The mere existence of the formation number is intriguing since it hints at the possibility that nature uses this time scale for some evolutionary incentives such as optimum ejection of blood from the left atrium to the heart's left ventricle.
—Gharib et al.1
About 500 years ago, Leonardo da Vinci conducted a haemodynamic study of the sinus of Valsalva in which he deduced and explained formation of a blood flow vortex.2 Fast forwarding to the present, Gharib et al.1 used an experimental arrangement of a piston producing a wide range of jets and vortex rings alongside those jets by pushing columns of fluid of length L through an orifice with diameter D. They referred to the length-to-diameter ratio, i.e. L/D, as the formation number. While settings with low L/D generated merely a single and rather weak vortex ring, settings with large L/D resulted in a vortex ring that could no longer grow and was followed by a less-efficient fluid transport by a trailing jet.3 The maximum growth of the vortex ring without a trailing jet was observed when the L/D ratio was within a limited value range of 3.6–4.6 for an otherwise wide variety of flow conditions.1 Because L can be expressed in terms of the mean piston velocity 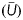 and duration of the piston stroke (t) as
and duration of the piston stroke (t) as 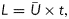 then the formation number can be defined as
then the formation number can be defined as 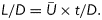 1 The right side of the equation, which incorporates time information, makes it clear why this dimensionless index is also referred to as vortex formation time (VFT).
1 The right side of the equation, which incorporates time information, makes it clear why this dimensionless index is also referred to as vortex formation time (VFT).
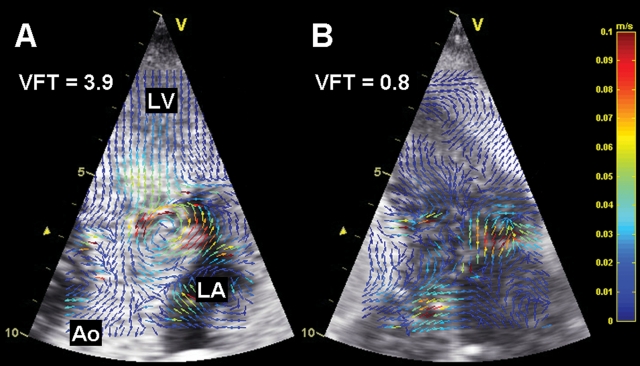
Poh et al.4 report VFT values for normal subjects as well as heart failure (HF) patients with and without preserved left ventricular (LV) ejection fraction (EF). On the basis of exclusion criteria, only data from subjects without significant primary valvular disease were used in the study, which is important because mitral pathology would impair correlation between valve kinematics and vortex-limiting formation time.5 The results of the current study indicate that VFT could be a predictor of adverse cardiovascular events in patients with HF. As the authors state, VFT is a composite index and prognostic significance of its component parameters has previously been demonstrated. However, the ‘whole’, i.e. VFT as the composite index, may be in some respect better than the combination of its component parts: experimental evidence suggests that the narrow range of optimal VFT values may represent a universal time scale for efficient vortex ring formation.1 Moreover, a diastolic vortex ring appears to function as a temporary kinetic energy reservoir5,6 that supports blood redirection into the outflow tract during the preejection phase7,8 and generates a force that initiates timely closure of the mitral valve.9 One could then speculate that, for example, formation of a diastolic vortex (or lack thereof) is an intraventricular fluid dynamics factor associated with improvement (or no improvement) of LV function or clinical status after cardiac resynchronization therapy. In this context, an experimental study by Sengupta et al.7 documents that conversion from a sinus rhythm to LV epicardial or right atrial pacing results in loss of a well-organized vortex and occurrence of blood turbulence during the preejection period. A different experimental study,10 in which measurement of D reflected early diastolic filling, demonstrates that acute elevation of LV afterload leads to a significant drop of the mean VFT value below the optimal range. Figure 1 is based on echocardiographic particle image velocimetry11–14 applied to contrast clips collected in this experimental study10 at baseline and during moderate LV afterload. The emerging echocardiographic imaging method complements the VFT parametric analysis by making loss of a vortex ring visually evident and providing local flow velocity vectors.
Figure 1.
Representative examples of flow analysis of early filling with echocardiographic particle image velocimetry. (A) Baseline. A well-developed vortex lasted ∼30 ms. Vortex formation time (VFT) was within an optimal range. (B) Moderate elevation of afterload. Vortex was not detected and VFT was below the optimal range. Ao, aorta; LA, left atrium; LV, left ventricle; contrast harmonic imaging with fundamental and harmonic frequencies of 1.7 and 3.4 MHz, respectively, and frame rate of 158 frames/s.
The current study by Poh et al. also demonstrates that VFT can separate control subjects from HF patients with preserved EF and HF patients with preserved EF from those with reduced EF. These findings are expectable because the component parts of the VFT index incorporate both systolic and diastolic functional indicators. Surprisingly, however, in all three subject groups, the mean VFT values were well below the normal range otherwise predicted by others in bench tests1 and confirmed by in vivo experiments10 and clinical studies.3,15 Values of VFT below the optimal range do not preclude formation of a vortex.5 Still, why would not at least normal control subjects have the value within the optimal VFT range? One explanation, which has also been offered by Poh et al., is in using the diameter of the mitral annulus as a surrogate of the exit diameter of the mitral valve orifice in a beating heart. Considering that VFT is inversely related to D and that the diameter of the mitral annulus will tend to overestimate the time history of the exit diameter represented by the mitral valve, then the VFT results will be, indeed, biased towards lower values.
VFT is a novel index that could become a universal indicator of biological fluid transport efficiency1,5 and, as the current work by Poh et al. corroborates, serve as a clinical diagnostic and prognostic marker. However, as the study by Poh et al. also reveals, practical issues related to the feasibility of obtaining component parameters of VFT, such as the mitral diameter, are yet to be reconciled. Flow tracking by echocardiographic particle image velocimetry could complement the ‘global’ analysis of haemodynamic conditions suitable for vortex formation, as calculated by VFT, in the similar way that tissue tracking echocardiography complements analysis of global LV function by EF. However, until four-dimensional echocardiographic imaging with scan rates high enough to resolve rapidly moving intracardiac jets and vortices becomes available, geometric assumptions will have to be taken into account when analysing the spatially complex intracardiac blood flow.
Conflict of interest: none declared.
Funding
M.B. is supported by a Grant EB009111 from the National Institutes of Health and a Grand-in-Aid Award 09GRNT2060631 from the American Heart Association.
References
- 1.Gharib M, Rambod E, Shariff K. A universal timescale for vortes ring formation. J Fluid Mech. 1998;360:121–40. [Google Scholar]
- 2.Gharib M, Kremers D, Koochesfahani MM, Kemp M. Leonardo's vision of flow visualization. Exp Fluids. 2002;33:219–23. [Google Scholar]
- 3.Gharib M, Rambod E, Kheradvar A, Sahn DJ, Dabiri JO. Optimal vortex formation as an index of cardiac health. Proc Natl Acad Sci USA. 2006;103:6305–8. doi: 10.1073/pnas.0600520103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poh KK, Lee LC, Shen L, Chong E, Tan YL, Chai P, et al. Left ventricular fluid dynamics in heart failure: echocardiographic measurement and utilities of vortex formation time. doi: 10.1093/ejechocard/jer288. EHJ Cardiovasc Imaging 2012; 10.1093/ehjci/jer288 [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Dabiri JO, Gharib M. The role of optimal vortex formation in biological fluid transport. Proc Biol Sci. 2005;272:1557–60. doi: 10.1098/rspb.2005.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedrizzetti G, Domenichini F. Nature optimizes the swirling flow in the human left ventricle. Phys Rev Lett. 2005;95:108101. doi: 10.1103/PhysRevLett.95.108101. [DOI] [PubMed] [Google Scholar]
- 7.Sengupta PP, Khandheria BK, Korinek J, Jahangir A, Yoshifuku S, Milosevic I, et al. Left ventricular isovolumic flow sequence during sinus and paced rhythms: new insights from use of high-resolution Doppler and ultrasonic digital particle imaging velocimetry. J Am Coll Cardiol. 2007;49:899–908. doi: 10.1016/j.jacc.2006.07.075. [DOI] [PubMed] [Google Scholar]
- 8.Domenichini F, Pedrizzetti G, Baccani B. Three-dimensional filling flow into a model left ventricle. J Fluid Mech. 2005;539:179–98. [Google Scholar]
- 9.Reul H, Talukder N, Muller EW. Fluid mechanics of the natural mitral valve. J Biomech. 1981;14:361–72. doi: 10.1016/0021-9290(81)90046-4. [DOI] [PubMed] [Google Scholar]
- 10.Jiamsripong P, Calleja AM, Alharthi MS, Dzsinich M, McMahon EM, Heys JJ, et al. Impact of acute moderate elevation in left ventricular afterload on diastolic transmitral flow efficiency: analysis by vortex formation time. J Am Soc Echocardiogr. 2009;22:427–31. doi: 10.1016/j.echo.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faludi R, Szulik M, D'Hooge J, Herijgers P, Rademakers F, Pedrizzetti G, et al. Left ventricular flow patterns in healthy subjects and patients with prosthetic mitral valves: an in vivo study using echocardiographic particle image velocimetry. J Thorac Cardiovasc Surg. 2010;139:1501–10. doi: 10.1016/j.jtcvs.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 12.Hong GR, Pedrizzetti G, Tonti G, Li P, Wei Z, Kim JK, et al. Characterization and quantification of vortex flow in the human left ventricle by contrast echocardiography using vector particle image velocimetry. JACC Cardiovasc Imaging. 2008;1:705–17. doi: 10.1016/j.jcmg.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukdadi OM, Kim HB, Hertzberg J, Shandas R. Numerical modeling of microbubble backscatter to optimize ultrasound particle image velocimetry imaging: initial studies. Ultrasonics. 2004;42:1111–21. doi: 10.1016/j.ultras.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Westerdale J, Belohlavek M, McMahon EM, Jiamsripong P, Heys JJ, Milano M. Flow velocity vector fields by ultrasound particle imaging velocimetry: in vitro comparison with optical flow velocimetry. J Ultrasound Med. 2011;30:187–95. doi: 10.7863/jum.2011.30.2.187. [DOI] [PubMed] [Google Scholar]
- 15.Belohlavek M, Jiamsripong P, Calleja AM, McMahon EM, Maarouf CL, Kokjohn TA, et al. Patients with Alzheimer disease have altered transmitral flow: echocardiographic analysis of the vortex formation time. J Ultrasound Med. 2009;28:1493–500. doi: 10.7863/jum.2009.28.11.1493. [DOI] [PubMed] [Google Scholar]