Abstract
Background
In transfusional siderosis, the iron binding capacity of plasma transferrin is often surpassed, with concomitant generation of non-transferrin-bound iron. Although implicated in tissue siderosis, non-transferrin-bound iron modes of cell ingress remain undefined, largely because of its variable composition and association with macromolecules. Using fluorescent tracing of labile iron in endosomal vesicles and cytosol, we examined the hypothesis that non-transferrin-bound iron fractions detected in iron overloaded patients enter cells via bulk endocytosis.
Design and Methods
Fluorescence microscopy and flow cytometry served as analytical tools for tracing non-transferrin-bound iron entry into endosomes with the redox-reactive macromolecular probe Oxyburst-Green and into the cytosol with cell-laden calcein green and calcein blue. Non-transferrin-bound iron-containing media were from sera of polytransfused thalassemia major patients and model iron substances detected in thalassemia major sera; cell models were cultured macrophages, and cardiac myoblasts and myocytes.
Results
Exposure of cells to ferric citrate together with albumin, or to non-transferrin-bound iron-containing sera from thalassemia major patients caused an increase in labile iron content of endosomes and cytosol in macrophages and cardiac cells. This increase was more striking in macrophages, but in both cell types was largely reduced by co-exposure to non-transferrin-bound iron-containing media with non-penetrating iron chelators or apo-transferrin, or by treatment with inhibitors of endocytosis. Endosomal iron accumulation traced with calcein-green was proportional to input non-transferrin-bound iron levels (r2=0.61) and also preventable by pre-chelation.
Conclusions
Our studies indicate that macromolecule-associated non-transferrin-bound iron can initially gain access into various cells via endocytic pathways, followed by iron translocation to the cytosol. Endocytic uptake of plasma non-transferrin-bound iron is a possible mechanism that can contribute to iron loading of cell types engaged in bulk/adsorptive endocytosis, highlighting the importance of its prevention by iron chelation.
Keywords: iron, chelators, thalassemia, macrophages, fluorescence
Introduction
The presence of iron forms in the plasma that are not tightly bound to transferrin has been described for various iron overload disorders.1–4 These forms, commonly referred to as non-transferrin bound iron or NTBI,1 appear in two pathological scenarios: i) when plasma transferrin binding capacity for iron is exceeded due to an outpouring of iron into plasma;5 or ii) when the iron transferred to plasma fails to be catalytically incorporated into transferrin due to aceruloplasminemia or atransferrinemia.6 Persistently high plasma NTBI levels can lead to uncontrolled ingress of labile iron into cells and ensuing tissue damage in organs such as liver, endocrine glands and heart.3,6–8 These properties have led NTBI to be considered an indicator of impending tissue iron overload and a target of chelation therapy.3,5,9 However, the identification of membrane-permeant iron species in plasma NTBI and their routes of ingress into particular cells have not been established, largely because of the heterogeneic nature of NTBI itself and its variable composition in different pathologies.5 In systemic iron overload, NTBI has been claimed to be associated with various plasma components, such as organic acids like citrate, phosphates and proteins.10–12 The results of such associations are heterogeneous mixtures of chemical composition that vary according to a variety of factors: the source and flux of incoming iron, previous chelation or phlebotomy or, conversely, with the frequency of blood transfusions or parenteral iron administration.
Previous experiments to define the pathways of NTBI entry into various cell types have examined: i) application of iron(III) complexed to various organic acids as model permeant substrates; ii) supplementation of agents capable of reducing ferric complexes so as to maintain it in the ferrous state, which is presumed to be the only ionic permeant form of the metal; and iii) avoidance of incorporating proteins in the transport assays, as these might reduce the chemical activity of iron or its salts and thereby prevent their cell uptake. This contrasts with the fact that, in thalassemic patients, most of the plasma NTBI is excluded by size filtration1,12 and that its chemical determination requires harsh extraction measures.10–12 This would indicate that in thalassemic plasma in general and, as we found in this study, in plasma from non-chelated patients, the chemically active forms of NTBI consist of high-molecular weight complexes which may be protein-associated. We predict that for macromolecular forms to gain access to cells, they would need to be initially transferred as fluid cargo or membrane adsorbed species that are taken up by endocytosis and subsequently by transmembrane mechanisms into the cytosol. In order to assess the initial steps of ingress of plasma NTBI into model cells,13,14 we used as NTBI substrate the original sera from thalassemia major patients who had only undergone sporadic treatment with iron chelators, so that levels of Fe-chelates in circulation were negligible. As model NTBI forms, we used whole human serum or only its albumin (HSA) fraction supplemented with iron-citrate or other iron sources. The ingress of iron into cell compartments was followed by time-lapse fluorescence imaging tracing in two formats: i) in endosomes by following the rise in fluorescence of Oxyburst-green, an endosomal macromolecular ROS indicator, and particularly its sensitivity to iron chelators; ii) in cytosol, by following the quenching of the fluorescent metalosensors calcein-green (CALG) or calcein-blue (CALB) and the reversal by permeating iron chelators as iron identification tools. Both confocal and epifluorescence microscopy were used to image cell fluorescence and, when applicable, flow cytometry was also used on suspended cells. Cell lines of macrophages, endocrine glands and heart were used as those are major cells types that accumulate iron in poly-transfused patients. Specific blockers of endocytosis were used as experimental tools to examine the involvement of endocytic steps in NTBI cell loading and iron chelators for associating fluorescence changes with labile iron.
Design and Methods
Information concerning sources of materials is available in the Online Supplementary Appendix.
Cell culture
RAW264.7 mouse macrophage cells and the RAW264.7 macrophage cell lines stably transfected with a functional NRAMP1 (RAW 37) or NRAMP1 antisense variant (RAW 21) (generously provided by Dr H Barton, University of Southampton, UK) were grown in 5% CO2 Dulbecco’s modified Eagle’s (DMEM) medium supplemented with 10% fetal calf serum, 4.5 g/L D-glucose, glutamine and antibiotics (Biological Industries, Kibbutz Bet Haemek, Israel).15 Cells were plated onto 12-well plates or onto microscopic slides glued onto perforated 3-cm diameter tissue culture plates. The plates were analyzed by confocal microscopy with an FV-1000 confocal microscope (Olympus, Japan) equipped with an IX81 inverted objective and placed in a thermostated CO2 environmental chamber, or by a Nikon TE 2000 microscope equipped with optigrid and autofocusing systems and a Hamamatsu Orca-Era CCD camera. The system was operated with a Volocity 4 operating system (Improvision, Coventry, UK) that was used for both image data acquisition and analysis. The NIH Image J program was also used.13,14
RAW cells and H9c2 cultured cardiomyoblasts were grown as previously described.13,14 Rat INS1 cells clone 832/13 (generously provided by Dr HE Hohmeier, Duke University, NC, USA) derived from rat pancreas were stably transfected with the human proinsulin gene. The cells were grown in RPMI containing 10% fetal bovine serum, 10 mM Hepes, 1mM Sodium Pyruvate and 50 μM β-Mercaptoethanol. Flow cytometric analysis was performed with an automated Eclipse instrument (iCyt, Champaign, IL, USA) on H9c2 cardiomyoblasts after trypsinization, as described elsewhere for other cells.13–15 We also used cultured HL-1 cardiac muscle cells derived from the AT-1 mouse atrial cardiomyocyte tumor lineage that continuously divide and spontaneously contract while maintaining a differentiated cardiac phenotype (generously provided by Dr WC Claycomb, LSU, New Orleans, USA).16
Measurement of DCI (directly chelatable iron) in serum samples
The concentration of DCI in the sera of patients was determined as described previously.17,18 The assay is based on the binding of NTBI in serum to fluorescein-DFO (Fl-DFO), causing quenching of its fluorescence. Briefly, each serum sample is measured under two separate conditions: A, with Fl-DFO only; and B, as in condition A but in the presence of a large excess of non-fluorescent DFO. This ensures that the change in fluorescence is due to the binding of iron to Fl-DFO rather than due to other unknown factors in the sample. The concentration of DCI is calculated using iron calibration solutions, from the difference between fluorescence under conditions A and B divided by the maximal fluorescence of the sample (under condition B). As there is a strong positive correlation between DCI and NTBI,17.18 sera were defined as NTBI-positive or negative if their DCI values were above or below 0.4 μM, respectively. The fact that inclusion of nitrilotriacetic acid in the DCI assay did not reveal additional DFO chelatable material was taken as an indication that the DCI values represented most of the NTBI fraction.18
Human sera samples
The sera samples used in this study were primarily from thalassemia major adolescents (age 14–21 years) living in the Gaza area, who were regularly transfused but only sporadically chelated and who had not undergone any chelation for at least six months prior to the present study. The study was approved by the Helsinki ethics committee of the European Hospital in Gaza and patients provided their written informed consent. All sera were initially tested for iron related parameters serum ferritin and transferrin saturation and for non-transferrin bound iron (NTBI) measured as directly chelatable iron (DCI).18 Sera with DCI over 1.2 μM were used as source of high NTBI (hNTBI) and those with values below 0.4 μM as low NTBI (lNTBI). Model NTBI-containing sera were prepared by supplementing 20 μM ferric citrate to human sera that had originally a lower than 70% transferrin saturation and thereby attained NTBI levels of 10–15 μM (measured as DCI, see below). All human sera were applied to cells in culture at 30% concentrations in DMEM media.
Measurement of labile iron in endosomes
Cells were incubated for up to 90 min in growth media containing the Oxyburst green probe (Oxyburst-green) (40 μg/mL) and either 30% human serum (with or without NTBI) or human serum albumin (40 μM) supplemented (or not) with 30 μM ferric citrate. After incubation, cells were washed and bathed in DMEM-HEPES medium and subsequently reacted with H2O2 (50 μM) for 10 min at 37°C.
Treatment of cells with different iron containing media
RAW264.7 cells were perfused with DMEM-HEPES (20 mM pH 7.4) medium containing either 30% human sera or human serum albumin (50 μM) or iron-saccharate (Venofer) (500 μM), in general for up to 3 h at 37°C, and subsequently washed with DMEM-HEPES alone. In some studies, CALG (5–30 μM) was added in order to trace iron within cells by addition of the permeant iron chelator SIH (50 μM), which reveals all Fe quenched complexes. The sera used were from: i) normal individuals (N); ii) from thalassemia major patients with high NTBI (hNTBI); and iii) from patients with low NTBI (lNTBI) but rendered hNTBI by incubating them with 10 μM ferric citrate (FC).
Measurement of cytosolic CALG or CALB fluorescence in cells exposed to NTBI-containing media
Cells which were pre-exposed (up to 3 h) to medium containing 30% sera with high NTBI (hNTBI) or low NTBI (lNTBI), or human serum albumin (HSA) supplemented or not with ferric citrate, were cytosolically loaded with CALG by 1 min incubation at 37°C with CALG-AM (1 μM) in DMEM-HEPES medium or with CALB via CALB-AM (10 μM at 37°C for 10 min). Cells were subsequently washed with HEPES-buffered saline (HBS, 130 mM NaCl, 20 mM Hepes, pH 7.4) and bathed at 37°C in DMEM-HEPES containing 0.5 mM probenecid (to minimize probe leakage).13,19,20 To assess fluorescence properties, the cells were analyzed either microscopically or by flow cytometry (following release by trypsinization). Epi-fluorescence microscopy analysis of CALG was carried out using EXC: 488nm and Em: 520 nm and for CALB EXC: 390 nm and Em: 430 nm).
Results
NTBI uptake into cells as revealed with cytosolic iron markers
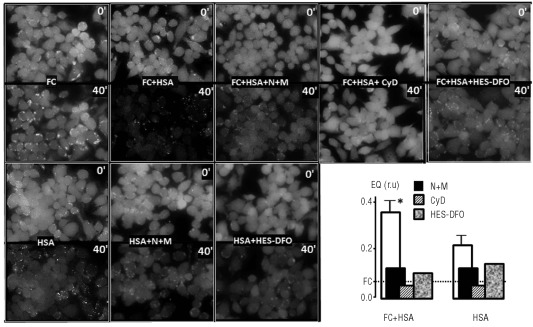
The mechanism of plasma NTBI uptake by cells depends both on the chemical nature of the substrate, the composition of the medium and the cell in question. The canonical mode of assessing transport of metals or molecules into cells is by tracing substrate ingress into the cytosol. For tracing ingress of labile iron into the cytosol we used CALG, a green fluorescent metal sensor that is loaded into cells via its CALG-AM precursor.13,20 The probe undergoes swift and stoichiometric quenching by interacting with labile iron and recovers its fluorescence when challenged with iron chelators. Using CALG-laden RAW macrophages and fluorescence microscopy live imaging, we found that ferric compounds such as ferric citrate, considered a major component of NTBI in iron overloaded sera,11,12 failed to evoke changes in cytosol fluorescence over a period of 90 min, unless the medium was supplemented with human serum albumin (HSA) (Figure 1 shows data for only two time points: 0 and 40 min).
Figure 1.
Uptake of ferric citrate into the cytosol of RAW cells. Effect of human serum albumin, inhibitors of endocytosis and impermeant iron chelators. RAW cells labeled in the cytosol with CALG via its AM precursor were followed for up to 1 h by epifluorescence microscopy while perfused in DMEM-HEPES supplemented with ferric citrate only (Fe:citrate10: 30 μM) (FC), or together with 50 μM human serum albumin (FC+HSA) and the indicated substances: nocodazole (N=30 μM), ML-9 (M=100 μM)(FC+HSA+N+M), cytochalasin D (cyD= 100 μM) (FC+HSA+CyD), and hydroxyethyl-starch-DFO (FC+HSA+HES-DFO 50 μM). Systems without added ferric citrate were HSA alone (50 μM) (HSA), HSA with nocodazole (N=30 μM) and ML-9 (M=100 μM)(HSA+N+M) and HSA with hydroxyethyl-starch-DFO (HSA+HES-DFO 50 μM). All incubations and perfusions were carried out at 37°C. The pictures show snapshots of the same field taken at 0 at 40 min, and the graph shows the mean fluorescence quenching (FQ) of each field (analyzed with NIH Image J; National Institutes of Health, Bethesda, MD, USA) normalized to the initial fluorescence intensity (control cells).
The degree of CALG quenching in the cytosol that resulted from exposure of cells to various types of iron-containing media is shown in Figure 1 (lower right panel). Several features can be observed: i) the change in fluorescence evoked by ferric citrate applied together with HSA was iron-related, as the addition of the impermeant iron chelator hydroxyethyl-starch-DFO to the medium essentially abrogated the change. Similarly, HSA alone led to a lower but significant cytosolic quenching that was related to contaminating iron, as hydroxyethyl-starch-DFO also abrogated it; ii) the observation that quenching evoked by both ferric citrate + HSA and HSA alone was markedly reduced when cells were pre-treated with blockers of endocytosis, nocodazole + ML9 or cytocholasin D, strongly suggested a cellular endocytic route in the process of iron delivery to cytosol from a surrogate NTBI source. The enhancing effect of HSA on endocytic uptake of iron can be interpreted in terms of the observations of Evans et al.12 showing that while ferric citrate consists of low-molecular weight forms (< 30 kD) in the absence of HSA, it acquires high-molecular weight properties (> 30 kD) immediately after the addition of HSA.
NTBI uptake into cells as revealed with endosomal iron markers
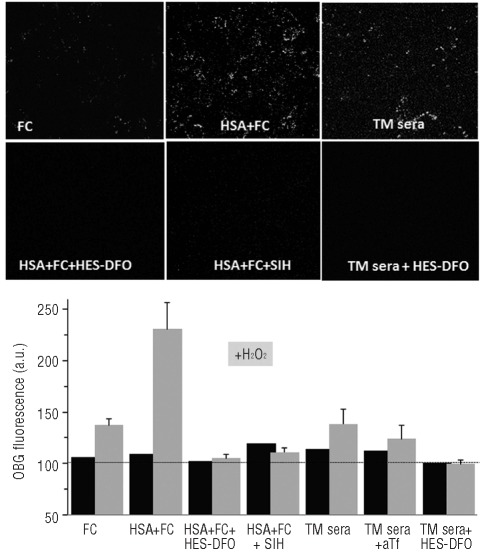
In order to assess if iron derived from native plasma/serum NTBI was initially taken up into the endosomal compartment, we supplemented NTBI-containing media with the albumin-tagged Oxyburst-green probe which is endocytosed and whose ability to fluoresce upon addition of H2O2 can be attributed to labile iron due to its sensitivity to iron chelators. RAW cells exposed to media containing Oxyburst-green showed a punctuated fluorescence when the medium contained ferric citrate + HSA but not ferric citrate alone. There was no difference between pictures of cells exposed only to ferric citrate (Figure 2) and those exposed only to HAS, patients’ sera or just control medium (data not shown). Importantly, the endosome associated fluorescence is attributable to endosomal labile iron, as the addition of a permeant chelator (SIH) after exposure to iron-containing media, but prior to hydrogen peroxide, abrogated the rise in fluorescence. More importantly, Oxyburst-green supplemented to sera from thalassemia major patients showed similar properties to those of HSA + ferric citrate, while hydroxyethyl-starch-DFO or apo-transferrin abrogated the fluorescence changes.
Figure 2.
Uptake of NTBI into endosomes of RAW cells as revealed with an endosomal ROS probe. RAW cells were incubated for 2–3 h in DMEM medium containing Oxyburst-green (OBG) (400 μg/mL) with 10 μM ferric citrate (FC), or 50 μM human serum albumin + 10 μM ferric citrate (FC+HSA), or 30% thalassemia major sera (TM sera), or HSA (50 μM) + 10 μM FC supplemented with either hydrox-yethyl-starch-DFO (HSA+FC+HES-DFO 50 μM) or the permeable chelator SIH (HSA+FC+SIH 50 μM), or TM sera with hydroxyethyl-starch-DFO (TM sera+HES-DFO 50 μM). After washing of cells, the fluorescence of Oxyburst-green was monitored before and 10 min after addition of H2O2 (50 μM). For this we used live epifluorescence microscopy adapted for pseudo-confocal imaging with an Optigrid system. The pictures shown in the upper panel are snapshot images taken after addition of H2O2 and the bar graph in the lower panel represents the mean fluorescence intensities of Oxyburst-green in endosomes of 4 cells per field (±SEM) for one of 3 representative experiments. The bar graph includes data obtained with TM sera + apo-transferrin (TM sera+aTf). Data are given in terms of arbitrary fluorescence units obtained by image analysis with Image J.
All this information links labile iron to the fluorescence changes occurring within endosomes and traces its origin to the NTBI-containing medium, either associated with native thalassemia major serum or with the artificially formulated NTBI in the form of HSA+ ferric citrate.
A similar approach to monitor iron ingress into cytosol following exposure to NTBI-containing medium was applied to H9c2 cardiomyoblasts and to HL-1 contractile cardiomyocytes. The cardiac cells are markedly less active in endocytosis than the RAW macrophages and accordingly, the follow up of NTBI uptake by cardiomyoblasts and cardiomyocytes demanded extended exposure of cells to NTBI-containing media so as to enable fluorescence detection of iron-dependent signals. An NTBI-evoked rise in fluorescence was observed: i) in endosomes using Oxyburst-green in conjunction with fluorescence microscopy (Online Supplementary Figures S1 and S2); and ii) in cytosol using CALG in conjunction with fluorescence microscopy (Online Supplementary Figure S3) or flow cytometry following release of attached cells by trypsinization (Online Supplementary Figure S4). The analysis revealed qualitatively similar features of NTBI uptake into endosomes (Online Supplementary Figures S1 and S2) and to cytosol (Online Supplementary Figures S3 and S4) of cardiomyoblasts and cardiomyocytes as compared to RAW macrophage cells (Figures 1 and 2), particularly their susceptibility to inhibitors of endocytosis and/or chelating agents.
NTBI uptake into cells as revealed with a fluorescent membrane impermeant iron marker
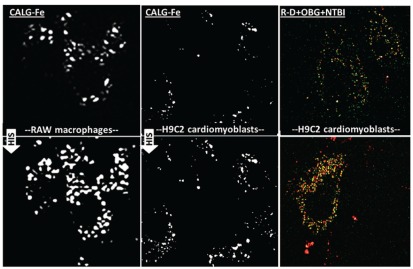
The implication of these results is that initial stages of cellular uptake of NTBI occur via endocytosis of whole complexes of iron together with its presumed ligands (citrate and albumin). In order to assess this possibility by an alternative means, we tried to generate a complex simulating NTBI by use of an iron-responsive fluorescent probe with iron-binding moieties similar to those of citrate. In this approach, ferric citrate is replaced with ferric-calcein green (CALG-Fe), a fluorescence-quenched NTBI surrogate whose metal-quenched fluorescence can be revealed in cell compartments by addition of permeant chelators such as SIH. We initially explored the possibility that CALG added to sera might show different fluorescence responses depending on the presence or absence of NTBI. For this we used two different cell lines representative of transfusional siderosis, macrophages and cardiomyoblasts and assessed uptake of the fluorescent (partially quenched by the complexed iron) NTBI simulator CALG-Fe into the endosomal compartment (Figure 3). Macrophages showed prominent acquisition of endosomal fluorescence following exposure to NTBI-containing media supplemented with CALG, possibly related to their high constitutive endocytic activity. A similar property was observed in macrophages when using polymeric forms of NTBI,12 such as the iron-saccharate Venofer (Sohn et al., unpublished observations, 2011) that is administered i.v. in various treatment regimens of iron deficiencies.
Figure 3.
Uptake of NTBI into the endosomal compartment of various cell types RAW, macrophages (left panels) and H9c2 cardiomyoblast cells (middle panels) were loaded with calcein-green-iron complexes (CALG-Fe 20 μM) for 3 h and washed extensively. SIH (50 μM) was added and the fluorescence in the same fields was inspected 10 min later (lower left and lower middle panels). H9c2 cardiomyoblasts were exposed for 18 h to Oxyburst-green (400 μg/mL) + Rhodamine-dextran (R-D) (30 μM) in growth medium supplemented with NTBI in the form of 30 μM ferric citrate + HSA (50 μM) (RD+OBG+NTBI) (upper right panel) and after washing they were challenged with H2O2 (50 μM) (lower right panel) as described in Figure 2 for RAW cells. Colocalization of Oxyburst-green and Rhodamine dextran appears as yellow spots.
In both macrophages and cardiomyoblasts, we found that the endosomal fluorescence rose following addition of the permeant chelator SIH, indicating the presence of chelatable iron. Moreover, similarly to RAW cells exposed to serum NTBI-containing medium and probed with Oxyburst-green, the H9c2 cardiomyoblasts also showed uptake of NTBI into a cell compartment shared with the fluid-endocytosis marker rhodamine-dextran (R-D).
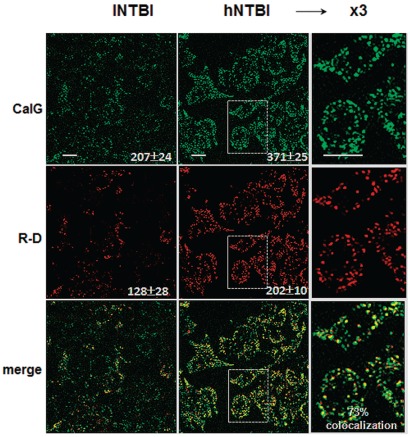
To confirm that the endosome-associated fluorescence originating from CALG-Fe, as observed in HSA-containing medium (Figure 3) can also be demonstrated with genuine sera from thalassemia major patients containing NTBI, we incubated RAW cells with NTBI-positive sera from thalassemia major patients supplemented with: i) the red fluorescent R-D as marker of fluid endocytosis; and ii) CALG as in situ green fluorescent marker of native plasma NTBI (Figure 4).
Figure 4.
Endocytosis of CALG and rhodamine-dextran by RAW cells in media containing sera from thalassemia major patients. RAW cells were incubated for 3 h with thalassemia major sera containing low and high NTBI (lNTBI or hNTBI) as indicated and both CALG (10 μM) and Rhodamine-dextran (R-D) (30 μM). After washing of cells and treatment with SIH 50 μM, the fluorescence of CALG and R-D was monitored by live epifluorescence microscopy equipped with an Optigrid system. The fluorescence intensity of CALG and R-D obtained from 5 different cell areas and the mean values obtained from 3 independent experiments are shown in the body of the figure. Colocalization of merged images of CALG and R-D (shown in the 3rd row of images) performed with the Volocity program was 73–75%.
We noted that both markers applied separately or together yielded similar cell patterns of green and of red fluorescence associated with the endosomal compartment, as highlighted in Figure 4 (right column). When both probes were applied on the same cultures, the calculated degree of probe co-localization (Figure 4, bottom panel) was 70–75% indicating similar uptake features of both markers. As both cell fluorescence signals were: i) markedly intensified when the thalassemia major sera used were from patients with high NTBI (hNTBI); and ii) green but not red fluorescence was reduced when sera were pre-treated with DFO (Sohn and Cabantchik, unpublished observations, 2011), we also concluded that sera from thalassemia major patients most likely have components that might promote endocytosis beyond what is observed with HSA alone.
NTBI uptake and the status of iron in thalassemia major sera as assessed with CALG
Initial studies on the interaction of CALG with iron overloaded sera revealed that a substantial fraction of the fluorescent probe CALG becomes adsorbed to serum components (Online Supplementary Figure S4). Incubation of 6 different thalassemia major sera with 20 μM CALG followed by ultrafiltration on 30 kD cut-off filters showed that 69% of CALG fluorescence was associated with the filter-retained, high molecular weight fraction, compared to 7% in the absence of serum. In addition, when CALG was added to thalassemia major sera it also underwent fluorescence quenching (and dequenching following addition of excess chelators such as SIH), in a manner roughly proportional to the NTBI content of the sera (Sohn et al., unpublished observations, 2011). Thus, CALG appears to bind labile iron in thalassemia major sera, similarly to labile iron in solution19 and concomitantly becomes associated with serum proteins, similarly to ferric citrate.12 Ultrafiltration experiments of CALG incubated with purified human serum albumin showed similar CALG-binding properties as whole serum (Online Supplementary Figure S4), indicating that albumin is the probable CALG-binding serum component. These observations, together with the fact that most of the NTBI fraction could not filtered (with or without CALG), led us to assume that exposure of cells to sera from thalassemia major patients doped with CALG might provide some clues as to the fate of NTBI uptake into cells.
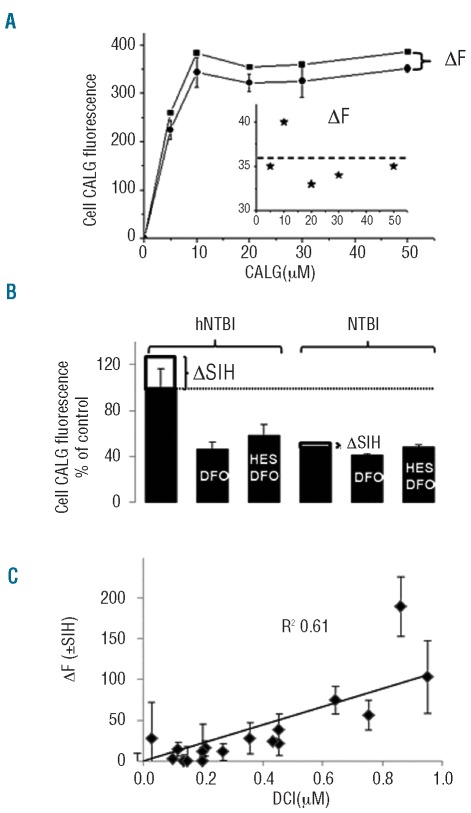
We found that endocytic CALG uptake into cells was saturable and did not increase further at concentrations of 10 μM probe or over for a particular hNTBI serum (Figure 5A). However, we noticed (Figure 3A inset) that the fraction revealed by SIH, namely CALG-Fe, was essentially independent of CALG concentration used in the range 5–50μM.
Figure 5.
CALG uptake into RAW cells exposed to thalassemia major sera is dependent on concentration of CALG and NTBI. (A) RAW cells were incubated for 3 h in DMEM medium containing 30% high-NTBI thalassemia major serum supplemented with the indicated concentrations of CALG and the mean fluorescence intensity measured in cells was plotted against CALG concentration (circles). The fluorescence intensity, ΔF, following addition of SIH (squares) was plotted against CALG (inset). (B) The uptake of CALG was measured in RAW cells exposed for 3 h to 30% thalassemia major sera with low NTBI (lNTBI) and high NTBI (hNTBI), as indicated, which were supplemented with 20 μM CALG without and with 50 μM DFO or hydroxyethyl-starch-DFO (HES-DFO). Fluorescence measurements were taken before and after addition of SIH (ΔF corresponds to ΔSIH) and the mean cell CALG fluorescence of 4 cells per field (±SD, from 3 independent experiments) was calculated with Image J program. (C) Correlation between endosomal CALG-Fe acquired by RAW cells and NTBI levels detected in thalassemia major sera. RAW cells exposed to sera from thalassemia major patients (n=20) were examined microscopically after 3 h incubation before and after addition of SIH, as described in Figure 1 (where ΔF corresponds essentially to ΔSIH). The mean cell CALG fluorescence (± SD) associated with the endosomal compartment was calculated from 4 different cells in a given field. The increment in fluorescence following SIH addition (ΔF+SIH) was plotted against serum DCI (in μM; a measure of serum NTBI, see Design and Methods section) and analyzed by linear regression analysis (slope 132±30 fluorescence units per μM Fe; R2=0.61).
In order to assess to what extent CALG fluorescence associated with endosomes depended on plasma NTBI, we compared the fluorescence intensity of cells exposed to sera with high NTBI and low NTBI following their pre-treatment with deferrioxamine (DFO) or its macromolecular conjugate hydroxyethyl-starch-DFO. As shown in Figure 5B, pre-treatment with these impermeant chelators markedly (about 50%) reduced CALG uptake and virtually eliminated the NTBI component revealed with SIH. We interpret these observations to indicate that the fluorescence associated with cells originated from both protein-associated CALG (DFO-insensitive fluorescence uptake) as well as CALG-Fe complexes formed in hNTBI thalassemia major serum that were taken up by cells in the 3 h incubation period. Conceivably, some of the CALG-Fe complexes dissociated in the endosomal compartment, so that SIH revealed only a residual fraction of CALG-Fe.
Finally, as CALG-Fe complexes are essentially dissociable,20,21 we considered the possibility that during the incubation of cells with CALG-supplemented thalassemia major sera, a fraction of the endosomal CALG-Fe undergoes dissociation followed by Fe translocation into the cytosol via NRAMP1 and/or 2 (DMT1), the two principal iron transporters in endocytic vesicles. That possibility was examined in two sublines of RAW cells over-expressing functional NRAMP1 (RAW 37) or non-functional anti-sense NRAMP1 (RAW 21).15,16 RAW 37 cells exposed to high NTBI sera showed higher levels of endosomal CALG than either WT or RAW 21 cells following 3 h incubation with CALG supplemented thalassemia major sera (Figure 6A). However, the fluorescence increase exerted by SIH, which reveals endosomal CALG-Fe complexes, showed higher values in WT and RAW 21 relative to RAW 37 cells. We suggest that this could be attributed to the egress of Fe dissociated from endosomal CALG-Fe complexes into the cytosol of NRAMP1-positive subline 37. As shown in, there was a time-dependent increase in endosomal CALG fluorescence concomitant with a time dependent decrease in cytosol CALB fluorescence, indicating translocation of Fe from endosomes into cytosol, particularly in the NRAMP1 expressing subline 37 (Figure 5B and Figure 6C and D).
Discussion
The appearance of plasma NTBI in systemic iron overload caused by iron hyperabsorption or blood hypertranfusions is a phenomenon that has pathophysiological, diagnostic and therapeutic implications.3,22 The pathological accumulation of iron in multiple tissues observed in iron overload conditions is thought to be caused by excessive influx of iron into plasma, as well as unbalanced erythrophagocytosis by reticuloendothelial cells in spleen and liver that lead to persistently high circulating NTBI levels which are responsible for the systemic iron dispersal. The hypothesis that tissue iron overload is caused by NTBI is largely based on the assumption that NTBI is randomly transported into cells by unregulated mechanisms, presumably via non-specific divalent cation transporters23,24 or calcium channels,25–27 subsequent to extracellular reduction of iron(III) to iron(II) by a cell-surface iron reductase such as Dcytb. Chronic exposure of cells in vitro to artificial iron complexes that presumably mimic NTBI (usually ferric citrate)10–12,28,29 has been shown to generate cellular iron overload as indicated by increased ferritin levels, ROS generation, protein and DNA oxidation, and other indicators of cell damage.30–32 However, the relevance of such models to cell iron overload in vivo is open to question because of various factors associated with the presence of NTBI in plasma: i) the association/complexation of NTBI with various acids and plasma proteins;28 ii) the changes in chemical composition of NTBI with changing iron concentrations12 and plasma oxidation;29,30 and iii) the changes in NTBI properties due to fluctuations in plasma component composition. Adsorption of NTBI to macromolecular plasma components, such as albumin,28,29 may restrict its access to iron reductases and divalent ion transporters at the cell surface due to steric hindrance. Moreover, in thalassemia or other iron overload disorders, it is not only difficult to experimentally simulate NTBI per se, but also to reproduce the possible chemical modifications of plasma components that become exposed to oxidative stresses due to a rise in labile iron and depletion of antioxidants.29,30 Despite these limitations, attempts have been made to assess NTBI transport by using NTBI-simulating complexes of radiolabeled iron in protein free settings and by artificial inclusion of reductants in order to render the iron transportable by various voltage activatable Ca channels25–27 or by a putative Zn transporter.23,24 The pathophysiological significance of such approaches is still a subject of debate, and likewise the effects of Ca-channel blockers on iron-associated cardiac damage.27
Considering the complex nature of plasma NTBI, we choice to use native NTBI containing sera from hyper-transfused thalassemia major patients in conjunction with two major strategies for tracing iron ingress into cell compartments. One was based on the Oxyburst-green probe that is taken up from medium into the endosomal compartment and which fluoresces when activated by hydrogen peroxide in a metal-dependent/chelator-sensitive manner (Figure 2, Online Supplementary Figures S1 and S2). The other strategy monitored labile iron ingress into the cytosolic CALG-laden or CALB-laden compartment by following metal evoked quenching of fluorescence (Figures 1 and 6, Online Supplementary Figures S3 and S4). Evidence that both strategies monitored processes that involved endocytosis of NTBI was based on the effects of inhibitors of endocytosis and on those of impermeant iron chelators, such as hydroxyethyl-starch-DFO (Figures 1 and 5, Online Supplementary Figures S1 and S2), whereas proof that the species monitored in endosomes or cytosol was labile iron leaned on the action of permeant iron chelators, such as SIH or deferiprone (Figures 3–6). In addition to thalassemia major sera containing NTBI, we also used ferric citrate, an accepted component of plasma NTBI, either by itself or supplemented to human sera or human serum albumin (Figures 1 and 2, Online Supplementary Figures S1–S3). While cell exposure to ferric-citrate alone at concentrations measured in thalassemia major sera failed to evoke significant iron-associated changes in endosomal or cytosolic iron pools, its addition together with human sera or serum albumin (HSA) renders it demonstrably accessible to cells: first by undergoing endocytosis and subsequently by releasing labile iron in endosomes and translocating it to the cytosol, in the case of RAW cells, via NRAMP1 (Figure 6). That the processes monitored by endosomal Oxyburst-green or cytosolic CALG were associated with NTBI and not with TBI was deduced from the fact that addition of apotransferrin to media containing native or artificial NTBI abrogated the processes in a similar way as hydroxyethyl-starch-DFO.
We also found that the proposed steps of NTBI uptake into RAW cells were largely recapitulated with thalassemia major sera probed with CALG, that reversibly binds iron,20,21 including components of plasma NTBI, and is endocytosed commensurately with NTBI levels (Figure 3). However, a key question is to what extent CALG added to sera reports NTBI ingress into cells rather than promotes NTBI ingress by binding to plasma NTBI, whether present as low-molecular weight complexes or bound to plasma proteins. Since endocytic uptake of CALG-Fe and thalassemia major sera with CALG is inhibited by impermeant iron chelators, while the uptake of rhodamine-dextran is unaffected by the same chelators, it may be concluded that CALG is a reporter rather than a promoter of endocytosis of NTBI. This is further supported by our observation that cellular uptake of CALG-Fe was negligible in the absence of serum or serum albumin. Furthermore, not only CALG-Fe, but also ferric-citrate in the presence of albumin, and NTBI in thalassemia major sera also exist in macromolecular forms12 that could be taken up by cells by a similar bulk mechanism of endocytosis, whether adsorptive or pinocytic, which would be in line with the same mechanistic concept.
Taken together, these data indicate that a major component of plasma NTBI ingress into cells is associated with bulk mechanisms of endocytosis that prevail in cells of various organs.33–37 The proposed mechanism explains how NTBI species bound to plasma components gain access to particular cells, but does not exclude others which comprise some plasma NTBI forms transportable by resident membrane transporters or channels. Endocytic uptake of NTBI, which is demonstrated primarily in macrophages, also operates, though to a lesser extent, in other cells such as cardiomyoblasts and cardiomyocytes (Figure 4, Online Supplementary Figures S1–S4) and insulinoma cells (Glickstein and Cabantchik, unpublished observations, 2011).The relative contribution of NTBI endocytosis to iron accumulation in the above organs will depend on both their endocytic/pinocytic activities and on the levels of NTBI in plasma that undergoes significant modifications in chronic iron overload. Macropinocytic uptake of a variety of macromolecules has only recently become recognized as a regulated pathway with features that distinguish it from clathrin-dependent endocytic processes, such as receptor-mediated endocytosis.33 Numerous stimuli regulate macropinocytosis, among which the best defined are growth factor signaling and surface binding of intracellular pathogens. Pinocytic activity is particularly high in macrophages, epithelial and endothelial cells, and cells of the immune system.33 However, it also prevails, though at relatively lower levels, in various cell types and tissues, including those susceptible to iron overload such as the heart35–36 and endocrine glands.37
A surprising observation in this study was the enhanced endocytosis of rhodamine-dextran in the presence of sera containing NTBI (Figure 4). Although the biochemical basis for this effect is still unclear, it could be a key component in the mechanism of tissue iron loading in iron overloaded thalassemia major patients. As the uptake of rhodamine-dextran added to thalassemia major plasma is not affected by DFO, the contribution of NTBI per se to endocytosis is likely to involve pre-oxidized plasma components, for which there is ample evidence in hypertranfused patients with inadequate chelation treatment.26 Furthermore, albumin, especially in its oxidized form, has been shown to avidly bind iron29 and has been suggested to be a possible plasma carrier of NTBI.29 Considering that the half-life of oxidized human albumin in mice is reduced by almost 50%, mainly due to liver clearance,30 it is conceivable that oxidized albumin or other plasma components are preferentially endocytosed by macrophages. In fact, macrophages are essential to the recycling of iron extracted from senescent erythrocytes and the control of systemic iron levels.38 These properties have implications for the pathophysiology and treatment of iron overload, and highlight the need to eliminate NTBI from the circulation by chelation as a means of reducing plasma protein oxidation and ensuing tissue iron overload. However, further studies are also required to assess whether a similar mode of NTBI ingress by endocytosis prevails in other disorders of systemic iron overload, such as hemochromatosis, or transfusional siderosis, such as myelodysplastic syndromes and sickle cell disease.
Acknowledgments
We thank Ms. Hava Glickstein for valuable technical assistance and the support of the Israel Science Foundation (141/06) and the Canadian Friends of the Hebrew University.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Hershko C, Peto TE. Non-transferrin plasma iron. Br J Haematol. 1987;66(2):149–51. doi: 10.1111/j.1365-2141.1987.tb01291.x. [DOI] [PubMed] [Google Scholar]
- 2.Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18(2):277–87. doi: 10.1016/j.beha.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Porter JB. Pathophysiology of iron overload. Hematol Oncol Clin North Am. 2005;19(Suppl 1):7–12. [Google Scholar]
- 4.Hider RC, Silva A, Podinovskaia M, Ma YM. Monitoring the efficiency of iron chelation therapy: the potential of nontransferrin-bound iron. Ann NY Acad Sci. 2010;1202:94–9. doi: 10.1111/j.1749-6632.2010.05573.x. [DOI] [PubMed] [Google Scholar]
- 5.Breuer W, Hershko C, Cabantchik ZI. The importance of non-transferrin bound iron in disorders of iron metabolism. Transfus Sci. 2000;23(3):185–92. doi: 10.1016/s0955-3886(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 6.Ponka P. Rare causes of hereditary iron overload. Semin Hematol. 2002;39(4):249–62. doi: 10.1053/shem.2002.35638. [DOI] [PubMed] [Google Scholar]
- 7.Brittenham GM. Iron-chelating therapy for transfusional iron overload. N Engl J Med. 2011;364(2):146–56. doi: 10.1056/NEJMct1004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu P, Olivieri N. Iron overload cardiomy-opathies: new insights into an old disease. Cardiovasc Drugs Ther. 1994;8(1):101–10. doi: 10.1007/BF00877096. [DOI] [PubMed] [Google Scholar]
- 9.Cabantchik ZI, Fibach E, Breuer W. Can labile plasma iron (LPI) and labile cell iron (LCI) levels serve as early indicators of chelation efficacy in iron overload? BloodMed.com. 2009. Available from: http://www.bloodmed.com/800000/mini-reviews1.asp?id=254.
- 10.Grootveld M, Bell JD, Halliwell B, Aruoma OI, Bomford A, Sadler PJ. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear resonance spectroscopy. J Biol Chem. 1989;264(8):4417–44. [PubMed] [Google Scholar]
- 11.Hider RC. Nature of nontransferrin-bound iron. Eur J Clin Invest. 2002;32(Suppl 1):50–4. doi: 10.1046/j.1365-2362.2002.0320s1050.x. [DOI] [PubMed] [Google Scholar]
- 12.Evans RW, Rafique R, Zarea A, Rapisarda C, Cammack R, Evans PJ, et al. Nature of non-transferrin-bound iron: studies on iron citrate complexes and thalassemic sera. J Biol Inorg Chem. 2008;13(1):57–74. doi: 10.1007/s00775-007-0297-8. [DOI] [PubMed] [Google Scholar]
- 13.Glickstein H, Ben El R, Shvartsman M, Cabantchik ZI. Intracellular labile iron pools as direct targets of iron chelators. A fluorescence study of chelator action in living cells. Blood. 2005;106(9):3242–50. doi: 10.1182/blood-2005-02-0460. [DOI] [PubMed] [Google Scholar]
- 14.Sohn YS, Breuer W, Munnich A, Cabantchik ZI. Redistribution of accumulated cell iron: a modality of chelation with therapeutic implications. Blood. 2008;111(3):1690–9. doi: 10.1182/blood-2007-07-102335. [DOI] [PubMed] [Google Scholar]
- 15.Nairz M, Fritsche G, Crouch ML, Barton HC, Fang FC, Weiss G, et al. Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition. Cell Microbiol. 2009;11(9):1365–81. doi: 10.1111/j.1462-5822.2009.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA. 1998;95(6):2979–84. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breuer W, Ermers MJ, Pootrakul P, Abramov A, Hershko C, Cabantchik ZI, et al. Desferrioxamine-chelatable iron, a component of serum non-transferrin-bound iron, used for assessing chelation therapy. Blood. 2001;97(3):792–8. doi: 10.1182/blood.v97.3.792. [DOI] [PubMed] [Google Scholar]
- 18.Breuer W, Ghoti H, Shattat A, Goldfarb A, Koren A, Rachmilewitz E, et al. Non-trans-ferrin bound iron in Thalassemia: differential detection of redox active forms in children and older patients. Am J Hematol. 2012;87(1):55–61. doi: 10.1002/ajh.22203. [DOI] [PubMed] [Google Scholar]
- 19.Epsztejn S, Kakhlon O, Breuer W, Glickstein H, Cabantchik ZI. A fluorescence assay for the labile iron pool (LIP) of mammalian cells. Anal Biochem. 1997;248(1):31–40. doi: 10.1006/abio.1997.2126. [DOI] [PubMed] [Google Scholar]
- 20.Espósito BP, Epsztejn S, Breuer W, Cabantchik ZI. A review of fluorescence methods for assessing labile iron in cells and biological fluids. Anal Biochem. 2002;304(1):1–18. doi: 10.1006/abio.2002.5611. [DOI] [PubMed] [Google Scholar]
- 21.Ali A, Zhang Q, Dai J, Huang X. Calcein as a fluorescent iron chemosensor for the determination of low molecular weight iron in biological fluids. Biometals. 2003;16(2):285–93. doi: 10.1023/a:1020642808437. [DOI] [PubMed] [Google Scholar]
- 22.Hershko C, Link G, Cabantchik I. Pathophysiology of iron overload. Ann NY Acad Sci. 1998;850:191–201. doi: 10.1111/j.1749-6632.1998.tb10475.x. [DOI] [PubMed] [Google Scholar]
- 23.Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA. 2006;103(37):13612–7. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwiczek S, Theurl I, Muckenthaler MU, Jakab M, Mair SM, Theurl M, et al. Ca2+ channel blockers reverse iron overload by a new mechanism via divalent metal transporter-1. Nat Med. 2007;13(4):448–54. doi: 10.1038/nm1542. [DOI] [PubMed] [Google Scholar]
- 25.Oudit GY, Sun H, Trivieri MG, Koch SE, Dawood F, Ackerley C, et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med. 2003;9(9):1187–94. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- 26.Oudit GY, Trivieri MG, Khaper N, Liu PP, Backx PH. Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J Mol Med. 2006;84(5):349–64. doi: 10.1007/s00109-005-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowe S, Bartfay WJ. Amlodipine decreases iron uptake and oxygen free radical production in the heart of chronically iron overloaded mice. Biol Res Nurs. 2002;3(4):189–97. doi: 10.1177/109980040200300404. [DOI] [PubMed] [Google Scholar]
- 28.Løvstad RA. Interactions of serum-albumin with the Fe(III)–citrate complex. Int JBiochem. 1993;25(7):1015–7. doi: 10.1016/0020-711x(93)90115-u. [DOI] [PubMed] [Google Scholar]
- 29.Silva AM, Hider RC. Influence of nonenzymatic post-translation modifications on the ability of human serum albumin to bind iron: implications for nontransferrin-bound iron speciation. Biochem Biophys Acta. 2009;1794(10):1449–58. doi: 10.1016/j.bbapap.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Livrea MA, Tesoriere L, Pintaudi AM, Calabrese A, Maggio A, Freisleben HJ, et al. Oxidative stress and antioxidant status in beta-thalassemia major: iron overload and depletion of lipid-soluble antioxidants. Blood. 1996;88(9):3608–14. [PubMed] [Google Scholar]
- 31.Walter PB, Fung EB, Killilea DW, Jiang Q, Hudes M, Madden J, et al. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135(2):254–63. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kakhlon O, Gruenbaum Y, Cabantchik ZI. Repression of ferritin expression increases the labile iron pool, oxidative stress and short-term growth of human erythroleukemia cells. Blood. 2001;97(9):2863–71. doi: 10.1182/blood.v97.9.2863. [DOI] [PubMed] [Google Scholar]
- 33.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10(4):364–71. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 34.Soeiro Mde N, Mota RA, Batista Dda G, Meirelles Mde N. Endocytic pathway in mouse cardiac cells. Cell Struct Funct. 2002;27(6):469–78. doi: 10.1247/csf.27.469. [DOI] [PubMed] [Google Scholar]
- 35.Swildens J, de Vries AA, Li Z, Umar S, Atsma DE, Schalij MJ, et al. Integrin stimulation favors uptake of macromolecules by cardiomyocytes in vitro. Cell Physiol Biochem. 2010;26(6):999–1010. doi: 10.1159/000324013. [DOI] [PubMed] [Google Scholar]
- 36.Cowan DB, Noria S, Stamm C, Garcia LM, Poutias DM, del Nido PJ, et al. Lipopolysaccharide internalization activates endotoxin-dependent signal transduction in cardiomyocytes. Circ Res. 2001;88(5):491–8. doi: 10.1161/01.res.88.5.491. [DOI] [PubMed] [Google Scholar]
- 37.Pow DV, Morris JF. Membrane routing during exocytosis and endocytosis in neuroen-docrine neurones and endocrine cells: use of colloidal gold particles and immunocyto-chemical discrimination of membrane compartments. Cell Tissue Res. 1991;264(2):299–316. doi: 10.1007/BF00313967. [DOI] [PubMed] [Google Scholar]
- 38.Soe-Lin S, Apte SS, Andriopoulos B, Jr, Andrews MC, Schranzhofer M, Kahawita T, et al. Nramp1 promotes efficient macrophage recycling of iron following erythrophagocytosis in vivo. Proc Natl Acad Sci USA. 2009;106(14):5960–5. doi: 10.1073/pnas.0900808106. [DOI] [PMC free article] [PubMed] [Google Scholar]