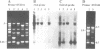Abstract
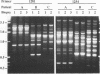
The RAPD (or AP-PCR) DNA fingerprinting method was used to distinguish among clinical isolates of Helicobacter pylori, a bacterium whose long term carriage is associated with gastritis, peptic ulcers and gastric carcinomas. This method uses arbitrarily chosen oligonucleotides to prime DNA synthesis from genomic sites to which they are fortuitously matched, or almost matched. Most 10-nt primers with > or = 60% G + C yielded strain-specific arrays of up to 15 prominent fragments, as did most longer (> or = 17-nt) primers, whereas most 10-nt primers with 50% G+C did not. Each of 64 independent H. pylori isolates, 60 of which were from patients in the same hospital, was distinguishable with a single RAPD primer, which suggests a high level of DNA sequence diversity within this species. In contrast, isolates from initial and followup biopsies were indistinguishable in each of three cases tested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATWOOD K. C., SCHNEIDER L. K., RYAN F. J. Selective mechanisms in bacteria. Cold Spring Harb Symp Quant Biol. 1951;16:345–355. doi: 10.1101/sqb.1951.016.01.026. [DOI] [PubMed] [Google Scholar]
- Barthel J. S., Westblom T. U., Havey A. D., Gonzalez F., Everett E. D. Gastritis and Campylobacter pylori in healthy, asymptomatic volunteers. Arch Intern Med. 1988 May;148(5):1149–1151. [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori: its role in disease. Clin Infect Dis. 1992 Sep;15(3):386–391. doi: 10.1093/clind/15.3.386. [DOI] [PubMed] [Google Scholar]
- Béji A., Mégraud F., Vincent P., Gavini F., Izard D., Leclerc H. GC content of DNA of Campylobacter pylori and other species belonging or related to the genus Campylobacter. Ann Inst Pasteur Microbiol. 1988 Sep-Oct;139(5):527–534. doi: 10.1016/0769-2609(88)90152-4. [DOI] [PubMed] [Google Scholar]
- Foxall P. A., Hu L. T., Mobley H. L. Use of polymerase chain reaction-amplified Helicobacter pylori urease structural genes for differentiation of isolates. J Clin Microbiol. 1992 Mar;30(3):739–741. doi: 10.1128/jcm.30.3.739-741.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcombe C. Helicobacter pylori: the African enigma. Gut. 1992 Apr;33(4):429–431. doi: 10.1136/gut.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudbier J. H., Langenberg W., Rauws E. A., Bruin-Mosch C. Genotypical variation of Campylobacter pylori from gastric mucosa. J Clin Microbiol. 1990 Mar;28(3):559–565. doi: 10.1128/jcm.28.3.559-565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Bickley J., Costas M., Morgan D. R. Genomic variation in Helicobacter pylori: application to identification of strains. Scand J Gastroenterol Suppl. 1991;181:43–50. doi: 10.3109/00365529109093207. [DOI] [PubMed] [Google Scholar]
- Simor A. E., Shames B., Drumm B., Sherman P., Low D. E., Penner J. L. Typing of Campylobacter pylori by bacterial DNA restriction endonuclease analysis and determination of plasmid profile. J Clin Microbiol. 1990 Jan;28(1):83–86. doi: 10.1128/jcm.28.1.83-86.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee W., Lambert J., Smallwood R., Schembri M., Ross B. C., Dwyer B. Ribotyping of Helicobacter pylori from clinical specimens. J Clin Microbiol. 1992 Jun;30(6):1562–1567. doi: 10.1128/jcm.30.6.1562-1567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990 Dec 25;18(24):7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., Pretzman C., Postic D., Saint Girons I., Baranton G., McClelland M. Genomic fingerprinting by arbitrarily primed polymerase chain reaction resolves Borrelia burgdorferi into three distinct phyletic groups. Int J Syst Bacteriol. 1992 Jul;42(3):370–377. doi: 10.1099/00207713-42-3-370. [DOI] [PubMed] [Google Scholar]
- Westblom T. U., Madan E., Subik M. A., Duriex D. E., Midkiff B. R. Double-blind randomized trial of bismuth subsalicylate and clindamycin for treatment of Helicobacter pylori infection. Scand J Gastroenterol. 1992;27(3):249–252. doi: 10.3109/00365529208999958. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]