Abstract
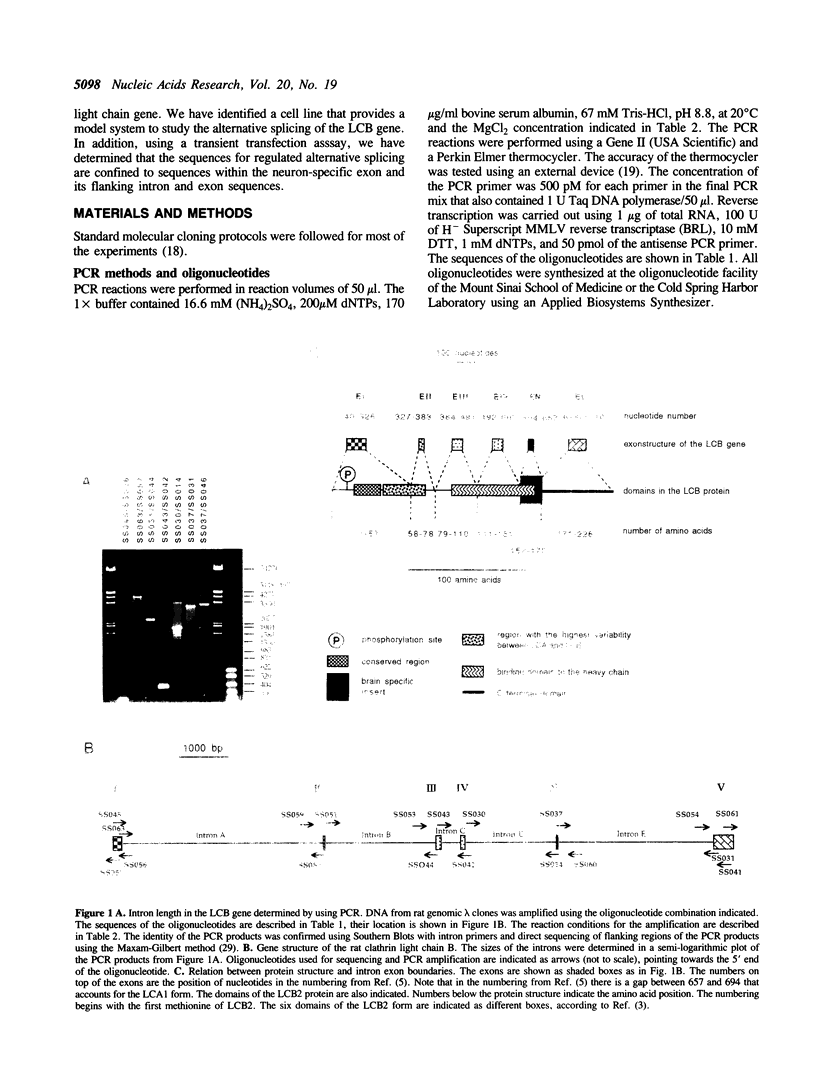
The clathrin light chains are components of clathrin coated vesicles, structural constituents involved in endocytosis and membrane recycling. The clathrin light chain B (LCB) gene encodes two isoforms, termed LCB2 and LCB3, via an alternative RNA splicing mechanism. We have determined the structure of the rat clathrin light chain B gene. The gene consists of six exons that extend over 11.9 kb. The first four exons and the last exon are common to the LCB2 and LCB3 isoforms. The fifth exon, termed EN, is included in the mRNA in brain, giving rise to the brain specific form LCB2 but is excluded in other tissues, generating the LCB3 isoform. Primary rat neuronal cell cultures express predominantly the brain specific LCB2 isoform, whereas primary rat cultures of glia express only the LCB3 isoform, suggesting that expression of the brain-specific LCB2 form is limited to neurons. Further evidence for neuronal localization of the LCB2 form is provided using a teratocarcinoma cell line, P19, which can be induced by retinoic acid to express a neuronal phenotype, concomitant with the induction of the LCB2 form. In order to determine the sequences involved in alternative splice site selection, we constructed a minigene containing the alternative spliced exon EN and its flanking intron and exon sequences. This minigene reflects the splicing pattern of the endogenous gene upon transfection in HeLa cell and primary neuronal cell cultures, indicating that this region of the LCB gene contains all the necessary information for neuron-specific splicing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adema G. J., van Hulst K. L., Baas P. D. Uridine branch acceptor is a cis-acting element involved in regulation of the alternative processing of calcitonin/CGRP-l pre-mRNA. Nucleic Acids Res. 1990 Sep 25;18(18):5365–5373. doi: 10.1093/nar/18.18.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Black D. L. Activation of c-src neuron-specific splicing by an unusual RNA element in vivo and in vitro. Cell. 1992 May 29;69(5):795–807. doi: 10.1016/0092-8674(92)90291-j. [DOI] [PubMed] [Google Scholar]
- Black D. L. Does steric interference between splice sites block the splicing of a short c-src neuron-specific exon in non-neuronal cells? Genes Dev. 1991 Mar;5(3):389–402. doi: 10.1101/gad.5.3.389. [DOI] [PubMed] [Google Scholar]
- Blum M. Regulation of neuroendocrine peptide gene expression. Methods Enzymol. 1989;168:618–633. doi: 10.1016/0076-6879(89)68045-7. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Hill B. L., Acton S. L., Näthke I., Wong D. H., Ponnambalam S., Parham P. Clathrin light chains: arrays of protein motifs that regulate coated-vesicle dynamics. Trends Biochem Sci. 1991 Jun;16(6):208–213. doi: 10.1016/0968-0004(91)90087-c. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M. Living with clathrin: its role in intracellular membrane traffic. Science. 1988 Dec 9;242(4884):1396–1402. doi: 10.1126/science.2904698. [DOI] [PubMed] [Google Scholar]
- Casper D., Mytilineou C., Blum M. EGF enhances the survival of dopamine neurons in rat embryonic mesencephalon primary cell culture. J Neurosci Res. 1991 Oct;30(2):372–381. doi: 10.1002/jnr.490300213. [DOI] [PubMed] [Google Scholar]
- Dinsmore J. H., Solomon F. Inhibition of MAP2 expression affects both morphological and cell division phenotypes of neuronal differentiation. Cell. 1991 Feb 22;64(4):817–826. doi: 10.1016/0092-8674(91)90510-6. [DOI] [PubMed] [Google Scholar]
- Emeson R. B., Hedjran F., Yeakley J. M., Guise J. W., Rosenfeld M. G. Alternative production of calcitonin and CGRP mRNA is regulated at the calcitonin-specific splice acceptor. Nature. 1989 Sep 7;341(6237):76–80. doi: 10.1038/341076a0. [DOI] [PubMed] [Google Scholar]
- Jackson A. P., Parham P. Structure of human clathrin light chains. Conservation of light chain polymorphism in three mammalian species. J Biol Chem. 1988 Nov 15;263(32):16688–16695. [PubMed] [Google Scholar]
- Jackson A. P., Seow H. F., Holmes N., Drickamer K., Parham P. Clathrin light chains contain brain-specific insertion sequences and a region of homology with intermediate filaments. Nature. 1987 Mar 12;326(6109):154–159. doi: 10.1038/326154a0. [DOI] [PubMed] [Google Scholar]
- Kelley K. A., Stamm S., Kozak C. A. Expression and chromosome localization of the murine cystic fibrosis transmembrane conductance regulator. Genomics. 1992 Jun;13(2):381–388. doi: 10.1016/0888-7543(92)90257-s. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T., Scarmato P., Harrison S. C., Monroe J. J., Chow E. P., Mattaliano R. J., Ramachandran K. L., Smart J. E., Ahn A. H., Brosius J. Clathrin light chains LCA and LCB are similar, polymorphic, and share repeated heptad motifs. Science. 1987 Apr 17;236(4799):320–324. doi: 10.1126/science.3563513. [DOI] [PubMed] [Google Scholar]
- Kuo H. C., Nasim F. H., Grabowski P. J. Control of alternative splicing by the differential binding of U1 small nuclear ribonucleoprotein particle. Science. 1991 Mar 1;251(4997):1045–1050. doi: 10.1126/science.1825520. [DOI] [PubMed] [Google Scholar]
- Lees-Miller J. P., Goodwin L. O., Helfman D. M. Three novel brain tropomyosin isoforms are expressed from the rat alpha-tropomyosin gene through the use of alternative promoters and alternative RNA processing. Mol Cell Biol. 1990 Apr;10(4):1729–1742. doi: 10.1128/mcb.10.4.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M. Regulation of alternative splicing. Genet Eng (N Y) 1990;12:139–181. doi: 10.1007/978-1-4613-0641-2_9. [DOI] [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 1990 Feb 25;18(4):1068–1068. doi: 10.1093/nar/18.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmato P., Kirchhausen T. Analysis of clathrin light chain-heavy chain interactions using truncated mutants of rat liver light chain LCB3. J Biol Chem. 1990 Mar 5;265(7):3661–3668. [PubMed] [Google Scholar]
- Schmauss C., McAllister G., Ohosone Y., Hardin J. A., Lerner M. R. A comparison of snRNP-associated Sm-autoantigens: human N, rat N and human B/B'. Nucleic Acids Res. 1989 Feb 25;17(4):1733–1743. doi: 10.1093/nar/17.4.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Stamm S., Gillo B., Brosius J. Temperature recording from thermocyclers used for PCR. Biotechniques. 1991 Apr;10(4):430-2, 434-5. [PubMed] [Google Scholar]
- Stamm S., Longo F. M. Direct sequencing of PCR products using the Maxam-Gilbert method. Genet Anal Tech Appl. 1990 Sep;7(5):142–143. doi: 10.1016/0735-0651(90)90021-7. [DOI] [PubMed] [Google Scholar]
- Wong D. H., Ignatius M. J., Parosky G., Parham P., Trojanowski J. Q., Brodsky F. M. Neuron-specific expression of high-molecular-weight clathrin light chain. J Neurosci. 1990 Sep;10(9):3025–3031. doi: 10.1523/JNEUROSCI.10-09-03025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]