Abstract
Introduction
Aggressive management of hepatic neuroendocrine (NE) metastases improves symptoms and prolongs survival. Because of the rarity of these tumors, however, the best method for hepatic artery embolization has not been established. We hypothesized that in patients with hepatic NE metastases, hepatic artery chemoembolization (HACE) would result in better symptom improvement and survival compared to bland embolization (HAE).
Methods
Retrospective review identified all patients with NE hepatic metastases managed by HACE or HAE at three institutions from January 1996 through December 2007.
Results
We identified 100 patients managed by HACE (n=49) or HAE (n=51) that were similar with respect to age, gender, and primary tumor type. The percentage of patients experiencing morbidity, 30-day mortality, and symptom improvement were similar between the two groups (HACE vs. HAE: 2.4% vs. 6.6%; 0.8% vs. 1.8%; and 88% vs. 83%, respectively.) No differences in the median overall survival were observed between HACE and HAE from the time of the first embolization procedure (25.5 vs. 25.7 months, p=0.79). Multivariate analysis revealed that resection of the primary tumor predicted survival (73.8 vs. 19.4 months, p<0.04).
Conclusions
These data suggest that morbidity, mortality, symptom improvement, and overall survival are similar in patients with hepatic neuroendocrine metastases managed by chemo- or bland hepatic artery embolization.
Keywords: Chemoembolization, Embolization, Hepatic artery, Metastasis, Neuroendocrine tumor, Liver
Introduction
Neuroendocrine (NE) tumors comprise a heterogeneous group of similarly behaving cancers that include gastrointestinal carcinoid and pancreatic islet cell tumors. Due to the indolent nature of NE tumors, patients frequently present late in the disease course once metastases have spread to regional lymph nodes, the liver, and/or bone. Accordingly, NE tumors are the second most common cause of isolated hepatic metastases after colorectal adenocarcinoma.1 The treatment of hepatic NE metastases frequently involves a multidisciplinary approach because aggressive management has been shown to improve symptoms and prolong survival.2–4 Medical treatments including somatostatin analogs, chemotherapy, and external beam radiation have limited effectiveness on slowing disease progression but have been shown to reduce symptoms and improve quality of life, particularly the somatostatin analogs.5–7 More invasive treatments for patients with advanced disease include surgical resection, cryo- and radiofrequency ablation, transplantation, and various forms of hepatic arterial embolization.8–10
Over the past several years, a number of controversies have developed over the optimal treatment for patients with this disease due, in part, to the rare nature of NE tumors and the resultant lack of level I evidence. One particular debate that has emerged is whether hepatic arterial embolization should be performed with or without localized, intra-arterial chemotherapy. Many practitioners believe that hepatic artery chemoembolization (HACE) is superior to bland embolization (HAE) in terms of symptom control and survival, but no study to date fully supports this belief. Only two reports have directly addressed this question; but both have failed to show a statistically significant difference between HACE and HAE in patients with NE hepatic metastases.11–12 Therefore, we designed a multi-institutional study to test the hypothesis that HACE would result in better symptom improvement and overall survival than HAE with equivalent morbidity and mortality in patients with hepatic NE metastases.
Materials and Methods
Patient Population
Retrospective review identified all patients with NE hepatic metastases treated by HACE or HAE at three institutions from January 1996 to December 2007. The participating institutions were Indiana University (IU), the University of Wisconsin (UW), and the Medical College of Wisconsin (MCW). Prospective cancer registries, interventional radiology databases, and hospital records at each organization were utilized to identify eligible patients. At all three institutions, electronic medical records, clinic charts, radiological studies, and pathology reports were reviewed to gather patient demographics, symptom reporting, tumor characteristics, and treatment information. The diagnosis of NE tumor was confirmed by pathologic review of tissue samples. Standard cross-sectional imaging techniques were used to verify the presence of liver metastases. Approval for this study was obtained from the respective institutional review boards at IU, UW, and MCW.
Treatment Groups
Patients were grouped with respect to the treatment modality received: HACE or HAE. At each institution, the attending interventional radiologist selected which method of embolization to perform: HACE or HAE. Four patients who underwent both procedures during their care were excluded from the analyses. In addition, three recent patients who were treated by yttrium-90 radioembolization were excluded. The procedures were performed with particle embolization with or without iodized oil using either polyvinyl alcohol, gel foam, or embospheres (Fig. 1). The chemotherapies administered varied among institutions and included cisplatin, adriamycin, and mitomycin C. The timing between treatments, total number of embolizations performed, and extent of each procedure was at the discretion of the attending physicians, surgeons, and interventional radiologists. Factors considered in these decisions were multiple, such as presence of symptoms, performance status, and response to prior treatments. If patients underwent two separate procedures to address bilobar metastases (i.e., one right and one left hepatic artery procedure), these procedures were classified separately in our analyses. At all three institutions, protocols in compliance with hospital and national standards of care were used to obtain informed consent and screen laboratory values and to administer post-procedure intravenous fluids, analgesics, antibiotics, steroids, and antiemetics.
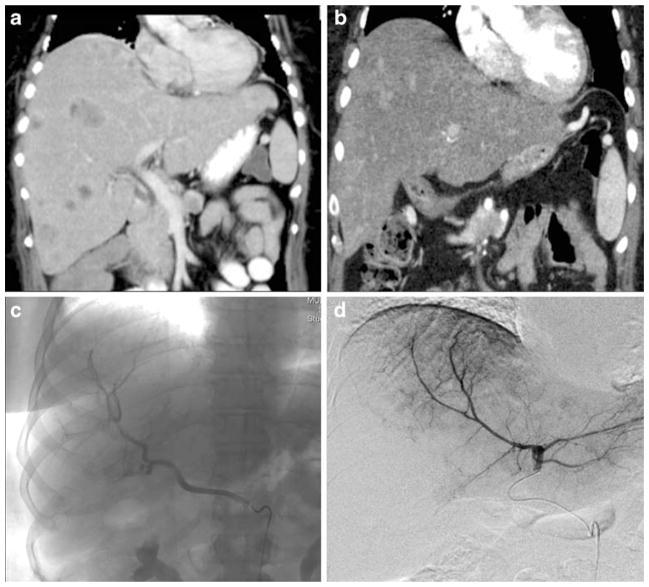
Figure 1.
CT imaging of a patient with multiple, bilobar NE hepatic metastases before (a) and after (b) bland HAE. This patient underwent sequential embolizations of her replaced right (c) and left (d) hepatic arteries with subsequent symptom improvement.
Outcomes
Our primary outcome measurement was overall survival which was calculated both from the time of diagnosis of metastatic disease and the time of first hepatic artery embolization until the time of death or date of last follow-up. In all patients, survival and follow-up data were obtained from respective hospital records including electronic medical records, clinic notes, cancer registries, and the Social Security Death Index database.
Improvement in symptoms was a secondary outcome measure in this study. We evaluated symptoms due to systemic hormone release, locoregional invasion, and mass effect. Therefore, symptoms were considered present if the patient reported flushing, diarrhea, or abdominal pain. Patients who reported complete alleviation or significant sustained relief of their symptoms were regarded as improved.
In addition, we analyzed procedure-related morbidity and mortality as secondary outcome measurements. Any complication that occurred during or within 30 days following an embolization procedure was considered morbidity. Patients who experienced “post-embolization syndrome” were not included in the morbidity analysis as symptoms were mild, common, and difficult to quantify retrospectively. Similarly, mortality was defined as a death by any cause within 30 days of an embolization.
Statistical Analysis
Groups were compared by Fisher’s exact or χ2 analysis where appropriate. Statistical significance was reached at p <0.05. Statistical Package for Social Sciences (SPSS) software version 11.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the survival data by the Kaplan–Meier actuarial method with statistical significance ascertained by log-rank analysis as well as to perform univariate and multivariate analyses.
Results
Demographics
One hundred patients (43 females and 57 males) with hepatic NE metastases managed by either HACE or HAE were identified at IU, UW, and MCW (Table 1). Mean age was 56 years (age range, 26–82 years). No significant differences in age or gender were found between the HACE and HAE treatment groups. The primary tumor type was carcinoid in 56 patients and islet cell in 44 patients. The two treatment groups, HACE and HAE, were similar with respect to the primary tumor type (% carcinoid 63 vs. 55, respectively) and location (% small bowel 61 and 51, respectively).
Table 1.
Patient Demographics and Characteristics
| HACE | HAE | p Value | |
|---|---|---|---|
| Number | 49 | 51 | |
| Age, years | 58±2 | 54±2 | 0.14 |
| Female, % | 47 | 37 | 0.33 |
| Tumor type | |||
| Carcinoid, % | 63 | 55 | 0.40 |
| Small bowel, % | 61 | 51 | |
| Colorectal, % | 2 | 0 | |
| Other, % | 0 | 4 | |
| Pancreatic islet cell, % | 37 | 45 | 0.31 |
| Extent of hepatic metastases | |||
| Bilobar, % | 90 | 94 | 0.43 |
| Tumor size, cm | 5.1±0.5 | 6.2±0.8 | 0.24 |
| Metastases number | 6.7±0.3 | 6.4±0.3 | 0.42 |
| >8 metastases, % | 61 | 53 | 0.43 |
| Synchronous, % | 80 | 78 | 0.84 |
| Treatments | |||
| Number of embolizations | 2.9±0.3 | 2.1±0.1 | 0.015 |
| Primary tumor resected, % | 67 | 49 | 0.07 |
| Liver resection or ablation, % | 41 | 4 | 0.0001 |
| Octreotide, % | 45 | 66 | 0.052 |
| Chemotherapy, % | 14 | 61 | 0.0001 |
Results reported as mean ± SEM where appropriate; Significant values in bold type.
We also found no differences between the groups with respect to tumor burden within the liver (% bilobar involvement for HACE vs. HAE, 90 vs. 94, respectively) or the disease presentation (% synchronous 80 vs. 78, respectively; Table 1). The size of the largest metastatic lesion was also comparable between the HACE and HAE groups [mean ± standard error of the mean (SEM), 5.1±0.5 and 6.1±0.7, respectively]. With respect to number of hepatic metastases, we limited the total to a maximum of eight lesions to improve reporting accuracy in those patients with “innumerable” metastases. The percentage of patients with eight or greater lesions in the HACE and HAE groups was 63 and 53, respectively (p=0.43). The mean number of metastatic liver lesions was similar between the HACE and HAE groups (mean ± SEM, 6.7±0.3 and 6.4±0.3, respectively).
Treatments
The 49 patients in the HACE group underwent a total of 123 chemoembolization procedures (mean ± SEM, 2.9± 0.3; Table 1). Compared to the 51 patients in the HAE group who underwent 106 bland embolizations (mean ± SEM, 2.1±0.1), the HACE group was treated with a statistically significant greater number of embolization procedures (p<0.02). While the percentage of patients who underwent surgical resection of their primary tumor was similar between the two groups (HACE vs. HAE, 67 and 49, respectively, p=0.07), the HACE group was more likely to undergo resection and/or ablation of their hepatic metastases (HACE vs. HAE, 41% and 4%, respectively, p<0.0001). In addition, the HACE group, when compared to the HAE group, was less likely to receive additional therapies, such as chemotherapy or octreotide (14% vs. 61%, p<0.0001 and 45% vs. 66%, p=0.052, respectively). The finding that patients in the HACE group underwent a greater number of surgical procedures addressing their hepatic metastases, but the fact that they received fewer chemotherapy or octreotide treatments, probably reflects differences in treatment algorithms among the three institutions (see Table 2).
Table 2.
Patient Treatments by Institution
| MCW | UW | IU | |
|---|---|---|---|
| Number | 45 | 21 | 34 |
| HACE, % | 100* | 13 | 3 |
| HAE, % | 0* | 87 | 97 |
| Primary tumor resected, % | 67 | 38 | 60 |
| Liver resection or ablation, % | 38* | 19 | 3 |
| Octreotide, % | 42 | 57 | 76* |
| Chemotherapy, % | 11 | 76* | 36 |
p<0.05 vs. others by chi square analysis
Because of the varying treatment philosophies for patients with significant hepatic NE metastases among the three participating hospitals, we analyzed the results by institution. Table 2 shows the differences in therapies at MCW, UW, and IU where patients are treated largely by HACE ± liver resection and/or ablation, HAE ± chemotherapy, and HAE alone, respectively. Therefore, patients at MCW were treated more aggressively with respect to their hepatic NE metastases. These patients were more likely to undergo potentially curative liver resection and/or ablation in addition to HACE and less likely to receive chemotherapy or octreotide in an attempt to slow tumor progression. At both UW and IU, patients were treated with HAE primarily for palliation of symptoms as opposed to tumor progression. Figure 2 depicts the Kaplan–Meier curve for overall survival from the time of metastases diagnosis and reveals no differences among the institutions involved in this study (p=0.23).
Figure 2.
Overall survival calculated from the time of metastases diagnosis reveals no differences among the three institutions involved in this study: IU (solid line—median survival 48.7 months), UW (dashed grey line—median survival 37.0 months), and MCW (dotted line—median survival 42.9 months; p=0.23).
Morbidity and Mortality
The morbidity and mortality between the two treatment groups were comparable (Table 3). In the HACE group, three complications occurred after 123 chemoembolization procedures (2.4%): a groin hematoma, acute renal failure, and a biloma. Seven patients in the HAE group experienced complications after 106 bland embolizations (6.6% per procedure, p=0.19 compared to HACE). These complications included three liver abscesses, two patients with ileus, one groin hematoma, and one patient with hypotension. One patient in the HACE group and two patients in the HAE group died within 30 days of a procedure (0.8% vs. 1.8%, respectively, p=0.60). The patient in the HACE group had widespread metastatic disease, and her death was not thought to be directly related to the procedure. One of the patients in the HAE group died within 30 days after choosing to go on hospice care. The other patient in the HAE group developed liver failure (thought to be secondary to tumor burden), pneumonia, and acute lung injury after his embolization and was unable to be resuscitated when he went into a dysrhythmia.
Table 3.
Tumor Response and Outcomes
| HACE | HAE | p Value | |
|---|---|---|---|
| Morbidity, % | 2.4 | 6.6 | 0.19 |
| 30-day mortality, % | 0.8 | 1.8 | 0.60 |
| Symptomatic, % | 76 | 69 | 0.34 |
| Symptoms improved, % | 86 | 83 | 0.70 |
| Survival—metastases dx | 0.64 | ||
| Median, months | 50.1 | 39.1 | |
| 5-year, % | 43 | 35 | |
| Survival—1st embolization | 0.79 | ||
| Median, months | 25.5 | 25.7 | |
| 5-year, % | 19 | 13 |
Symptom Control
The HACE and HAE groups were similar with respect to both the presence of symptoms and improvement of symptoms after embolization (Table 3). Of the 49 patients in the HACE group and the 51 patients in the HAE group, 37 and 35 patients in each group, respectively, had tumor-related symptoms (76% and 69%, respectively, p=NS). Eighty-six percent of patients in the HACE group (32/37) improved after therapy compared to 83% of HAE patients (29/35, p=NS).
Survival
Median survival for the entire cohort was 32.4 months (range 1.3–177). Overall survival from the time of diagnosis of hepatic NE metastases was not statistically significantly different in the HACE group compared to the HAE group (Table 3, pFig. 3a). The median survival calculated from the time of metastasis diagnosis was slightly longer in the HACE group at 50.1 compared to 39.1 months for the HAE group, but this difference was not statistically different (=0.62). The probability of 1-, 2-, and 5-year survival for HACE patients from the time of diagnosis of metastases was 78%, 69%, and 45%, respectively, while HAE patients’ survival was 82%, 71%, and 33%.
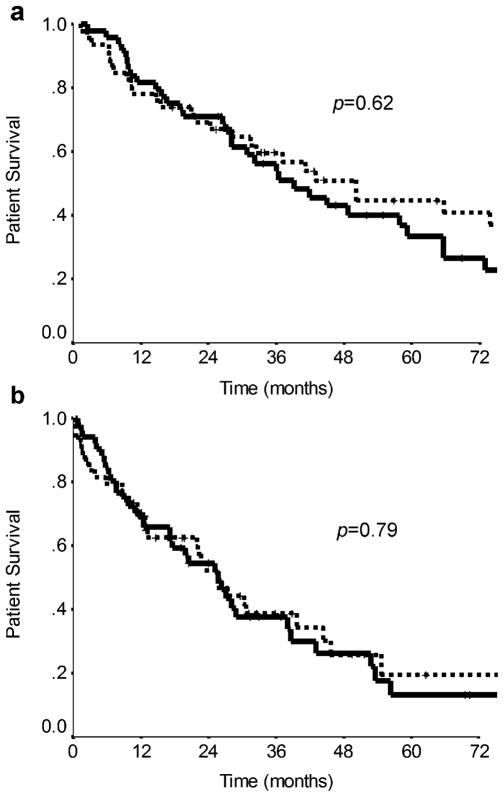
Figure 3.
Kaplan–Meier curves comparing overall survival from a the time of diagnosis of metastases (median survival 50.1 vs. 39.1 months, respectively, p=0.62) and b the time of first embolization procedure (median survival 25.5 vs. 25.7 months, respectively, p=0.79) between patients treated by HACE (dotted line) or HAE (solid line).
When analyzed from the time of first embolization procedure, the overall survival for the two treatment groups was also similar (Table 3, pFig. 3b). The median survival for the HACE and HAE groups from first embolization was 25.5 and 25.7 months, respectively (=0.79). The HACE and HAE groups had 1-, 2-, and 5-year survival probabilities of 69%, 52%, and 19% and 70%, 54%, and 13%, respectively. Comparison of the median overall survivals from the time of diagnosis of metastases to the time of first embolization procedure revealed a similar delay of 24.6 and 19.3 months for the HACE and HAE groups, respectively. This delay likely represents the time elapsed during surgical resection of the primary lesion and/or hepatic metastases with an associated recovery period(s) as well as the time to development of symptoms from the metastatic lesions.
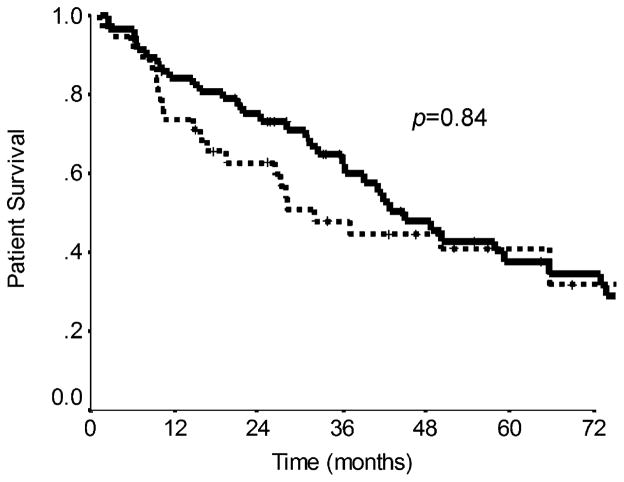
Further univariate analysis of the entire cohort examining factors predictive of overall survival found that only resection of the primary tumor significantly increased survival. The median survival for patients undergoing resection of their primary tumor versus those tumors left intact revealed 73.1 versus 28.0 months (p=0.0002; Table 4). The cumulative overall survival rates at 1, 2, and 5 years were 89%, 80%, and 55% for patients whose primary tumor was resected as opposed to 67%, 53%, and 16% for patients who did not undergo surgery. Figure 4 depicts the Kaplan–Meier curve for overall survival plotted as a function of primary tumor resection. Additional factors that were examined for predictive effects on overall survival but were not significant included age, gender, tumor type (carcinoid vs. islet cell), disease presentation (synchronous vs. metachronous), extent of liver involvement (unilobar vs. bilobar), resection and/or ablation of liver metastases, octreotide, chemotherapy, and embolization type (HACE vs. HAE; Table 4). Only resection or ablation of liver metastases (p=0.11) trended toward significance and was included in a multivariate analysis. Resection of the primary tumor was not predictive of poor survival in the multivariate model (p=0.08).
Table 4.
Univariate Analysis for Factors Predictive of Overall Survival
| Factor | Number | Median survival, months | p Value |
|---|---|---|---|
| Age (continuous)a | 95 | 0.42 | |
| Gender | |||
| Male | 53 | 41.7 | 0.30 |
| Female | 42 | 50.1 | |
| Tumor type | |||
| Carcinoid | 57 | 45.0 | 0.84 |
| Islet cell | 38 | 32.0 | |
| Disease presentation | |||
| Synchronous | 77 | 37.0 | 0.53 |
| Metachronous | 18 | 48.7 | |
| Liver involvement | |||
| Unilobar | 8 | 81.6 | 0.47 |
| Bilobar | 87 | 41.8 | |
| Primary tumor resection | |||
| Yes | 55 | 73.1 | 0.0002 |
| No | 39 | 28.0 | |
| Resection/ablation mets | |||
| Yes | 22 | 65.6 | 0.11 |
| No | 73 | 36.5 | |
| Octreotide | |||
| Yes | 46 | 48.6 | 0.85 |
| No | 37 | 37.0 | |
| Chemotherapy | |||
| Yes | 25 | 39.1 | 0.71 |
| No | 50 | 43.0 | |
| Embolization type | |||
| HACE | 46 | 50.1 | 0.62 |
| HAE | 49 | 39.1 | |
Significant values in bold type.
Log transformation
Figure 4.
Overall survival in patients who underwent resection of their primary tumor (solid line) was significantly longer compared to those whose primary tumors remained intact (dotted line) (median survival 73.1 vs. 28.0 months, respectively, p=0.0002). Survival was calculated from the time of diagnosis of metastatic disease.
Discussion
As a result of the rarity and often unpredictable biologic behavior of NE tumors with hepatic metastases, the appropriate treatment algorithm for these patients has yet to be defined. The majority of patients with this disease undergo a wide variety of treatments ranging from somatostatin analogs to hepatic artery embolization to liver transplantation. In this series, we reviewed 100 patients from three institutions with hepatic NE metastases who were similar with respect to age, gender, primary tumor type, and extent of hepatic tumor burden (Table 1). We compared these patients based on the type of hepatic artery embolization procedure they received: chemoembolization-HACE (n=49) or bland embolization-HAE (n=51). The morbidity and 30-day mortality were similar between the two groups (Table 3). We observed no differences in the rate of symptoms at presentation or symptom relief after either procedure (Table 3). Overall survival from the time of diagnosis of hepatic metastases and the time of first embolization procedure was also comparable between HACE and HAE (Table 3, pFig. 3A and B). The only factor found to be predictive of survival for all patients in a univariate analysis was resection of the primary tumor (= 0.002, Table 4, pFig. 4). However, in multivariate analysis that included those factors with a ≤ 0.15 (resection and/or ablation of hepatic metastases and resection of the primary tumor), resection of the primary tumor was no longer an independent predictor of a better prognosis (p=0.08). Further analysis of the patients revealed that differences in treatment philosophies of patients with NE hepatic metastases exist among the institutions involved; however, despite these differences, overall survival was similar at all three institutions (Table 2, Fig. 2).
Treatment of hepatic NE metastases with bland HAE has been reported since the early to mid-1980s in multiple case series.13–16 However, the last 25 years has seen tremendous improvements in techniques, technologies, and aggressiveness of treatment strategies in this patient population. More recent studies examining the efficacy of HAE alone report morbidity (major complications) in the range of 0% to 17% and mortality ranging from 0% to 6%.17–23 Our results of 6% morbidity and 1.8% 30-day mortality after HAE are consistent with these previous reports. Some of these same analyses also revealed symptomatic improvement after HAE ranging from 64% to 91%, which is consistent with the 83% observed in this study.3,20–22 Likewise, for patients receiving HAE, we found the median overall survival from the time of first embolization procedure (25.7 months) to be similar to that reported in the literature (range 20–80 months).3,17,19,21,22
Studies examining the effectiveness and safety of adding intra-arterial chemotherapy to hepatic artery embolization began to appear in the literature about 10 years later in the early 1990s.24–26 The morbidity, mortality, symptomatic control, and survival with HACE were similar to HAE in these reports; but, until recently, the largest case series was a single institution report of 23 patients.24,27,28 In 2003, Gupta et al., then, reported 81 patients who underwent either HAE or HACE demonstrating a 63% reduction in tumor-related symptoms and 31-month median overall survival from the time of first embolization procedure. However, in this report, the two treatment types were examined together.29 More recently, Ho et al. described a cohort of 46 patients who underwent primarily HACE (93% of procedures) of whom 78% had improved symptoms and median survival was 32 months.30 The morbidity and mortality reported in this cohort were 9.7% and 4.3%, respectively.30 In the current study, we found a 25.5-month median overall survival for patients treated by HACE, which is slightly shorter but still comparable to these previous reports. Similarly, our 2.4% morbidity and 0.8% 30-day mortality rates per procedure after HACE are somewhat improved over these same studies.
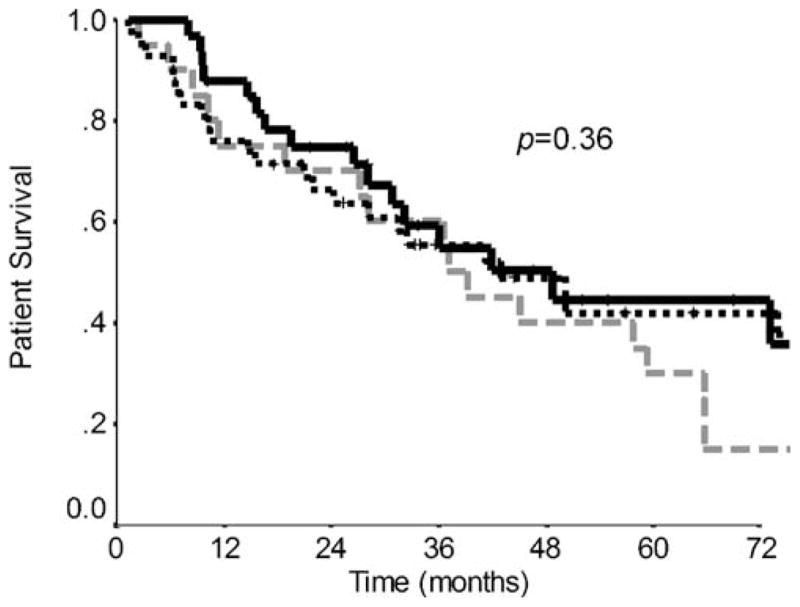
Most recently, two larger series have been reported that directly compare HACE and HAE.11,12 The largest of these studies from Gupta et al. examined 69 patients with carcinoids and 54 patients with islet cell tumors who were examined separately because the overall survival for patients with carcinoids was significantly longer (33.8 vs. 23.2 months, p=0.012).11 Whether or not these heterogeneous NE tumors should be considered as a single cohort or separately is unclear. Other studies have shown differences in survival between carcinoid and islet cell tumors similar to Gupta et al., all of which reveal a more favorable prognosis for patients with carcinoids.12, 17, 31–34 However, our analyses and others have found no significant differences in overall survival for these two types of NE tumors (carcinoid vs. islet cell: 45.0 vs. 32.0 months, p=0.83, Fig. 5).2, 30 Consequently, we chose to analyze carcinoids and islet cell tumors together.
Figure 5.
Patient overall survivals by primary tumor type: median survival for carcinoids (solid line) was 45.0 months while islet cell tumors (dotted line) had a median survival of 32.0 months. This difference was not statistically significant (p=0.84).
The literature comparing HACE to HAE shows similar toxicity profiles, morbidity, and mortality for these two procedures.11,12 The results we reported here confirm these findings of previous authors. Ruutiainen et al. also described similar rates of symptom improvement after HACE and HAE, 92% vs. 93%, respectively, which is comparable to our data (Table 3).12 The only two other series that have examined overall survival comparing HACE to HAE in patients with hepatic NE metastases (including carcinoid and islet cell tumors) both reported prolonged overall survival for patients treated with HACE (31.5 and 44 months) compared to those treated with HAE (18.2 and 39 months).11,12 However, these differences did not reach statistical significance in either study due, in part, to the small number of patients. In our cohort, the median survival from first embolization was nearly identical for patients treated by HACE and HAE (25.5 vs. 25.7 months, p=0.79, Fig. 3b). These results taken together suggest that the addition of intra-arterial chemotherapy to embolization does not provide a survival benefit over bland HAE. On the other hand, HACE does not confer increased risks to the patient since morbidity and mortality are comparable to HAE, and HACE offers equal expectations of symptom control.
The efficacy of systemic chemotherapy, including streptozotocin, fluorouracil, doxorubicin, irinotecan, interferon-α, oxaliplatin, capcitabine, and others, has been extensively examined in patients with hepatic NE metastases. Both single and multi-agent regimens have limited effectiveness in patients with unresectable disease due to poor response rates coupled with significant toxicity.35 Reported median overall survival for patients with metastatic NE tumors treated with chemotherapy ranges from 15 to 23 months.36,37 Longer median overall survivals of up to 40 months in a subset patients with well-differentiated NE tumors or up to 37 months in patients with pancreatic islet cell tumors also have been reported.38,39 In addition, newer agents, including anti-angiogenic drugs such as vascular endothelial growth factor monoclonal antibodies, tyrosine kinase inhibitors, epidermal growth factor receptor inhibitors, and mammalian target of rapamycin inhibitors are currently under investigation and show promising initial results especially in patients with islet cell tumors.40–42 In 1994, Moertel et al. demonstrated that the addition of systemic chemotherapy in patients undergoing HAE improved the rate and duration of tumor response.31 Therefore, some authors have supported a theoretical advantage of HACE in patients with islet cell tumors, which tend to respond better to systemic chemotherapy.11 In the current study, we found no differences in survival in patients who received systemic chemotherapy in addition to either type of embolization procedure compared to patients who did not undergo chemotherapy, even when carcinoid and islet cell tumors were analyzed separately (carcinoid only: 57.8 vs. 41.1 months, respectively, p=0.84; islet cell only: 28.0 vs. 50.1 months, respectively, p=0.62). However, the administration of systemic chemotherapy was not standardized in this study. Furthermore, when islet cell tumors were analyzed alone, no significant differences in overall survival were found between patients treated with HACE and HAE, though HACE-treated patients did tend to live longer (50.1 vs. 27.1 months, respectively, p=0.45).
While either method of embolic therapy appears to provide a survival benefit over no treatment or systemic chemotherapy alone, surgical resection of the primary and any associated metastatic disease, even if incomplete, remains the recommended approach. Our study found that resection of the primary tumor significantly prolonged survival, 73.1 vs. 28.0 months (p=0.0002; Table 4, Fig. 4). Other studies also have revealed that primary tumor resection is a favorable prognostic variable;11,43 however, the predictive value of primary tumor resection on survival may reflect a selection bias towards healthier patients that are able to undergo surgery.
Correspondingly, several authors have shown that surgical debulking or cytoreduction with either hepatic resection or ablation results in improved symptom control and survival when compared to embolization alone.2–4,22 While our data were not statistically significant, resection or ablation of the hepatic metastases did provide a survival advantage over no surgical debulking (65.6 vs. 36.5 months, respectively, p=0.11). Despite a greater number of liver resections and/or ablations in the HACE group (Table 1), we found no significant survival benefit in these patients. Studies comparing curative and palliative resection of hepatic NE metastases show a clear survival benefit when curative resection is achieved (85% vs. 63% 5-year survival in one study).4 Analyses that have not distinguished the curative intent of the liver resection still report 5-year survivals of at least 70%.2,4,22 Meanwhile, reports of HACE and cytoreduction used in combination reveal 5-year survivals in the range of 40% to 50%.2,4 Theses rates are better than what we observed with a 19% 5-year survival in patients undergoing HACE; however, only 41% of these patients underwent hepatic resection and/or ablation in addition to HACE (Table 1). In addition, survival was calculated from the time of first embolization procedure and not the time of hepatic resection as in the other studies. Therefore, we continue to recommend an aggressive treatment strategy with resection of the primary tumor and surgical debulking of hepatic NE metastases when possible, combined with either HAE or HACE.2,22,44
Our study has several limitations including nonrandomized retrospective design, inherent selection bias, changes in technology over the 11-year study period, and lack of standardized treatment protocols at the three institutions. Additionally, reporting of symptoms and symptom improvement were subjective and not collected with a validated assessment tool. Biochemical markers were also not routinely measured and, therefore, could not be analyzed as part of this study. Because whole-body octreotide or bone scans were not performed on all patients, we were unable to collect reliable data with respect to the presence of extrahepatic metastases; therefore, we did not analyze whether the presence of extrahepatic disease influenced survival. In addition, the quality, availability, and reporting of CT scans precluded our ability to stratify patients accurately with respect to the percentage of liver involvement. The presence of extrahepatic metastases and greater than 50% liver involvement are both factors that have been shown to predict survival in other studies.2,11
The addition of HACE to HAE in patients with hepatic NE metastases did not prolong survival or improve symptoms. However, the morbidity and mortality associated with HACE were similar to HAE and did not pose additional risks to the patient. As with any rare disease, the design of a prospective, randomized trial examining these therapies will be difficult but should be undertaken. Our results support resection of the primary carcinoid or islet cell tumor, when possible, even in the presence of metastatic disease. In conclusion, we continue to support an aggressive approach in patients with NE hepatic metastases including HACE or HAE in addition to surgical resection and somatostatin analogs.
Acknowledgments
This work was supported by the American College of Surgeons Resident Research Scholarship and National Institutes of Health Grant T32 CA009614 Physician Scientist Training in Cancer Medicine.
Footnotes
Presented at Society for Surgery of the Alimentary Tract, May 21, 2008, San Diego, CA, USA.
Contributor Information
Susan C. Pitt, Department of Surgery, Indiana University, 535 Barnhill Dr., RT103D, Indianapolis, IN 46, USA. Department of Surgery, University of Wisconsin, Madison, WI, USA
Jaime Knuth, Department of Surgery, Indiana University, 535 Barnhill Dr., RT103D, Indianapolis, IN 46, USA.
James M. Keily, Department of Surgery, Medical College of Wisconsin, Milwaukee, WI, USA
John C. McDermott, Department of Radiology, University of Wisconsin, Madison, WI, USA
Sharon M. Weber, Department of Surgery, University of Wisconsin, Madison, WI, USA
Hebert Chen, Department of Surgery, University of Wisconsin, Madison, WI, USA.
William S. Rilling, Department of Radiology, Medical College of Wisconsin, Milwaukee, WI, USA
Edward J. Quebbeman, Department of Surgery, Medical College of Wisconsin, Milwaukee, WI, USA
David M. Agarwal, Department of Radiology, Indiana University, 535 Barnhill Dr., RT103D, Indianapolis, IN 46, USA
Henry A. Pitt, Email: hapitt@iupui.edu, Department of Surgery, Indiana University, 535 Barnhill Dr., RT103D, Indianapolis, IN 46, USA
References
- 1.Chen H, Hardacre J, Uzar A, Cameron J, Choti M. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998;187:88–93. doi: 10.1016/S1072-7515(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 2.Touzios JG, Kiely JM, Pitt SC, Rilling WS, Quebbeman EJ, Wilson SD, et al. Neuroendocrine hepatic meastases: does aggressive management improve survival? Ann Surg. 2005;241:776–785. doi: 10.1097/01.sla.0000161981.58631.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musunuru S, Chen H, Rajpal S, Stephani N, McDermott JC, Holen K, et al. Metastatic neuroendocrine hepatic tumors: resection improves survival. Arch Surg. 2006;141:1000–1004. doi: 10.1001/archsurg.141.10.1000. [DOI] [PubMed] [Google Scholar]
- 4.Yoa KA, Talamonti MS, Nemeck A, Angelos P, Chrisman H, Skarda J, et al. Indications and results of liver resection and hepatic chemoembolization for metastastic gastrointestinal neuroendocrine tumors. Surgery. 2001;130:677–685. doi: 10.1067/ msy.2001.117377. [DOI] [PubMed] [Google Scholar]
- 5.Woodside KJ, Townsend CM, Jr, Evers MB. Current management of gastrointestinal carcinoid tumors. J Gastrointest Surg. 2004;8:742–756. doi: 10.1016/j.gassur.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Schupak KD, Wallner KE. The role of radiation therapy in the treatment of locally unresectable or metastatic carcinoid tumors. Int J Radiol Oncol Biol Phys. 1991;20:489–495. doi: 10.1016/0360-3016(91)90061-8. [DOI] [PubMed] [Google Scholar]
- 7.Townsend CM, Jr, Thompson JC. The clinical use of gastrointestinal hormones for alimentary tract disease. Adv Surg. 1996;29:79–92. [PubMed] [Google Scholar]
- 8.Que FG, Nagorney DM, Batts KP, et al. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg. 1995;169:36–42. doi: 10.1016/S0002-9610(99)80107-X. [DOI] [PubMed] [Google Scholar]
- 9.O’Toole D, Maire F, Ruszniewski P. Ablative therapies for liver metastases of digestive endocrine tumors. Endocr Relat Cancer. 2003;10:463–468. doi: 10.1677/erc.0.0100463. [DOI] [PubMed] [Google Scholar]
- 10.Le Treut YP, Delpero JR, Dousset B, et al. Results of liver transplantation in the treatment of metastatic neuroendocrine tumors: a 31-case French multicentric report. Ann Surg. 1997;225:355–364. doi: 10.1097/00000658-199704000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Johnson MM, Murthy R, Ahrar K, Wallace MJ, Madhoff DC, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rate and survival. Cancer. 2005;104:1590–1602. doi: 10.1002/cncr.21389. [DOI] [PubMed] [Google Scholar]
- 12.Ruutiainen AT, Soulen MC, Tuite CM, Clark TWI, Mandschein JI, Stavropoulos SW, et al. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J Vasc Interv Radiol. 2007;18:847–855. doi: 10.1016/j.jvir.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Lunderquist A, Ericsson M, Nobin A, Sandén G. Gelfoam powder embolization of the hepatic artery in liver metastases of carcinoid tumors. Radiologe. 1982;22:65–70. [PubMed] [Google Scholar]
- 14.Mårtensson H, Nobin A, Bengmark S, Lunderquist A, Owman T, Sandén G. Embolization of the liver in the management of metastatic carcinoid tumors. J Surg Oncol. 1984;27:152–158. doi: 10.1002/jso.2930270305. [DOI] [PubMed] [Google Scholar]
- 15.Stöckman F, Von Romatowski HJ, Reimold WV, Schuster R, Cruetzfeldt W. Hepatic artery embolization for the treatment of endocrine gastrointestinal tumors with liver metastases. Z Gastroenterol. 1984;22:652–660. [PubMed] [Google Scholar]
- 16.Odurny A, Birsch SJ. Hepatic arterial embolisation in patients with metastatic carcinoid tumours. Clin Radiol. 1985;36:597–602. doi: 10.1016/S0009-9260(85)80241-5. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson BK, Larsson EG, Skogseid BM, Löfberg Am, Lörelius LE, Oberg KE. Liver embolizations of patients with malignant neuroendocrine gastrointestinal tumors. Cancer. 1998;83:2293–2301. doi: 10.1002/(SICI)1097-0142(19981201)83:11<2293::AID-CNCR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Brown KT, Koh BY, Brody LA, Getrajdman GI, Susman J, Fong Y, et al. Particle embolization of hepatic neuroendocrine metastases for control of pain and hormonal symptoms. J Vasc Interv Radiol. 1999;10:397–403. doi: 10.1016/s1051-0443(99)70055-2. [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll. 2000;190:432–435. doi: 10.1016/S1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 20.Schell SR, Camp ER, Caridi JG, Hawkins IF., Jr Hepatic artery embolization for control of symptoms, octreotide requirements, and tumor progression in metastatic carcinoid tumors. J Gastrointest Surg. 2002;6:664–670. doi: 10.1016/S1091-255X(02)00044-6. [DOI] [PubMed] [Google Scholar]
- 21.Strosberg JR, Choi J, Cantor AB, Kvols LK. Selective hepatic artery embolization for treatment of patients with metastatic carcinoid and pancreatic endocrine tumors. Cancer Control. 2006;13:72–78. doi: 10.1177/107327480601300110. [DOI] [PubMed] [Google Scholar]
- 22.Osborne DA, Zervos EE, Strosberg J, Boe BA, Malafa M, Rosemurgy AS, et al. Improved outcome with cytoreduction versus embolization for symptomatic hepatic metastases of carcinoid and neuroendocrine tumors. Ann Surg Oncol. 2006;13:572–581. doi: 10.1245/ASO.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 23.Granberg D, Eriksson LG, Welin S, Kindmark H, Janson ET, Skogseid B, et al. Liver embolization with trisacryl gelatin microshperes (embosphere) in patients with neuroendocrine tumors. Acta Radiol. 2007;48:180–185. doi: 10.1080/02841850601080440. [DOI] [PubMed] [Google Scholar]
- 24.Stokes KR, Stuart K, Clouse ME. Hepatic arterial chemoembolization for metastatic endocrine tumors. J Vasc Interv Radiol. 1993;4:341–345. doi: 10.1016/s1051-0443(93)71871-0. [DOI] [PubMed] [Google Scholar]
- 25.Therasse E, Breittmayer F, Roche A, DeBaere T, Indushekar S, Ducreux M, et al. Transcatheter chemoembolization of progressive carcinoid liver metastases. Radiology. 1993;189:541–7. doi: 10.1148/radiology.189.2.7692465. [DOI] [PubMed] [Google Scholar]
- 26.Clouse ME, Perry L, Stuart K, Stokes KR. Hepatic arterial chemoembolization for metastatic neuroendocrine tumors. Digestion. 1994;199(55):92–97. doi: 10.1159/000201208. [DOI] [PubMed] [Google Scholar]
- 27.Drougas JG, Anthony LB, Blair TK, Lopez RR, Wright JK, Jr, Chapman WC, et al. Hepatic arterial chemoembolization for management of patients with advanced metastatic carcinoid tumors. Am J Surg. 1998;175:408–412. doi: 10.1016/S0002-9610(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 28.Dominguez S, Denys A, Madiera I, Hammel P, Vilgrain V, Menu Y, et al. Hepatic arterial chemoembolization with streptozocin in patients with metastatic digestive endocrine tumors. Eur J Gastroenterol Hepatol. 2000;12:151–157. doi: 10.1097/00042737-200012020-00004. [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Yao JC, Ahrar K, Wallace MJ, Morello FA, Madoff DC, et al. Hepatic arterial embolizationand chemoembolization for treatment of patients with metastatic carcinoid tumors: the M.D. Anderson experience. Cancer J. 2003;9:261–267. doi: 10.1097/ 00130404-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Ho AS, Picus J, Darcy MD, Tan B, Gould JE, Pilgram TK, et al. Long-term outcome after chemoembolization and embolization of hepatic metastatic lesions from neuroendocrine tumors. AJR Am J Roentgenol. 2007;188:1201–1207. doi: 10.2214/AJR.06.0933. [DOI] [PubMed] [Google Scholar]
- 31.Moertel CG, Johnson CM, McKusick MA, Martin JK, Jr, Nagorney DM, Kvols LK, et al. The management of patients with advanced carcinoid tumors and islet cell carcinomas. Ann Intern Med. 1994;120:302–309. doi: 10.7326/0003-4819-120-4-199402150-00008. [DOI] [PubMed] [Google Scholar]
- 32.McDermott EW, Guduric B, Brennan MF. Prognostic variables in patients with gastrointestinal carcinoid tumours. Br J Surg. 1994;81:1007–1009. doi: 10.1002/bjs.1800810725. [DOI] [PubMed] [Google Scholar]
- 33.Shabani KO, Souba WW, Finkelstein DM, Stark PC, Elgadi KM, Tenabe KK, et al. Prognosis and survival in patients with gastrointestinal tract carcinoid tumors. Ann Surg. 1999;229:815–823. doi: 10.1097/00000658-199906000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caprotti R, Angelini C, Mussi C, et al. Gastrointestinal carinoids. Prognosis and survival Minerva Chir. 2003;58:523–532. [PubMed] [Google Scholar]
- 35.Woodside KJ, Townsend CM, Jr, Evers MB. Current management of gastrointestinal carcinoid tumors. J Gastrointest Surg. 2004;8:742–756. doi: 10.1016/j.gassur.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Andreyev HJ, Scott-Mackie P, Cunningham D, Nicolson V, Norman AR, Badve SS, et al. Phase II study of continuous infusion fluorouracil and interferon alpha-2b in the palliation of malignant neuroendocrine tumors. J Clin Oncol. 1995;13:1486–1492. doi: 10.1200/JCO.1995.13.6.1486. [DOI] [PubMed] [Google Scholar]
- 37.Ducreux MP, Boige V, Leboulleux S, et al. A phase II study irinotecan with 5-fluorouracil and leucovorin in patients with pretreated gastroenteropancreatic well-differentiated endocrine carcinomas. Oncology. 2006;70:134–140. doi: 10.1159/ 000093004. [DOI] [PubMed] [Google Scholar]
- 38.Kouvaraki MA, Anjani JA, Hoff P, Wolff R, Evans DB, Lozano R, et al. Fuorouracil, doxorubicin, and streptozosin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762–4771. doi: 10.1200/JCO.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 39.Bajetta E, Catena L, Procopio G, De Dosso S, Bichisao E, Ferrari L, et al. Are capecitabine and oxaliplatin (XELOX) suitable treatment for progressing low-grade and high-grade neuroendocrine tumours? Cancer Chemother Pharmacol. 2007;59:637–642. doi: 10.1007/s00280-006-0306-6. [DOI] [PubMed] [Google Scholar]
- 40.Durán I, Salazar R, Casanovas O, Arruzubi V, Vilar E, Siu LL, et al. New drug development in digestive neuroendocrine tumors. Ann Oncol. 2007;18:1307–1313. doi: 10.1093/annonc/mdm009. [DOI] [PubMed] [Google Scholar]
- 41.Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumors. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 42.Pinchot SN, Pitt SC, Sippel RS, Kunnimalaiyaan M, Chen H. Novel target for the treatment and palliation of gastrointestinal neuroendocrine tumors. Curr Opin Investig Drugs. 2008;9:576–582. [PMC free article] [PubMed] [Google Scholar]
- 43.Chu QD, Hill HC, Douglass HO, Jr, Driscoll D, Smith JL, Nava HR, et al. Predictive factors associated with long-term survival in patients with neuroendocrine tumors of the pancreas. Ann Surg Oncol. 2002;9:855–862. doi: 10.1007/BF02557521. [DOI] [PubMed] [Google Scholar]
- 44.Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resction to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/ S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]