Abstract
Background
According to WHO 2008 guidelines, the required percentage of cells manifesting dysplasia in the bone marrow to qualify as significant is 10% or over in one or more hematopoietic cell lineages, but this threshold is controversial. No ‘normal’ values have been established. Therefore, we investigated dyshematopoiesis in bone marrow aspirate squash preparations of 120 healthy bone marrow donors.
Design and Methods
Bone marrow squash slides of 120 healthy unrelated bone marrow donors were examined independently by 4 experienced morphologists. Samples were taken from the first aspiration during the harvest. Bone marrow preparation and assessment were performed according to WHO recommendations and ICSH guidelines.
Results
More than 10% dysmyelopoiesis could be detected in 46% of bone marrow aspirate squash preparations with 26% in 2 or more cell lineages and 7% in 3 cell lineages in healthy bone marrow donors. Donors under the age of 30 years exhibited more dysgranulopoietic changes and dysmegakaryopoietic changes (P<0.001) compared to the older donors. Female donors showed more dysgranulopoietic changes than male donors (P=0.025). The concordance rate between the 4 investigators was modest in dysgranulopoiesis but poor in dyserythropoiesis and dysmegakaryopoiesis.
Conclusions
The poor reliability of the 10% cut off was partly related to the proximity of the current criteria to the observed cut-off mean values of the normal population. These findings question the current WHO threshold of the 10% or over necessary for the percentage of cells manifesting dysplasia to be considered significant, and suggest that either a higher threshold would be more appropriate or different thresholds should be set for each lineage.
Keywords: dysplasia, hematopoiesis, cut off, significant, percentage, bone marrow donors
Introduction
Myelodysplastic syndromes are characterized by dysplastic and ineffective clonal hematopoiesis and constitute a neoplastic condition with the potential to transform to acute leukemia. Dyshematopoietic changes are observed in many primary or acquired hematologic disorders, e.g. congenital dyserythropoietic anemias, thalassemias, congenital dyserythropoietic porphyria1 or hereditary sideroblastic anemia2 or acquired MDS, as well as secondary disorders. Secondary myelodysplasia-like changes are found in severe illness,3 after exposure to drugs,4 toxins,5,6 autoimmune diseases,7 vitamin B12, folate and copper deficiency,8,9 parvovirus B19 infection10 or excessive alcohol intake.11,12 They are neither neoplastic nor pre-leukemic. If the underlying cause is recognized, treated or removed the morphological changes are reversible.
The morphological definition of MDS is based on the percentage of blasts in the BM and PB, the type and degree of dysplasia, and the presence of ring sideroblasts. The required percentage of cells manifesting dysplasia to qualify as significant is 10% or over in one or more hematopoietic cell lineages.13–18 Matsuda et al. describe pseudo Pelger-Huët anomalies of 10% and over, micromegakaryocytes of 10% or over, and dysmegakaryopoiesis of 40% or over in MDS as unfavorable prognostic factors for overall survival (OS) and leukemia-free survival (LFS). They proposed modifications to the morphological criteria for RCMD with dysgranulopoiesis of 10% or over, dysmegakaryopoiesis of 40% or over, and micromegakaryocytes of 10% or over.15
In 1996, Bain19 investigated freshly obtained, non-anticoagulated bone marrow smears of 50 Caucasian subjects aged between 21 and 56 years, 30 men and 20 women. Study subjects were in good health, and free of current infection and allergic conditions. In that study, 20 megakaryocytes and 100 erythroblasts were scored for dysplastic features, and cells of the granulocyte series encountered during the differential count were examined for any evidence of dysplasia. Bain described dysplastic changes in megakaryopoiesis (above all non-lobulated, multinucleated megakaryocytes; always below 10%), erythropoiesis (binuclearity and cytoplasmic bridging; always below 10%) and granulopoiesis (only one single neutrophil resembling a Pelger-Hüet neutrophil in one subject, otherwise no hypogranular or pseudo-Pelger neutrophils and no dysplastic granulocyte precursors). Bain19 observed minor degrees of dyserythropoiesis and occasional non-lobulated or multinucleated megakaryocytes to be quite frequent in healthy subjects with normal iron stores. Poor granulation of megakaryocyte cytoplasm is also common in MDS and has been found to be highly specific,20 but its evaluation was not part of this study as this is not proposed under WHO 2008 guidelines.13
Approximately 180 bone marrow harvests from healthy unrelated donors are performed every year at Dresden University Hospital, Germany. We gathered marrow squash slides from over 400 healthy subjects harvested over a 4-year period at our institution to investigate myelopoiesis.
Design and Methods
Bone marrow donors
In a retrospective study, we investigated dyshematopoiesis in bone marrow (BM) particle squash slides of 120 healthy unrelated BM donors randomly selected from all accrued samples. The donors had not received growth factors. All volunteers underwent a pre-donation health check-up about four weeks prior to the harvest that included health history, physical examination and chest X-ray. Laboratory testing included full automated peripheral blood counts, chemistry tests including ferritin, and infectious disease markers (EBV, CMV, HSV1/2, HIV, Treponema Pallidum, Hepatitis A, B, C).
Bone marrow harvests were performed under general anesthesia. For this study, samples were taken from the first aspirate performed during the harvest. The study was approved by the institutional review board (EK240102007) of the Technical University of Dresden and procedures were in accordance with the Helsinki Declaration of 1975, as revised in 2000.
Selection of samples
Samples were randomly selected slides from a total of 445 voluntary BM donor harvests performed between July 2005 and October 2009. In order to balance subgroup analyses, random samples were selected for equal numbers of males and females, smokers and non-smokers, and in the three age groups (≤30, >31–40, >40 years).
Medical record review
All medical records were reviewed. Laboratory results were collected directly from the medical chart or the central laboratory server of the university hospital. Smoking status was categorized as follows: non-smoker, occasional smoker, up to 10 cigarettes per day, 11–20 cigarettes per day, and more than 20 cigarettes per day. Regular alcohol intake was defined as daily consumption of alcohol.
Slide preparation
Slide preparations were made by experienced laboratory technicians from freshly obtained bone marrow specimens (the first 4 mL of aspirated bone marrow in a 20 mL tube by BRAUN® Germany, mixed with 1 mL Di-Na-EDTA 1.107% (AlleMan Pharma GmbH) with the final concentration of EDTA being approximately 0.22%) with exposure to anticoagulants for less than two hours as recommended by the WHO.13 The BM nucleated differential cell count (NDC) was performed according to ICSH guidelines.21 BM aspirate squash slides were investigated by conventional cytochemical methods (May-Grunwald-Giemsa, myeloperoxidase, alpha-naphthyl butyrate esterase and iron staining) as previously desribed.21–23 The prepared slides were centrally stored in the local department.
Bone marrow evaluation
The BM squash slides were examined independently by 4 experienced morphologists. Each investigator assessed cellularity and performed a 200 cell differential cell count. In addition, 100 erythroid cells, 100 granulopoietic cells and 30 megakaryocytes (as recommended by the WHO)13 were assessed for the evaluation of dysplasia by each investigator. Granulation was evaluated behind the particles, cells had to be well spread, and the concomitant appearance of normally granulated neutrophils beside hypogranular or agranulated neutrophils made artefactual staining error less likely.
Evaluation of dysplastic features in erythropoiesis included megaloblastic changes, karyorrhexis (increased pyknosis), bi- and multinuclearity, nuclear lobulation, irregularity or fragmentation, internuclear bridges and ring sideroblasts (an erythroblast with at least 5 siderotic granules covering at least a third of the circumference of the nucleus)24 in granulopoiesis cytoplasmic hypo- or agranularity, nuclear hypolobation (pseudo Pelger-Huët) and hypersegmentation, pseudo Chédiak-Higashi granules, small size and Auer rods. In megakaryocytopoiesis micromegakaryocytes (cells about the size of a myeloblast with one or two small round nuclei), non-or hypolobated nuclei in megakaryocytes of all sizes, and multiple, widely-separated nuclei.
Statistical analysis
The counts of cells with defined dysplastic features in each lineage were added together in order to calculate the frequency of dysplastic cells per sample and investigator. The frequencies of dysmyelopoiesis described by the 4 investigators were then averaged per sample for erythropoiesis, granulopoiesis and megakaryopoiesis. The aggregated 800 cell count of all 4 investigators was averaged. Average values on dysmyelopoietic findings were further classified dichotomously whether significant dysplasia was present or absent by strictly applying the less than 10% WHO cut off. Samples with 10% or more dysplastic cells in the respective lineage were considered as having significant dysplasia. The rating of bone marrow cellularity was averaged over the 4 investigators. With respect to the presence of significant dysplasia and bone marrow cellularity, inter-observer concordance was assessed as percent agreement per sample.
Frequencies are reported together with approximate confidence intervals (Agresti-Coull).
A linear mixed regression model was used to assess the impact of age, sex, autologous blood donation, smoking status and regular alcohol intake on the frequency of dysplastic findings. Smoking status was categorized as non-smoking versus smoking to any degree. All variables apart from age were entered as categorical factors. The investigator was entered as a random effect.
PASW statistics 17 software (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
Donors’ characteristics
The final set of slides for cytomorphology review was selected from 120 bone marrow donors, 57 females (47.5%) and 63 males (52.5%). The median age of the whole cohort was 32 years (range 18–51 years). Sixty-six (55%) donors were non-smokers, 4 (3%) donors reported occasional smoking, 16 (13%) donors consumed up to 10 cigarettes per day, 18 (15%) donors 11–20 cigarettes per day, and 7 (6%) donors more than 20 cigarettes per day. Quantitative information was not available for 7 (6%) smokers. In 2 cases, no information on nicotine abuse was available. The donations dated from July 2005 to October 2009. Ninety-one out of 120 donors had an autologous blood donation (500 mL) a median of 21 days (interquartile range 17–26 days, minimum 8 days, maximum 138 days) before the intervention (Table 1). So far, no donor of this cohort has developed MDS, cytopenia or acute leukemia during follow up.
Table 1.
Donors’ characteristics.
Laboratory results
Seventeen percent of all donors had slightly elevated white bood cell counts (normal range 3.8–9.8×109/L) with a maximum leukocyte count of 13.3×109/L. No donor was leukopenic. One percent of all donors had elevated platelet counts (normal range 150–400×109/L) with a maximum platelet count of 442×109/L, and one donor was thrombopenic with 130×109/L.
Three percent of males were anemic (normal hemoglobin range 13.2–18.6 g/dL) and 8% of male donors had a pathologically low ferritin level (normal range 30–400 μg/L). One (0.8%) male donor had an elevated ferritin level (maximum 405 μg/L). Three percent of females were anemic (normal hemoglobin range 11.4–16.5 g/dL) and 13% of female donors had a pathologically low ferritin level (normal range 15–150 μg/L). No donor had an elevated hemoglobin level. No female donor had an elevated ferritin level. MCV was below the normal range in 3% of all donors and elevated in 3% of all donors. MCH was low in 4% of all donors but not elevated in any donor. All donors had normal vitamin B12 and folate levels.
Four percent of male donors had pathologically elevated gamma-glutamyl-transferase activity (GGT) and none of the male donors had pathologically elevated aspartate-aminotransferase (ASAT). Three percent of female donors had elevated GGT and 5% had elevated ASAT (Table 1).
Bone marrow cellularity
Thirteen percent of BM sections were considered hypocellular, 7% of samples were assessed as hypo-to normocellular, 79% of samples were normocellular and one sample (1%) was evaluated as normo-to hypercellular. All investigators agreed on cellularity of the sample in 65% of the slides (58% normocellular, 7% hypocellular). Dual observer concurrence was made in 9 (7.5%) samples while only one investigator had a differing opinion in the remaining 27.5% of samples.
Dysgranulopoiesis
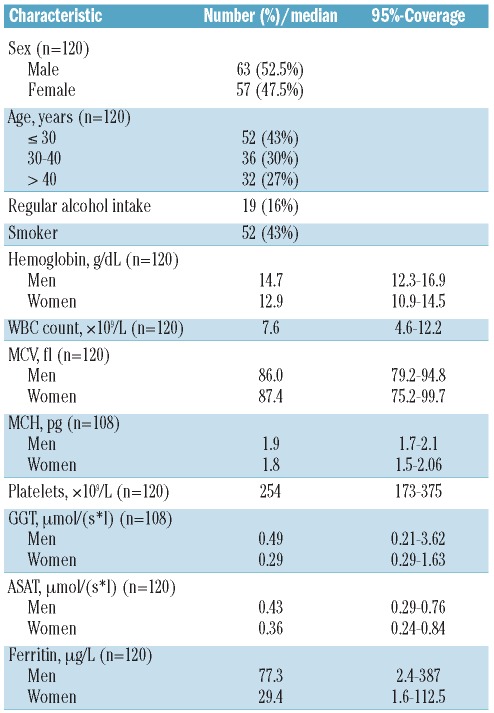
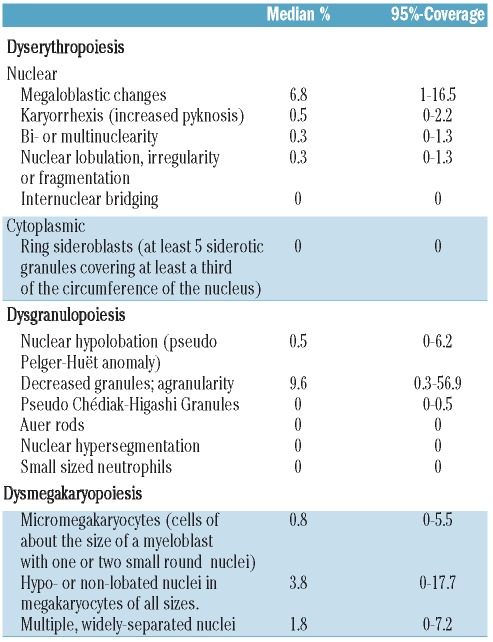
The median proportion of cells with dysgranulopoietic changes was 10% (range 0.5–73%). Hypogranulation was the most prevalent dysgranulopoietic finding (93%). Hypolobulation (pseudo Pelger-Huët anomaly) was rare (7%) and irregular hypersegmentation was extremely rare (<1%). Sixty-four donors (53%, 95% CI, 44–62%) showed a 10% or over dysgranulopoiesis. Samples from 4 donors (3%) showed more than 50% dysgranulopoietic changes. No Auer rods or excess of blasts were found (Table 2, Figure 1A and B).
Table 2.
Morphological manifestations of dysplasia evaluated in the donor bone marrow (according to WHO 2008; 100 cells of granulopoiesis and erythropoiesis and 30 megakaryocytes evaluated by each investigator; results averaged).
Figure 1.
Dysgranulopoiesis and dyserythropoiesis: hypogranulated neutrophils (A–B). Erythroblasts with megaloblastoid changes (C–D). Shown at 100 × original magnification.
Dyserythropoiesis
The median proportion of cells with dyserythropoietic changes was 7.9% (range 1.5–18%). Megaloblastoid changes predominated (87%), while karyorrhexis (6%), multinuclearity (3%) and nuclear fragmentation (4%) were less frequent findings (Table 2, Figure 1C and D). Forty-one donors (34%, 95% CI 26–43%) showed 10% or over dyserythropoietic changes. None of the donors had over 50% dyserythropoiesis. Ring sideroblasts could not be found within the iron stain.
Dysmegakaryopoiesis
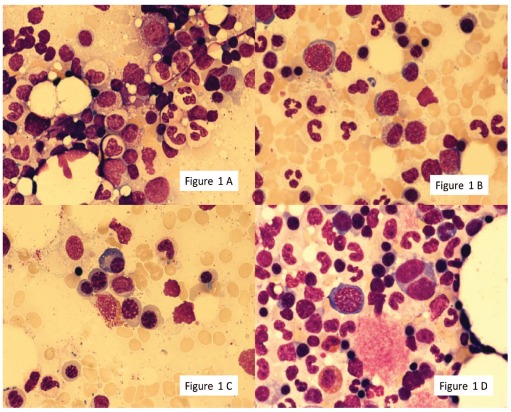
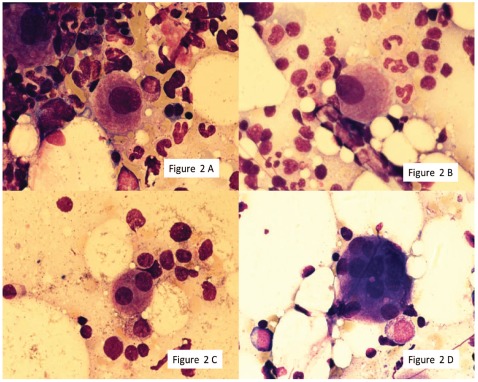
The median proportion of cells with dysmegakaryopoietic changes was 7.5% (range 0–40%). Multinucleation was the most prominent finding accounting for 60% of dysplastic changes. Nuclear hypolobation contributed 27% dysmegakaryopoietic changes and micromegakaryocytes represented 13% of dysplastic findings. Thirty-six donors (30%, 95% CI 23–39%) showed 10% or over; no donor showed more than 50% dysplastic changes (Table 2, Figure 2A-D).
Figure 2.
Dysmegakaryopoiesis: hypolobated megakaryocyte (AB), binucleated micromegakaryocyte (C), multinucleated megakaryocyte. Shown at 100 × original magnification.
Factors associated with dysmyelopoiesis
We found no significant impact of sex, smoking status, alcohol intake and previous autologous blood donation on rates of dyserythropoiesis. The relationship of age to the frequency of all dysplastic changes was the borderline of significance with a trend to less dysplasia in older donors compared to younger ones (P=0.053). This effect, however, was rather modest. The median frequency of dysplastic changes in erythropoietic cells was 8.9%, 7.0% and 8.5% for patients aged 18–30, 31–40 and over 40 years, respectively (Figure 3A).
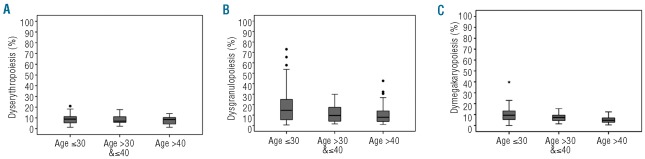
Figure 3.
Dyshematopoiesis and age. The percentage of cells with dysplastic features assessed in 120 donors are categorized by donor age for erythropoiesis (A), granulopoiesis (B), and megakaryopoiesis (C). (A) Dyserythropoiesis: age group ≤ 30: median: 8.9, 25–75 percentile: 5.3–11.3; age group > 30 & ≤ 40: median: 7.0, 25–75 percentile: 5.8–11.7; age group > 40: median: 8.5, 25–75 percentile: 4.0–10.4. (B) Dysgranulopoiesis: age group ≤ 30: median: 14.5, 25–75 percentile: 5.5–26.0; age group > 30 & ≤ 40: median: 9.6, 25–75 percentile: 4.0–17.5; age group > 40: median: 7.9, 25–75 percentile: 3.8–14.8. (C) Dysmegakaryopoiesis: age group ≤ 30: median: 9.4, 25–75 percentile: 5.5–13.6; age group > 30 & ≤ 40: median: 7.4, 25–75 percentile: 4.4–9.5; age group > 40: median: 4.8, 25–75 percentile: 3.0–6.9.
We found a strong correlation of age and the frequency of dysgranulopoietic findings (P<0.001). Younger donors more frequently showed granulopoietic dysplasia compared with older donors. The median frequency of dysplastic changes in granulopoietic cells was 14.5%, 9.6% and 7.9% for donors aged 18–30, 31–40 and over 40 years, respectively (Figure 3B). In addition, female donors exhibited significantly higher frequencies of dysgranulopoiesis compared with male donors (P=0.025). Nicotine or alcohol abuse and previous autologous blood donation were not associated with the frequency of dysgranulopoietic findings.
In megakaryopoiesis, age was also inversely correlated (P<0.001) with the frequency of dysmegakaryopoietic findings (Figure 3C) and no other factors were associated with this variable. The median frequency of dysplastic changes in megakaryopoietic cells was 9.4%, 7.4% and 4.8% for donors aged 18–30, 31–40 and over 40 years, respectively.
Multilineage dysplasia
Of all BM donors, 55 (46%, 95%-CI 37–55%) had significant dysplastic findings in at least one lineage. Thirty-one (26%, 95%-CI 19–34%) BM donors showed over 10% dysplastic changes in two lineages (erythropoiesis and granulopoiesis in 12 donors, erythropoiesis and megakaryopoiesis in 5 donors, and granulopoiesis and megakaryopoiesis in 14 donors) and 8 donors (7%, 95%-CI 3–13%) showed dysplastic changes in all three cell lineages.
Investigator reliability
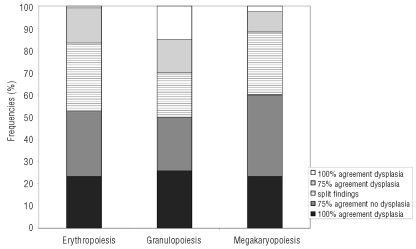
The concordance rate of morphological evaluation according to the WHO classification between the 4 investigators was poor for all lineages (Figure 4). The best agreement was observed for the assessment of dysgranulopoiesis. The investigators fully agreed on the presence or absence of dysgranulopoiesis in 41% of the samples, compared to 26% in megakaryopoiesis and 24% in erythropoiesis. However, this high percentage of agreement was also due to full agreement on the presence of significant dysgranulopoiesis in 15% of the samples. In contrast, the investigators fully agreed on the presence of dyserythropoiesis in only one sample (1%) and on dysmegakaryopoiesis in 3 samples (2.5%). There was the full agreement of all 4 investigators on the absence of significant dysplastic findings in 23%, 26% and 23% of the samples with respect to erythropoiesis, granulopoiesis and megakaryopoiesis.
Figure 4.
Investigator concordance (threshold for dysplasia 10%). The concordance rate of morphological evaluation according to the WHO classification between the four investigators was poor for all lineages. Best rates of agreement were observed for the assessment of dysgranulopoiesis. Full agreement on the presence or absence of dysgranulopoiesis could be stated in 41% of the samples, compared to 26% in megakaryopoiesis and 24% in erythropoiesis. This high percentage of agreement was also due to full agreement on the presence of significant dysgranulopoiesis in 15% of the samples. Full agreement on the presence of dyserythropoiesis was seen in only one sample (1%) and on dysmegakaryopoiesis in 3 samples (2.5%). Full agreement of all four investigators on the absence of significant dysplastic findings could be stated for 23%, 26% and 23% of the samples with respect to erythropoiesis, granulopoiesis and megakaryopoiesis, respectively.
The poor inter-observer concordance was partly related to the closeness of the current WHO criteria cut off to the mean values observed in normal individuals in this study. Raising the threshold from 10% to an arbitrary threshold of 20% for all lineages led to a higher concordance rate. With this higher threshold, 4 investigators fully agreed on the absence of significant dysplasia in 82%, 54% and 81% of samples for erythropoiesis, granulopoiesis and megakaryopoiesis, respectively.
Discussion
Previous investigations on dysplastic bone marrow changes were mainly carried out on patients with acute myeloid leukemia or within only a small population of healthy subjects.17–19 In the daily practice of marrow cytology, the currently defined WHO threshold for defining significant disease seems questionable. Validated normal values do not exist.
In our study of dysmyelopoiesis in bone marrow of healthy bone marrow donors, clinically significant dyserythropoiesis (≥ 10%) was found in 34% of the cases, dysgranulopoiesis (≥10%) in 53% of all donors and dysmegakaryopoiesis (≥10%) with 30% of all donors showing 10% or more dysplastic changes. Except for granulopoiesis, none of the donors showed more than 50% dysplastic changes. The dysgranulopoietic changes were mainly hypogranularity with only occasional agranular or hypolobated neutrophils. Assessment of hypogranularity is subjective and investigator concordance rate was modest in dysgranulopoiesis but poor in dyserythropoiesis and dysmegakaryopoiesis, as already described by Bain.25
The poor discrimination between normal and dysplastic is related to poor inter-observer concordance and the proximity of the currently accepted WHO 10% cut off to the mean observed values found in this study of normal healthy bone marrow donors. Raising the threshold from 10% to an arbitrary threshold of 20% for all lineages, would lead to a lower abnormality rate. With this higher threshold, the assessment of the 4 investigators would have scored the marrows as normal in 82%, 54% and 81% of samples for erythropoiesis, granulopoiesis and megakaryopoiesis, respectively.
The poor rate of concordance between the 4 morphologists reflects contemporary practice and problems of diagnosing myelodysplasia. It agrees with earlier data by Ramos in which poor correlation coefficients for dyserythropoiesis (R=0.27) and dysgranulopoiesis (R=0.45) were reported in 24 MDS bone marrows independently reviewed by 5 morphologists.26
It should be mentioned that only particle squash slides and not BMA smears had been prepared, reflecting daily routine practice in many hospitals where either BMA squash slides or BMA smears are prepared and not (as recommended) both kinds of slides.21 While evaluation for megakaryocytic dysplasia may be better in BMA squash preparations than in smears, BM smears appear to be better for assessing nuclear hypo- or agranulation in neutrophils. But assessment of the neutrophils behind the particles might have, at least in part, compensated for this fact.
Several reasons for dyshematopoiesis (artefactual or transient) within the bone marrow should be taken into account, e.g. EDTA storage artefacts, stress, infections, chronic diseases and medications. Acute or chronic infections and chronic disease were excluded from the pre-donation health check up. None of the donors reported a regular intake of medications associated with myelodysplasia.
The fact that the obtained bone marrow was processed immediately should have minimized possible storage artefacts resulting from EDTA-anticoagulated bone marrow or blood.21,27,28 When assessing bone marrow of patients with possible MDS, prolonged exposure to EDTA (>2 h) must be excluded, otherwise dysplastic-like changes might be due to sequestrine induced artifacts.
Ninety-one of the bone marrow donors had given an autologous blood donation four weeks before the intervention, which showed no differences in dysmyelopoiesis compared to those having no blood donation. Recent blood donation is, therefore, not responsible for the frequent observation of dyshematopoietic changes.
Another relevant point might be the potential mental stress a bone marrow donor encounters before the intervention. This adrenergic stimulation may explain the slightly elevated white blood cell count in some cases. However, patients with suspected hematologic disorders and a planned diagnostic bone marrow also experience mental stress and, in this setting, the results are normally not questioned.
So far, no donor of this cohort has developed MDS, cytopenia or acute leukemia during follow up.
The most difficult decision on finding dysplasias in the bone marrow is when to define it as ‘MDS’, especially when the blast count is below 5% and reactive or toxic reasons have been excluded. In the absence of cytopenias, it has been proposed that marked dysplasia (> 10%) in one or more major cell lineages should be defined as ‘IDUS’ (‘idiopathic dysplasia of undetermined significance’).29 Several of our donors would meet these criteria, but the term and its significance should be questioned in this setting.
Many studies have been performed on the epidemiology of myelodysplastic syndromes.11,30–36 Aul et al. proposed that the rising incidence of MDS in recent years might not be due to changes in etiological factors, but more likely an increased awareness on the part of physicians and extended use of diagnostic procedures in elderly patients.30 Here, the unexpected finding that donors below the age of 30 years exhibited more dysgranulopoietic and dysmegakaryopoietic changes compared to the older donors cannot be explained, but might lose its significance in the more elderly (>60 years) who do not usually belong to the regular bone marrow donor pool. Several independent studies describe smoking as a relevant epidemiological factor for developing MDS. Du et al. found smoking to be a significant factor for MDS, but not alcohol.35 Strom et al. could show a joint effect between smoking and chemical exposure with the highest risk among smokers exposed to solvent/agricultural chemicals.31 Recently, Sekeres described advanced age, male gender, smoking and previous exposure to chemotherapy, radiation therapy or exposure to industrial chemicals as the most common risk factors for developing MDS.36 In our study, no significant differences in dysmyelopoiesis could be attributed to smoking habits. Interestingly, more dysgranulopoiesis was observed in females than males. The mean donor age was 33 years (the oldest donor was 51 years old) and the impact of smoking might depend on the duration and cumulative cigarette consumption (pack-years) and would, therefore, be mainly relevant in even older subjects. Regular low to moderate alcohol intake might show a significant impact on dysmyelopoiesis, but the number of donors admitting regular alcohol intake was too small to exclude an influence of that factor on dysmyelopoiesis.
The fact that in our study the counting of pseudo Pelger-Huët anomalies and micromegakaryocytes did not exceed 10% or more and dysmegakaryopoiesis 40% or more agrees with the findings of Matsuda et al. on patients with MDS, thus proposing modifications on morphological criteria for refractory cytopenia with multilineage dysplasia (RCMD) with dysgranulopoiesis of 10% or over, dysmegakaryopoiesis of 40% or over and micromegakaryocytes of 10% or over.15 These data also support the need to distinguish between dysplastic features being more typical for MDS (e.g. pseudo Pelger-Huët, micromegakaryocytes) and those found in many non-malignant conditions (e.g. megaloblastic changes) as already proposed by Bain22,25 and other groups.
Further studies should include an international multicenter program to define and refine normal values for dysmyelopoiesis in the bone marrow and to define new thresholds for significant dysplasias.
In summary, although reactive changes cannot be ruled out, these data suggest that the 10% cut off for dyshematopoietic cells in the bone marrow is questionable in patients without cytopenia and should be revisited for future consensus recommendations.
Acknowledgments
Special thanks go to the excellent work of our medical-technical assistants (Christiane Külper, Kerstin Mende, Jana Bornhäuser, Heidrun Zengler, Rita Scheffler, Bianca Umlauft).
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Kushner JP, Pimstone NR, Kjeldsberg CR, Pryor MA, Huntley A. Congenital erythropoietic porphyria, diminished activity of uroporphyrinogen decarboxylase and dyserythropoiesis. Blood. 1982;59(4):725–37. [PubMed] [Google Scholar]
- 2.Wickramasinghe SN, Wood WG. Advances in the understanding of the congenital dyserythropoietic anaemias. Br J Haematol. 2005;131(4):431–46. doi: 10.1111/j.1365-2141.2005.05757.x. [DOI] [PubMed] [Google Scholar]
- 3.Amos RJ, Deane M, Ferguson C, Jeffries G, Hinds CJ, Amess JA. Observations on the haemopoietic response to critical illness. J Clin Pathol. 1990;43(10):850–6. doi: 10.1136/jcp.43.10.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson MA, Davis A, Elliott P, Cole-Sinclair M. Linezolid-induced dyserythropoiesis: chloramphenicol toxicity revisited. Intern Med J. 2005;35(10):626–8. doi: 10.1111/j.1445-5994.2005.00912.x. [DOI] [PubMed] [Google Scholar]
- 5.Westhoff DD, Samaha RJ, Barnes A., Jr Arsenic intoxication as a cause of mega-loblastic anemia. Blood. 1975;45(2):241–6. [PubMed] [Google Scholar]
- 6.Pye KG, Kelsey SM, House IM, Newland AC. Severe dyserythropoiesis and autoimmune thrombocytopenia associated with ingestion of kelp supplements. Lancet. 1992;339(8808):1540. doi: 10.1016/0140-6736(92)91305-r. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez-Balderas FJ, Morales-Polanco MR, Gutierrez L. Acute sideroblastic anemia in active systemic lupus erythematosus. Lupus. 1994;3(3):157–9. doi: 10.1177/096120339400300305. [DOI] [PubMed] [Google Scholar]
- 8.Gregg XT, Reddy V, Prchal JT. Copper deficiency masquerading as myelodysplastic syndrome. Blood. 2002;100(4):1493–5. doi: 10.1182/blood-2002-01-0256. [DOI] [PubMed] [Google Scholar]
- 9.Huff JD, Keung YK, Thakuri M, Beaty MW, Hurd DD, Owen J, et al. Copper deficiency causes reversible myelodysplasia. Am J Hematol. 2007;82(7):625–30. doi: 10.1002/ajh.20864. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter SL, Zimmerman SA, Ware RE. Acute parvovirus B19 infection mimicking congenital dyserythropoietic anemia. J Pediatr Hematol Oncol. 2004;26(2):133–5. doi: 10.1097/00043426-200402000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Michot F, Gut J. Alcohol-induced bone marrow damage. A bone marrow study in alcohol-dependent individuals. Acta Haematol. 1987;78(4):252–7. doi: 10.1159/000205888. [DOI] [PubMed] [Google Scholar]
- 12.Ballard HS. Alcohol-associated pancytopenia with hypocellular bone marrow. Am J Clin Pathol. 1980;73(6):830–4. doi: 10.1093/ajcp/73.6.830. [DOI] [PubMed] [Google Scholar]
- 13.Swerdlow SH, Campo E, Harris NL, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2008. [Google Scholar]
- 14.Rosati S, Mick R, Xu F, Stonys E, Le Beau MM, Larson R, et al. Refractory cytopenia with multilineage dysplasia: further characterization of an ‘unclassifiable’ myelodys-plastic syndrome. Leukemia. 1996;10(1):20–6. [PubMed] [Google Scholar]
- 15.Matsuda A, Germing U, Jinnai I, Iwanaga M, Misumi M, Kuendgen A, et al. Improvement of criteria for refractory cytopenia with multilineage dysplasia according to the WHO classification based on prognostic significance of morphological features in patients with refractory anemia according to the FAB classification. Leukemia. 2007;21(4):678–86. doi: 10.1038/sj.leu.2404571. [DOI] [PubMed] [Google Scholar]
- 16.Verburgh E, Achten R, Louw VJ, Brusselmans C, Delforge M, Boogaerts M, et al. A new disease categorization of low-grade myelodysplastic syndromes based on the expression of cytopenia and dysplasia in one versus more than one lineage improves on the WHO classification. Leukemia. 2007;21(4):668–77. doi: 10.1038/sj.leu.2404564. [DOI] [PubMed] [Google Scholar]
- 17.Goasguen JE, Matsuo T, Cox C, Bennett JM. Evaluation of the dysmyelopoiesis in 336 patients with de novo acute myeloid leukemia: major importance of dysgranulopoiesis for remission and survival. Leukemia. 1992;6(6):520–5. [PubMed] [Google Scholar]
- 18.Gahn B, Haase D, Unterhalt M, Drescher M, Schoch C, Fonatsch C, et al. De novo AML with dysplastic hematopoiesis: cytogenetic and prognostic significance. Leukemia. 1996;10(6):946–51. [PubMed] [Google Scholar]
- 19.Bain BJ. The bone marrow aspirate of healthy subjects. Br J Haematol. 1996;94(1):206–9. doi: 10.1046/j.1365-2141.1996.d01-1786.x. [DOI] [PubMed] [Google Scholar]
- 20.Wong KF, Chan JK. Are ‘dysplastic’ and hypogranular megakaryocytes specific markers for myelodysplastic syndrome? Br J Haematol. 1991;77(4):509–14. doi: 10.1111/j.1365-2141.1991.tb08618.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Erber WN, Porwit A, Tomonaga M, Peterson LC. ICSH guidelines for the standardization of bone marrow specimens and reports. Int J Lab Hematol. 2008;30(5):349–64. doi: 10.1111/j.1751-553X.2008.01100.x. [DOI] [PubMed] [Google Scholar]
- 22.Bain BJ, Clark DM, Wilkins B. Bone marrow pathology. 4th ed. Chichester, UK; Hoboken, NJ: Wiley-Blackwell; 2010. [Google Scholar]
- 23.Lewis SM, Bain BJ, Bates I. Practical Haematology. 10th edn. Churchill Livingstone; London: 2006. pp. 1–722. [Google Scholar]
- 24.Mufti GJ, Bennett JM, Goasguen J, Bain BJ, Baumann I, Brunning R, et al. Diagnosis and classification of myelodysplastic syndrome: International Working Group on Morphology of myelodysplastic syndrome (IWGM-MDS) consensus proposals for the definition and enumeration of myeloblasts and ring sideroblasts. Haematologica. 2008;93(11):1712–7. doi: 10.3324/haematol.13405. [DOI] [PubMed] [Google Scholar]
- 25.Bain BJ. Leukaemia diagnosis. 4th ed. Chichester, West Sussex; Hoboken, NJ: Wiley-Blackwell; 2010. [Google Scholar]
- 26.Ramos F, Fernandez-Ferrero S, Suarez D, Barbon M, Rodriguez JA, Gil S, et al. Myelodysplastic syndrome: a search for minimal diagnostic criteria. Leuk Res. 1999;23(3):283–90. doi: 10.1016/s0145-2126(98)00166-0. [DOI] [PubMed] [Google Scholar]
- 27.Löffler H, Rastetter J, Haferlach T, editors. Atlas der klinischen Hämatologie. 6 Auflage ed. Springer Verlag; 2004. [Google Scholar]
- 28.Bain BJ. Blood cells: a practical guide. 4th ed. Malden, Mass: Blackwell; 2006. [Google Scholar]
- 29.Valent P, Horny HP. Minimal diagnostic criteria for myelodysplastic syndromes and separation from ICUS and IDUS: update and open questions. Eur J Clin Invest. 2009;39(7):548–53. doi: 10.1111/j.1365-2362.2009.02151.x. [DOI] [PubMed] [Google Scholar]
- 30.Aul C, Gattermann N, Schneider W. Age-related incidence and other epidemiological aspects of myelodysplastic syndromes. Br J Haematol. 1992;82(2):358–67. doi: 10.1111/j.1365-2141.1992.tb06430.x. [DOI] [PubMed] [Google Scholar]
- 31.Strom SS, Gu Y, Gruschkus SK, Pierce SA, Estey EH. Risk factors of myelodysplastic syndromes: a case-control study. Leukemia. 2005;19(11):1912–8. doi: 10.1038/sj.leu.2403945. [DOI] [PubMed] [Google Scholar]
- 32.Strom SS, Velez-Bravo V, Estey EH. Epidemiology of myelodysplastic syndromes. Semin Hematol. 2008;45(1):8–13. doi: 10.1053/j.seminhematol.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112(1):45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 34.Ma X, Lim U, Park Y, Mayne ST, Wang R, Hartge P, et al. Obesity, lifestyle factors, and risk of myelodysplastic syndromes in a large US cohort. Am J Epidemiol. 2009;169(12):1492–9. doi: 10.1093/aje/kwp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du Y, Fryzek J, Sekeres MA, Taioli E. Smoking and alcohol intake as risk factors for myelodysplastic syndromes (MDS) Leuk Res. 2010;34(1):1–5. doi: 10.1016/j.leukres.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Sekeres MA. The epidemiology of myelodysplastic syndromes. Hematol Oncol Clin North Am. 2010;24(2):287–94. doi: 10.1016/j.hoc.2010.02.011. [DOI] [PubMed] [Google Scholar]