) | |
|---|---|---|---|
| Mismatch negativity | |||
| Fz | −2.12 (1.22) | −1.35 (0.87) | −0.94 (0.78) |
| Midline electrode cluster | −1.82 (0.99) | −1.18 (0.76) | −0.85 (0.67) |
| Left electrode cluster | −1.65 (0.84) | −0.98 (0.76) | −0.65 (0.62) |
| Right electrode cluster | −1.83 (1.11) | −1.13 (0.79) | −0.88 (0.71) |
| P3a | |||
| Fz | 1.25 (1.05) | 0.39 (0.91) | 0.75 (0.89) |
| Midline electrode cluster | 1.00 (0.83) | 0.38 (0.79) | 0.61 (0.70) |
| Left electrode cluster | 0.87 (0.65) | 0.36 (0.74) | 0.59 (0.75) |
| Right electrode cluster | 0.95 (0.77) | 0.38 (0.77) | 0.66 (0.74) |
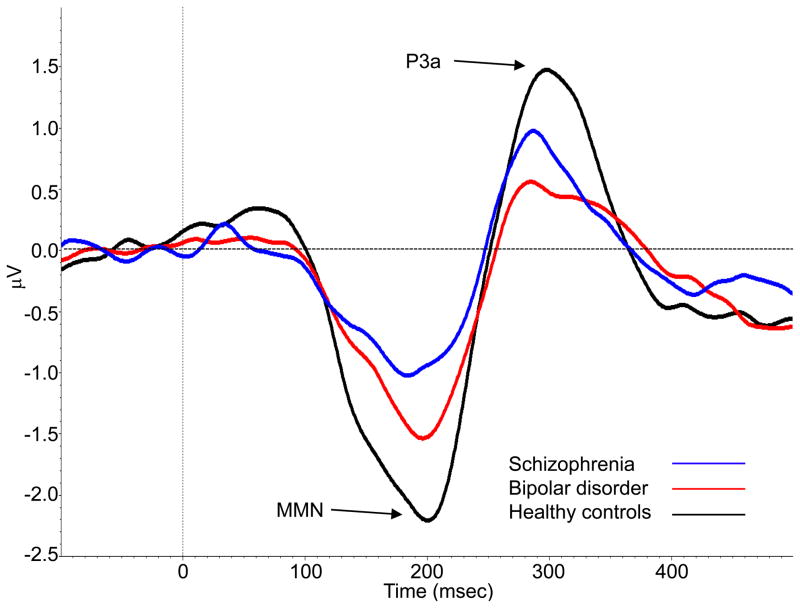
Examining P3a at electrode Fz, the ANOVA revealed a significant main effect of group [F(2,106) = 7.42, p = 0.001]. LSD-corrected pairwise comparisons showed that patients with bipolar disorder (M = 0.39, SD = 0.91) and schizophrenia (M = 0.75, SD = 0.89) had significantly reduced P3a amplitude at Fz (p < 0.001 and p = 0.049, respectively) relative to healthy controls (M = 1.25, SD = 1.05); the difference in P3a amplitude was not significant between the two patient groups. Examining P3a over the midline region, the ANOVA revealed a significant main effect of group [F(2,106) = 5.59, p = 0.005]. The group contrasts with the midline cluster were similar to those with Fz alone; patients with bipolar disorder (M = 0.38, SD = 0.78) had significantly reduced P3a amplitude (p = 0.001) relative to healthy controls (M = 1.00, SD = 0.77). The difference between patients with schizophrenia (M = 0.61, SD = 0.78) and healthy controls, which was significant for Fz, was a trend for the midline cluster (p = 0.06). Mean MMN and P3a amplitudes for each group at Fz and the midline electrode cluster are presented in Table 2. Figure 1 shows the grand average waveforms with MMN and P3a amplitudes at the midline region.
Fig. 1.
Grand average mismatch negativity (MMN) and P3a waveforms for patients with bipolar disorder (red), patients with schizophrenia (blue), and healthy controls (black) at the midline region. MMN was measured as the mean activity between 135–205 msec and P3a was measured as the mean activity between 250–350 msec.
Laterality analyses
The repeated-measures ANOVA for MMN revealed significant main effects of group [F(2,106) = 12.61, p < 0.001] and region [F(1,106) = 10.54, p = 0.002] but no significant group × region interaction. The main effect for group was addressed with the Fz and midline analyses above. Averaged across groups, MMN was significantly smaller over the left (M = −1.09, SD = 0.78) relative to the right (M = −1.28, SD = 0.90) region.
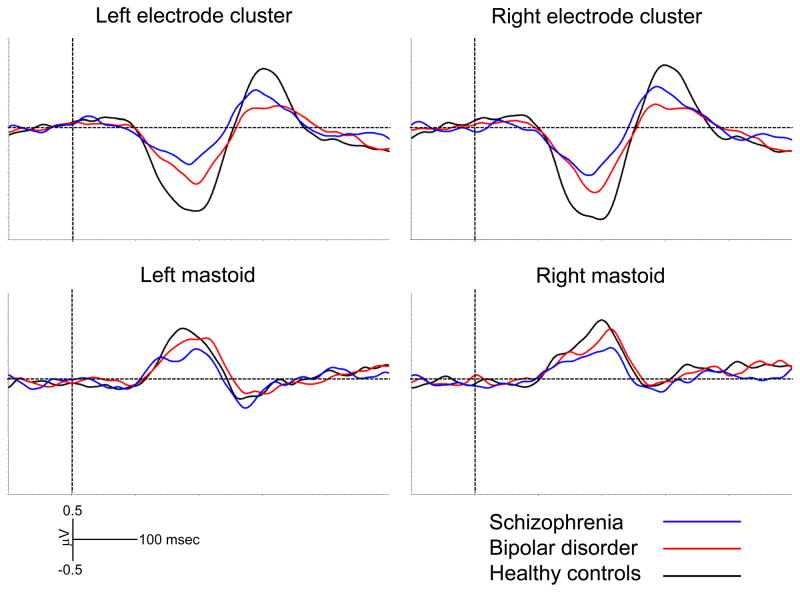
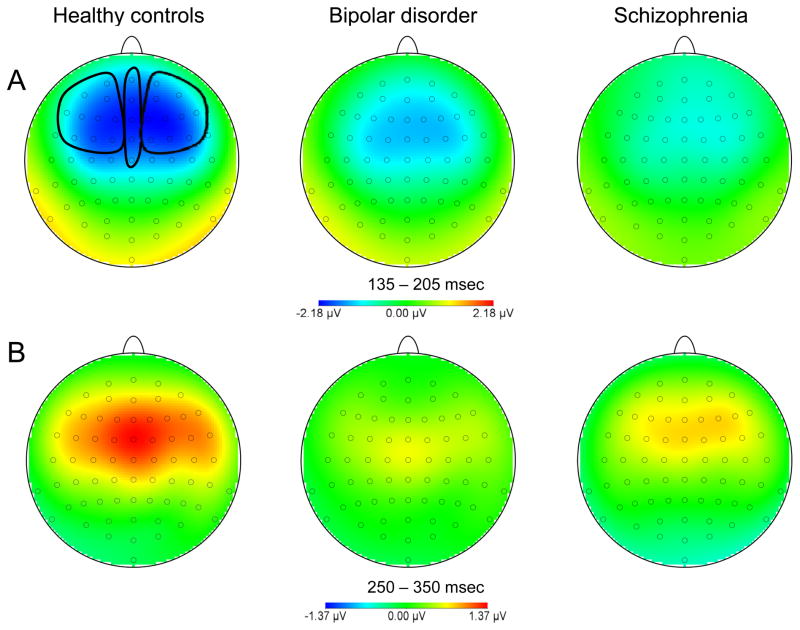
The repeated-measures ANOVA for P3a showed a significant main effect of group [F(2,106) = 5.42, p = 0.006], but no significant main effect of region and no significant group × region interaction. Mean MMN and P3a amplitudes for each group at the left and right electrode clusters are presented in Table 2. Figure 2 shows the grand average waveforms at the lateral regions and the mastoids. Topographical maps of MMN and P3a activity are shown in the top and bottom panels, respectively, of Figure 3.
Fig. 2.
Grand average mismatch negativity (MMN) and P3a waveforms at the left and right regions, as well as the left and right mastoids.
Fig. 3.
Topographical maps of mismatch negativity (MMN) (A) and P3a (B) activity for each group.
Subgroup analyses within bipolar disorder
The t-tests comparing patients with bipolar I (n = 34) and bipolar II (n = 18) disorder revealed no significant differences in MMN at Fz [t(50) = 0.87, p = 0.39] or over the midline region [t(50) = 1.24, p = 0.22]. Similarly, no significant group differences were observed in P3a at Fz [t(50) = −0.30, p = 0.76] or over the midline region [t(50) = −0.21, p = 0.84]. Mean MMN and P3a amplitudes for each bipolar disorder subgroup are presented in Table 3.
Table 3.
Mean and standard deviation (SD) for the mismatch negativity and P3a amplitudes for the bipolar I and II disorder subgroups
| Bipolar I (n = 34) | Bipolar II (n = 18) | |
|---|---|---|
| Mismatch negativity | ||
| Fz | −1.27 (0.81) | −1.49 (0.98) |
| Midline electrode cluster | −1.09 (0.73) | −1.36 (0.81) |
| Left electrode cluster | −0.99 (0.73) | −0.96 (0.84) |
| Right electrode cluster | −0.98 (0.76) | −1.41 (0.80) |
| P3a | ||
| Fz | 0.36 (0.98) | 0.45 (0.80) |
| Midline electrode cluster | 0.36 (0.84) | 0.41 (0.69) |
| Left electrode cluster | 0.30 (0.80) | 0.48 (0.62) |
| Right electrode cluster | 0.41 (0.81) | 0.33 (0.73) |
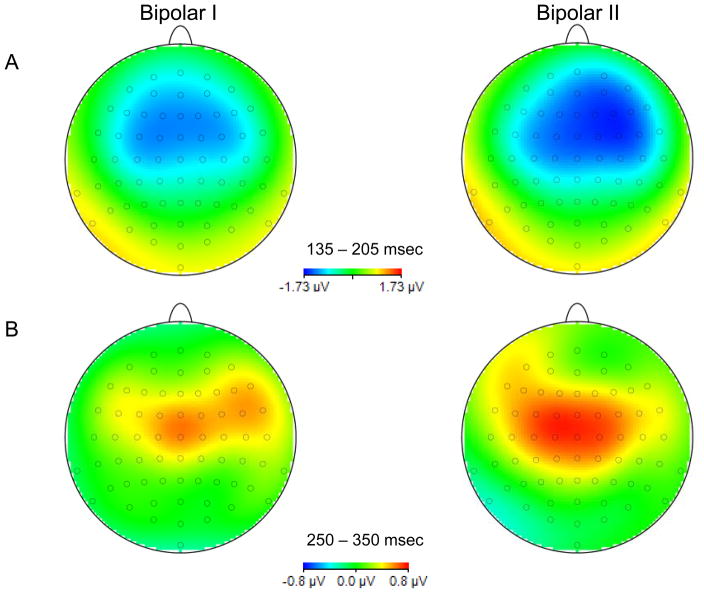
The repeated-measures ANOVA for MMN revealed a significant main effect of region [F(1, 50) = 9.18, p = 0.004] and a significant group × region interaction [F(1,50) = 9.88, p = 0.003], but no significant main effect of group. Averaged across groups, MMN was significantly smaller over the left (M = −0.97, SD = 0.79) relative to the right (M = −1.19, SD = 0.79) region. The group × region interaction revealed no differences in amplitude between the left and right regions for bipolar I disorder patients, but significant differences between the left (M = −0.96, SD = 0.84) compared to the right (M = −1.41, SD = 0.80) region in bipolar II disorder patients [t(17) = 3.51, p = 0.003]. Topographical maps of MMN and P3a activity for patients with bipolar I and bipolar II disorder are shown in the top and bottom panels, respectively, of Figure 4.
Fig. 4.
Topographical maps of mismatch negativity (MMN) (A) and P3a (B) activity for patients with bipolar I and bipolar II disorder.
The repeated-measures ANOVA for P3a revealed no significant main effects of group or region and no significant group × region interaction.
Effects of medications on MMN and P3a
The t-tests comparing patients with bipolar disorder taking lithium (n = 12) and those not taking lithium (n = 40) revealed no significant differences in MMN at Fz [t(50) = −0.59, p = 0.56] or over the midline region [t(50) = −0.43, p = 0.67], and no significant differences in P3a at Fz [t(50) = −1.85, p = 0.07] or over the midline region [t(50) = −1.67, p = 0.10].
Similarly, there were no significant differences between patients with bipolar disorder taking second-generation antipsychotics (n = 30) and those not taking second-generation antipsychotics (n = 22) in MMN at Fz or over the midline region, as well as P3a at Fz or over the midline region (all p-values > 0.25).
Discussion
The results of this study revealed significant MMN and P3a deficits in patients with bipolar disorder and schizophrenia compared to healthy controls. The bipolar disorder group’s mean MMN was intermediate between that of the schizophrenia and healthy control groups’. This pattern was reversed for P3a, in which the schizophrenia group’s mean P3a amplitude was intermediate between that of the bipolar disorder and healthy control groups’. The bipolar disorder and schizophrenia groups did not differ significantly from each other at either component, but the difference was at trend level for the MMN. Further, there was a main effect for laterality across all three groups (with the left MMN response being smaller). Within patients with bipolar disorder, there was a group-by-laterality interaction, such that patients with bipolar II disorder showed the laterality effect, but patients with bipolar I disorder did not. There were no MMN or P3a differences in patients with bipolar disorder taking versus not taking lithium, or those taking or not taking antipsychotic medications. Taken together, these results point to dysfunctional preattentive auditory processing in patients with bipolar disorder that is similar to what is typically seen in patients with schizophrenia.
While we found medium to large (d = 0.73) (57) MMN deficits in our bipolar disorder sample, one relatively large study (31) and three relatively small studies (30, 32, 33) did not. Our finding is consistent with Andersson et al.’s (29) finding of reduced MMN, though that study used only patients with bipolar II disorder and our study included both bipolar I and bipolar II disorder patients. The one previous well-powered study on this topic (31), failed to find MMN deficits in bipolar disorder, and there are some methodological differences with our study that could explain the inconsistencies. For example, the Hall et al. (31) study used a more rapidly-paced paradigm with a shorter interstimulus interval and briefer stimuli, and a different method to reference the EEG recording. That study also used a specialized sample ascertainment method (all patients were recruited with family members) which might have yielded a different subgroup of patients with bipolar disorder than the ones in our study.
Despite different conclusions with previous studies of MMN and bipolar disorder, the effect sizes in this study are actually comparable to previous studies. For example, the effect size between the bipolar disorder and schizophrenia groups was d = 0.50, and the schizophrenia patients had a large effect size of d = 1.15 compared with healthy controls. These differences are comparable to the mean effect sizes across the three studies (30, 32, 33) that used a similar three-group design (d’s of 0.55 and 0.83 for the same group comparisons).
For P3a, the bipolar disorder group exhibited large (d = 0.87) P3a reductions at Fz relative to the healthy control group and medium (d = 0.40) reductions relative to the schizophrenia group. The reduced P3a response in the patients with bipolar disorder suggests an impaired covert orienting response or an inability to shift the attention to meaningful auditory stimuli (37). Only one other study, to our knowledge, examined P3a in bipolar disorder (bipolar II disorder only), and found an intact P3a response (29). Whether P3a deficits are a consistent finding in patients with bipolar disorder remains to be seen. The magnitude of the P3a deficits in the schizophrenia group was nonsignificantly smaller than that in the bipolar disorder group.
The finding of smaller MMN response over the left hemisphere relative to the right hemisphere across groups is consistent with studies of lateralization of MMN in normal subjects and patients with schizophrenia. There is evidence in healthy controls that MMN generators are stronger over right auditory cortex (58–60) and that the right hemisphere makes larger contributions to processing duration changes (e.g., 61, 62). Similarly, patients with schizophrenia were found to have larger MMN over the right compared with left hemisphere (63), and a stronger MMN dipole in the right hemisphere based on a magnetoencephalography study (64).
The finding of abnormal laterality in patients with bipolar I disorder is difficult to interpret because there is little information in the literature about differential cerebral asymmetry in the two subtypes of bipolar disorder. One study of diffusion tensor imaging reported that patients with bipolar I disorder had abnormal lateralized asymmetry in fractional anisotropy compared with controls (65). A study of magnetic resonance imaging reported a larger left ventricular size in patients with bipolar I disorder compared with both patients with bipolar II disorder and healthy control subjects (66). The lack of MMN laterality in the bipolar I disorder group, in contrast to all of the other groups, highlights the importance of distinguishing bipolar I from bipolar II disorder in EEG studies and supports the continued examination of the neurobiological and pathophysiological differences between these two subtypes.
Some limitations to be noted in our study include the fact that most of our patients with schizophrenia were taking antipsychotic medications at the time of EEG testing. However, given that antipsychotic medications did not have an effect on MMN or P3a in the patients with bipolar disorder, and previous studies have shown a lack of an effect of antipsychotics on MMN in patients with schizophrenia (e.g., 36), it is unlikely that they had a strong impact on the findings in the patients with schizophrenia. It also remains unclear whether MMN and P3a impairments are associated with clinical variables such as history of psychotic symptoms, because we had too few bipolar disorder subjects with a history of psychosis. Moreover, a longitudinal design will be needed to determine whether these deficits are stable over time and whether they become more pronounced during acute manic/hypomanic and depressive periods.
Although NMDA-receptor functioning was not directly assessed in our study, the MMN and P3a deficits in our bipolar disorder sample suggest that NMDA abnormalities are present in this disorder, given the involvement of NMDA in modulation of these components. Identifying common neurobiological disturbances between bipolar disorder and schizophrenia allows us to better define the boundaries between these two disorders, clarify etiology, and propose new treatment targets (67). Future studies will benefit from examining the relationship of MMN and P3a in bipolar disorder to clinical symptoms, higher-order cognitive processes, and social functioning.
In summary, we found impairments in both the MMN and P3a ERP components in bipolar disorder. At the earlier stage of automatic sensory discrimination reflected by MMN, bipolar disorder patients showed deficits that were similar to patients with schizophrenia, but to a lesser degree. This finding suggests that both groups of patients may have problems detecting changes in their auditory environment. At the slightly later stage of orienting to salient auditory stimuli reflected by P3a, bipolar disorder patients showed pronounced deficits, and these impairments were nonsignificantly larger than those in schizophrenia.
Acknowledgments
This work was supported by a grant from the National Institute of Mental Health (MH089634 to MFG). The authors wish to thank Crystal Gibson, Cory Tripp, Katie Weiner, Mark McGee, Christen Waldon, and Amanda Bender for their assistance with recruitment and testing.
Footnotes
Disclosures
The authors of this paper do not have any commercial or other relationships that could constitute a conflict of interest.
References
- 1.Brandon NJ, Millar JK, Korth C, Sive H, Singh KK, Sawa A. Understanding the role of DISC1 in psychiatric disease and during normal development. J Neurosci. 2009;29:12768–12775. doi: 10.1523/JNEUROSCI.3355-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook EH, Jr, Scherer SW. Copy-number variations associated with neuropsychiatric conditions. Nature. 2008;455:919–923. doi: 10.1038/nature07458. [DOI] [PubMed] [Google Scholar]
- 3.Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stefansson H, Ophoff RA, Steinberg S, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Ann Rev Clin Psychololgy. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JS, Kornhuber HH, Schmid-Burgk W, Holzmuller B. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci Lett. 1980;20:379–382. doi: 10.1016/0304-3940(80)90178-0. [DOI] [PubMed] [Google Scholar]
- 7.Law AJ, Deakin JF. Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport. 2001;12:2971–2974. doi: 10.1097/00001756-200109170-00043. [DOI] [PubMed] [Google Scholar]
- 8.McCullumsmith RE, Kristiansen LV, Beneyto M, Scarr E, Dean B, Meador-Woodruff JH. Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Res. 2007;1127:108–118. doi: 10.1016/j.brainres.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 10.Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans; psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 11.Strelnikov K. Can mismatch negativity be linked to synaptic processes? A glutamatergic approach to deviance detection. Brain Cogn. 2007;65:244–251. doi: 10.1016/j.bandc.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci USA. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heekeren K, Daumann J, Neukirch A, et al. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacol. 2008;199:77–88. doi: 10.1007/s00213-008-1129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naatanen R, Gaillard AW, Mantysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol. 1978;42:313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- 15.Naatanen R, Jiang D, Lavikainen J, Reinikainen K, Paavilainen P. Event-related potentials reveal a memory trace for temporal features. Neuroreport. 1993;5:310–312. doi: 10.1097/00001756-199312000-00033. [DOI] [PubMed] [Google Scholar]
- 16.Sculthorpe LD, Ouellet DR, Campbell KB. MMN elicitation during natural sleep to violations of an auditory pattern. Brain Res. 2009;1290:52–62. doi: 10.1016/j.brainres.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Fischer C, Morlet D, Giard M. Mismatch negativity and N100 in comatose patients. Audiol Neurootol. 2000;5:192–197. doi: 10.1159/000013880. [DOI] [PubMed] [Google Scholar]
- 18.Draganova R, Eswaran H, Murphy P, Huotilainen M, Lowery C, Preissl H. Sound frequency change detection in fetuses and newborns, a magnetoencephalographic study. Neuroimage. 2005;28:354–361. doi: 10.1016/j.neuroimage.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Arch Gen Psychiatry. 2000;57:1131–1137. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- 20.Kiang M, Braff DL, Sprock J, Light GA. The relationship between preattentive sensory processing deficits and age in schizophrenia patients. Clin Neurophysiol. 2009;120:1949–1957. doi: 10.1016/j.clinph.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. Am J Psychiatry. 2010;167:818–827. doi: 10.1176/appi.ajp.2010.09030338. [DOI] [PubMed] [Google Scholar]
- 22.Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry. 1991;30:1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- 23.Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry. 2006;59:762–772. doi: 10.1016/j.biopsych.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 24.Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 25.Braff DL, Greenwood TA, Swerdlow NR, Light GA, Schork NJ. Advances in endophenotyping schizophrenia. World Psychiatry. 2008;7:11–18. doi: 10.1002/j.2051-5545.2008.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawakubo Y, Kamio S, Nose T, et al. Phonetic mismatch negativity predicts social skills acquisition in schizophrenia. Psychiatry Res. 2007;152:261–265. doi: 10.1016/j.psychres.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62:127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 28.Light GA, Swerdlow NR, Braff DL. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J Cogn Neurosci. 2007;19:1624–1632. doi: 10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson S, Barder HE, Hellvin T, Løvdahl H, Malt UF. Neuropsychological and electrophysiological indices of neurocognitive dysfunction in bipolar II disorder. Bipolar Disord. 2008;10:888–899. doi: 10.1111/j.1399-5618.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 30.Catts SV, Shelley AM, Ward PB, et al. Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am J Psychiatry. 1995;152:213–219. doi: 10.1176/ajp.152.2.213. [DOI] [PubMed] [Google Scholar]
- 31.Hall MH, Schulze K, Rijsdijk F, et al. Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley Bipolar Twin and Family Study. Psychol Med. 2009;39:1277–1287. doi: 10.1017/S0033291709005261. [DOI] [PubMed] [Google Scholar]
- 32.Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umbricht D, Koller R, Schmid L, et al. How specific are deficits in mismatch negativity generation to schizophrenia? Biol Psychiatry. 2003;53:1120–1131. doi: 10.1016/s0006-3223(02)01642-6. [DOI] [PubMed] [Google Scholar]
- 34.Korostenskaja M, Dapsys K, Siurkute A, Maciulis V, Ruksenas O, Kahkonen S. Effects of olanzapine on auditory P300 and mismatch negativity (MMN) in schizophrenia spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:543–548. doi: 10.1016/j.pnpbp.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 35.Umbricht D, Javitt D, Novak G, et al. Effects of clozapine on auditory event-related potentials in schizophrenia. Biol Psychiatry. 1998;44:716–725. doi: 10.1016/s0006-3223(97)00524-6. [DOI] [PubMed] [Google Scholar]
- 36.Umbricht D, Javitt D, Novak G, et al. Effects of risperidone on auditory event-related potentials in schizophrenia. Int J Neuropsychopharmacol. 1999;2:299–304. doi: 10.1017/S1461145799001595. [DOI] [PubMed] [Google Scholar]
- 37.Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci Biobehav Rev. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- 38.Watson TD, Petrakis IL, Edgecombe J, Perrino A, Krystal JH, Mathalon DH. Modulation of the cortical processing of novel and target stimuli by drugs affecting glutamate and GABA neurotransmission. Int J Neuropsychopharmacol. 2009;12:357–370. doi: 10.1017/S1461145708009334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller-Gass A, Macdonald M, Schroger E, Sculthorpe L, Campbell K. Evidence for the auditory P3a reflecting an automatic process: elicitation during highly-focused continuous visual attention. Brain Res. 2007;1170:71–78. doi: 10.1016/j.brainres.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 40.Grillon C, Courchesne E, Ameli R, Geyer MA, Braff DL. Increased distractibility in schizophrenic patients. Electrophysiologic and behavioral evidence. Arch Gen Psychiatry. 1990;47:171–179. doi: 10.1001/archpsyc.1990.01810140071010. [DOI] [PubMed] [Google Scholar]
- 41.Grzella I, Muller BW, Oades RD, et al. Novelty-elicited mismatch negativity in patients with schizophrenia on admission and discharge. J Psychiatry Neurosci. 2001;26:235–246. [PMC free article] [PubMed] [Google Scholar]
- 42.Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol Med. 2012;42:85–97. doi: 10.1017/S0033291711001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turetsky B, Colbath EA, Gur RE. P300 subcomponent abnormalities in schizophrenia: II. Longitudinal stability and relationship to symptom change. Biol Psychiatry. 1998;43:31–39. doi: 10.1016/s0006-3223(97)00261-8. [DOI] [PubMed] [Google Scholar]
- 44.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition. New York: New York State Psychiatric Institute; 1997. [Google Scholar]
- 45.First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. New York: New York State Psychiatric Institute; 1996. [Google Scholar]
- 46.Wilkinson GS. Wide Range Achievement Test–Revision 3. Wilmington: Jastak Association; 1993. [Google Scholar]
- 47.Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Brief Psychiatric Rating Scale (BPRS) expanded version: scales, anchor points, and administration manual. Int J Meth Psychiatric Res. 1993;3:227–243. [Google Scholar]
- 48.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 50.Goodman SH, Sewell DR, Cooley EL, Leavitt N. Assessing levels of adaptive functioning: the Role Functioning Scale. Community Ment Health J. 1993;29:119–131. doi: 10.1007/BF00756338. [DOI] [PubMed] [Google Scholar]
- 51.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 52.Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Res. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- 53.Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- 54.Picton TW, Bentin S, Berg P, et al. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiol. 2000;37:127–152. [PubMed] [Google Scholar]
- 55.Michie PT, Innes-Brown H, Todd J, Jablensky AV. Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biol Psychiatry. 2002;52:749–758. doi: 10.1016/s0006-3223(02)01379-3. [DOI] [PubMed] [Google Scholar]
- 56.Wynn JK, Sugar C, Horan WP, Kern R, Green MF. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biol Psychiatry. 2010;67:940–947. doi: 10.1016/j.biopsych.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 58.Doeller CF, Opitz B, Mecklinger A, Krick C, Reith W, Schroger E. Prefrontal cortex involvement in preattentive auditory deviance detection: neuroimaging and electrophysiological evidence. Neuroimage. 2003;20:1270–1282. doi: 10.1016/S1053-8119(03)00389-6. [DOI] [PubMed] [Google Scholar]
- 59.Grimm S, Roeber U, Trujillo-Barreto NJ, Schroger E. Mechanisms for detecting auditory temporal and spectral deviations operate over similar time windows but are divided differently between the two hemispheres. Neuroimage. 2006;32:275–282. doi: 10.1016/j.neuroimage.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 60.Shtyrov Y, Kujala T, Lyytinen H, Ilmoniemi RJ, Naatanen R. Auditory cortex evoked magnetic fields and lateralization of speech processing. Neuroreport. 2000;11:2893–2896. doi: 10.1097/00001756-200009110-00013. [DOI] [PubMed] [Google Scholar]
- 61.De Sanctis P, Molholm S, Shpaner M, Ritter W, Foxe JJ. Right hemispheric contributions to fine auditory temporal discriminations: high-density electrical mapping of the duration mismatch negativity (MMN) Front Integr Neurosci. 2009;3:5. doi: 10.3389/neuro.07.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mathiak K, Rapp A, Kircher TT, et al. Mismatch responses to randomized gradient switching noise as reflected by fMRI and whole-head magnetoencephalography. Hum Brain Mapp. 2002;16:190–195. doi: 10.1002/hbm.10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- 64.Pekkonen E, Katila H, Ahveninen J, Karhu J, Huotilainen M, Tiihonen J. Impaired temporal lobe processing of preattentive auditory discrimination in schizophrenia. Schizophr Bull. 2002;28:467–474. doi: 10.1093/oxfordjournals.schbul.a006954. [DOI] [PubMed] [Google Scholar]
- 65.Versace A, Almeida JR, Hassel S, et al. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry. 2008;65:1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hauser P, Matochik J, Altshuler LL, et al. MRI-based measurements of temporal lobe and ventricular structures in patients with bipolar I and bipolar II disorders. J Affect Disord. 2000;60:25–32. doi: 10.1016/s0165-0327(99)00154-8. [DOI] [PubMed] [Google Scholar]
- 67.Sanislow CA, Pine DS, Quinn KJ, et al. Developing constructs for psychopathology research: research domain criteria. J Abnorm Psychol. 2010;119:631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]