Abstract
Background
Because of its rarity, adenocarcinoma of the small intestine is frequently compared to adenocarcinoma of the colon, though the validity of this comparison is not known.
Methods
Patients with small (SBA) and large bowel adenocarcinoma (LBA) diagnosed between 1988–2007 were identified from the SEER registry. Age-standardized incidence and mortality rates were determined. Cancer-specific survival (CSS) stratified by stage and by number of assessed lymph nodes was calculated.
Results
A total of 4,518 and 261,521 patients with SBA and LBA, respectively, were identified. In comparison to LBA, patients with SBA were younger and presented with higher stage and histological grade. The age-standardized incidence rates decreased for LBA (−1.24%/year) but increased for SBA (+1.47%/year). While age-standardized mortality rates decreased for both LBA and SBA, the decreases were more pronounced for LBA. Five-year cancer-specific survival (CSS) was worse for resected SBA compared with resected LBA, though this difference diminished when comparing cases having ≥8 lymph nodes assessed. The relative reduction in CSS when selecting ≥8 lymph nodes was much greater for duodenal as opposed to jejunal/ileal subsite of the small bowel. With nodal selection the absolute difference in CSS between LBA and SBA for stages I, II, and III was 13%, 15.9%, and 18.5%, respectively.
Conclusions
Adequate nodal assessment is much less common in SBA than LBA; and it appears that SBA, in particular duodenal adenocarcinoma, is understaged. Even after corrections to minimize the effect of stage migration and inadequate lymph node evaluation, SBA demonstrated distinctly worse CSS than LBA.
Keywords: small intestinal adenocarcinoma, colon cancer, seer, prognosis
INTRODUCTION
Adenocarcinoma of the small intestine is a rare, orphan malignancy. Though it is the second most common malignancy of the small intestine, only 2,000 to 3,000 new cases will be diagnosed in the United States in 2009.1, 2 At present the clinical management for patients with small bowel adenocarcinoma (SBA) is based upon the management paradigms used in large bowel adenocarcinoma (LBA) (i.e. colon cancer), but the validity of such an approach is not known.
Adenocarcinomas of the small bowel are commonly perceived as having a clinical behavior similar to that of adenocarcinomas of the large bowel. In particular, both tumors share similarities in staging, prognostic factors, and metastatic site predilection.1, 3–5 Moreover, in patients with metastatic disease, response rates to cytotoxic chemotherapy are comparable for both sites, and recent data supports a fluoropyrimidine and oxaliplatin as standard frontline therapy for SBA.6
Carcinogenesis for SBA appears to occur via a similar phenotypic adenoma to carcinoma transformation as occurs in colorectal cancers.7, 8 From a molecular perspective a number of alterations, such as 18q loss9, p53 loss10, 11, and activating mutations in kras12, 13, occur at similar rates in both SBA and LBA. Surprisingly, mutations in the adenomatous polyposis coli (APC) gene differ markedly between SBA (7–13%)12, 14 and LBA (60–68%)15, 16. The limited rate of APC mutations in SBA correlates with the lower rate of adenomas within the small intestine.17 This is likely part of the reason for the most dramatic difference between adenocarcinomas of the small and large intestine: the 50-fold difference in incidence between these two sites.18, 19 Based upon these molecular findings, it may be hypothesized that the difference in incidence for these two cancers relates to a difference in adenoma initiation rather than a biological difference between these two adenocarcinomas.
We therefore sought to conduct a population based comparison of adenocarcinomas of the small and large intestine in order to explore the differences and similarities in clinical features, presentation, incidence, and mortality for these two tumor types. As clinical management for SBA rests upon the comparisons to LBA, the validity of this comparison has direct clinical ramifications.
METHODS
Patients
Data from the Surveillance, Epidemiology and End Results (SEER) cancer registry, version 2009, spanning the years 1988 to 2007, were used in this study. The SEER registry collects data on patient demographics, primary tumor site, tumor morphology, disease stage at diagnosis (per the AJCC, since 1988), first course of treatment, patient follow-up for vital status, and cause of death.
Eligible patients were aged 18 to 90 years with a histological diagnosis of SBA [duodenum (C17.0), jejunum (C17.1), ileum (C17.2), small bowel not otherwise specified (C17.9)] or LBA [cecum (C18.0), ascending colon (C18.2), hepatic flexure of colon (C18.3), transverse colon (C18.4), splenic flexure of colon (C18.6), sigmoid colon (C18.7), overlapping lesion of colon (C18.8), and colon not otherwise specified (C18.9)]. All patients had undergone cancer-directed surgery, defined as local excision or radical resection with a specimen available for pathological review. Exclusion criteria included: in situ disease and lack of histology, survival time <1 month, cancer reporting from a nursing home, hospice, autopsy, or death certificate, and for stage I,II or III cases if incomplete data regarding tumor and nodal stage precluded AJCC 7th edition stage assignment. Surgery was not an inclusion criteria for stage IV cases. The majority of exclusions, 14.4% (n=760) of the small bowel cohort and 16.8% (n=52,896) of the large bowel cohort, were due to lack of information to permit staging for analysis. Stage stratified outcomes for LBA and SBA were directly compared using the AJCC 7th edition staging system.20
Statistical Analysis
Survival outcomes were determined using SEER data through December 2007 and CSS was estimated using the Kaplan-Meier method. For cancer-specific survival (CSS) analyses, cases were censored if the patient was alive at follow-up or if death was from causes other than intestinal or pancreatic cancer for patients with SBA and from causes other than intestinal cancer for patients with LBA. A total of 2,232 patients with SBA and 76,828 patients with LBA died of their disease over the study time period. Based on our previous work, the eight-node cut point for adequate lymph node (LN) evaluation was used in this analysis.21 Multivariate Cox regression analysis was employed to calculate the adjusted survival based on the regression model and stratified by tumor stage. Covariates adjusted in the model included clinically and demographic relevant factors such as age, sex, race, tumor stage, tumor site, and tumor grade.
Trends in incidence and mortality rate over time were determined using annual percent change (APC) which is calculated by fitting a regression line to the natural logarithm of the rates (r) using the calendar year (x) as a regressor variable (e.g. log(r)=mx+b). APC significance testing compared the obtained APC to an APC of 0, which represents a lack change in the rates over time. All rates were age-adjusted based on the 2000 US standard population.
Statistical analyses were performed using Stata MP version 10.1 (release 2009; College Station, TX). Because the study used preexisting data with no personal identifiers, it was exempt from review by our Institutional Review Board.
RESULTS
Patient Population
A total of 4,518 cases of SBA and 261,521 cases of LBA met the inclusion criteria within the SEER registry from 1988 to 2007 (Table 1). The median age was 67 (interquartile range [IQR]: 56–76) and 71 years (IQR: 62–79), P<0.01, for SBA and LBA, respectively. Patients with SBA were more likely to be males (54% vs. 49%, P<0.01) and to be identified as of black race (16% vs. 10%, P<0.01) when compared to LBA. Patients with SBA were also more likely to present with stage IV disease (32% vs. 20%, P<0.01) and with high grade tumors (33% vs. 21%, P<0.01). The total number of LNs assessed among patients with SBA was positively associated with tumor size (P=0.001). Also the total number of assessed LNs was greater among patients with LBA, although the difference decreased with advancing stage. Within SBA, the median number and interquartile range (IQR) of the total number of assessed LNs was greatest for the ileal subsite (8, 3–15) as compared to jejunal (6, 2–10; P<0.01) and duodenal (4, 0–11; P<0.01) subsites.
TABLE 1.
Patient and tumor characteristics of small and large intestine adenocarcinomas
| Patient Characteristics |
SBA Stage I N=468 |
LBA Stage I N=51,221 |
SBA Stage II N=1,360 |
LBA Stage II N=87,085 |
SBA Stage III N=1,238 |
LBA Stage III N=71,726 |
SBA Stage IV N=1,452 |
LBA Stage IV N=51,489 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| Age | P <.01 | P <.01 | P <.01 | P <.01 | |||||||||||||
| <50 years | 51 | 11 | 2,667 | 5 | 199 | 15 | 5,491 | 6 | 201 | 16 | 6,418 | 9 | 181 | 12 | 5,537 | 11 | |
| 50–75 years | 245 | 52 | 27,534 | 54 | 757 | 56 | 41,808 | 48 | 700 | 57 | 38,001 | 53 | 843 | 58 | 29,023 | 56 | |
| >75 years | 172 | 37 | 21,020 | 41 | 404 | 30 | 39,786 | 46 | 337 | 27 | 27,307 | 38 | 428 | 29 | 16,929 | 33 | |
| Year of diagnosis | P =0.01 | P <.01 | P =0.01 | P <.01 | |||||||||||||
| 1988–1992 | 46 | 10 | 6,288 | 12 | 151 | 11 | 13,328 | 15 | 132 | 11 | 9,498 | 13 | 170 | 12 | 7,417 | 14 | |
| 1993–1997 | 64 | 14 | 8,157 | 16 | 223 | 16 | 16,719 | 19 | 217 | 18 | 13,088 | 18 | 235 | 16 | 9,529 | 19 | |
| 1998–2002 | 135 | 29 | 16,148 | 32 | 412 | 30 | 27,182 | 31 | 384 | 31 | 22,571 | 31 | 437 | 30 | 15,360 | 30 | |
| 2003–2007 | 223 | 48 | 20,628 | 40 | 574 | 42 | 29,856 | 34 | 505 | 41 | 26,569 | 37 | 610 | 42 | 19,183 | 37 | |
| Gender | P =0.16 | P <.01 | P <.01 | P <.01 | |||||||||||||
| Male | 249 | 53 | 25,616 | 50 | 697 | 51 | 41,378 | 48 | 676 | 55 | 34,293 | 48 | 796 | 55 | 25,930 | 50 | |
| Female | 219 | 47 | 25,605 | 50 | 663 | 49 | 45,707 | 52 | 562 | 45 | 37,433 | 52 | 656 | 45 | 25,559 | 50 | |
| Race | P <.01 | P <.01 | P <.01 | P <.01 | |||||||||||||
| White | 359 | 77 | 43,390 | 85 | 1,062 | 78 | 73,459 | 84 | 994 | 80 | 58,309 | 81 | 1,107 | 76 | 41,101 | 80 | |
| Black | 77 | 16 | 4,383 | 9 | 215 | 16 | 7,600 | 9 | 168 | 14 | 7,390 | 10 | 253 | 17 | 6,767 | 13 | |
| Other | 32 | 7 | 3,448 | 7 | 83 | 6 | 6,026 | 7 | 76 | 6 | 6,027 | 8 | 92 | 6 | 3,621 | 7 | |
| Tumor Grade | P <.01 | P <.01 | P <.01 | P <.01 | |||||||||||||
| Well-Moderate | 280 | 60 | 42,406 | 83 | 887 | 65 | 68,923 | 79 | 635 | 51 | 49,129 | 68 | 590 | 41 | 29,212 | 57 | |
| Poor | 86 | 18 | 4,267 | 8 | 382 | 28 | 15,289 | 18 | 508 | 41 | 20,159 | 28 | 514 | 35 | 14,484 | 28 | |
| Unknown | 102 | 22 | 4,548 | 9 | 91 | 7 | 2,873 | 3 | 95 | 8 | 2,438 | 3 | 348 | 24 | 7,793 | 15 | |
| Site | |||||||||||||||||
| Duodenum | 320 | 68 | 591 | 43 | 691 | 56 | 773 | 53 | |||||||||
| Jejunum | 52 | 11 | 316 | 23 | 242 | 20 | 256 | 18 | |||||||||
| Ileum | 67 | 14 | 234 | 17 | 184 | 15 | 170 | 12 | |||||||||
| Small bowel NOS | 29 | 6 | 219 | 16 | 121 | 10 | 253 | 17 | |||||||||
| Total LN assessed | P <.01 | P <.01 | P <.01 | ||||||||||||||
| Median (IQR) | 0 (0–6) | 9 (5–15) | 4 (0–10) | 12 (7–18) | 8 (3–13) | 12 (8–18) | |||||||||||
| Positive LN | - | - | P <.01 | ||||||||||||||
| Median (IQR) | 0 | 0 | 0 | 0 | 2(1–4) | 2(1–4) | |||||||||||
Abbreviations: SBA, small bowel adenocarcinoma; LBA, large bowel adenocarcinoma; NOS, not otherwise specified; LN, lymph node; IQR, interquartile range.
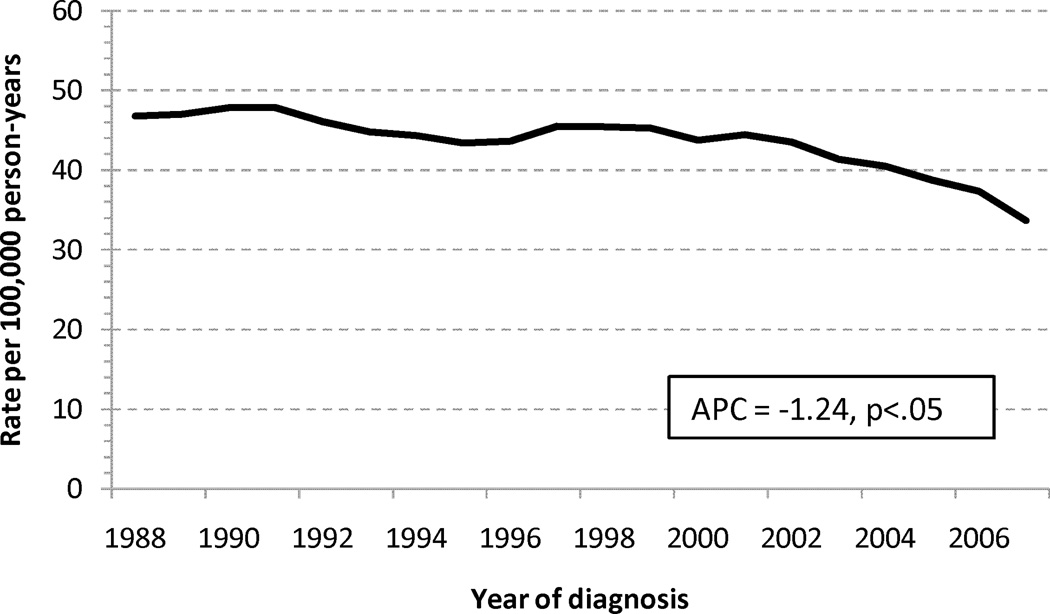
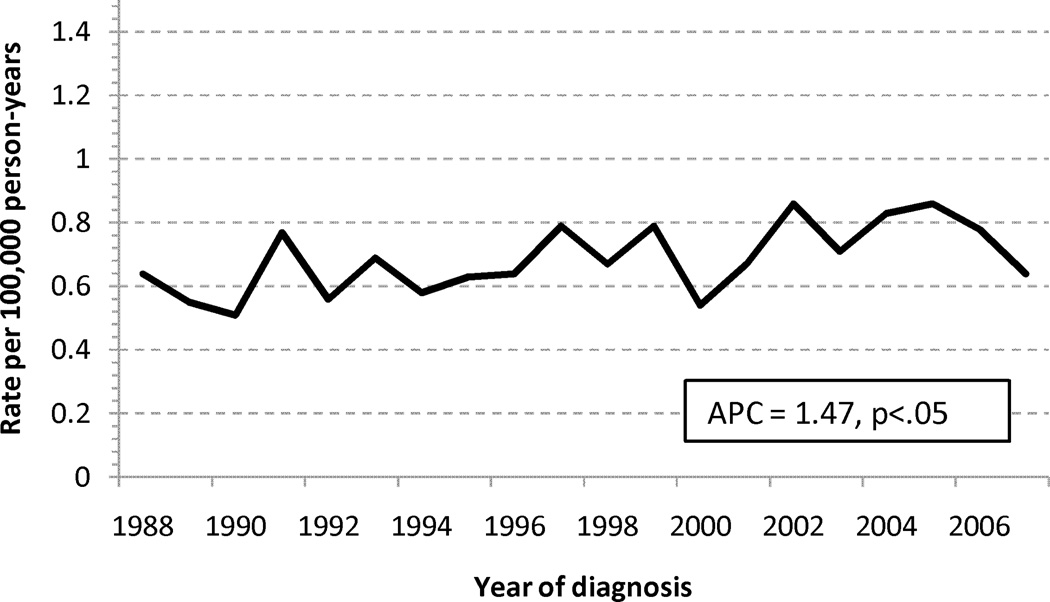
Trends in Incidence
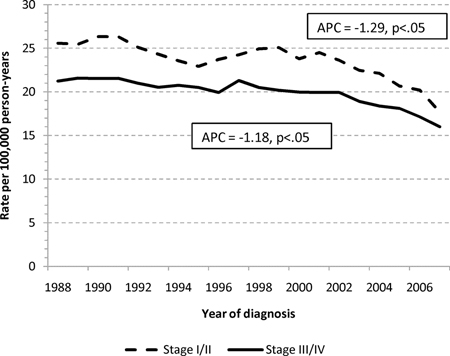
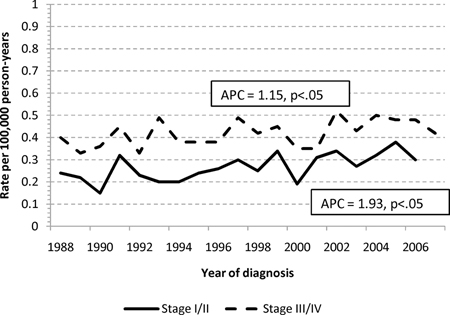
The age-standardized incidence rates for adenocarcinomas of the small and large intestine are shown in Figure 1. The annual percentage change in incidence was decreasing for LBA (−1.24%/calendar year), but increasing for SBA, (+1.47%/calendar year). When stratified into early (I-II) or advanced (III–IV) stage cohorts, the increasing incidence for SBA was more pronounced for early stage disease (1.93%/yr vs. 1.15%/yr), and the decreasing incidence for LBA was slightly more pronounced for early stage disease (−1.29%/yr vs. −1.18%/yr), Appendix 1.
FIGURE 1.
Age-standardized incidence rates for adenocarcinoma of the (a) large and (b) small intestine. Abbreviations: SBA, small bowel adenocarcinoma; LBA, large bowel adenocarcinoma; APC, annual percentage change
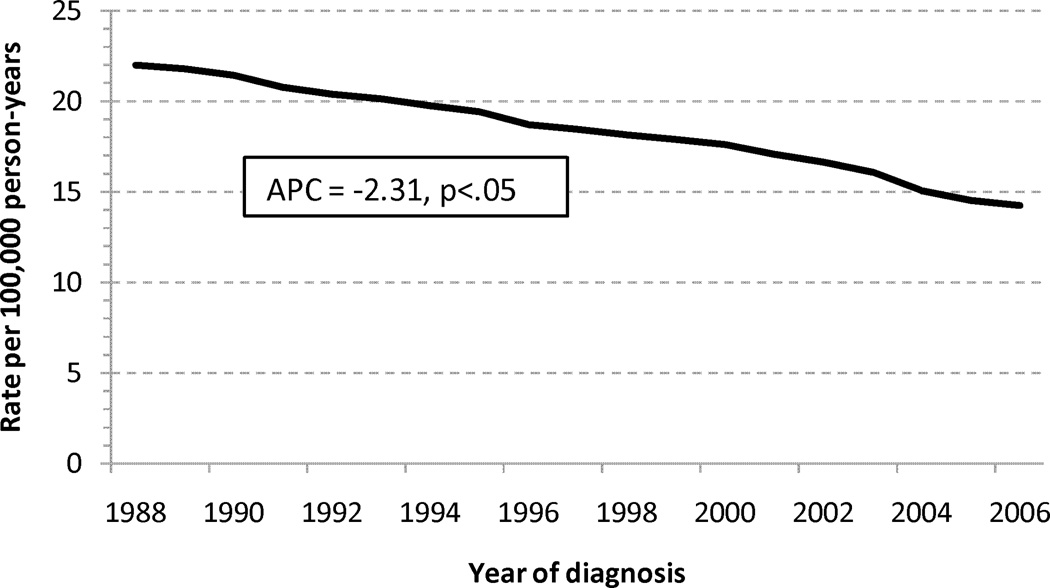
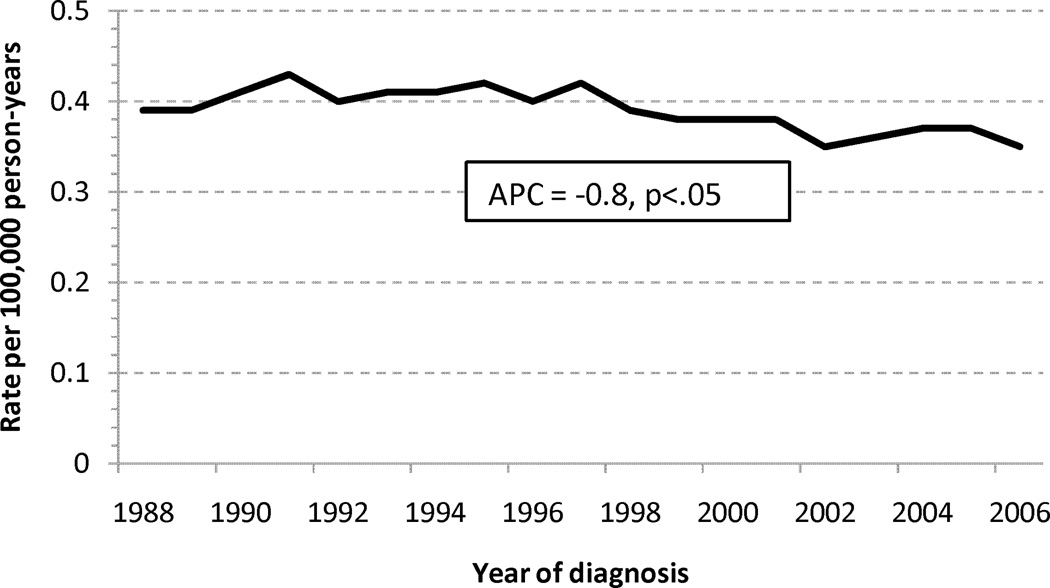
Trends in Mortality
A consistent decline in age-standardized mortality rates was seen for LBA with an APC of −2.31%/yr (Figure 2A). SBA age-standardized mortality rates appear relatively stable, with a slightly decreasing rate noted since approximately 1998 (Figure 2B).
FIGURE 2.
Age-standardized mortality rates for adenocarcinoma of the (a) large and (b) small intestine. Abbreviations: SBA, small bowel adenocarcinoma; LBA, large bowel adenocarcinoma; APC, annual percentage change
Stage Stratified Mortality
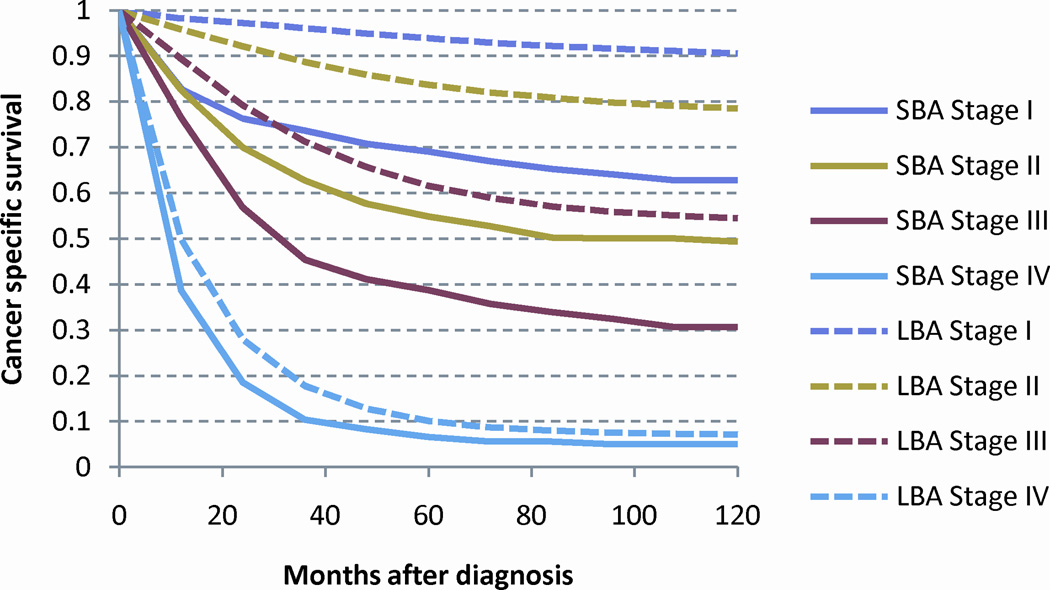
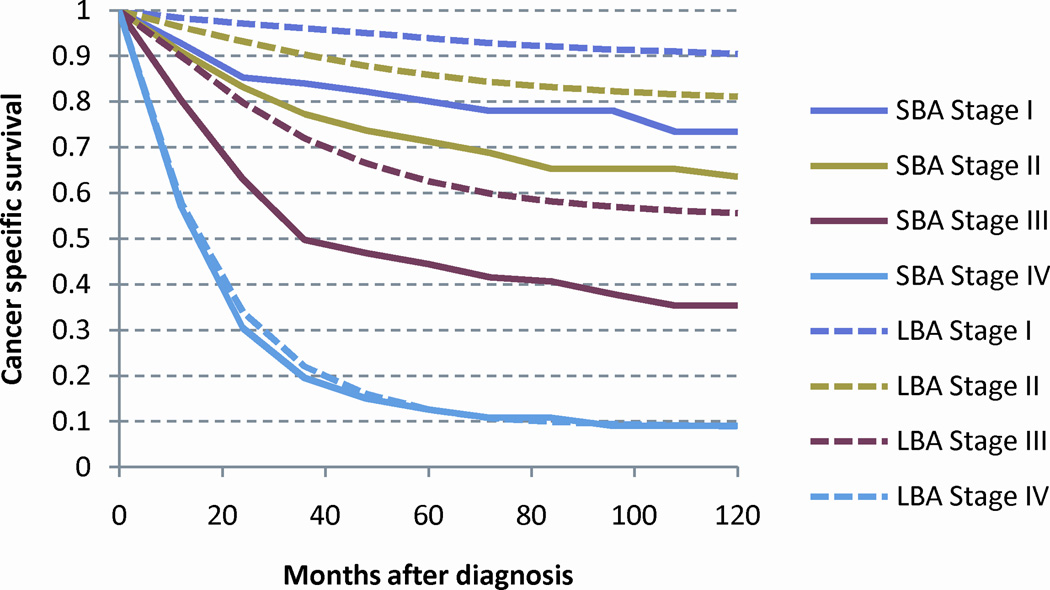
As the risk of death from cancer is strongly related to stage at presentation, CSS was calculated by the Kaplan-Meier method for each stage (Figure 3A). SBA demonstrated significantly worse outcomes than for LBA. As a prior analysis has identified ≥ 8 assessed LNs to be a significant stage-stratified discriminator of outcomes among patients with SBA, we compared this subset of patients to those patients with LBA.21 When only patients with ≥ 8 assessed LNs were analyzed, the difference in CSS between SBA and LBA reduced with LN selection resulting in a relative reduction in the difference between stage I, II and III SBA and LBA of 53%, 45%, and 19%, respectively (Figure 3B).
FIGURE 3.
Stage stratified cancer specific survival for adenocarcinoma of the large and small intestine: (a) all patients and (b) patients with ≥ 8 lymph nodes examined. Abbreviations: SBA, small bowel adenocarcinoma; LBA, large bowel adenocarcinoma.
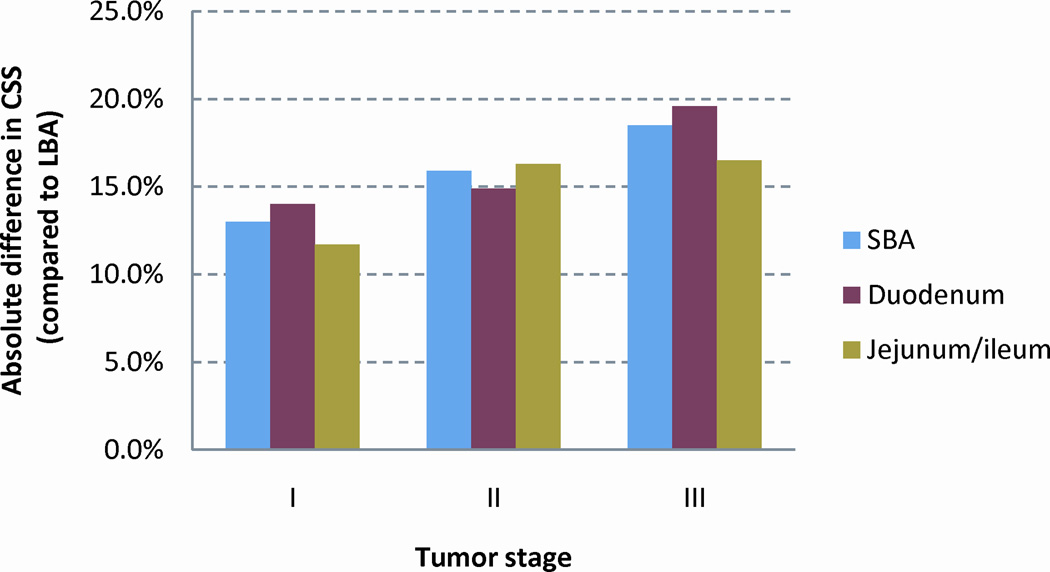
As the number of assessed LNs varied by small bowel subsites, we compared the subsites of the small bowel, duodenal and jejunal/ileal, with LBA (Table 2). The improvement in CSS with the selection ≥ 8 assessed LNs differed by small bowel subsite and disease stage. Interestingly, the relative reduction in cancer specific mortality with the selection of ≥ 8 assessed LNs was greatest for stages I and II duodenal adenocarcinoma (Figure 4A). However, after the selection of ≥ 8 assessed LNs, the CSS was similar for duodenal and jejunal/ileal subsites, and the difference in CSS when compared to LBA was similar across stage I, II, and III disease (Figure 4B).
TABLE 2.
Five-year cancer specific survival stratified by stage for all cases and those with ≥8 LN assessed.
| STAGE | LBA | SBA | Duodenal | Jejunal/Ileal | |||
|---|---|---|---|---|---|---|---|
| All Patients | CSS | CSS | Difference* | CSS | Difference* | CSS | Difference* |
| I | 93.2% | 65.3% | 27.9% | 59.8% | 33.4% | 80.4% | 12.8% |
| II | 83.8% | 55.0% | 28.8% | 47.6% | 36.2% | 62.8% | 21.0% |
| III | 62.7% | 40.0% | 22.7% | 38.6% | 24.1% | 42.2% | 20.5% |
| Patients with ≥8 LN | |||||||
| I | 93.3% | 80.3% | 13.0% | 79.3% | 14.0% | 81.6% | 11.7% |
| II | 85.8% | 69.9% | 15.9% | 70.9% | 14.9% | 69.5% | 16.3% |
| III | 63.6% | 45.1% | 18.5% | 44.0% | 19.6% | 47.1% | 16.5% |
In comparison to LBA; Cancer specific survival adjusted by age, sex, race, tumor grade;
Abbreviations: SBA, small bowel adenocarcinoma; LBA, large bowel adenocarcinoma; LN, lymph node.
FIGURE 4.
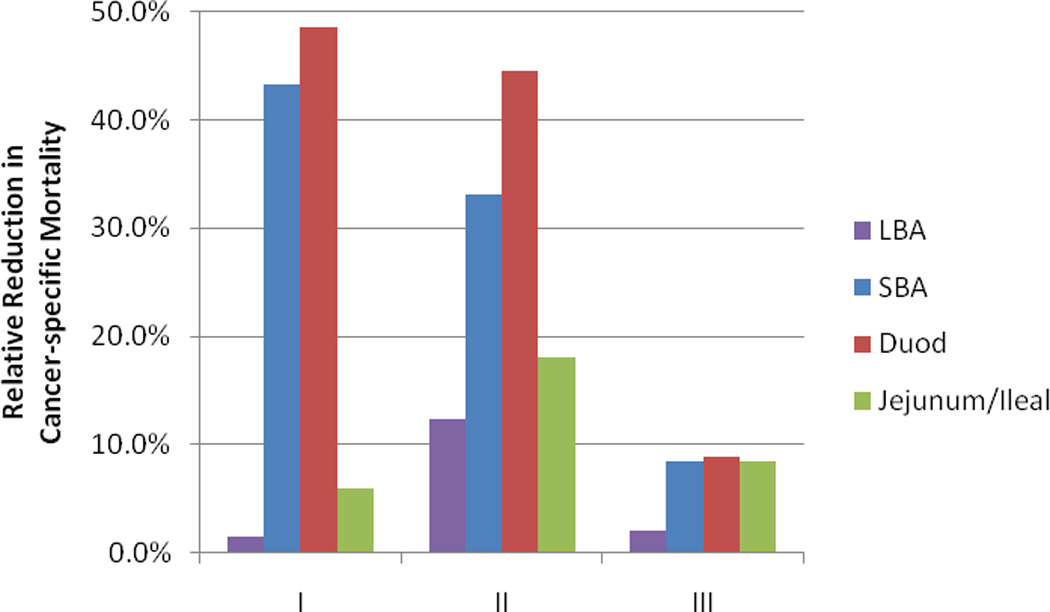
(a) The relative reduction in cancer specific mortality with the selection of cases with ≥ 8 lymph nodes assessed and (b) the absolute difference in CSS between SBA and LBA for cases with ≥ 8 lymph nodes assessed. Abbreviations: SBA, small bowel adenocarcinoma; LBA, large bowel adenocarcinoma; CSS, cancer specific survival.
DISCUSSION
This study provides new insights into the rare malignancy of small intestinal adenocarcinoma. Although improvements in survival have occurred for both adenocarcinomas of the small and large intestine, the improvements for SBA are markedly smaller than those for LBA. In contrast to LBA the incidence of SBA continued to climb over time. Stage-per-stage, survival among patients with resected LBA was better than that for patients with resected SBA. A large part of this effect may be explained by stage migration because after stratification for patients with ≥8 LN assessed, there was a marked reduction in the adjusted difference between SBA and LBA. The effect was particularly large for stage I and II duodenal adenocarcinomas suggesting a high degree of understaging for this small bowel subsite. In cases with ≥ 8 LNs assessed the absolute difference in CSS between LBA and SBA remained relatively stable across stages, with an overall difference of 13% for stage I, 15.9% for stage II, and 18.5% for stage III. These findings suggest that distinct differences in biology and treatment patterns may exist between these two intestinal malignancies, and strongly supports further research to understand the molecular mechanisms of adenocarcinoma of the small intestine.
This study did identify some demographic differences between patients with SBA and LBA. Younger age and poorly differentiated histology were more commonly seen in SBA than LBA. A higher rate of microsatellite instability may partially explain this difference, with rates in SBA ranging from 18 to 35%.22, 23 Interestingly, two prior studies have demonstrated a markedly higher rate of microsatellite instability in SBA patients with coexisting celiac disease, with rates of 67 and 73%.24, 25
Although the exact explanation for the divergence in age-standardized incidence trends for SBA and LBA cannot be determined from this data, the impact of successful screening for colorectal polyps and cancer is likely at least partly responsible.26, 27 Aside from upper endoscopy for patients with familial adenomatous polyposis (FAP), no known screening recommendations exist for patients at risk for SBA. However the rising incidence of SBA may in part be related to increased detection during abdominal imaging (e.g. CT scan) obtained for workup of symptoms or for other disease.
Whether a portion of the divergence in incidence trends reflects the role of differing etiologic and environmental risk factors for carcinogenesis in the small and large intestine cannot be determined in this study. However, prior studies have suggested a number of similarities regarding risk factors for both small and large intestinal adenocarcinoma. Two population based studies have demonstrated an increased risk for SBA following a diagnosis of LBA and vice versa.28, 29 Epidemiological studies have demonstrated associations between SBA and the use of tobacco30, 31, consumption of animal fat32 and alcohol30, 33, each of which are also associated with risk for LBA. In addition, the inheritable colorectal cancer syndromes of hereditary nonpolyposis colorectal cancer and FAP, as well as inflammatory bowel disease, are strong risk factors for developing adenocarcinoma in both the small and large intestine.
Improvements in mortality were seen for both SBA and LBA during the study period, though the reduction for SBA was smaller and only seen after approximately 1998. For both LBA and SBA this improvement in outcome is likely multi-factorial and may be related to changes in supportive care, surgical technique, adjuvant therapy, and systemic chemotherapy. While limited data exists regarding benefits of adjuvant chemotherapy for SBA, a recent analysis of the National Cancer Database demonstrated an increasing rate of adjuvant chemotherapy use from 8% of cases in 1985 to 24% in 2005.1 Interestingly the greater increase in incidence of stage I and II as compared to stage III and IV SBA indicates a shift towards earlier diagnosis. However, the percentage of patients diagnosed with stage I/II disease remains dramatically lower for SBA than LBA, 40% as compared to 52%, respectively. The recent incorporation of capsule endoscopy, has provided an improved method of evaluating the small intestine and radiographic CT imaging technology for the evaluation of abdominal complaints has also improved. Irrespective of the reasons for the decline in mortality for this rare cancer, this finding is encouraging and represents the first demonstration of improving outcomes for this cancer.
Perhaps the most interesting observation arises from the comparison of survival outcomes of patients with SBA who underwent surgical resection with evaluation of ≥ 8 LNs to stage matched patients with LBA. For both LBA and SBA the correlation between increasing nodal assessment and improved survival has been demonstrated. 21, 34 As is seen in colorectal cancer, this association is likely due to the combined effects of improved stage-assignment and improved oncologic clearance of micrometastatic disease as well as patient and tumor biology related factors. How these various factors impact the LN yields for SBA is not known but the same factors are likely relevant, as studies in other tumor types have noted the importance of these same factors.35, 36 In a prior SEER registry study, assessment of ≥8 LNs was identified to be the optimal cut-point for discriminating differences in cancer-specific survival.21 We therefore utilized this cut-point in the present analysis as a means to minimize the potential effects of stage-migration. Thus, among patients who had ≥8 LNs evaluated at the time of resection, the risk of unresected residual nodal metastasis and stage miss-assignment should be minimized and the survival comparisons are expected to reflect the true natural history of the disease.
The greater relative reduction in CSS for stages I (23%) and II (27.1%) than for stage III (12.8%) SBA following stratification for ≥8 LNs, suggests that stage migration with improved LN assessment is occurring in stage I and II disease. This finding is consistent with a prior study.37 However, in our analysis we found that the understaging of SBA was strongly dependent upon small bowel subsite, with duodenal subsite being predominantly responsible for this effect. In fact, for the jejunal/ileal subsite the selection of ≥8 LNs assessed, had minimal impact on stage I disease (relative reduction in CSS of 1.5%) and similar impact for stage II and III disease (relative reduction in CSS of 10.7% and 11.6%, respectively).
It is interesting that despite the variable reduction in CSS among the subsets analyzed with the selection of ≥8 LNs, the absolute difference in CSS between LBA and SBA was similar across both subsites of the small bowel and stages of disease in cases with ≥ 8 LNs assessed. This consistent difference between LBA and SBA may suggest an underlying biological difference between small and large bowel adenocarcinoma or a difference in treatment patterns between these two sites. As evidence for improved outcome with adjuvant therapy has been demonstrated for LBA but not for SBA, it is likely that more patients with LBA in this dataset received adjuvant chemotherapy. However, given that chemotherapy use is stage-related, the fact that our findings were consistent for all stages suggests that this was not a significant limitation in the analysis.
Epidemiologic studies of cancer specific survival are reliant on the accuracy of cause of death ascertainment. Although prior studies of the SEER registry have indicated the accuracy of cause of death coding, we identified a high percentage of patients with SBA having a cause of death attributed to either colon or pancreatic cancer.38, 39 This indicates the difficulty in cause assignment due to the rarity of SBA and suggests that the anatomic proximity and treatment similarities between these sites may lead to misassignment.
As with most registry-based observational studies, this analysis has both strengths and limitations. As an observational study, our analysis was not designed to prove an underlying biologic relationship between SBA and LBA, but rather to provide an exploratory analysis highlighting the similarities and differences that may or may not support a comparative approach in clinical practice. SEER does not provide information regarding comorbidities, pathological margin status, detailed surgical procedure performed, nor receipt of chemotherapy. Though 14% of our small bowel cohort was excluded due to lack of staging information, this number was similar to our large bowel cohort (17%) and the major reason for both tumors types was lack of nodal nodal information. As the median number of assessed LNs was greater for LBA as for SBA, a greater selection bias was placed upon SBA when selecting for cases with ≥8 LN. It is plausible that surgical differences would exist between SBA and LBA, as SBA is a rare cancer and the criteria for and expertise with performing an optimal cancer-directed surgery for SBA have not been well established. In addition, as the surgical approach and patient selection criteria are different for duodenal as compared to non-duodenal sites, it is likely that this difference in complexity is likely partially reflected in the lower rate of duodenal as opposed to non-duodenal cases with ≥8 LN, 26% and 35%, respectively. Despite these limitations, the SEER registry, which, captures data from 26% of the cancer cases within the U.S., represents one of the largest datasets of this rare cancer and provides valuable information beyond the single institutional retrospective studies that currently dominate the literature.
The differences in survival outcomes for patients with SBA or LBA highlight the underlying biologic differences between these two cancers. However, the marked reduction in this effect after stratification for adequate LN assessment shows that the prognosis for adequately treated and evaluated SBA may not be as poor as once thought. Adequate nodal assessment is much less common in SBA than LBA, and it appears that a high degree of understaging may be occurring for stage I and II small bowel adenocarcinomas, particularly of the duodenum. Comparative analysis between a potentially related rare and common cancer, provides a novel way to gain insights into a cancer with limited available information.
ACKNOWLEDGMENTS
This work was supported by the Kavanagh Family Foundation (M.J.O), American Society of Clinical Oncology Foundation Career Development Award (G.J.C.) and the National Cancer Institute K07-CA133187 (G.J.C.)
APPENDIX 1 FIGURE
Age-standardized incidence rates for adenocarcinoma of the (a) large and (b) small intestine stratified into early (stage I and II) and advanced (stage III and IV) disease. Abbreviations: SBA, small bowel adenocarcinoma; LBA, large bowel adenocarcinoma; APC, annual percentage change difference in CSS when compared to LBA.
Footnotes
Conflicts of Interest: none
REFERENCES
- 1.Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249(1):63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal S, McCarron EC, Gibbs JF, et al. Surgical management and outcome in primary adenocarcinoma of the small bowel. Ann Surg Oncol. 2007;14(8):2263–2269. doi: 10.1245/s10434-007-9428-2. [DOI] [PubMed] [Google Scholar]
- 4.Howe JR, Karnell LH, Menck HR, Scott-Conner C. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985–1995. Cancer. 1999;86(12):2693–2706. doi: 10.1002/(sici)1097-0142(19991215)86:12<2693::aid-cncr14>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Wu TJ, Yeh CN, Chao TC, et al. Prognostic factors of primary small bowel adenocarcinoma: univariate and multivariate analysis. World J Surg. 2006;30(3):391–398. doi: 10.1007/s00268-005-7898-6. discussion 399. [DOI] [PubMed] [Google Scholar]
- 6.Overman MJ, Varadhachary GR, Kopetz S, et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27(16):2598–2603. doi: 10.1200/JCO.2008.19.7145. [DOI] [PubMed] [Google Scholar]
- 7.Sellner F. Investigations on the significance of the adenoma-carcinoma sequence in the small bowel. Cancer. 1990;66(4):702–715. doi: 10.1002/1097-0142(19900815)66:4<702::aid-cncr2820660419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Perzin KH, Bridge MF. Adenomas of the small intestine: a clinicopathologic review of 51 cases and a study of their relationship to carcinoma. Cancer. 1981;48(3):799–819. doi: 10.1002/1097-0142(19810801)48:3<799::aid-cncr2820480324>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Blaker H, von Herbay A, Penzel R, et al. Genetics of adenocarcinomas of the small intestine: frequent deletions at chromosome 18q and mutations of the SMAD4 gene. Oncogene. 2002;21(1):158–164. doi: 10.1038/sj.onc.1205041. [DOI] [PubMed] [Google Scholar]
- 10.Nishiyama K, Yao T, Yonemasu H, et al. Overexpression of p53 protein and point mutation of K-ras genes in primary carcinoma of the small intestine. Oncol Rep. 2002;9(2):293–300. [PubMed] [Google Scholar]
- 11.Zhang MQ, Chen ZM, Wang HL. Immunohistochemical investigation of tumorigenic pathways in small intestinal adenocarcinoma: a comparison with colorectal adenocarcinoma. Mod Pathol. 2006;19(4):573–580. doi: 10.1038/modpathol.3800566. [DOI] [PubMed] [Google Scholar]
- 12.Blaker H, Helmchen B, Bonisch A, et al. Mutational activation of the RAS-RAF-MAPK and the Wnt pathway in small intestinal adenocarcinomas. Scand J Gastroenterol. 2004;39(8):748–753. doi: 10.1080/00365520410005847. [DOI] [PubMed] [Google Scholar]
- 13.Rashid A, Hamilton SR. Genetic alterations in sporadic and Crohn's-associated adenocarcinomas of the small intestine. Gastroenterology. 1997;113(1):127–135. doi: 10.1016/s0016-5085(97)70087-8. [DOI] [PubMed] [Google Scholar]
- 14.Arai M, Shimizu S, Imai Y, et al. Mutations of the Ki-ras, p53 and APC genes in adenocarcinomas of the human small intestine. Int J Cancer. 1997;70(4):390–395. doi: 10.1002/(sici)1097-0215(19970207)70:4<390::aid-ijc3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 15.Miyaki M, Konishi M, Kikuchi-Yanoshita R, et al. Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res. 1994;54(11):3011–3020. [PubMed] [Google Scholar]
- 16.Miyoshi Y, Nagase H, Ando H, et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1(4):229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- 17.River L, Silverstein J, Tope JW. Benign neoplasms of the small intestine; a critical comprehensive review with reports of 20 new cases. Surg Gynecol Obstet. 1956;102(1):1–38. [PubMed] [Google Scholar]
- 18.O’Riordan BGVM, Herrera L. Small bowel tumors: and overview. Dig Dis. 1996;14:245–257. doi: 10.1159/000171556. [DOI] [PubMed] [Google Scholar]
- 19.DeSesso JM, Jacobson CF. Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem Toxicol. 2001;39(3):209–228. doi: 10.1016/s0278-6915(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 20.Edge SBBD, Compton CC, et al. AJCC Cancer Staging Manual. Seventh ed. Springer; 2010. [Google Scholar]
- 21.Overman MJ, Hu CY, Wolff RA, Chang GJ. Prognostic value of lymph node evaluation in small bowel adenocarcinoma: analysis of the surveillance, epidemiology, and end results database. Cancer. doi: 10.1002/cncr.25324. [DOI] [PubMed] [Google Scholar]
- 22.Planck M, Ericson K, Piotrowska Z, et al. Microsatellite instability and expression of MLH1 and MSH2 in carcinomas of the small intestine. Cancer. 2003;97(6):1551–1557. doi: 10.1002/cncr.11197. [DOI] [PubMed] [Google Scholar]
- 23.Overman MJ, Pozadzides J, Kopetz S, et al. Immunophenotype and molecular characterisation of adenocarcinoma of the small intestine. Br J Cancer. 102(1):144–150. doi: 10.1038/sj.bjc.6605449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diosdado B, Buffart TE, Watkins R, et al. High-resolution array comparative genomic hybridization in sporadic and celiac disease-related small bowel adenocarcinomas. Clin Cancer Res. 16(5):1391–1401. doi: 10.1158/1078-0432.CCR-09-1773. [DOI] [PubMed] [Google Scholar]
- 25.Potter DD, Murray JA, Donohue JH, et al. The role of defective mismatch repair in small bowel adenocarcinoma in celiac disease. Cancer Res. 2004;64(19):7073–7077. doi: 10.1158/0008-5472.CAN-04-1096. [DOI] [PubMed] [Google Scholar]
- 26.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91(5):434–437. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 27.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(27):1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 28.Neugut AI, Jacobson JS, Suh S, et al. The epidemiology of cancer of the small bowel. Cancer Epidemiol Biomarkers Prev. 1998;7(3):243–251. [PubMed] [Google Scholar]
- 29.Scelo. Associations between small intestine cancer and other primary cancers: an international population-based study. Int J Cancer. 2006;118 doi: 10.1002/ijc.21284. [DOI] [PubMed] [Google Scholar]
- 30.Chen CC, Neugut AI, Rotterdam H. Risk factors for adenocarcinomas and malignant carcinoids of the small intestine: preliminary findings. Cancer Epidemiol Biomarkers Prev. 1994;3(3):205–207. [PubMed] [Google Scholar]
- 31.Wu AH, Yu MC, Mack TM. Smoking, alcohol use, dietary factors and risk of small intestinal adenocarcinoma. Int J Cancer. 1997;70(5):512–517. doi: 10.1002/(sici)1097-0215(19970304)70:5<512::aid-ijc4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Lowenfels AB, Sonni A. Distribution of small bowel tumors. Cancer Lett. 1977;3(1–2):83–86. doi: 10.1016/s0304-3835(77)94394-4. [DOI] [PubMed] [Google Scholar]
- 33.Kaerlev L, Teglbjaerg PS, Sabroe S, et al. Is there an association between alcohol intake or smoking and small bowel adenocarcinoma? Results from a European multi-center case-control study. Cancer Causes Control. 2000;11(9):791–797. doi: 10.1023/a:1008920502888. [DOI] [PubMed] [Google Scholar]
- 34.Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99(6):433–441. doi: 10.1093/jnci/djk092. [DOI] [PubMed] [Google Scholar]
- 35.Herr HW, Faulkner JR, Grossman HB, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol. 2004;22(14):2781–2789. doi: 10.1200/JCO.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 36.Petrik DW, McCready DR, Sawka CA, Goel V. Association between extent of axillary lymph node dissection and patient, tumor, surgeon, and hospital factors in patients with early breast cancer. J Surg Oncol. 2003;82(2):84–90. doi: 10.1002/jso.10198. [DOI] [PubMed] [Google Scholar]
- 37.Nicholl MB, Ahuja V, Conway WC, et al. Small bowel adenocarcinoma: understaged and undertreated? Ann Surg Oncol. 17(10):2728–2732. doi: 10.1245/s10434-010-1109-x. [DOI] [PubMed] [Google Scholar]
- 38.Howlader N, Ries LA, Mariotto AB, et al. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 102(20):1584–1598. doi: 10.1093/jnci/djq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lund JL, Harlan LC, Yabroff KR, Warren JL. Should cause of death from the death certificate be used to examine cancer-specific survival? A study of patients with distant stage disease. Cancer Invest. 28(7):758–764. doi: 10.3109/07357901003630959. [DOI] [PMC free article] [PubMed] [Google Scholar]