Abstract
Background
Iron metabolism during pregnancy maintains fetal iron levels at the expense of the mother. The mechanism behind this regulation is still not clear despite recent advances. Here we examine the role of maternal and fetal Hfe, its downstream signaling molecule, hepcidin and dietary iron in the regulation of placental iron transfer.
Design and Methods
Hfe wild-type, knockout and heterozygote dams were fed iron deficient (12.5 ppm), adequate (50 ppm) and replete (150 ppm) iron diets and mated with heterozygote males to produce pups of all genotypes. Dams and pups were sacrificed at Day 18 of gestation; serum, placenta, body and liver iron parameters were measured. Protein and mRNA levels of various iron transporter genes were determined in duodenum, liver and placenta by Western blotting and real time PCR.
Results
Maternal liver iron levels were dependent on both dietary iron intake and Hfe genotype. Increasing iron levels in the maternal diet resulted in increased total iron in the fetus, primarily in the liver. However, fetuses of Hfe-knockout mothers showed further elevation of liver iron levels, concomitant with elevated expression of Tfr1, Dmt1 and Fpn in the placenta. Hfe-knockout fetuses that express low levels of liver hepcidin accumulated more iron in their liver than wild-type fetuses due to increased ferroportin levels in the placenta.
Conclusions
Maternal and fetal status, as well as dietary iron, is important in regulating iron transfer across placenta. Maternal Hfe regulates iron transfer by altering gene expression in the placenta. Fetal Hfe is important in regulating placental iron transfer by modulating fetal liver hepcidin expression.
Keywords: iron, fetal Hfe, dietary, genotype, maternal
Introduction
Iron homeostasis is important for human health because its deficiency or overload results in anemia or hemochromatosis, respectively. Iron deficiency anemia is the most common nutritional deficiency, affecting nearly 2 billion people worldwide; pregnant women, women of child-bearing age and children are most vulnerable.1 Iron deficiency in the neonatal and early postnatal period has been associated with impaired brain function and other deleterious neurological effects.2 The majority of fetal iron is acquired in the third trimester of gestation; therefore, understanding the mechanism of iron transport across the placenta in this gestational period is important.
Iron transfer from mother to fetus is a regulated process involving iron uptake from the maternal circulation, its transport across the placenta and subsequent transfer into the fetal circulation.3 The mechanisms behind iron transfer from mother to fetus are beginning to be clarified but much is still unknown. As pregnancy progresses, the amount of iron transferred from mother to fetus increases and at term is directed against a concentration gradient. Syncytiotrophoblast cells of the placenta form the barrier between mother and fetus with the apical surface adjacent to the maternal circulation and the basolateral surface bordering the fetal circulation. Proteins localized at the apical membrane of syncytiotrophoblasts include transferrin receptor 1 (TfR1) and HFE.4–8 Diferric transferrin from the maternal circulation binds to TfR1 at the apical surface of the syncytiotrophoblast and this complex is internalized by endocytosis.9,10 Acidification of the endosomal compartments causes dissociation of iron from TfR1; iron is subsequently released into the cytoplasm, possibly by DMT1.11 Transfer of iron to the fetal circulation probably occurs via ferroportin (FPN) as this has been shown to localize to the basal membrane of syncytiotrophoblast cells.12
HFE is a negative regulator of iron absorption. Mutations in the HFE gene result in hereditary hemochromatosis (HH) which is characterized by increased iron absorption and transferrin saturation, leading to hepatic iron overload.13 There have been no studies on the relationship between the HH mutation and levels of iron in newborn infants, so the functional consequences of an absence of HFE in placenta are not known. However, it has been shown that in placenta, HFE is associated with TfR1 at the apical membrane of syncytiotrophoblast cells7,8 where it is predicted to compete with transferrin for the same binding site on TfR1.14 HFE is an upstream regulator of liver hepcidin which has been demonstrated to be a negative regulator of intestinal dietary iron absorption and efflux of recycled iron from macrophages. Hepcidin regulates iron levels by binding to and causing the internalization of FPN in hepatocytes, macrophages and enterocytes.15,16 The loss of HFE in humans and mice results in low hepcidin expression in the liver.17–20 The precise mechanism by which this occurs is not known; however, it has been proposed that binding of diferric transferrin to the TfR1-HFE complex regulates hepcidin synthesis by releasing HFE to bind to TfR2.21,22 As pregnancy progresses, maternal hepcidin levels decrease but return to normal soon after birth.23 Transgenic mice that over-express hepcidin in the liver show a reduction in placental Tfr1 levels; the offspring of these mice are also severely anemic and die shortly after birth.24,25 How hepcidin regulates iron transfer across the placenta in order to cause fetal anemia is still unknown.
In this study, we investigated the role of, on the one hand, maternal and fetal Hfe genotype and, on the other hand, dietary iron levels in iron transfer from mother to fetus. Our data demonstrate that fetal Hfe status is important in the regulation of placental iron transfer by the modulation of fetal hepcidin expression. Maternal Hfe genotype also affects iron transfer to the fetus; this is likely due to increased iron stores at higher dietary iron levels and modulation of gene expression in the placenta.
Design and Methods
Experimental animals
All experimental procedures were approved and conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986. Hfe knockout mice (129/Ola-C57BL/6 mixed background strain) were derived by replacing a 2.5 kb BglII fragment of the Hfe gene with a 2 kb pgk-neor gene as described previously.26 Knockout mice (KOs) were mated with wild-type (WT) mice to produce heterozygotes (Hets). Hets were crossed with each other to produce KO, WT and Het pups. Subsequently, these mice with the same genetic background, of all three genotypes were used for the following experiments.
After weaning, animals were fed regular chow (RM1 diet, SDS, UK). One week after weaning, animals were placed on low (12.5 ppm), or adequate (50 ppm) iron diets. The experimental diets used were based on a dried egg albumin diet27 and conformed to American Institute of Nutrition guidelines for laboratory animals.28 FeSO4 was added to achieve total levels of Fe. Dietary ingredients were purchased from Mayjex, Ltd. (Chalfont-St Peter, UK), BDH Chemicals (Poole, UK), or Sigma (Poole, UK). Females of all genotypes were mated with Het males three weeks later and remained on their respective diet throughout pregnancy. Pregnancy was confirmed by detection of a vaginal plug and this day was denoted Day 0. On Day 18 of gestation, dams from each group were anesthetized with pentobarbitone. The fetuses were delivered by caesarean section, decapitated and weighed before dissection. Fetal sex was not determined at any point in this study. Maternal and fetal livers, maternal duodenum and placenta were removed, weighed and rapidly frozen in liquid nitrogen before being stored at −80°C.
Iron, non-heme iron and serum iron measurements
Whole fetuses (minus liver) were dried at 50°C for 72 h and subsequently weighed. The dried carcasses were digested in 1% (v/v) nitric acid (VWR) for 24 h at room temperature before heating to 80°C for a further 24 h to ensure complete digestion. The digests were centrifuged at 3000g and supernatants were analyzed for Fe using ICP-MS. Briefly, supernatants were diluted 1:50 using 1% nitric acid and 0.1% TX100 containing ~5 ppb of the internal standards scandium and rhodium. The Fe concentrations were measured from calibration curves (0–2500 ppb) of the standards.
Livers were dried at 50°C for 72 h and subsequently weighed. Quantitative measurement of non-heme iron was performed according to the method of Torrance and Bothwell.29 Results were reported as micrograms iron/gram of tissue dry weight. Serum iron was measured using an iron binding assay kit (Pierce). Results were reported as micrograms iron/deciliter.
RNA extraction and RT-PCR
Duodenal, hepatic and placental RNA was extracted with TRIzol (Invitrogen) according to the manufacturer’s instructions. Total RNA (1 μg) was reverse transcribed using the Verso cDNA reverse transcription kit (Thermofisher Scientific) according to the manufacturer’s instructions. Real time PCR reactions were run using a Lightcycler (Roche) with β-actin as an internal standard. Each reaction was performed in duplicate and contained 10 pmoles of specific primers, 1 × SYBR Green Mastermix (Qiagen) and 1 μL of cDNA in a 20 μL reaction. Samples without cDNA were included as negative controls. Mouse PCR primer-pairs were as follows: β-actin: 5′-GACGGCCAAGTCATCACTATT-3′ (forward); 5′-CCACAGGATTCCATACCCAAGA-3′ (reverse); Tfr1: 5′-CAGAAAGTTCCTCAGCTCAACCA-3′ (forward); 5′-GTTCAATTCAACGTCATGGGTAAG-3′ (reverse); Total (IRE plus non-IRE form) Dmt1: 5′-GGCTTTCTTATGAGCATTGCCTA-3′ (forward); 5′-GGAGCACCCAGAGCAGCTTA-3′ (reverse); ferroportin: 5′-CCAGCATCAGAACAAACACG-3′ (forward); 5′-ACTGCAAAGTGCCACATCC-3′ (reverse); Hamp-1: 5′-CCTATCTCCATCAACAGATG-3′ (forward); 5′-AACAGATAC-CACACTGGGAA-3′ (reverse); ceruloplasmin: 5′-TGCAAACC-TATTCCCTCATAAAAGT-3′ (forward); 5′-GGCACTCAACAT-CAAAAGTCCC-3′ (reverse). Cycle threshold (Ct) values were obtained for each gene of interest and the β-actin internal standard. Gene expression was normalized to β-actin and represented as ΔCt values. For each sample the mean of the ΔCt values was calculated. Relative gene expression was normalized to controls with an arbitrary expression level of 1.0.
Western blot analysis
Placental protein samples (40 μg) were heated in 1 × sample loading buffer (0.125M Tris-HCl, pH 6.8, 4% SDS, 20% (v/v) glycerol, 0.6M β-mercaptoethanol and 0.02% bromophenol blue) at 95°C for 5 min, resolved on a 10% SDS polyacrylamide gel and transferred to polyvinylidene difluoride membranes (BioRad, Hercules, USA). Membranes were blocked for 1 h at room temperature with 5% non-fat dry milk in PBS-T (PBS/0.1% Tween 20) and incubated at 4°C overnight with 1:500 dilution of ferroportin antibody (Alphadiagnostics, San Antonio, USA) and 1:10,000 dilution of β-actin antibody (Abcam, Cambridge, UK). Cross reactivity was detected using an HRP-linked secondary antibody (1:4000; GE Healthcare, Buckingham, UK) and ECL reagent (Pierce, Rockford, USA). Band densities were quantified using Quantity One 1-D Analysis software (BioRad).
Statistical analysis
All of the results are presented as mean ±SEM. Statistical analysis was calculated by two-tailed unpaired Student’s t-tests. P≤0.05 was considered significant.
Results
Duodenal and hepatic iron transporter gene expression increases during pregnancy
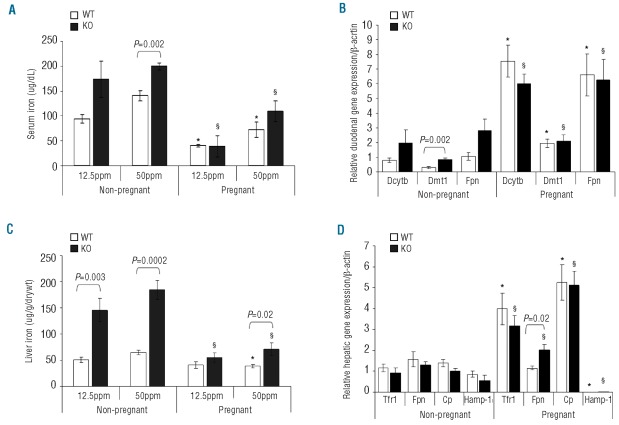
Serum iron levels were measured in both non-pregnant and pregnant WT and KO dams to observe the effects pregnancy has on circulating iron levels and to see whether there are differences between WT and KO dams. Serum iron levels decreased markedly during pregnancy, and both WT and KO dams had significantly lower levels compared with their non-pregnant counterparts on both iron deficient and adequate diets (Figure 1A). No difference was observed between pregnant WT and KO dams at either 12.5 or 50 ppm dietary iron (iron deficient and iron adequate diets, respectively). In the non-pregnant state, serum iron levels at 50 ppm were significantly higher in KO than WT dams. This difference was not observed at 12.5 ppm dietary iron.
Figure 1.
(A) Serum iron levels in non-pregnant and pregnant WT and KO dams ingesting 12.5ppm and 50ppm dietary iron. Mean±SEM presented: *P<0.05 for WT non-pregnant vs. pregnant at 12.5 and 50ppm dietary iron (n≥4); §P<0.05 for KO non-pregnant vs. pregnant at 12.5 and 50 ppm dietary iron (n≥4). (B) Duodenal transporter mRNA levels in non-pregnant and pregnant WT and KO dams ingesting 50ppm dietary iron. Mean ± SEM presented: *P<0.05; WT non-pregnant vs. pregnant for Dcytb, Dmt1 and Fpn (n≥4); §P<0.05; KO non-pregnant vs. pregnant for Dcytb, Dmt1 and Fpn (n ≥ 4). (C). Non-heme liver iron levels in non-pregnant and pregnant WT and KO dams ingesting 12.5ppm and 50ppm dietary iron. Mean ± SEM presented: *P<0.001 for WT non-pregnant vs. pregnant at 50ppm dietary iron (n ≥ 4); §P<0.001 for KO non-pregnant vs. pregnant at 12.5 ppm and 50 ppm dietary iron (n≥4). (D). Hepatic transporter mRNA levels in non-pregnant and pregnant WT and KO dams ingesting 50ppm dietary iron. Mean ± SEM presented: *P<0.05; WT non-pregnant vs. pregnant for Tfr1 and Cp (n ≥ 4); §P<0.05; KO non-pregnant vs. pregnant for Tfr1, Fpn and Cp (n ≥ 4)
In order to assess whether the differences in serum iron levels during pregnancy were attributable to differences in iron transporter gene expression, we measured duodenal Dcytb, Dmt1 and Fpn mRNA levels. During pregnancy, regardless of maternal genotype, all three genes were significantly up-regulated compared to their non-pregnant counterparts (Figure 1B); however, this was only true at 50ppm dietary iron. No difference was observed at 12.5 ppm between pregnant and non-pregnant dams (data not shown). The levels of Dcytb, Dmt1 and Fpn expression were the same in both WT and KO pregnant dams at 50 ppm dietary iron suggesting that during pregnancy the increased demand for iron overrides the genotypic difference. In the non-pregnant state, only Dmt1 appeared to be significantly up-regulated in the duodenum of KO dams compared with WT.
We measured non-heme liver iron levels in non-pregnant and pregnant WT and KO dams ingesting 12.5 and 50 ppm iron diets to determine whether iron storage during pregnancy is genotype and diet specific. During pregnancy, liver iron levels decreased markedly in both WT and KO dams ingesting an iron adequate diet. Although we found decreased liver iron levels at 50 ppm during pregnancy, KO dams still retained more iron compared to their WT pregnant counterparts (Figure 1C). Interestingly, at 12.5 ppm dietary iron, no difference was observed between non-pregnant and pregnant WT dams, indicating that once a certain minimum limit is obtained, no further iron is mobilized from maternal liver. This suggests there is a lower limit threshold for liver iron loss during pregnancy. Liver iron levels in non-pregnant KO dams were much higher than in WT dams at both diets tested (Figure 1C).
The expression levels of Tfr1, Fpn, ceruloplasmin (Cp) and Hamp-1 were assessed in the livers of non-pregnant and pregnant dams to see whether their mRNA levels were altered during pregnancy. The expression of Tfr1 and Cp increased during pregnancy for both WT and KO dams at 50 ppm iron; conversely Hamp-1 levels were barely detectable during pregnancy in both WT and KO dams (Figure 1D). No change was observed in any of the genes tested at 12.5 ppm (data not shown). Liver Fpn expression was up-regulated in KO dams during pregnancy at 50 ppm dietary iron, suggesting that maternal liver Fpn expression was up-regulated to increase iron supply to the fetus; no change was observed in WT dams.
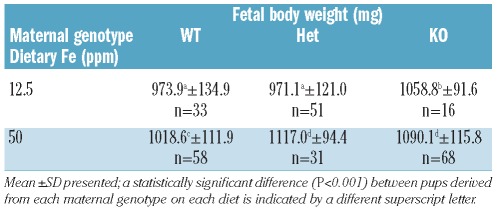
Maternal Hfe genotype and dietary iron intake determine fetal development
Measurement of fetal body weight showed that growth was dependent on maternal diet, increasing with increasing dietary iron intake. Body weight was also influenced by maternal Hfe status. Overall, KO dams appeared to produce pups with higher body weight than WT. It would, therefore, appear that the Hfe status of the mothers and the iron content of their diet are essential for maintaining high fetal body weight during pregnancy (Table 1). Dietary iron and Hfe genotype did not alter the number of fetuses born per litter (data not shown).
Table 1.
Total body weight (in milligrams) of fetuses born from dams of varying genotypes (WT, Het, KO) fed varying iron diets (12.5 and 50 ppm) during pregnancy.
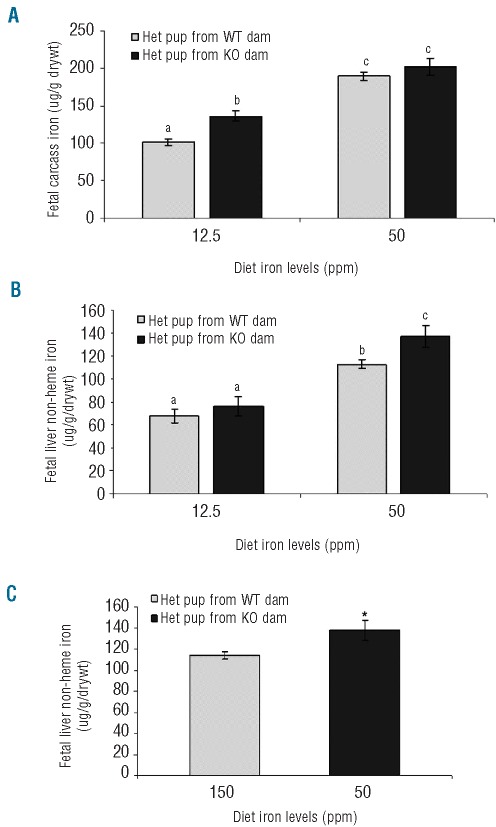
Maternal Hfe status affects iron transport across the placenta and fetal liver iron stores
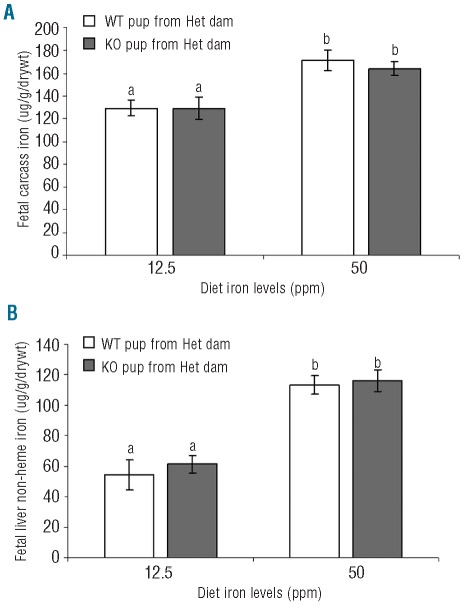
What effects do maternal genotype and diet have on iron transfer to the fetus? We wanted to see whether loss of maternal Hfe could promote increased iron transport across the placenta. To address this, we mated WT and KO dams with Het males and measured body iron in addition to non-heme liver iron in Het fetuses. Our results show that maternal genotype affects the amount of iron being transferred to the fetus (Figure 2). At 12.5 ppm iron, the amount of iron in Het pups born from KO dams was significantly higher than those born from WT dams (Figure 2A). This, however, was not a factor when dietary iron levels were adequate at 50 ppm. Iron levels in fetuses were higher in pups derived from dams fed an adequate iron diet than those fed an iron deficient diet.
Figure 2.
(A) Fetal carcass iron (total body iron minus liver) and (B) non-heme liver iron levels of Het pups derived from either WT or KO dams fed 12.5 or 50 ppm iron diets. Mean ±SEM presented: bars with different letter superscripts are significantly different (P<0.001, n≥7). (C) Non-heme liver iron levels of Het pups derived from WT dams on 150 ppm diet and Het pups derived from KO dams on 50 ppm diet. Mean ±SEM presented: *P=0.02; n≥27.
Non-heme iron was measured in fetal liver in order to assess fetal iron storage during the latter stages of gestation (Figure 2B). It appeared that little iron was stored in the liver of fetuses when the dams were fed a low-iron diet, independent of maternal genotype. However, when dietary iron was adequate, liver iron levels were significantly increased in fetuses from both WT and KO dams compared with those on an iron deficient diet. In addition, fetal liver iron levels in pups derived from KO dams were significantly higher than those derived from WT dams on the adequate diet.
In order to assess whether the effects of iron transport to the fetus were due to maternal serum and liver iron levels or Hfe genotype, WT dams were iron loaded to the same degree as KO dams. WT dams were placed on 150 ppm iron whereas KO dams were fed a diet consisting of 50 ppm iron. Serum iron levels of both WT and KO dams were equivalent at 104.4±18.0 and 108.8±20.6 mg/dL, respectively (mean ±SEM; P=0.8). Liver iron levels of both WT and KO dams were also equivalent at 66.3 ±5.3 and 70.8±12.2 μg/g/drywt, respectively (mean ±SEM; P=0.9). Liver iron levels of Het pups were then assessed (Figure 2C). Fetal liver iron levels were significantly higher in Het pups derived from KO dams on 50 ppm iron than Het pups derived from WT dams on 150 ppm iron, suggesting an effect of loss of Hfe rather than maternal serum and liver iron levels on iron transfer to the fetus.
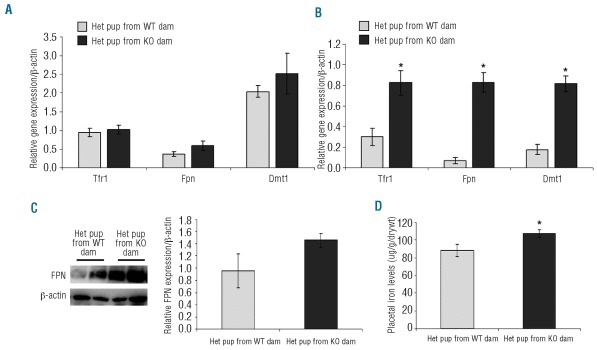
Iron transporter expression in KO placentas is elevated at adequate dietary iron levels
The expression of various key iron regulatory genes was measured in the placenta of Het pups derived from both WT and KO dams on low and adequate iron diets in order to clarify the molecular mechanisms of iron acquisition from the mother (Figure 3). The levels of Tfr1, Fpn and Dmt1 expression were unchanged at 12.5 ppm iron; maternal genotype had no effect (Figure 3A). This, however, was not the case at 50 ppm iron where Tfr1, Fpn and Dmt1 expression in the placenta were elevated approximately 3-fold in pups derived from KO dams compared with WT (Figure 3B). Placental FPN protein levels were also measured by Western blotting (Figure 3C). It appears that there are higher FPN protein levels in placenta of Het pups derived from KO dams at 50 ppm than WT. Placental non-heme iron levels were also measured (Figure 3D) and higher iron levels were observed in Het pups from KO dams when fed an adequate diet.
Figure 3.
Placental gene expression in Het pups derived from WT and KO dams fed (A) 12.5 ppm and (B) 50 ppm dietary iron. Mean ±SEM presented; *P<0.05, n≥5. (C) Western blot analysis of placental ferroportin expression (upper panel) of Het pups derived from WT and KO dams fed 50 ppm iron diet. Lanes 1 and 2: Het pups from WT dams. Lanes 3 and 4: Het pups from KO dams. Band intensity was quantified by densitometry, corrected for loading using β-actin as a control and represented graphically, n=3. (D) Non-heme placental iron levels of Het pups derived from WT and KO dams on 50 ppm iron diet. Mean ±SEM presented: *P=0.04; n=8.
Fetal genotype controls iron absorption from mothers fed a high iron diet
If maternal genotype is a factor affecting iron transfer across placenta, could fetal genotype also play a role? To address this, Het dams were mated with Het males and total body iron as well as non-heme liver iron was measured in fetuses with a WT and KO genotype (Figure 4). We found that there was less iron in fetuses from dams on a low iron diet than those from dams on an adequate diet (Figure 4A). In addition, fetal genotype appeared to have no effect on liver iron storage at either low or adequate iron diets (Figure 4B). Here again, there was less liver iron in those fetuses derived from dams on a low iron diet than those on the adequate diet. At these dietary iron concentrations, we also found no difference in the levels of placental Tfr1, Fpn and Dmt1 mRNA expression in both KO and WT pups (data not shown). At the protein level, also no difference in FPN expression was detected (data not shown).
Figure 4.
(A) Fetal carcass iron (total body iron minus liver) and (B) non-heme liver iron levels of WT and KO pups born from Het dams fed 12.5 or 50 ppm iron diets. Mean ±SEM presented: bars with different letter superscripts are significantly different (P<0.05, n ≥ 7).
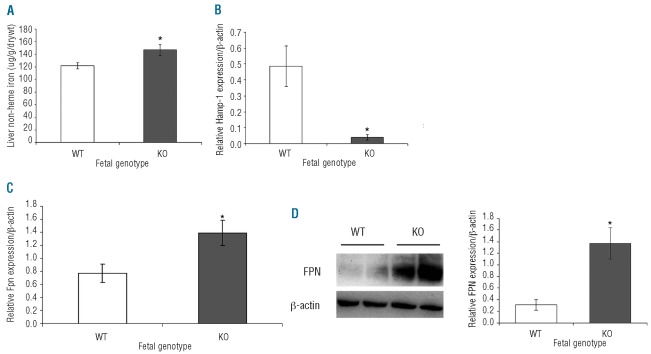
When Het dams were fed a high iron diet consisting of 150 ppm iron, fetal iron measurements showed no difference between KO or WT pups (data not shown). However, when fetal non-heme liver iron was measured, KO pups had higher levels than WT (Figure 5A) suggesting increased iron acquisition and storage in these fetuses. This suggests strongly that fetal iron regulatory genes might regulate iron absorption from the mother. Consequently, hepcidin mRNA was measured in the livers of WT and KO pups (Figure 5B). Liver hepcidin expression was barely detectable in KO pups compared with WT. In addition, Fpn mRNA levels were measured in both WT and KO pup placenta. Fpn mRNA levels were found to be significantly lower in WT than in KO pups (Figure 5C). Placental FPN protein expression mirrored its mRNA expression, being lower in WT than in KO pups (Figure 5D); this was confirmed by quantification of band intensity (Figure 5E).
Figure 5.
Fetal iron regulation. (A) Fetal non-heme iron, (B) fetal liver hepcidin mRNA levels and (C) placental ferroportin mRNA levels of WT and KO pups born from Het dams fed 150 ppm iron diet. (D) Western blot analysis of placental ferroportin protein expression (upper panel) of WT and KO pups born from Het dams fed 150 ppm iron diet. Lanes 1 and 2: WT pups derived from Het dams. Lanes 3 and 4: KO pups derived from Het dams. Band intensity was quantified by densitometry, corrected for loading using β-actin as a control and represented graphically, n=3. Mean ±SEM presented. Statistically significant values between WT and KO pups are indicated by an asterisk above the bar (P<0.03, n≥3).
Discussion
Most of the iron transferred to the developing fetus takes place during the last trimester of pregnancy. It is clear that iron transfer is strictly regulated. In a previous study using iron supplementation and deficiency during pregnancy, we were able to infer by correlation data that fetal liver plays a central role in regulating the fetal iron supply.30 In this study, using a mating strategy between Hfe−/−, Hfe+/− and Hfe+/+ mice, we have been able to confirm that fetal iron levels are indeed involved. This regulation by fetal liver iron levels involves expression of hepcidin and its negative effect on placental ferroportin protein levels, thereby modulating iron efflux. In the case of Hfe KO fetuses, placental ferroportin levels are increased due to low expression of hepcidin, thereby resulting in increased iron levels when dams are fed an iron replete diet. In addition to this, we have also shown that maternal Hfe status plays a role in the transfer of iron across placenta, but this is independent of maternal hepcidin expression during pregnancy.
As in previous studies, we have also shown that deletion of Hfe in non-pregnant mice results in increased serum iron and hyper accumulation of iron in the liver.26,31 However, during pregnancy, increases in serum iron and liver iron are observed only when dietary iron is adequate. When dietary iron levels are low (12.5 ppm), maternal liver iron levels drop to low levels. What is interesting is that maternal iron levels in WT dams are the same when they are fed both the iron deficient and adequate diets, a 4-fold difference in dietary iron levels, whereas fetal iron levels are significantly higher in iron adequate fed dams. Therefore, we can conclude that during iron deficiency, the mother maintains its liver iron at the expense of the fetus, as has been suggested previously.32,33 Previous studies have shown that iron supplementation and adequate iron ingestion during the first two trimesters of pregnancy are associated with better pregnancy outcome and higher fetal weights;34–37 this present study confirms this. Our results demonstrate that the combination of maternal iron ingestion and Hfe status affects fetal body weight with dams ingesting an iron deficient diet producing fetuses with the lowest body weight. Pups derived from KO dams appeared to have higher body weights than those derived from WT dams when fed both deficient and adequate diets. This has also been demonstrated recently in a human study; mothers with HFE variants were more likely to give birth to babies of a higher weight than controls; although this was true only for babies with HFE mutations.38 In agreement with this, we found that when high maternal iron was ingested, KO pups had higher weights than either WT or Het pups regardless of maternal genotype (data not shown). Hfe KO dams, therefore, appear to be better able to provide iron to their pups than their WT counterparts.
In order to understand the mechanism of regulation of iron homeostasis during pregnancy, we studied gene expression of the main iron transporter genes in the liver and intestine. In the liver, during pregnancy, iron transporter gene expression altered to compensate for a loss in iron stores by increasing Tfr1, Fpn and Cp mRNA levels. We also tested the role of genotype and found that there was differential expression of only Fpn in WT and KO dam liver. The increase in Fpn expression in KO dams may serve to release hepatic iron for delivery to the fetus. Increased Tfr1 expression in maternal liver during pregnancy was similar in both WT and KO dams. An increase in maternal liver Tfr1 expression has previously been shown to be inversely correlated with maternal iron status during pregnancy in the rat.23, 34 Increased CP oxidase activity in serum has also been previously reported during iron deficiency in pregnancy.39,40 In support of this, we found increased Cp mRNA levels in both WT and KO pregnant dams compared with their non-pregnant counterparts. This may serve to change the valency of iron as it shuttles between the mother and fetus. During the third trimester of pregnancy, hepcidin levels are barely detectable23 and no difference in hepcidin mRNA expression was observed between pregnant WT and KO dams in this study.
Depletion of liver iron stores during pregnancy has been shown to induce rapid iron intake by the maternal gut.30,41 In support of this, we found increased expression of Dmt1, Dcytb and Fpn in the duodenum of pregnant WT and KO mice compared to their non-pregnant counterparts. Previous studies in rats have shown increased Dcytb and Dmt1 mRNA levels in the last trimester of pregnancy; however, no changes in Fpn expression were found.23 We also found that non-pregnant KO dams showed increased Dmt1 expression; this may account for the increased iron absorption seen in the KO mouse, as has been described previously.42 We did not find any differences in duodenal gene expression for Dmt1, Dcytb and Fpn during pregnancy between WT and KO dams, suggesting that pregnancy and maintenance of maternal iron levels override all other factors.
Maternal Hfe status may be critical for regulating iron transport across the placenta to the fetus; the heterozygote offspring of KO dams had higher iron levels than heterozygote pups from WT dams at both dietary levels. In addition, when WT dams were loaded to the same degree as KO dams, higher liver iron levels were observed in pups derived from KO dams, indicating that iron transfer to the fetus is not due solely to maternal iron levels but is likely attributable to loss of Hfe in the dam. In an attempt to explain this observation, we measured the expression levels of major iron transport genes Tfr1, Dmt1 and Fpn in the placenta. When maternal dietary iron intake was limited, expression of these genes was maximal regardless of maternal genotype. When maternal dietary iron was adequate, Tfr1, Dmt1 and Fpn expression was down-regulated in the placentas of pups derived from WT dams. Tfr1 has been shown to be up-regulated in the placentas of iron deficient rats43 and this was true for those pups derived from WT dams on 12.5 ppm versus 50 ppm (cf Tfr1 Figure 3A and B; Tfr1 arbitrary value of 1 at 12.5 ppm vs. 0.3 at 50 ppm). This regulation, however, was lost in those pups derived from KO dams that had equivalent levels of Tfr1 and Fpn mRNA at both dietary iron levels. FPN protein levels were also increased in placenta of pups derived from KO dams on an adequate diet compared with those derived from WT, indicative of increased iron transfer to the fetus; this accounts for the increased liver iron stores seen in those pups derived from KO dams ingesting 50 ppm iron. Interestingly, placental non-heme iron levels were lower in pups derived from WT than those derived from KO on 50 ppm dietary iron. It would appear that KO dams are unable to regulate the amount of iron transported across the placenta; this is manifested in the inability to down-regulate both Tfr1 and Fpn in the placenta when dietary iron levels are adequate and when placental iron and fetal iron stores are high. The driving force behind this increase in placental gene expression in pups derived from KO dams is still not clear; however, it is unlikely to be due to maternal hepcidin levels as the hepatic expression of hepcidin was negligible in both maternal genotypes. We propose that some other as yet unidentified factor under the regulation of the Hfe gene is involved in these changes in placental gene expression.
Interestingly, fetal genotype also appears to play a role in iron acquisition. Our results show that KO pups accumulate more iron in the liver than their WT counterparts when maternal dietary intake is high. WT pups produce hepcidin in the liver whereas KO pups fail to do so. It has been suggested previously that liver iron levels in the fetus regulate iron uptake across placenta;30 our data support this. Our results suggest that normal regulation of iron absorption in KO pups is lost, thus enabling increased iron transport even though liver iron stores are near full capacity. Fetal hepcidin has been shown to be decreased in the livers of iron deficient rats compared with rats on an iron-supplemented diet;30,44 this indicates that, in WT animals, fetal hepcidin could contribute to the regulation of iron transport. Our data shows that this effect of fetal hepcidin at higher dietary iron levels may be directed primarily at placental ferroportin. Martin et al.24 showed that hepcidin transgenic embryos were anemic because they expressed reduced Tfr1 levels in the placenta but with no change in ferroportin levels. It would be interesting to look at fetal iron metabolism in Hamp KO pups and assess gene changes in placentas of these fetuses.
Taken together, our results show that adequate iron intake during pregnancy is crucial for maintaining maternal iron stores and healthy fetal development. Maternal Hfe appears to play a major role in iron acquisition by the fetus. Hfe KO fetuses lose the ability to control their iron absorption when maternal dietary intake is high. This may be partly due to dynamic changes in hepcidin-ferroportin interaction in the placenta. Taken together, our data suggests that there may be an evolutionary advantage to the Hfe mutation during pregnancy: high maternal iron produces healthier offspring with the caveat that if this mutation is inherited, it may not be so advantageous later in life.
Acknowledgments
We thank Michelle Murphy and Helen Hayes for technical help throughout the study.
Footnotes
Funding: this work was supported by a Biotechnological and Biological Sciences Research Council grant (BB/D521322/1) to SKSS.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.de Benoist B, McLean E, Egli I, Cogswell M, editors. WHO Global Database on Anaemia. Geneva: World Health Organization; 2008. Worldwide prevalence of anaemia 1993–2005. [Google Scholar]
- 2.Georgieff MK. The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem Soc Trans. 2008;36(6):1267–71. doi: 10.1042/BST0361267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srai SK, Bomford A, McArdle HJ. Iron transport across cell membranes: molecular understanding of duodenal and placental iron uptake. Best Pract Res Clin Haematol. 2002;15(2):243–59. doi: 10.1016/s1521-6926(02)90003-4. [DOI] [PubMed] [Google Scholar]
- 4.Enns CA, Sussman HH. Similarities between the transferrin receptor proteins on human reticulocytes and human placentae. J Biol Chem. 1981;256(24):12620–3. [PubMed] [Google Scholar]
- 5.Enns CA, Sussman HH. Physical characterization of the transferrin receptor in human placentae. J Biol Chem. 1981;256(19):9820–3. [PubMed] [Google Scholar]
- 6.Enns CA, Shindelman JE, Tonik SE, Sussman HH. Radioimmunochemical measurement of the transferrin receptor in human trophoblast and reticulocyte membranes with a specific anti-receptor antibody. Proc Natl Acad Sci USA. 1981;78(7):4222–5. doi: 10.1073/pnas.78.7.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkkila S, Waheed A, Britton RS, Bacon BR, Zhou XY, Tomatsu S, et al. Association of the transferrin receptor in human placenta with HFE, the protein defective in hereditary hemochromatosis. Proc Natl Acad Sci USA. 1997;94(24):13198–202. doi: 10.1073/pnas.94.24.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruper Y, Bar J, Bacharach E, Ehrlich R. Transferrin receptor co-localizes and interacts with the hemochromatosis factor (HFE) and the divalent metal transporter-1 (DMT1) in trophoblast cells. J Cell Physiol. 2005;204(3):901–12. doi: 10.1002/jcp.20349. [DOI] [PubMed] [Google Scholar]
- 9.McArdle HJ, Morgan EH. Transferrin and iron movements in the rat conceptus during gestation. J Reprod Fertil. 1982;66(2):529–36. doi: 10.1530/jrf.0.0660529. [DOI] [PubMed] [Google Scholar]
- 10.McArdle HJ, Douglas AJ, Morgan EH. Transferrin binding by microvillar vesicles isolated from rat placenta. Placenta. 1984;5(2):131–8. doi: 10.1016/s0143-4004(84)80056-9. [DOI] [PubMed] [Google Scholar]
- 11.Georgieff MK, Wobken JK, Welle J, Burdo JR, Connor JR. Identification and localization of divalent metal transporter-1 (DMT-1) in term human placenta. Placenta. 2000;21(8):799–804. doi: 10.1053/plac.2000.0566. [DOI] [PubMed] [Google Scholar]
- 12.Bastin J, Drakesmith H, Rees M, Sargent I, Townsend A. Localisation of proteins of iron metabolism in the human placenta and liver. Br J Haematol. 2006;134(5):532–43. doi: 10.1111/j.1365-2141.2006.06216.x. [DOI] [PubMed] [Google Scholar]
- 13.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13(4):399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 14.Giannetti AM, Björkman PJ. HFE and transferrin directly compete for transferrin receptor in solution and at the cell surface. J Biol Chem. 2004;279(24):25866–75. doi: 10.1074/jbc.M401467200. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 16.Delaby C, Pilard N, Gonçalves AS, Beaumont C, Canonne-Hergaux F. Presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and down-regulated by hepcidin. Blood. 2005;106(12):3979–84. doi: 10.1182/blood-2005-06-2398. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad KA, Ahmann JR, Migas MC, Waheed A, Britton RS, Bacon BR, et al. Decreased liver hepcidin expression in the Hfe knockout mouse. Blood Cells Mol Dis. 2002;29(3):361–6. doi: 10.1006/bcmd.2002.0575. [DOI] [PubMed] [Google Scholar]
- 18.Bridle KR, Frazer DM, Wilkins SJ, Dixon JL, Purdie DM, Crawford DH, et al. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet. 2003;361(9358):669–73. doi: 10.1016/S0140-6736(03)12602-5. [DOI] [PubMed] [Google Scholar]
- 19.Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood. 2005;105(4):1803–6. doi: 10.1182/blood-2004-08-3042. [DOI] [PubMed] [Google Scholar]
- 20.Constante M, Jiang W, Wang D, Raymond VA, Bilodeau M, Santos MM. Distinct requirements for Hfe in basal and induced hepcidin levels in iron overload and inflammation. Am J Physiol Gastrointest Liver Physiol. 2006;291(2):G229–37. doi: 10.1152/ajpgi.00092.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietrangelo A, Trautwein C. Mechanisms of disease: The role of hepcidin in iron homeostasis--implications for hemochromatosis and other disorders. Nat Clin Pract Gastroenterol Hepatol. 2004;1(1):39–45. doi: 10.1038/ncpgasthep0019. [DOI] [PubMed] [Google Scholar]
- 22.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 23.Millard KN, Frazer DM, Wilkins SJ, Anderson GJ. Changes in the expression of intestinal iron transport and hepatic regulatory molecules explain the enhanced iron absorption associated with pregnancy in the rat. Gut. 2004;53(5):655–60. doi: 10.1136/gut.2003.031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin ME, Nicolas G, Hetet G, Vaulont S, Grandchamp B, Beaumont C. Transferrin receptor 1 mRNA is downregulated in placenta of hepcidin transgenic embryos. FEBS Lett. 2004;574(1–3):187–91. doi: 10.1016/j.febslet.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA. 2002;99(7):4596–601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahram S, Gilfillan S, Kühn LC, Moret R, Schulze JB, Lebeau A, et al. Experimental hemochromatosis due to MHC class I HFE deficiency: immune status and iron metabolism. Proc Natl Acad Sci USA. 1999;96(23):13312–17. doi: 10.1073/pnas.96.23.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams RB, Mills CF. The experimental production of zinc deficiency in the rat. Br J Nutr. 1970;24(4):989–1003. doi: 10.1079/bjn19700102. [DOI] [PubMed] [Google Scholar]
- 28.American Institute of Nutrition. Second report of the ad hoc committee on standards for nutritional studies. J Nutr. 1980;131:741–4. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 29.Torrance JD, Bothwell TH. Tissue iron stores. Methods Hematol. 1980;1:90–115. [Google Scholar]
- 30.Gambling L, Czopek A, Andersen HS, Holtrop G, Srai SK, Krejpcio Z, et al. Fetal iron status regulates maternal iron metabolism during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R1063–70. doi: 10.1152/ajpregu.90793.2008. [DOI] [PubMed] [Google Scholar]
- 31.Fleming RE, Holden CC, Tomatsu S, Waheed A, Brunt EM, Britton RS, et al. Mouse strain differences determine severity of iron accumulation in Hfe knockout model of hereditary hemochromatosis. Proc Natl Acad Sci USA. 2001;98(5):2707–11. doi: 10.1073/pnas.051630898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Georgieff MK, Mills MM, Gordon K, Wobken JD. Reduced neonatal liver iron concentrations after uteroplacental insufficiency. J Pediatr. 1995;127(2):308–11. doi: 10.1016/s0022-3476(95)70317-9. [DOI] [PubMed] [Google Scholar]
- 33.Halvorsen S. Iron balance between mother and infant during pregnancy and breast-feeding. Acta Paediatr. 2000;89(6):625–7. doi: 10.1080/080352500750043882. [DOI] [PubMed] [Google Scholar]
- 34.Gambling L, Andersen HS, Czopek A, Wojciak R, Krejpcio Z, McArdle HJ. Effect of timing of iron supplementation on maternal and neonatal growth and iron status of iron-deficient pregnant rats. J Physiol. 2004;561(1):195–203. doi: 10.1113/jphysiol.2004.068825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM. Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. Am J Clin Nutr. 2003;78(4):773–81. doi: 10.1093/ajcn/78.4.773. [DOI] [PubMed] [Google Scholar]
- 36.Aranda N, Ribot B, Garcia E, Viteri FE, Arija V. Pre-pregnancy iron reserves, iron supplementation during pregnancy, and birth weight. Early Hum Dev. 2011 doi: 10.1016/j.earlhumdev.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Gambling L, Charania Z, Hannah L, Antipatis C, Lea RG, McArdle HJ. Effect of iron deficiency on placental cytokine expression and fetal growth in the pregnant rat. Biol Reprod. 2002;66(2):516–23. doi: 10.1095/biolreprod66.2.516. [DOI] [PubMed] [Google Scholar]
- 38.Dorak MT, Mackay RK, Relton CL, Worwood M, Parker L, Hall AG. Hereditary hemochromatosis gene (HFE) variants are associated with birth weight and childhood leukemia risk. Pediatr Blood Cancer. 2009;53(7):1242–8. doi: 10.1002/pbc.22236. [DOI] [PubMed] [Google Scholar]
- 39.Gambling L, Dunford S, McArdle HJ. Iron deficiency in the pregnant rat has differential effects on maternal and fetal copper levels. J Nutr Biochem. 2004;15(6):366–72. doi: 10.1016/j.jnutbio.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Fosset C, McGaw BA, Abramovich D, McArdle HJ. Interrelations between ceruloplasmin and Fe status during human pregnancy. Biol Trace Elem Res. 2004;98(1):1–12. doi: 10.1385/BTER:98:1:01. [DOI] [PubMed] [Google Scholar]
- 41.O’Brien KO, Zavaleta N, Abrams SA, Caulfield LE. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr. 2003;77(4):924–30. doi: 10.1093/ajcn/77.4.924. [DOI] [PubMed] [Google Scholar]
- 42.Fleming RE, Migas MC, Zhou X, Jiang J, Britton RS, Brunt EM, Tomatsu S, Waheed A, Bacon BR, Sly WS. Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: increased duodenal expression of the iron transporter DMT1. Proc Natl Acad Sci USA. 1999;96(6):3143–8. doi: 10.1073/pnas.96.6.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gambling L, Danzeisen R, Gair S, Lea RG, Charania Z, Solanky N, et al. Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochem J. 2001;356(3):883–9. doi: 10.1042/0264-6021:3560883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hossain MB, Kelleher SL, Lonnerdal B. Maternal iron and zinc supplementation during pregnancy affects body weight and iron status in rat pups at weaning. J Nutr. 2011;141(5):798–804. doi: 10.3945/jn.110.135681. [DOI] [PubMed] [Google Scholar]