Abstract
Background
Due to increased rates of secondary solid organ cancer in patients with severe aplastic anemia who received an irradiation-based conditioning regimen, we decided some years ago to use the combination of cyclophosphamide and antithymocyte globulin. We report the long-term follow up of patients who underwent hematopoietic stem cell transplantation from an HLA-matched sibling donor after this conditioning regimen.
Design and Methods
We analyzed 61 consecutive patients transplanted from June 1991 to February 2010, following conditioning with cyclophosphamide (200 mg/kg) and antithymocyte globulin (2.5 mg/kg/day × 5 days).
Results
Median age was 21 years (range 4–43); 41 of the 61 patients were adults. Median duration of the disease before hematopoietic stem cell transplantation was 93 days. All but 2 patients received bone marrow as the source of stem cells and all but 2 engrafted. Cumulative incidence of acute grade II–IV graft-versus-host disease was 23% (95%CI 13–34) and 18 developed chronic graft-versus-host disease (cumulative incidence 32% at 72 months, 95% CI 20–46). In multivariate analysis, a higher number of infused CD3 cells was associated with an increased risk of developing chronic graft-versus-host disease (P=0.017). With a median follow up of 73 months (range 8–233), the estimated 6-year overall survival was 87% (95% CI 78–97). At 72 months, the cumulative incidence of avascular necrosis was 21% and 12 patients presented with endocrine dysfunction (cumulative incidence of 19%). Only one patient developed a secondary malignancy (Hodgkin’s lymphoma) during follow up.
Conclusions
Cyclophosphamide and antithymocyte globulin is an effective conditioning regimen for patients with severe aplastic anemia and is associated with low treatment-related mortality. Long-term complications include avascular necrosis and endocrine dysfunction.
Keywords: hematopoietic stem cell transplantation, severe aplastic anemia, long-term outcomes
Introduction
Acquired aplastic anemia is a life-threatening hematopoietic disorder characterized by hypocellular bone marrow and peripheral blood pancytopenia. Autologous recovery of hematopoiesis in patients who failed to engraft after conditioning and stem cell transplantation, as well as responsiveness of patients to immunosuppressive therapies,1 provided evidence of the pivotal role of the immune system in the disease pathophysiology. Treatment strategy is based on severity of the disease, patient age and availability of an HLA-identical related donor.2 Immunosuppressive therapy (IST) with cyclosporine (CsA) and antithymocyte globulin (ATG) is currently used as front-line therapy for young patients who do not have an available HLA-identical related donor and for older patients. Response rate after IST ranges from 57 to 77% at six months and 5-year overall survival (OS) ranges from 60 to 90%.3,4 However, relapse occurs in one-third of cases3 and late events such as secondary malignancies or clonal evolution occur in 10%.5
Hematopoietic stem cell transplantation (HSCT) from an HLA-identical related donor is the treatment of choice for young patients with severe aplastic anemia (SAA). Storb et al. demonstrated excellent outcomes after CY-ATG with rates of engraftment of 95% and OS of almost 90%.6,7 Studies conducted by the European group for Blood and Marrow Transplantation8 and the International Bone Marrow Transplant Registry (IBMTR) found that the use of peripheral blood stem cells (PBSC) was associated with an increased risk of chronic graft-versus-host disease (GvHD) and lower 5-year overall survival compared to marrow grafts.9 The combination of CsA and methotrexate is recommended as prophylaxis for GvHD.10
Due to increased rates of secondary solid-organ cancer in patients with severe aplastic anemia who received an irradiation-based conditioning regimen, we decided some years ago to use the combination of CY and ATG. Although several studies have confirmed that HSCT after conditioning with CY plus ATG is associated with excellent OS rates,3,11,12 the long-term follow up of those patients is still limited, with the exception of the Seattle cohort.6,13 Long-term survivors after HSCT are exposed to late complications including abnormal immune reconstitution, non-malignant organ or tissue dysfunction, delayed infections and also secondary cancers. The aim of our study was, therefore, to analyze the long-term follow up of all consecutive patients in our center who received an HSCT from an HLA-identical related donor for SAA after conditioning with CY and ATG over a 20-year period.
Design and Methods
Inclusion criteria
The study included all consecutive patients with acquired SAA who: i) received a first HSCT from an HLA-matched sibling donor; ii) after a conditioning regimen based on Cy (200mg/kg) and ATG (2.5 mg/kg/day × 5 days); iii) with CsA and Mtx (Days 1, 3, 6 and 11) as GvHD prophylaxis from June 1991 to February 2010. Patients with Fanconi anemia and congenital bone marrow failure were excluded. The study protocol was approved by the review board of Hôpital Saint Louis and the study was carried out in accordance with the Declaration of Helsinki.
End point definitions
Neutrophil recovery was defined as the first of three consecutive days with an absolute neutrophil count (ANC) of at least 0.5×109/L, and the incidence of platelet recovery as the first of seven consecutive days of an unsupported platelet count of at least 20×109/L. Primary graft failure was defined as no sign of neutrophil recovery, as well as transient engraftment of donor cells 60 days after transplantation. Early clinical outcomes were defined as complications occurring in the first three months after transplantation. Acute GvHD and chronic GvHD were diagnosed and graded according to published criteria.14,15 Chronic GvHD was evaluated in all survivors 100 days after transplantation. Chimerism analyses were routinely evaluated by PCR of short tandem repeat sequences at three months in all but 2 patients transplanted after 2002. Full donor chimerism was defined as the presence of 95% or over of cells of donor origin, mixed chimerism if over 5% and less than 95% of donor cells, and autologous recovery if 5% or under of donor cells. Long-term complications and immune reconstitution were also evaluated. Late clinical outcomes were: occurrence of bacterial, viral and fungal infections, secondary malignancy (excluding in situ carcinoma and basal cell carcinoma), osteonecrosis, cardiovascular complications and endocrine dysfunction occurring more than three months after BMT. The CD3, CD4 and CD8 T cells, B cells and NK lymphocytes were enumerated from fresh whole blood EDTA samples by direct 3- or 4-color immunofluorescence with a Facscalibur analyzer (Becton Dickinson, San José, California, USA) as previously described.16 Percentages and absolute counts were determined. Lymphocyte subset populations were quantified three, six, 12 and 24 months after transplant.
Statistical analysis
Data are presented as median (range) or count (percentage). All time-to-event outcomes were counted from the date of transplant to the date of event or date of last follow up.
Neutrophil and platelet recovery, acute and chronic GvHD, infections and non-infectious complications were analyzed using a competing risks model where death was considered as the competing event. In addition, raw proportions of neutrophil and platelet recovery are reported. Cumulative incidence functions were estimated using the usual methodology.17 Since during the study no patient died while on immunosuppressive therapy, duration of immunosuppressive therapy was simply analyzed as a time-to-event end point. Factors associated with GvHD were analyzed using Cox’s proportional hazards models for the cause-specific hazard.18 Univariate analyses were carried out first, and then a multivariate analysis was used to identify a set of factors independently associated with chronic GvHD. In the latter analysis, all factors with P≤0.20 in the univariable analyses were considered but no model selection was subsequently undertaken. The proportional hazards assumption was checked by examination of Schoenfeld residuals and Grambsch and Therneau’s lack-of-fit test.19
Survival functions were estimated using Kaplan-Meier product-limit estimator and compared by log rank tests.17–19 Survival and cumulative incidence results are presented as estimates and 95% confidence intervals (95% CI), and results of Cox’s models as hazard ratios (95%CI). Given the median follow up, it was decided to present probability estimates at 72 months except otherwise stated.
Immune reconstitution was analyzed by modeling cell counts 3–24 months after transplant using linear mixed models with random intercept and slopes. Whenever necessary, cell counts were log-transformed to obtain approximately normally distributed residuals. Model goodness-of-fit was assessed by examination of residuals, and the covariance structure of random effects was assessed by Akaike’s information criterion and the likelihood ratio test.
All tests were two-sided and P values ≤ 0.05 were considered significant. Analyses were performed using the R statistical software version 2.10.1.20
Results
Patients’, disease and graft characteristics
Sixty-one consecutive patients underwent transplantation from an HLA-identical sibling donor in our center. All patients had acquired SAA and 80% had idiopathic aplastic anemia. Five patients had an aplastic anemia; PNH syndrome with small PNH clones (<10%) and without hemolysis. Median age was 21 years (range 4–43) with 41 adults. Thirteen patients were transplanted from 1991 to 1995, 21 from 1996 to 2002, and 27 after 2003. Details of patients’, diseases, prior therapies before transplants and graft characteristics are set out in Table 1.
Table 1.
Patients’ and graft characteristics.
Engraftment and chimerism studies
Neutrophil engraftment was observed in 59 patients (97%) at a median time from transplant of 22 days (inter-quartile range 19–26, IQR). Fifty-nine (97%) patients achieved an unsupported platelet count of at least 20×109/L (58 (95%) at least 50×109/L). Median (IQR) time to platelet recovery (>20×109/L) was 20 days (range 16–26) and 25 days (>50×109/L) (range 21–37), respectively. Primary graft failure occurred in 2 patients. No secondary graft failure was observed.
Chimerism was evaluated at three months post-HSCT in 25 of the 27 patients. Full donor chimerism was present in 14 (56%) patients and mixed donor chimerism in 11 (44%). Average chimerism was 90% (range 37–100).
Graft-versus-host disease
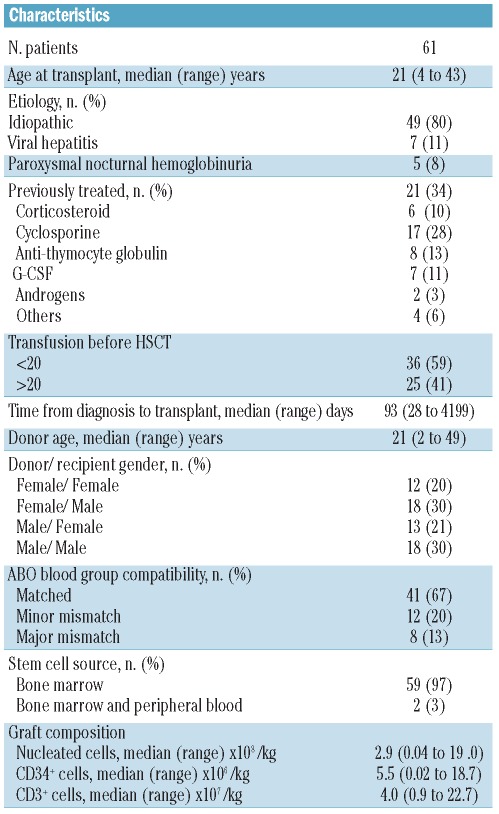
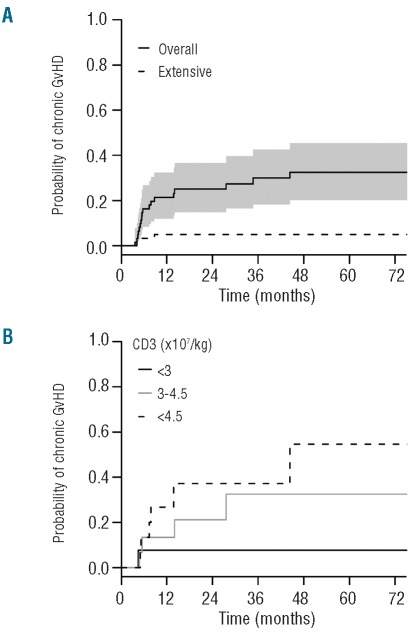
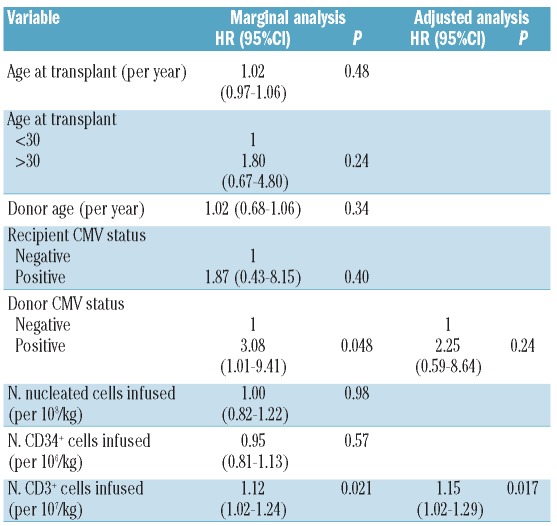
The cumulative incidence (CI) of acute GvHD grades 2–3 was 16% (range 8–27) and 23% (range 13–34) at three and 72 months, respectively. No acute GvHD grade 4 was observed. Fifty-nine patients were assessable for chronic GvHD; the CI at 72 months was 32% (range 20–46) (Figure 1A). Fifteen patients developed limited and 3 out of 18 developed extensive cGVHD. In univariate analysis, factors associated with higher cumulative incidence of chronic GvHD were positive donor CMV status (HR 3.08, 95% CI 1.01–9.41, P=0.048) and higher number of infused CD3 cells (HR 1.12, 95% CI 1.02–1.24, P=0.021). In multivariate analysis, the number of infused CD3 cells remained independently associated with a higher incidence of cGvHD (HR 1.15, 95% CI 1.02–1.29, P=0.017) (Table 2 and Figure 1B).
Figure 1.
Cumulative incidence of chronic GvHD. (A) The 72-month cumulative incidence of chronic GvHD was 32% (range 20–46). The solid line represents all chronic GvHD and the dashed line extensive chronic GvHD. The shaded area represents the pointwise 95% confidence interval. (B) The 72-month cumulative incidence of chronic GvHD is represented for patients who received less than 3.106/kg infused CD3+ cells, between 3 and 4.5 106/kg infused CD3+ cells and more than 4.5 106/kg infused CD3+ cells.
Table 2.
Factors associated with chronic GvHD.
Overall survival
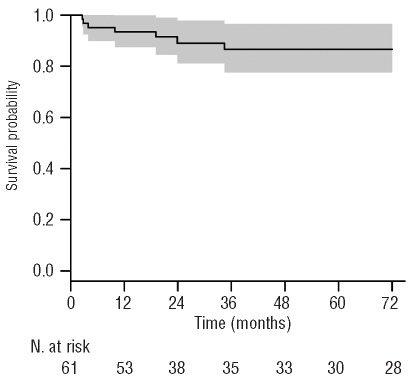
After a median follow up of 73 months (range 8–233), the estimated probability of 6-year OS was 87.5% (95% CI range 78–97) (Figure 2). Seven patients died during the study. The main cause of death was fungal infections (3 out of 7). Of note, these 3 patients died before 2000 and no patients died of fungal infection thereafter. Two patients died after a second transplant for rejection, one from steroid-resistant acute GvHD and one from EBV-associated Hodgkin’s lymphoma complications. Six deaths occurred within the first two years after HSCT.
Figure 2.
The 6-year probability of overall survival adjusted for waiting time to transplantation. The shaded area represents the point-wise 95% confidence interval.
Late complications
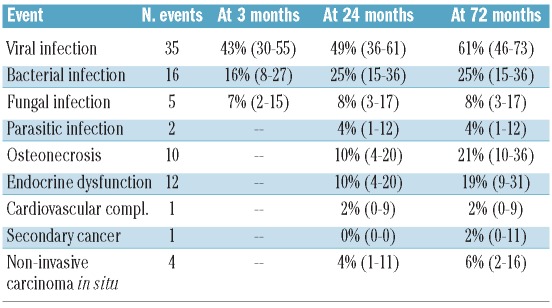
Late effects after HSCT are shown in Table 3. Ten patients developed an avascular necrosis during follow up. Age at transplant (≥ 21 years) and acute GvHD were associated with AVN: HR 8.7 (95% CI 1.1–69.2; P=0.04) and 3.6 (95% CI 1–13.5; P=0.06), respectively (Online Supplementary Table S1). One patient developed a secondary malignancy (EBV-associated Hodgkin’s lymphoma). Of note, 2 patients developed basal cell carcinoma and 2 others uterine cervical carcinoma in situ. Chronic GvHD was not significantly associated with higher incidence of cancer. Twelve patients developed endocrine dysfunction (3 had diabetes, 4 hypothyroidism, 4 dyslipidemia, one Cushing’s syndrome and one hypogonadism) and one patient was diagnosed with a cardiovascular complication (myocardial infarction). However, 4 patients presented with hypertension during follow up. The cumulative incidence of bacterial, fungal and viral infections at 72 months were 25 (95% CI 15–36), 8 (95% CI 3–17) and 61% (95% CI 46–73), respectively (Online Supplementary Figure S1). No difference was found in post transplant outcomes between patients who received prior therapies before HSCT and patients who were transplanted upfront (Online Supplementary Table S2). Accordingly, no correlation was identified between complication occurrence and the transplant periods (1991 to 1995, 1996 to 2002, and 2003 to 2010) (Online Supplementary Table S3).
Table 3.
Cumulative incidence of events.
Immune reconstitution
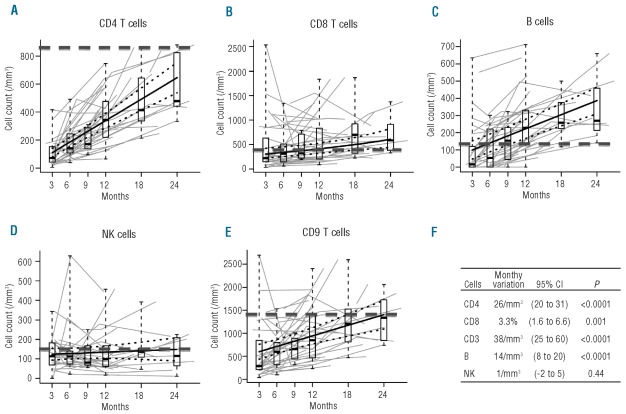
Immune reconstitution was analyzed 3–24 months post transplant since 2000. The evolution of CD3, CD4 and CD8 T cells, B cells and NK cells for 26 patients are shown in Figure 3A-E. Analysis showed a significant mean increase of CD3, CD4, CD8 and B-cell counts over the period of analysis, while no significant variation in NK cells was found. For CD4 T cells and B cells, the inter-patient variability in slope was limited as compared to the population-average estimate, and an increase was observed for almost all patients. On the contrary, the inter-patient variability in slope was much higher for CD3 and CD8 T-cell counts where patients could show different variation patterns.
Figure 3.
Immune reconstitution between 3 and 24 months post transplant. Thick plain lines represent model-based estimated average variation and thick dotted lines their 95% confidence interval. Box and whisker plots summarize the cell counts around 3, 6, 9, 12, 18 and 24 months. They display the median, first and third quartile (boxes), and outer whiskers extend to the whole range of observed data. Thin gray lines present individual patients’ data. Black dotted line represents the median of normal values in a healthy population of each kind of cells. (A) Immune reconstitution of CD4 T cells. (B) Immune reconstitution of CD8 T cells. (C) Immune reconstitution of B cells. (D) Immune reconstitution of NK cells. (E) Immune reconstitution of CD3 T cells. (F) Monthly variation of CD3, CD4, CD8, B ad NK cells over the analysis period.
Pregnancy
Among the 54 survivors, 9 post transplant pregnancies were reported in 8 patients. Eight pregnancies resulted in live births: 5 children in 4 male patients and 3 in female patients; one female patient developed a molar pregnancy. Median age of these patients at HSCT was 20 years (range 7–32); median age at pregnancy was 28 years (range 18–36).
Discussion
Two main conclusions can be drawn from this study. Firstly, we report excellent outcomes for patients with SAA who underwent HSCT using Cy ATG conditioning and CsA plus Mtx GVHD prophylaxis at our center. Secondly, the long-term survival in those patients is mainly affected by endocrine dysfunction and avascular necrosis but not by secondary malignancy, as was the case using irradiation in the conditioning regimen.21
It is likely that modifications in supportive care contributed to these excellent results. These modifications include the development of new antimicrobial agents and the introduction of screening and preemptive therapy for CMV reactivation. Indeed, the observation that 3 out of 7 patients died of fungal infection before 2000 while no deaths occurred from the same cause after this date illustrates the impact of improvements in antimicrobial therapy on outcomes in this particular population. Two other patients died because of engraftment failure; we consider this to be extremely low for SAA patients.6,7 One patient received several courses of IST with more than 50 red blood cell and platelet transfusions before HSCT while the second patient had transfusion-induced sensitization, a common cause of graft rejection.22–24 We also report low mortality due to GvHD: only one patient. This represents a major improvement in the treatment of a disease in which we do not need a graft-versus-leukemia effect. Until recently, GvHD represented the leading cause of death in patients with SAA after HSCT with or without infection.25–27 In our cohort, GvHD prophylaxis with CsA plus Mtx was associated with a low risk of acute GvHD and all but 3 chronic GvHD were limited.24,25 The only factor associated with an increased risk of chronic GvHD was a higher dose of infused CD3 cells in the graft which, to our knowledge, has never been reported.
Because of these excellent outcomes, we examined the occurrence of late complications. Studies in large series on the long-term follow up of patients transplanted for SAA are limited to that completed by the Seattle group13 and to our previous experience that mainly included irradiated patients.27 Avascular osteonecrosis was the most frequently encountered complication: CI was 21% at 72 months. The frequency of avascular necrosis ranges between 4 to 15% in different studies depending on the conditioning regimen and characteristics of transplant (HLA-identical sibling or unrelated donor).28 A higher risk of avascular necrosis has already been described in patients with SAA compared to patients with other diagnoses who undergo HSCT.27,29 We found a higher risk in older patients and in those with acute GvHD (probably related to corticosteroids), as previously reported.29–31 As proposed in the Petropoulou study, preemptive therapy with bisphosphonates should probably be given to those patients at higher risk of bone complications.32 The other major non-malignant complication was endocrine dysfunction, which until now has largely been underestimated. The CI of such complications reached 20% at 72 months, split between dyslipidemia, thyroid dysfunction (hypothyroidism), diabetes and gonadal dysfunction. Long-term metabolic abnormalities (diabetes, dyslipidemia and hypertension) emerged as significant late complications33 and, therefore, need specific management. These complications are of major importance since very late cardiovascular complications are increasingly reported.34,35 In our cohort, only one patient suffered myocardial infarction; this may merely reflect the relatively short follow-up period for this particular complication.
An important observation of this study is the extremely low rate of secondary malignancy; this concerned only one patient who developed Hodgkin’s Lymphoma and died. However, it should be noted that 2 patients who presented with basal cell carcinoma and 2 others diagnosed with cervical carcinoma in situ were not considered to have developed secondary malignancy. Indeed, these cancers are usually excluded from such an analysis mainly because their surgical excision may not always have been reported. Therefore, we decided to exclude these cancers to allow for a meaningful comparison with previously published studies. It is noteworthy that none of our patients developed squamous cell carcinoma of the oral cavity that we had previously reported in irradiated patients with chronic GvHD.27
We also assessed long-term immune reconstitution. To our knowledge, there have been no reports of long-term immune reconstitution after HSCT for SAA. However, this analysis was completed in only one-third of the study cohort because of the lack of regular monitoring of immune reconstitution before 2000. T lymphocytes (CD3+) normalized only by 24 months with an earlier recovery of B cells (at 9 months) and CD8+ cells (at 18 months) whereas NK cell counts remained stable from the third month onwards. In contrast, the CD4 T-cell count after transplant was still lower at 24 months compared to healthy subjects. This may explain the high incidence of viral infections, which reached over 60% at six years in this population. Our results differ from those of Storek et al. who report a low rate of clinically significant infection and conclude that T-cell subsets return to normal levels within five years after transplantation.36 However, among the patients who were assessed for leukocyte subsets by cytofluoremetry, 23 of 33 were transplanted for SAA and 19 received only cyclophosphamide as conditioning regimen. The long-term recovery of the immune system following allogeneic marrow transplantation would probably be different after another conditioning regimen, especially those including ATG.
A major finding of this study is the absence of an impact of prior therapy or prior transfusions on the long-term outcome of SAA patients after BMT. Previous studies have reported that an interval from diagnosis to transplantation of more than one year and the number of transfusions (> 20) before transplantation increased the risk of death after BMT for SAA.26,37–40 However, in our study, although the number of patients is limited, prior IST-treatment was not associated with a higher CI of GvHD nor other complications, nor with decreased survival (72 month-OS of 80% and 89%, respectively, in IST and non-IST treated patients, P=0.42).
In conclusion, we confirm that HSCT after CY-ATG conditioning offers effective therapy for patients with SAA. However, the late complications observed may encourage exploration of the use of new conditioning regimens and GvHD prophylaxis. Marsh et al. recently suggested a novel alemtuzumab-based conditioning regimen using fludarabine and CY before HSCT for SAA. Although encouraging OS and GvHD rates are reported, this series has very limited follow up.41 At present, long-term survival is now an expected outcome after HSCT for SAA, therefore, improving long-term health conditions must be a priority for further research in this field.
Acknowledgments
The authors declare no financial interests. We thank Duncan Purtill for editing the manuscript.
Footnotes
Funding: RPL received a bursary award from the AA and Myelodysplastic Syndrome International Foundation and a grant from France HPN.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Mathe G, Amiel JL, Schwarzenberg L, Choay J, Trolard P, Schneider M, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2(5702):131–6. doi: 10.1136/bmj.2.5702.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frickhofen N, Heimpel H, Kaltwasser JP, Schrezenmeier H German Aplastic Anemia Study G. Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood. 2003;101(4):1236–42. doi: 10.1182/blood-2002-04-1134. [DOI] [PubMed] [Google Scholar]
- 3.Bacigalupo A. Treatment strategies for patients with severe aplastic anemia. Bone Marrow Transplant. 2008;42(Suppl 1):S42–S4. doi: 10.1038/bmt.2008.113. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289(9):1130–5. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 5.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108(8):2509–19. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storb R, Leisenring W, Anasetti C, Appelbaum FR, Buckner CD, Bensinger WI, et al. Long-term follow-up of allogeneic marrow transplants in patients with aplastic anemia conditioned by cyclophosphamide combined with antithymocyte globulin. Blood. 1997;89(10):3890–1. [PubMed] [Google Scholar]
- 7.Storb R, Blume KG, O’Donnell MR, Chauncey T, Forman SJ, Deeg HJ, et al. Cyclophosphamide and antithymocyte globulin to condition patients with aplastic anemia for allogeneic marrow transplantations: the experience in four centers. Biol Blood Marrow Transplant. 2001;7(1):39–44. doi: 10.1053/bbmt.2001.v7.pm11215697. [DOI] [PubMed] [Google Scholar]
- 8.Cohen A, Bekassy AN, Gaiero A, Faraci M, Zecca S, Tichelli A, et al. Endocrinological late complications after hematopoietic SCT in children. Bone Marrow Transplant. 2008;41(Suppl 2):S43–8. doi: 10.1038/bmt.2008.54. [DOI] [PubMed] [Google Scholar]
- 9.Schrezenmeier H, Passweg JR, Marsh JC, Bacigalupo A, Bredeson CN, Bullorsky E, et al. Worse outcome and more chronic GVHD with peripheral blood progenitor cells than bone marrow in HLA-matched sibling donor transplants for young patients with severe acquired aplastic anemia. Blood. 2007;110(4):1397–400. doi: 10.1182/blood-2007-03-081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locatelli F, Bruno B, Zecca M, Van-Lint MT, McCann S, Arcese W, et al. Cyclosporin A and short-term methotrexate versus cyclosporin A as graft versus host disease prophylaxis in patients with severe aplastic anemia given allogeneic bone marrow transplantation from an HLA-identical sibling: results of a GITMO/EBMT randomized trial. Blood. 2000;96(5):1690–7. [PubMed] [Google Scholar]
- 11.Locasciulli A, Oneto R, Bacigalupo A, Socie G, Korthof E, Bekassy A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2007;92(1):11–8. doi: 10.3324/haematol.10075. [DOI] [PubMed] [Google Scholar]
- 12.Marsh JC, Ball SE, Darbyshire P, Gordon-Smith EC, Keidan AJ, Martin A, et al. Guidelines for the diagnosis and management of acquired aplastic anaemia. Br J Haematol. 2003;123(5):782–801. doi: 10.1046/j.1365-2141.2003.04721.x. [DOI] [PubMed] [Google Scholar]
- 13.Deeg HJ, Leisenring W, Storb R, Nims J, Flowers ME, Witherspoon RP, et al. Long-term outcome after marrow transplantation for severe aplastic anemia. Blood. 1998;91(10):3637–45. [PubMed] [Google Scholar]
- 14.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 15.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Corre E, Carmagnat M, Busson M, de Latour RP, Robin M, Ribaud P, et al. Long-term immune deficiency after allogeneic stem cell transplantation: B-cell deficiency is associated with late infections. Haematologica. 2010;95(6):1025–9. doi: 10.3324/haematol.2009.018853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalbfleisch P. In: The Statistical Analysis of Failure Time Data. Sons JW, editor. New York: 1980. [Google Scholar]
- 18.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. 1978;(34):541–54. [PubMed] [Google Scholar]
- 19.Grambsch T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994:515–56. [Google Scholar]
- 20.R: A Language and Environment for Statistical Computing [database on the Internet] R Foundation for Statistical Computing; 2009. Available from: URL http://www.R-project.org. [Google Scholar]
- 21.Gluckman E, Socie G, Devergie A, Bourdeau-Esperou H, Traineau R, Cosset JM. Bone marrow transplantation in 107 patients with severe aplastic anemia using cyclophosphamide and thoraco-abdominal irradiation for conditioning: long-term follow-up. Blood. 1991;78(9):2451–5. [PubMed] [Google Scholar]
- 22.Horowitz MM. Current status of allogeneic bone marrow transplantation in acquired aplastic anemia. Semin Hematol. 2000;37(1):30–42. doi: 10.1016/s0037-1963(00)90028-3. [DOI] [PubMed] [Google Scholar]
- 23.Storb R, Epstein RB, Rudolph RH, Thomas ED. The effect of prior transfusion on marrow grafts between histocompatible canine siblings. J Immunol. 1970;105(3):627–33. [PubMed] [Google Scholar]
- 24.Storb R, Prentice RL, Thomas ED. Marrow transplantation for treatment of aplastic anemia. An analysis of factors associated with graft rejection. N Engl J Med. 1977;296(2):61–6. doi: 10.1056/NEJM197701132960201. [DOI] [PubMed] [Google Scholar]
- 25.Socie G, Stone JV, Wingard JR, Weisdorf D, Henslee-Downey PJ, Bredeson C, Cahn JY. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341(1):14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 26.Bacigalupo A, Brand R, Oneto R, Bruno B, Socié G, Passweg J, et al. Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy--The European Group for Blood and Marrow Transplantation experience. Seminar Hematol. 2000;37(1):69–80. doi: 10.1016/s0037-1963(00)90031-3. [DOI] [PubMed] [Google Scholar]
- 27.Ades L, Mary JY, Robin M, Ferry C, Porcher R, Esperou H, et al. Long-term outcome after bone marrow transplantation for severe aplastic anemia. Blood. 2004;103(7):2490–7. doi: 10.1182/blood-2003-07-2546. [DOI] [PubMed] [Google Scholar]
- 28.Campbell S, Sun CL, Kurian S, Francisco L, Carter A, Kulkarni S, et al. Predictors of avascular necrosis of bone in long-term survivors of hematopoietic cell transplantation. Cancer. 2009;115(18):4127–35. doi: 10.1002/cncr.24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Socie G, Cahn JY, Carmelo J, Vernant JP, Jouet JP, Ifrah N, et al. Avascular necrosis of bone after allogeneic bone marrow transplantation: analysis of risk factors for 4388 patients by the Societe Francaise de Greffe de Moelle (SFGM) Br J Haematol. 1997;97(4):865–70. doi: 10.1046/j.1365-2141.1997.1262940.x. [DOI] [PubMed] [Google Scholar]
- 30.Marsh JC, Zomas A, Hows JM, Chapple M, Gordon-Smith EC. Avascular necrosis after treatment of aplastic anaemia with anti-lymphocyte globulin and high-dose methylprednisolone. Br J Haematol. 1993;84(4):731–5. doi: 10.1111/j.1365-2141.1993.tb03153.x. [DOI] [PubMed] [Google Scholar]
- 31.Socie G, Selimi F, Sedel L, Frija J, Devergie A, Esperou Bourdeau H, et al. Avascular necrosis of bone after allogeneic bone marrow transplantation: clinical findings, incidence and risk factors. Br J Haematol. 1994;86(3):624–8. doi: 10.1111/j.1365-2141.1994.tb04795.x. [DOI] [PubMed] [Google Scholar]
- 32.Petropoulou AD, Porcher R, Herr AL, Devergie A, Brentano TF, Ribaud P, et al. Prospective assessment of bone turnover and clinical bone diseases after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;89(11):1354–61. doi: 10.1097/TP.0b013e3181d84c8e. [DOI] [PubMed] [Google Scholar]
- 33.Baker KS, Ness KK, Steinberger J, Carter A, Francisco L, Burns LJ, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109(4):1765–72. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tichelli A, Bucher C, Rovo A, Stussi G, Stern M, Paulussen M, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110(9):3463–71. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 35.Tichelli A, Passweg J, Wojcik D, Rovo A, Harousseau JL, Masszi T, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93(8):1203–10. doi: 10.3324/haematol.12949. [DOI] [PubMed] [Google Scholar]
- 36.Storek J, Joseph A, Espino G, Dawson MA, Douek DC, Sullivan KM, et al. Immunity of patients surviving 20 to 30 years after allogeneic or syngeneic bone marrow transplantation. Blood. 2001;98(13):3505–12. doi: 10.1182/blood.v98.13.3505. [DOI] [PubMed] [Google Scholar]
- 37.Passweg JR, Socie G, Hinterberger W, Bacigalupo A, Biggs JC, Camitta BM, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90(2):858–64. [PubMed] [Google Scholar]
- 38.Bacigalupo A, Oneto R, Bruno B, Socie G, Passweg J, Locasciulli A, et al. Current results of bone marrow transplantation in patients with acquired severe aplastic anemia. Report of the European Group for Blood and Marrow transplantation. On behalf of the Working Party on Severe Aplastic Anemia of the European Group for Blood and Marrow Transplantation. Acta Haematol. 2000;103(1):19–25. doi: 10.1159/000041000. [DOI] [PubMed] [Google Scholar]
- 39.Bacigalupo A. Guidelines for the treatment of severe aplastic anemia. Working Party on Severe Aplastic Anemia (WPSAA) of the European Group of Bone Marrow Transplantation (EBMT) Haematologica. 1994;79(5):438–44. [PubMed] [Google Scholar]
- 40.Locasciulli A, van’t Veer L, Bacigalupo A, Hows J, Van Lint MT, Gluckman E, et al. Treatment with marrow transplantation or immunosuppression of childhood acquired severe aplastic anemia: a report from the EBMT SAA Working Party. Bone Marrow Transplantation. 1990;6(3):211–7. [PubMed] [Google Scholar]
- 41.Marsh JC, Gupta V, Lim Z, Ho AY, Ireland R, Hayden J, et al. Alemtuzumab with fludarabine and cyclophosphamide reduces chronic graft versus host disease after allogeneic stem cell transplantation for acquired aplastic anemia. Blood. 2011;118(8):2351–7. doi: 10.1182/blood-2010-12-327536. [DOI] [PubMed] [Google Scholar]