Abstract
Background
There are few psychosocial interventions specifically focused on improved treatment adherence in people with bipolar disorder (BD). Customized adherence enhancement (CAE) is a needs-based, manualized approach intended to improve medication adherence in individuals with BD. This was a six-month prospective trial of a CAE among 43 medication non-adherent individuals with BD who were receiving treatment in a community mental health clinic (CMHC).
Methods
CAE was flexibly administered in modules applied as indicated by an initial adherence vulnerabilities screening. Screening identified reasons for non-adherence and modules were then administered using pre-set criteria. CAE effects were evaluated at six-week, three-month, and six-month follow-up. The six-month follow-up was our primary time point of interest. The primary outcome was change from baseline in adherence using the Tablets Routine Questionnaire (TRQ) and pill counts. Secondary outcomes included change from baseline in BD symptoms [Hamilton Depression Rating Scale (HAM-D), Young Mania Rating Scale (YMRS), and Brief Psychiatric Rating Scale (BPRS)].
Results
Subjects completed 86% of scheduled sessions with only two individuals (5%) not participating in any sessions. The number of dropouts at six months was 12 (28%). Mean baseline non-adherence by TRQ was 48% [standard error (SE) 4.8%] missed tablets within the past week, and 51% (4.1%) missed tablets within the past month. At six-month follow-up, mean TRQ non-adherence improved to 25% (6.8%) missed tablets for the past week (p = 0.002), and 21% (5.5%) for the past month (p < 0.001). Symptoms improved with change in baseline mean (SE) BPRS of 43.6 (1.8) versus endpoint of 36.1 (2.3) (p = 0.001), and baseline mean (SE) HAM-D of 17.8 (1.1) versus endpoint of 15.3 (1.6) (p = 0.044).
Conclusion
CAE was associated with improvements in adherence, symptoms, and functional status. Controlled trials are needed to confirm these preliminary findings.
Keywords: adherence, antipsychotic medications, bipolar disorder, compliance, mania, mood stabilizers
A cornerstone of treatment uniformly recommended by guidelines on illness management for individuals with bipolar disorder (BD) is mood stabilizing medication such as lithium, anticonvulsants, or atypical antipsychotic drugs (1–4). However, approximately one in two individuals with BD are non-adherent with medication (5–8), often leading to substantial personal and financial burden through relapse and increased resource use (9–15). Gonzalez-Pinto et al. (16) reported a 5.2-fold increased suicide rate in BD patients with poor adherence compared to adherent BD patients. Sub-optimal adherence occurs across the full spectrum of classes of pharmacologic agents for BD (5, 6, 17, 18), and the majority of reports suggest that BD medication adherence rates are similar for older compounds such as lithium compared to the newer compounds (19). Some sub-groups of individuals with BD, such as minorities, are at particularly high risk for non-adherence and poor outcomes (20). Additionally, there is increasing awareness that medication may be neuroprotective for people with BD, minimizing the neuronal atrophy and degeneration seen over the life-course (21–26), and that non-adherent individuals may be particularly prone to these sequelae.
Reasons for non-adherence among individuals with BD are multi-dimensional, including patient knowledge, attitudes, and expectation towards illness and treatment, comorbidities, relationship with care providers, and social pressures or stigma (8, 27–30). Adherence attitudes and behaviors reflect the unique features of the individual, their environment, and their personal treatment experience (31–33). Previous work by these investigators found that inaccurate/incomplete information on BD treatments, problems with medication routines, fear of side effects and substance abuse are key impediments to adherence for many individuals with BD (5, 6, 17, 18, 27, 29, 31–51).
While an ample literature documents the importance of targeting adherence to improve outcomes in BD, the literature on interventions to improve treatment adherence is surprisingly limited (19, 48, 52–54). Current evidence-based psychosocial approaches for BD (55–59) have generally not focused specifically upon adherence, but rather deal with the full scope of problem areas for individuals with BD (19). Additionally, these interventions are characterized by high time duration and intensity, are not targeted for poorly adherent patients, and may be best suited for implementation in resource-heavy specialty settings (58, 59). A 2010 publication by an internationally-recognized BD research group (19) noted only five published interventional studies specifically focused on BD adherence, with the largest study including only 60 subjects, follow-up ranging from three to six months, and only two of these studies using a controlled methodology. The authors of this recent review advocated for a ‘needs-based approach’ that matches briefer interventions to core problem areas for BD patients (19). Given the urgent need for interventions to enhance adherence among BD patients that: (i) are at high risk for future non-adherence based upon past treatment history; (ii) may not have access to (or interest in) long-term, high-intensity, specialized care; and (iii) are informed by mixed–methods (qualitative and quantitative) findings in non-adherent and adherent patients with BD, our group has developed a needs-based approach intended to specifically address reasons for sub-optimal adherence in vulnerable BD patients.
This was a prospective trial of a psychosocial intervention [customized adherence enhancement (CAE)] among 43 medication non-adherent individuals with BD who were receiving care in a community mental health clinic (CMHC). We hypothesized that CAE would improve medication treatment adherence in this high-risk group.
Methods
Study overview
This NIMH-funded study (NIMH R34MH078967) was a six-month, prospective, uncontrolled trial evaluating the feasibility, acceptability and preliminary efficacy of CAE in 43 poorly adherent BD patients. CAE was prospectively and flexibly administered as a series of psychosocial intervention modules applied as indicated by response to an initial adherence vulnerabilities screening. Vulnerability screening identified reasons for non-adherence and intervention modules were then administered using pre-set criteria. CAE modules were implemented over a four-week period and the effects of CAE were evaluated at six-week, three-month, and six-month follow-up. Primary outcome was change from baseline in adherence. Secondary outcomes were change in BD symptoms, global psychopathology and functional status. The study was approved by the local Institutional Review Board (IRB).
Participants
Prospective study participants were referred from CMHC treating clinicians or were self-referred in response to posted IRB-approved study advertisements. Inclusion criteria were that individuals were ages 18 and older; had type I or type II BD confirmed by the Mini-International Neuropsychiatric Interview (MINI) (60); were poorly adherent with evidence-based BD medication (lithium, antipsychotics, or anticonvulsants) defined as missing 30% of more of medication within the past week or past 30 days as measured by the Tablet Routines Questionnaire (TRQ) or by pill counts; had BD for at least two years; were prescribed evidence-based mood stabilizing medication for at least six months and were receiving care at the enrollment site CMHC. To ensure a broad representation of ‘real-world’ non-adherent BD patients, only individuals who were unable or unwilling to participate in interviews and those at immediate high risk for suicide were excluded.
Intervention
CAE is a manualized behavioral intervention delivered as a series of four modules whose use is determined based upon an individual’s identified treatment adherence vulnerabilities and which are described in greater detail elsewhere (61). The modules are Psychoeducation on BD, Modified Motivational Enhancement Therapy (MET), coaching on Communication with Providers, and on Medication Routines. Adherence vulnerabilities (attitudes consistent with medication treatment non-adherence) and reasons for non-adherence were evaluated with standardized measures—the Attitudes towards Mood Stabilizer Questionnaire (AMSQ) (62–64) and the Rating of Medication Influences scale (ROMI) (65). Based upon the results of the adherence vulnerabilities screen, individuals received one or more behavioral treatment modules.
CAE was delivered over four weekly, individual, in-person, 60-minute sessions and up to two follow-up telephone sessions. The CAE intervention is intended to be a brief adjunct to standard mental health treatments, and all individuals continued to receive treatment with their regular mental health provider(s). Providers received information on patient enrolment and the specific CAE module assignment determined at baseline. CAE was delivered by a Ph.D. level psychologist (senior interventionist) and two non-Ph.D. interventionists. An intervention manual provided explicit guidelines regarding how modules could be co-administered in single or multiple sessions to minimize redundancy and time/effort burden.
Fidelity to the intervention
In order to increase generalizability to interventionists of diverse background and training, the investigators chose two non-Ph.D. interventionists to learn and implement CAE, a pre-Master’s level psychology trainee, and a PGY3 psychiatry resident. The three-month training included the following steps: (i) observation of the senior interventionist; (ii) videotaped practice with actors; and (iii) evaluation on pre-set fidelity criteria with ≥ 80% minimum cut-off. The fidelity criteria included: (i) establishes and maintains constructive rapport with the participant; (ii) administers the correct module in the corresponding session according to the Module Integration Chart; (iii) completes the session on time; (iv) utilizes the manual script; (v) completes worksheets according to the manual; (vi) addresses the main reasons for the individual's difficulties with adherence as it relates to the module; (vii) allots sufficient time for and appropriately answers questions or describes concepts that are unclear to the participant; and (viii) sets up next follow-up time/visit, provides contact information, and reviews recommended procedures for rescheduling if needed. Independent administration of junior interventionists was only permitted once the fidelity cut-offs were met.
Measures
The primary outcome was change in adherence behavior and adherence attitudes. Secondary outcomes were change from baseline in BD symptoms, global psychopathology and functional status. All outcome measures were evaluated at baseline, at six weeks, three months, and six months follow-up. While individuals were not paid to participate in the CAE intervention, they did receive a nominal compensation for participation in the research assessments. The compensation, given regardless of CAE session attendance, was $20 for each screening/baseline, six-week, and three-month interview and $40 for the final/six-month interview.
Treatment adherence behavior
Adherence behavior was evaluated with the Tablet Routines Questionnaire (TRQ), (62, 64) and by pill counts. For the TRQ and pill counts, adherence was assessed for each evidence based BD treatment agent (medication prescribed for three months or longer on a daily basis). For individuals on more than one agent to stabilize mood, adherence was assessed for each individual agent, and then an average adherence was calculated.
TRQ
The self-reported TRQ shows a high correlation with lithium levels (62, 64) and identifies partial and full adherence in the past seven and past 30 days. The TRQ has demonstrated statistically significant association with past non-adherence, repeated past non-adherence, any non-adherence in the past month, and non-adherence in the past week (χ2 = 7.2, df = 6, p = 0.03). Compared with non-adherence in the past two years, missing 30% or more of prescribed mood stabilizers in the past week has a specificity of 100% and a sensitivity of 65%. Compared with non-adherence in the past week, it has a specificity of 87% and a sensitivity of 84% (62). For this study, the TRQ was slightly modified to derive an exact proportion of medication taken in the past seven and the past 30 days.
Pill counts
Participants were asked to bring in their bottles of active medications. The pill-counts were then used to determine the proportion of medications taken to medications prescribed.
Treatment adherence attitudes
Adherence attitudes were measured with the 10-item version of the Drug Attitude Inventory (DAI) (66) and the Morisky Scale (67). While originally developed to assess patients with schizophrenia being treated with antipsychotic medications, the DAI has been widely utilized with other seriously mentally ill populations (68). The DAI is a simple, true-false format questionnaire that assesses domains of patient’s attitudes including positive and negative experience, locus of control, and attitudes towards health. The Morisky Scale is a four-item measure (Cronbach’s α = 0.61) based on a subject’s self-report of adherence. A higher score indicates poorer treatment adherence.
BD symptoms, global psychopathology, and functional status
Symptoms were measured with the Hamilton Rating Scale for Depression (HAM-D) (21), the Young Mania Rating Scale (YMRS) (22), and the Brief Psychiatric Rating Scale (BPRS) (23). Global psychopathology was measured with the Clinical Global Impression for Bipolar Disorder [(CGI-BP): severity of overall bipolar illness] (69). Functional status was measured with the Global Assessment of Functioning (GAF) (70).
Data analysis
Descriptive analyses examined changes over time for the entire sample receiving CAE looking at percentage change in treatment adherence with medication (TRQ score) at three months and six months compared with baseline. Given extensive missing pill count data due to participants failing to bring their pill bottles to study visits, the TRQ was used as the primary measure of adherence change. Paired t-tests or Wilcoxon signed-rank tests (WSRT) were used to test significance for change in adherence, depending on whether the normality assumption for the difference values appeared to be satisfied. Median scores are reported as well. In some cases, a floor effect was present in the post-treatment measurements (such as when being fully adherent), which is a reason for discrepancies in mean versus median values when they arose.
Longitudinal mixed models were used to analyze repeated measurements of TRQ non-adherence at baseline, six weeks, three months, and six months. Models of TRQ non-adherence for both the past month and the past week as a dependent variable were fit with various repeated covariance structures such as compound symmetry and first-order autoregressive structures. Time was viewed as a categorical variable and fit as a fixed effect. Type I error (alpha) was set to 0.01 to allow for multiple comparisons.
Results
Screening and enrollment
Among the 96 individuals screened for the study, 44 (45.8%) met inclusion criteria and of those, 43 were enrolled in the study. The most common reasons for screen failure were being lost to follow-up after initial contact (n = 16), not being a patient at the CMHC (n = 10), and not being prescribed evidence-based mood stabilizing medication for at least six months (n = 7). Table 1 shows demographic and clinical characteristics of enrolled participants. The majority were minorities (mainly African American) comprising 60.4% (n = 26) of the sample. Baseline illness severity was generally in the moderately–severe range with a mean BPRS of 43.6 and standard error (SE) = 1.8, mean HAM-D of 17.8 (SE = 1.1), mean GAF of 51.6 (SE = 1.2), and mean CGI of 4.4 (SE = 0.16).
Table 1.
Baseline demographic and clinical characteristics of sub-optimally adherent community mental health clinic patients with bipolar disorder participating in a prospective trial of customized adherence enhancement
| Variable | N | % |
|---|---|---|
| Gender (female) | 30 | 69.8 |
| Race | ||
| Caucasian | 17 | 39.5 |
| African American | 25 | 58.1 |
| Native American | 1 | 2.3 |
| Ethnicity (Hispanic) | 3 | 7.0 |
| Diagnosis | ||
| Bipolar I disorder | 39 | 90.7 |
| Bipolar II disorder | 4 | 9.3 |
| Mean | Standard deviation | |
| Age | 38.4 | 11.2 |
| HAM-D | 17.8 | 7.2 |
| YMRS | 14.2 | 7.8 |
| BPRS | 43.6 | 12.0 |
| CGI-BPa | 4.4 | 1.0 |
| TRQ (% non-adherence) | ||
| Last 7 days | 48.0 | 31.7 |
| Last 30 days | 51.4 | 27.1 |
| Pill counts (% non-adherence)b | 58.2 | 26.5 |
| DAI | 6.5 | 2.1 |
| GAF | 51.6 | 8.0 |
HAM-D = Hamilton Depression Rating Scale; YMRS = Young Mania Rating Scale; BPRS = Brief Psychiatric Rating Scale; CGI-BP = Clinical Global Impression for Bipolar Disorder; TRQ = Tablets Routine Questionnaire; DAI = Drug Attitude Inventory; GAF = Global Assessment of Functioning.
Severity of overall bipolar illness.
Last 30 days.
CAE module assignment
There were 21 (48.8%) individuals assigned to receive all four CAE treatment modules, 19 (44.2%) assigned to receive three modules, two (4.7%) assigned to receive two modules, and one individual (2.3%) was assigned a single module. Module assignment was 97.7% (n = 42) for Psychoeducation, 95.3% (n = 41) for Medication Routines, 86% (n = 37) for Communication with Providers, and 60.5% (n = 26) for MET.
Participation and retention
Participation was generally good, with subjects completing 85.5% of scheduled sessions. Only two (4.7%) of the enrolled participants did not participate in any sessions. Total dropout/lost to follow-up at the three-month and six-month time points was 10 (23.3%) and 12 (27.9%), respectively.
Improvement in adherence
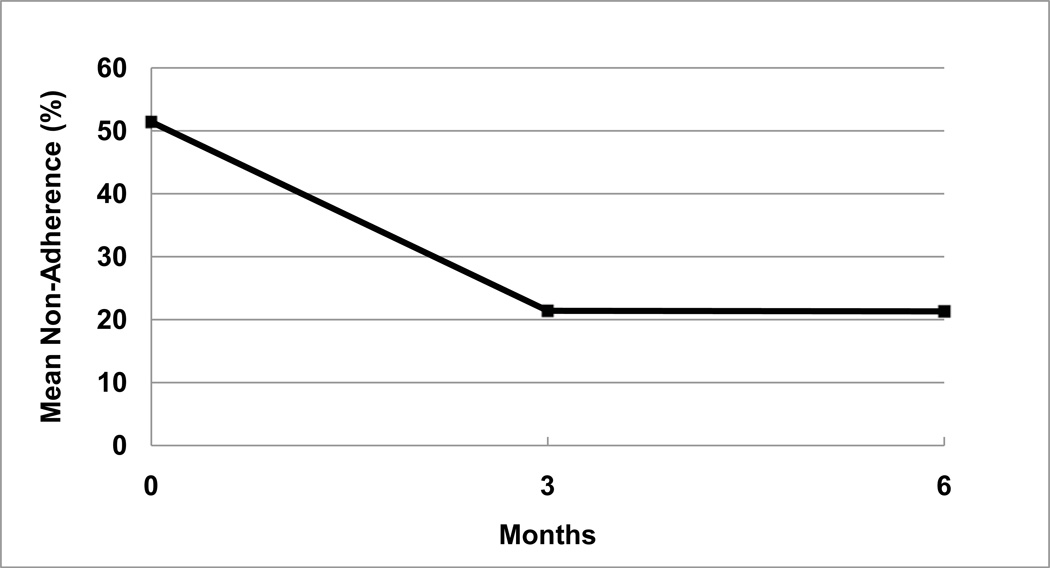
As noted in Table 2, mean non-adherence at baseline as measured with the TRQ was 48.0% [SE = 4.8%, median (MDN) = 43.0%] missed tablets within the past week, and 51.4% (SE = 4.1%, MDN = 43.0%) missed tablets within the past month. Over a three-month period, mean TRQ non-adherence improved to 24% (SE = 6.4%, MDN = 0%) missed tablets for the past week, and 21.4% (SE = 5.6%, MDN = 0%) for the past month, n = 33. Using WSRT, tests of median differences being zero were rejected in both cases at p = 0.002 (Z = −3.054) and p < 0.001 (Z = −3.753) respectively, indicating significant improvement in adherence. As noted in Table 2 and in Figure 1, over a six-month period, mean TRQ non-adherence improved from baseline to 25.2% missed tablets (SE = 6.8%, MDN = 3.5%) for the past week, and 21.3% missed tablets (SE = 5.5%, MDN = 7.0%) for the past month, n = 28. Tests of median differences being zero were also rejected in both cases at p = 0.01 (WSRT, Z = −2.561) and p < 0.001 (WSRT, Z = −3.679) respectively, indicating significant sustained improvement in adherence. Longitudinal mixed models for TRQ adherence for both the past month and the past week as dependent variables were fit. Using compound symmetry as the repeated covariance structure, which appeared to fit well, the fixed effect of time was found to be statistically significant for both TRQ past month (p < 0.001) and TRQ past week (p = 0.002), indicating improved adherence over time.
Table 2.
Improvements in treatment adherence among sub-optimally adherent community mental health clinic patients with bipolar disorder participating in a prospective trial of customized adherence enhancement
| Variable | Baseline Mean (SE), median |
Six-week Mean (SE), median |
Three-month Mean (SE), median |
Six-month Mean (SE), median |
|---|---|---|---|---|
| Non-adherence TRQ (last week)a | 48.0 (4.8), 43.0 | 23.5 (5.1), 14.0 | 24.0 (6.4), 0 | 25.2 (6.8), 3.5 |
| Non-adherence TRQ (last month)a | 51.4 (4.1), 43.0 | 20.7 (4.2), 14.0 | 21.4 (5.6), 0 | 21.3 (5.5), 7.0 |
| Pill countsa | 57.6 (7.6), 47.0 | 58.8 (20.7), 72.0 | 38.0 (19.2), 29.0 | 35.3 (9.9), 27.5 |
| Morisky Rating Scaleb | 3.0 (0.2), 3.0 | – | – | 1.3 (0.3), 1.0 |
| Drug Attitude Inventoryc | 6.5 (0.3), 7.0 | 7.5 (0.3), 8.0 | 7.8 (0.4), 8.0 | 8.1 (0.4), 9.0 |
Tablets Routine Questionnaire (TRQ) and pill count values indicate the percent of non-adherence or missed tablets, so that smaller values indicate better adherence.
Lower Morisky Rating Scale values indicate better adherence.
Higher values on the Drug Attitude Inventory scale indicate better adherence.
Fig. 1.
Adherence improvement with customized adherence enhancement in patients with bipolar disorder.
Mean baseline past month non-adherence as measured by pill counts was somewhat higher than TRQ, 57.6% (SE = 7.6%), but only one-third of participants provided pill bottles, resulting in substantial missing data. There were only two participants who brought in pill bottles at both baseline and three-month visits and there was only one participant who brought in pill bottles at both baseline and at six-month visits, limiting ability to assess change using pill counts.
The Morisky Rating Scale improved from a baseline mean of 3.0 (SE = 0.17, MDN = 3.0) to an endpoint mean at six-month of 1.3 (SE = 0.26, MDN = 1.0; WSRT, Z = −3.923, p < 0.001). The DAI improved from a baseline of 6.5 (SE = 0.32, MDN = 7.0) to a three-month mean value of 7.8 (SE = 0.39, MDN = 8.0; WSRT, Z = 2.815, p = 0.005) and a six-month mean value of 8.1 [SE = 0.40, MDN = 9.0; t(30) = 4.252, p < 0.001].
Improvement in symptoms, global psychopathology, and functioning
Secondary outcome measures were BD symptoms (YMRS, HAM-D, and BPRS), global psychopathology (CGI: severity of overall bipolar illness), and functional status (GAF). Depression and mania symptoms did not show significant improvement in change from baseline as measured by three-month mean HAM-D scores, with baseline mean of 17.8 (SE = 1.1, MDN = 18.5) versus endpoint mean of 16.2 [SE = 1.2, MDN = 16.0; t(31) = −1.182, p = 0.246], and YMRS scores, with baseline mean of 14.2 (SE = 1.2, MDN = 14.0) versus endpoint mean of 11.2 (SE = 1.4, MDN = 9.0; WSRT, Z = −1.638, p = 0.101). There was improvement in BPRS scores, with baseline mean of 43.6 (SE = 1.8, MDN = 42.5) versus endpoint mean of 37.3 (SE = 2.1, MDN = 36.0; WSRT, Z = −2.931, p = 0.003). CGI scores did not significantly improve from a baseline mean of 4.4 (SE = 0.16, MDN = 4.0) to endpoint of 3.9 [SE = 0.21, MDN = 4.0; t(31) = −1.717, p = 0.096]. GAF scores also did not significantly improve from a baseline mean of 51.6 (SE = 1.2, MDN = 51.0) to endpoint mean of 55.7 (SE = 1.3, MDN = 51.0; WSRT, Z = 1.797, p = 0.072).
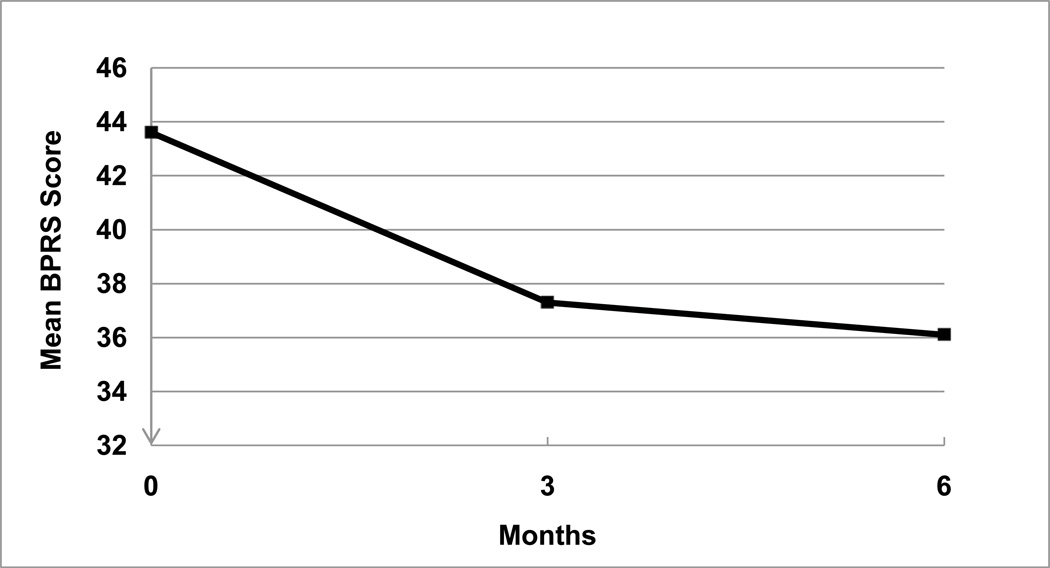
In contrast to the modest change in symptoms and functioning at three-month follow-up, clinical outcomes at six months showed clear improvement. Figure 2 shows change over six month in BPRS. Change from baseline in six-month mean BPRS scores went from baseline mean of 43.6 (SE = 1.8, MDN = 42.5) to an endpoint mean of 36.1 (SE = 2.3, MDN = 36.0; WSRT, Z = −3.267, p = 0.001), YMRS from baseline mean of 14.2 (SE = 1.2, MDN = 14.0) to endpoint mean of 9.6 [SE = 1.0, MDN = 9.0; t(29) = −3.404, p = 0.002], and HAM-D from baseline mean of 17.8 (SE = 1.1, MDN = 18.5) to endpoint mean of 15.3 (SE = 1.6, MDN = 15.0; WSRT, Z = −2.010, p = 0.044). CGI scores improved from a baseline mean of 4.4 (SE = 0.16, MDN = 4.0) to endpoint of 3.6 [SE = 0.24, MDN = 3.0; t(29) = −3.657, p = 0.001]. GAF scores improved from baseline mean of 51.6 (SE = 1.2, MDN = 51.0) to endpoint mean of 58.0 [SE = 1.7, MDN = 60.0; t(29) = 3.671, p = 0.001].
Fig. 2.
Change in Brief Psychiatric Rating Scale (BPRS) scores among bipolar disorder patients on customized adherence enhancement.
Participant acceptability
Participants were queried on perceived content comprehensiveness, relevance, benefit, and relative burden as well as acceptability of the treatment format (their satisfaction with length, timing, scheduling and logistics). Among the 32 participants who completed the CAE intervention, 81.3% (n = 26) strongly agreed, and the remainder agreed that CAE was useful. Most (96.9%, n = 31) strongly agreed or agreed that CAE addressed all important issues specific to their situation. Most individuals (81.3%, n = 26) felt that CAE benefit exceeded burden and most (87.5%, n = 28) felt that CAE was about right with respect to number of sessions and timing. Overall, there is strong evidence that the participants found the CAE intervention to be a positive experience.
Discussion
This prospective trial of a manualized psychosocial intervention designed to improve treatment adherence among sub-optimally adherent CMHC patients with BD was associated with improved adherence at three- and six-month follow-up. While BD symptoms, global psychopathology and functioning were mostly unchanged at three-month follow-up, by six-month follow-up these measures did show significant improvement. While interpretation of study findings must be balanced with considerations of study limitations such as the uncontrolled design, relatively short follow-up period, single site setting, and the fact that study participants were all either self-referred or referred by providers, the positive findings in this fairly ill and vulnerable BD population are encouraging. Improvements in adherence behaviors and attitudes among formerly non-adherent patients who participated in CAE appeared to eventually lead to improved BD symptoms and functioning. We have recently published a second CAE pilot (55) that was nearly identical to the study presented here with the exceptions that it was only three months in duration, enrolled poorly adherent people with BD at our academic facility who were on maintenance antipsychotic medications, and the pilot study was funded by a pharmaceutical company (AstraZeneca). The second CAE pilot similarly suggested that CAE significantly improved adherence rates and BD symptoms.
The literature on interventions to improve treatment adherence is limited (34, 48, 52–53, 71–72). Currently accepted evidence-based psychosocial approaches for individuals with BD (55–59) have generally not focused specifically upon medication treatment adherence enhancement, but rather deal with the full scope of problem areas for individuals with BD. Additionally, these interventions require a significant time commitment, are not targeted towards poorly adherent patients, and may be best suited for implementation in resource-heavy specialty settings where highly trained or speciality staff access are readily available (57, 58).
Our data suggest that patients participating in CAE had substantial and clinically significant change in adherence going from a baseline of missing approximately 50% of prescribed BD medication treatment to missing only approximately 20% of prescribed BD medications at endpoint. Clinically relevant BD non-adherence has been defined as missing more than 20% of prescribed medication (41, 62). This improvement in medication –taking may have been the reason why patients had eventual improvement in symptoms and functional status. Although groups receiving differing numbers of modules are too small to draw definitive conclusions, it does not appear that TRQ improvement was a purely attentional effect. When we combined our data from the study presented here and our second CAE pilot (60) we found that there was no significant interaction between number of modules assigned and improvement on TRQ [tests done for both baseline to three month (full sample)] [F(2,60) = 1.35, p = 0.27] and baseline to six month [F(2,25) = 0.126, p = 0.882]. In the majority of cases (> 90%) the CAE modules were administered over four in-person sessions. As with number of modules, analysis of number of sessions did not appear to be related to TRQ improvement.
Unfortunately, the relatively short-term (six months) duration of the study does not permit an assessment of the trajectory of adherence as a lifestyle resulting from the intervention. While it is possible that improved adherence could reduce future potential for other negative outcomes known to be associated with non-adherence, such as relapse or suicide, it is not possible to make definitive conclusions regarding longer-term effects of CAE from our six-month study. Additionally, it is possible that the improvements in adherence and other clinical outcomes could have been due to other treatments received by these individuals at the CMHC.
The CAE intervention was readily taught and implemented in this study using non-Ph.D. interventionist staff, and appears practical to apply in typical clinical settings. The relative brevity of CAE may make the intervention acceptable to individuals who may not have access to (or interest in) long-term, high intensity, and specialized care. While there are differing levels of risk for future non-adherence and it is likely that the most non-adherent individuals with BD would not agree to participate in a research study, our findings suggest that the CAE approach is able to engage and improve outcomes for at least some poorly adherent patients with BD.
An additional limitation of our CAE pilot is that we did not collect cost data. An adherence enhancement intervention that is likely to advance the care of patients with BD must not only improve adherence and mental health status, but must be cost-effective. While we believe that the format of CAE is potentially cost-effective this needs to be evaluated in future controlled studies.
Conclusions
Results of this six-month prospective trial suggest that this manualized CAE intervention may be helpful for BD patients who are known to be sub-optimally adherent with prescribed BD medications. The intervention can be readily implemented in a CMHC setting, is able to engage a good proportion of non-adherent individuals, and can be delivered by non-Ph.D. interventionist staff. Given that nine out of every ten individuals with BD has seriously considered medication withdrawal, and at least a third of individuals with BD fail to take more than 70% of their prescribed medication (72, 73), approaches that are flexible and patient-focused, taking into account reasons for non-adherence for a specific individual, have the potential to improve health outcomes for high risk or vulnerable individuals with BD. Public health implications of effective adherence enhancing interventions could be substantial.
Acknowledgement
This study was supported by a grant from the National Institute of Mental Health (R34MH078967).
Footnotes
Portions of this data were presented at the 16th Biennial Winter Workshop in Psychoses 2011, January 30–February 2, 2011, Innsbruck, Austria.
Disclosures
MS has received funding for research from GlaxoSmithKline, AstraZeneca, Janssen, Merck, and Pfizer; and has been a consultant for Cognition Group and United Biosource (Bracket). CT has received funding for research from AstraZeneca. JL, WM-G, EF-C, CSB, and KAC do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision) Am J Psychiatry. 2002;159:1–50. [PubMed] [Google Scholar]
- 2.Keck PE, Jr, Perlis RH, Otto MW, Carpenter D, Ross R, Docherty JP. The Expert Consensus Guidelines: Treatment of Bipolar Disorder. Postgraduate Medicine Report: Special Report. 2004 Dec;:1–120. [Google Scholar]
- 3.Yatham LN, Kennedy SH, O'Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: consensus and controversies. Bipolar Disord. 2005;7(Suppl. 3):5–69. doi: 10.1111/j.1399-5618.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin GM, Young AH. The British Association for Psychopharmacology guidelines for treatment of bipolar disorder: a summary. J Psychopharmacol. 2003;17:3–6. [PubMed] [Google Scholar]
- 5.Sajatovic M, Valenstein M, Blow FC, Ganoczy D, Ignacio RV. Treatment adherence with antipsychotic medications in bipolar disorder. Bipolar Disord. 2006;8:232–241. doi: 10.1111/j.1399-5618.2006.00314.x. [DOI] [PubMed] [Google Scholar]
- 6.Sajatovic M, Valenstein M, Blow F, Ganoczy D, Ignacio R. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr Serv. 2007;58:855–863. doi: 10.1176/ps.2007.58.6.855. [DOI] [PubMed] [Google Scholar]
- 7.Lingam R, Scott J. Treatment non-adherence in affective disorders. Acta Psychiatr Scand. 2002;105:164–172. doi: 10.1034/j.1600-0447.2002.1r084.x. [DOI] [PubMed] [Google Scholar]
- 8.Perlick DA, Rosenheck RA, Kaczynski R, Kozma L. Medication non-adherence in bipolar disorder: a patient-centered review of research findings. Clinical Approaches in Bipolar Disorders. 2004;3:56–64. [Google Scholar]
- 9.Muller-Oerlinghausen B, Muser-Causemann B, Volk J. Suicides and parasuicides in a high-risk patient group on and off lithium long-term medication. J Affect Disord. 1992;25:261–269. doi: 10.1016/0165-0327(92)90084-j. [DOI] [PubMed] [Google Scholar]
- 10.Mander AJ, Loudon JB. Rapid recurrence of mania following abrupt discontinuation of lithium. Lancet. 1988;2:15–17. doi: 10.1016/s0140-6736(88)92947-9. [DOI] [PubMed] [Google Scholar]
- 11.Mander AJ. Use of lithium and early relapse in manic-depressive illness. Acta Psychiatr Scand. 1988;78:198–200. doi: 10.1111/j.1600-0447.1988.tb06323.x. [DOI] [PubMed] [Google Scholar]
- 12.Strakowski SM, Keck PE, Jr, McElroy SL, et al. Twelve-month outcome after a first hospitalization for affective psychosis. Arch Gen Psychiatry. 1998;55:49–55. doi: 10.1001/archpsyc.55.1.49. [DOI] [PubMed] [Google Scholar]
- 13.Tohen M, Greil W, Calabrese JR, et al. Olanzapine versus lithium in the maintenance treatment of bipolar disorder: a 12-month, randomized, double-blind, controlled clinical trial. Am J Psychiatry. 2005;162:1281–1290. doi: 10.1176/appi.ajp.162.7.1281. [DOI] [PubMed] [Google Scholar]
- 14.Svarstad BL, Shireman TI, Sweeney JK. Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatr Serv. 2001;52:805–811. doi: 10.1176/appi.ps.52.6.805. [DOI] [PubMed] [Google Scholar]
- 15.Scott J. Predicting medication non-adherence in severe affective disorders. Acta Neuropsychiatrica. 2000;12:128–130. doi: 10.1017/S0924270800035584. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Pinto A, Gonzalez C, Enjuto S, et al. Psychoeducation and cognitive-behavioral therapy in bipolar disorder: an update. Acta Psychiatr Scand. 2004;109:83–90. doi: 10.1046/j.0001-690x.2003.00240.x. [DOI] [PubMed] [Google Scholar]
- 17.Sajatovic M, Chen P, Dines P, Shirley ER. Psychoeducational approaches to medication adherence in patients with bipolar disorder. Dis Mang Health Outcomes. 2007;15:181–192. [Google Scholar]
- 18.Sajatovic M, Blow FC, Kales HC, Valenstein M, Ganoczy D, Ignacio RV. Age comparison of treatment adherence with antipsychotic medications among individuals with bipolar disorder. Int J Geriatr Psychiatry. 2007;22:992–998. doi: 10.1002/gps.1777. [DOI] [PubMed] [Google Scholar]
- 19.Berk L, Hallam KT, Colom F, et al. Enhancing medication adherence in patients with bipolar disorder. Hum Psychopharmacol. 2010;25:1–16. doi: 10.1002/hup.1081. [DOI] [PubMed] [Google Scholar]
- 20.Zeber JE, Copeland LA, Good CB, Fine MJ, Bauer MS, Kilbourne AM. Therapeutic alliance perceptions and medication adherence in patients with bipolar disorder. J Affect Disord. 2008;107:53–62. doi: 10.1016/j.jad.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 23.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 24.Shaltiel G, Chen G, Manji HK. Neurotrophic signaling cascades in the pathophysiology and treatment of bipolar disorder. Curr Opin Pharmacol. 2007;7:22–26. doi: 10.1016/j.coph.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Bearden CE, Thompson PM, Dalwani M, et al. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry. 2007;62:7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sassi RB, Brambilla P, Hatch JP, et al. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry. 2004;56:467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Sajatovic M, Biswas K, Kilbourne AK, Fenn H, Williford W, Bauer MS. Factors associated with prospective long-term treatment adherence among individuals with bipolar disorder. Psychiatr Serv. 2008;59:753–759. doi: 10.1176/ps.2008.59.7.753. [DOI] [PubMed] [Google Scholar]
- 28.Maarbjerg K, Aagaard J, Vestergaard P. Adherence to lithium prophylaxis: I. Clinical predictors and patient's reasons for nonadherence. Pharmacopsychiatry. 1988;21:121–125. doi: 10.1055/s-2007-1014662. [DOI] [PubMed] [Google Scholar]
- 29.Sajatovic M, Ignacio RV, West JA, et al. Predictors of nonadherence among individuals with bipolar disorder receiving treatment in a community mental health clinic. Compr Psychiatry. 2009;50:100–107. doi: 10.1016/j.comppsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeber JE, Miller AL, Copeland LA, et al. Medication adherence, ethnicity, and the influence of multiple psychosocial and financial barriers. Adm Policy Ment Health. 2011;38:86–95. doi: 10.1007/s10488-010-0304-1. [DOI] [PubMed] [Google Scholar]
- 31.Sajatovic M, Jenkins JH, Safavi R, et al. Personal and societal construction of illness among individuals with rapid-cycling bipolar disorder: a life-trajectory perspective. Am J Geriatr Psychiatry. 2008;16:718–726. doi: 10.1097/JGP.0b013e3180488346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sajatovic M, Davies M, Bauer MS, et al. Attitudes regarding the collaborative practice model and treatment adherence among individuals with bipolar disorder. Compr Psychiatry. 2005;46:272–277. doi: 10.1016/j.comppsych.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Clatworthy J, Bowskill R, Rank T, Parham R, Horne R. Adherence to medication in bipolar disorder: a qualitative study exploring the role of patients' beliefs about the condition and its treatment. Bipolar Disord. 2007;9:656–664. doi: 10.1111/j.1399-5618.2007.00434.x. [DOI] [PubMed] [Google Scholar]
- 34.Sajatovic M, Davies M, Hrouda DR. Enhancement of treatment adherence among patients with bipolar disorder. Psychiatr Serv. 2004;55:264–269. doi: 10.1176/appi.ps.55.3.264. [DOI] [PubMed] [Google Scholar]
- 35.Sajatovic M, Bauer MS, Kilbourne AM, Vertrees JE, Williford W. Self-reported medication treatment adherence among veterans with bipolar disorder. Psychiatr Serv. 2006;57:56–62. doi: 10.1176/appi.ps.57.1.56. [DOI] [PubMed] [Google Scholar]
- 36.Gianfrancesco FD, Rajagopalan K, Sajatovic M, Wang RH. Treatment adherence among patients with bipolar or manic disorder taking atypical and typical antipsychotics. J Clin Psychiatry. 2006;67:222–232. doi: 10.4088/jcp.v67n0208. [DOI] [PubMed] [Google Scholar]
- 37.Gianfrancesco FD, Sajatovic M, Rajagopalan K, Wang RH. Antipsychotic treatment adherence and associated mental health care use among individuals with bipolar disorder. Clin Ther. 2008;30:1358–1374. doi: 10.1016/s0149-2918(08)80062-8. [DOI] [PubMed] [Google Scholar]
- 38.Gianfrancesco FD, Sajatovic M, Rajagopalan K, Wang RH. The association between treatment adherence and antipsychotic dose among individuals with bipolar disorder. Int Clin Psychopharmacol. 2008;23:305–316. doi: 10.1097/YIC.0b013e32830b0f88. [DOI] [PubMed] [Google Scholar]
- 39.Gianfrancesco FD, Sajatovic M, Tafesse E, Wang RH. Association between antipsychotic combination therapy and treatment adherence among individuals with bipolar disorder. Ann Clin Psychiatry. 2009;21:3–16. [PubMed] [Google Scholar]
- 40.Velligan D, Sajatovic M, Valenstein M, et al. Methodological challenges in psychiatric treatment adherence research. Clin Schizophr Relat Psychoses. 2010;4:74–91. [PubMed] [Google Scholar]
- 41.Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(Suppl. 4):1–46. [PubMed] [Google Scholar]
- 42.Sajatovic M, Velligan D, Weiden PJ, Valenstein M, Ogedegbe G. Measurement of psychiatric treatment adherence. J Psychosom Res. 2010;69:591–599. doi: 10.1016/j.jpsychores.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kriegshauser K, Sajatovic M, Jenkins JH, et al. Gender differences in subjective experience and treatment of bipolar disorder. J Nerv Ment Dis. 2010;198:370–372. doi: 10.1097/NMD.0b013e3181da8ef7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sajatovic M, Elhaj O, Youngstrom EA, et al. Treatment adherence in individuals with rapid cycling bipolar disorder: results from a clinical-trial setting. J Clin Psychopharmacol. 2007;27:412–414. doi: 10.1097/01.jcp.0000280310.50871.ff. [DOI] [PubMed] [Google Scholar]
- 45.Davies MA, McBride L, Sajatovic M. The collaborative care practice model in the long-term care of individuals with bipolar disorder: a case study. J Psychiatr Ment Health Nurs. 2008;15:649–653. doi: 10.1111/j.1365-2850.2008.01287.x. [DOI] [PubMed] [Google Scholar]
- 46.Sajatovic M, Jenkins JH. Is antipsychotic medication stigmatizing for people with mental illness? Int Rev Psychiatry. 2007;19:107–112. doi: 10.1080/09540260701278911. [DOI] [PubMed] [Google Scholar]
- 47.Sajatovic M, Jenkins JH, Cassidy KA, Muzina DJ. Medication treatment perceptions, concerns and expectations among depressed individuals with Type I Bipolar Disorder. J Affect Disord. 2009;115:360–366. doi: 10.1016/j.jad.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sajatovic M, Davies MA, Ganocy SJ, et al. A preliminary trial of Life Goals Therapy (LGP) vs. treatment as usual among individuals with bipolar disorder. Psychiatr Serv. 2009;60:1182–1189. doi: 10.1176/appi.ps.60.9.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Busby K, Sajatovic M. Patient, treatment, and systems-level factors in non-adherence: A summary of the literature. CNS Neurosci Therapeu. 2010;16:308–315. doi: 10.1111/j.1755-5949.2010.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from the expert consensus guidelines. J Psychiatr Pract. 2010;16:306–324. doi: 10.1097/01.pra.0000388626.98662.a0. [DOI] [PubMed] [Google Scholar]
- 51.Devulapalli K, Ignacio RV, Weiden P, et al. Why do persons with bipolar disorder stop their medication? Psychopharmacol Bull. 2010;43:5–7. [PMC free article] [PubMed] [Google Scholar]
- 52.Riley W, Velligan DI, Sajatovic M, et al. Adherence to psychiatric treatments. Curr Med Literature: Psychiatry. 2009;20:89–96. [Google Scholar]
- 53.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 54.Gaudiano BA, Weinstock LM, Miller IW. Improving treatment adherence in patients with bipolar disorder and substance abuse: rationale and initial development of a novel psychosocial approach. J Psychiatr Pract. 2011;17:5–20. doi: 10.1097/01.pra.0000393840.18099.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miklowitz DJ, Simoneau TL, George EL, et al. Family-focused treatment of bipolar disorder: 1-year effects of a psychoeducational program in conjunction with pharmacotherapy. Biol Psychiatry. 2000;48:582–592. doi: 10.1016/s0006-3223(00)00931-8. [DOI] [PubMed] [Google Scholar]
- 56.Colom F, Vieta E, Martinez-Aran A, et al. A randomized trial on the efficacy of group psychoeducation in the prophylaxis of recurrences in bipolar patients whose disease is in remission. Arch Gen Psychiatry. 2003;60:402–407. doi: 10.1001/archpsyc.60.4.402. [DOI] [PubMed] [Google Scholar]
- 57.Lam DH, Watkins ER, Hayward P, et al. A randomized controlled study of cognitive therapy for relapse prevention for bipolar affective disorder: outcome of the first year. Arch Gen Psychiatry. 2003;60:145–152. doi: 10.1001/archpsyc.60.2.145. [DOI] [PubMed] [Google Scholar]
- 58.Miklowitz DJ, Otto MW, Wisniewski SR, et al. Psychotherapy, symptom outcomes, and role functioning over one year among patients with bipolar disorder. Psychiatr Serv. 2006;57:959–965. doi: 10.1176/ps.2006.57.7.959. [DOI] [PubMed] [Google Scholar]
- 59.Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry. 2007;64:419–426. doi: 10.1001/archpsyc.64.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl. 20):22–33. [PubMed] [Google Scholar]
- 61.Sajatovic M, Levin J, Tatsuoka C, et al. Customized adherence enhancement for individuals with bipolar disorder receiving atypical antipsychotic therapy. Psychiatr Serv. 2012;63:176–178. doi: 10.1176/appi.ps.201100133. [DOI] [PubMed] [Google Scholar]
- 62.Scott J, Pope M. Nonadherence with mood stabilizers: prevalence and predictors. J Clin Psychiatry. 2002;63:384–390. doi: 10.4088/jcp.v63n0502. [DOI] [PubMed] [Google Scholar]
- 63.Harvey NS. The development and descriptive use of the Lithium Attitudes Questionnaire. J Affect Disord. 1991;22:211–219. doi: 10.1016/0165-0327(91)90067-3. [DOI] [PubMed] [Google Scholar]
- 64.Peet M, Harvey NS. Lithium maintenance: 1. A standard education programme for patients. Br J Psychiatry. 1991;158:197–200. doi: 10.1192/bjp.158.2.197. [DOI] [PubMed] [Google Scholar]
- 65.Weiden P, Rapkin B, Mott T, et al. Rating of medication influences (ROMI) scale in schizophrenia. Schizophr Bull. 1994;20:297–310. doi: 10.1093/schbul/20.2.297. [DOI] [PubMed] [Google Scholar]
- 66.Awad AG. Subjective response to neuroleptics in schizophrenia. Schizophr Bull. 1993;19:609–618. doi: 10.1093/schbul/19.3.609. [DOI] [PubMed] [Google Scholar]
- 67.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 68.Sajatovic M, Ramirez L. Rating Scales in Mental Health. Cleveland: Lexi-Comp, Inc.; 2001. [Google Scholar]
- 69.Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73:159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 70.Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF) Br J Psychiatry. 1995;166:654–659. doi: 10.1192/bjp.166.5.654. [DOI] [PubMed] [Google Scholar]
- 71.Scott J, Pope M. Self-reported adherence to treatment with mood stabilizers, plasma levels, and psychiatric hospitalization. Am J Psychiatry. 2002;159:1927–1929. doi: 10.1176/appi.ajp.159.11.1927. [DOI] [PubMed] [Google Scholar]
- 72.Scott J, Tacchi MJ. A pilot study of concordance therapy for individuals with bipolar disorders who are non-adherent with lithium prophylaxis. Bipolar Disord. 2002;4:386–392. doi: 10.1034/j.1399-5618.2002.02242.x. [DOI] [PubMed] [Google Scholar]
- 73.Jamison KR, Gerner RH, Goodwin FK. Patient and physician attitudes toward lithium: relationship to compliance. Arch Gen Psychiatry. 1979;36:866–869. doi: 10.1001/archpsyc.1979.01780080040011. [DOI] [PubMed] [Google Scholar]