Abstract
Background
This study evaluates the correlation between imatinib trough plasma concentrations (Cmin) and clinical response and safety in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase in the Tyrosine Kinase Inhibitor OPtimization and Selectivity (TOPS) trial.
Design and Methods
Patients were randomized 1:2 to 400 mg/day or 800 mg/day imatinib. Imatinib Cmin levels were collected at pre-dose before treatment, and at the end of months 1 (day 29), 6, 9, and 12.
Results
Imatinib Cmin were stable over time in the 400 mg/day dose arm, but showed a slight decrease in the 800 mg/day arm due to dose adjustments between months 1–6. The overall median imatinib Cmin levels were 1040, 1200, 1935, and 2690 ng/mL for the actual 300, 400, 600, and 800 mg/day doses, respectively. The rates of major molecular response (MMR) at 3, 6, 9, and 12 months, and complete cytogenetic response (CCyR) at 6 and 12 months were significantly lower among patients with the lowest imatinib Cmin levels at Day 29 (<1165 ng/mL, 25th percentile). There was an apparent association between high imatinib Cmin and the occurrence of grade 3/4 neutropenia and all-grade rash, diarrhea, arthralgia/myalgia, and all-cause edema.
Conclusions
Imatinib Cmin levels were relatively stable over time and proportional to the dose administered. Patients with an imatinib Cmin above 1165 ng/mL on Day 29 achieved MMR faster and had higher MMR and CCyR rates at 12 months. There appeared to be an association between imatinib Cmin and the frequency of some adverse events. This trial was registered at http://www.clinicaltrials.gov as NCT00124748.
Keywords: imatinib, pharmacokinetics, tyrosine kinase inhibitor, chronic myeloid leukemia
Introduction
Imatinib (Gleevec®/Glivec®, Novartis Pharmaceuticals Corporation, East Hanover, NJ) at 400 mg/day is approved as initial therapy for patients with newly diagnosed Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP) based on results of the International Randomized Study of Interferon and STI571 (IRIS) trial, which established the superiority of imatinib over interferon-alpha plus cytarabine.1–4 Long-term results from IRIS show that hematologic and cytogenetic responses to imatinib are durable, with cumulative complete cytogenetic response (CCyR) of 83%, cumulative major molecular response (MMR) of 86%, and overall survival (OS) of 85% at 8-year follow up.5 Previous pharmacokinetic (PK) analyses have suggested that achieving and maintaining an imatinib plasma level of at least 1000 ng/mL is associated with better clinical response.6–9
Several studies have suggested that the use of 800 mg/day imatinib as starting dose or dose escalation above the standard 400 mg/day dose might have better efficacy in newly diagnosed patients with CML-CP, particularly among higher risk patients.10–16 The phase III, randomized, open-label TOPS (Tyrosine Kinase Inhibitor Optimization and Selectivity) trial of imatinib at 400 mg/day versus 800 mg/day in patients with newly diagnosed, previously untreated CML-CP showed that both MMR and CCyR occurred faster among patients randomly assigned to imatinib 800 mg/day compared with those in the 400 mg/day arm, although there was no significant difference in MMR and CCyR rates at 12 months and there were more frequent grade 3/4 adverse events (AEs) on the 800 mg/day arm.17 The present study evaluates the dose-exposure relationship and the correlation between imatinib Cmin levels, clinical responses, and safety parameters within the first 12 months of the TOPS study.
Design and methods
Patients and definitions of response on TOPS
Patients with newly diagnosed Ph+ CML-CP were randomized 1:2 to imatinib 400 mg/day (once daily) or 800 mg/day (400 mg twice daily). The eligibility criteria and study design of the TOPS study have been previously reported.17
Cytogenetic response was assessed by bone marrow evaluations at baseline and every six months thereafter until patients achieved CCyR. Complete cytogenetic response was defined as 0% Ph+ metaphase cells out of at least 20 examined. Once patients achieved a CCyR, evaluations were performed annually. Major molecular response was defined as a standardized ratio of BCR-ABL to control gene ratio of 0.1% or under by real-time quantitative polymerase chain reaction (RQ-PCR) in peripheral blood expressed according to the international scale.18–20 Major molecular response was assessed at baseline, months 1, 2, 3, 6, 9, and 12, and every three months thereafter.
Pharmacokinetic sampling
Imatinib Cmin levels were collected at pre-dose before treatment, and at the end of months 1 (day 29), 6, 9, and 12 for all patients, whenever possible. The trough sample was defined as the blood sample collected in the morning prior to the morning dose, generally between 8 and 11 am (i.e. within 24±3 h from the last dose for once-daily dosing or 12±3 h from the last dose for twice-daily dosing). On the day of PK sampling, a 3 mL blood sample was collected from patients before treatment without modification of their daily dosing schedule. Plasma concentrations of imatinib and CGP74588 (the N-demethylated metabolite) were measured by the Drug Metabolism and Pharmacokinetics Department at Novartis Pharmaceuticals Corporation using liquid chromatography and tandem mass spectrometry with deuterated imatinib as the internal standard.21 The assay was fully validated, with a limit of quantification of 20 ng/mL for both imatinib and its primary active metabolite CGP74588.
Pharmacokinetic data analysis
Imatinib and CGP74588 trough plasma concentrations (Cmin) and the metabolite to imatinib Cmin concentration ratios were summarized by dose and by month using the following summary statistics: mean, median, standard deviation, and coefficient of variation. Since dose adjustments were allowed in the study, the dose-to-Cmin proportionality was examined over the actual doses received. Summary statistics or simple correlation analysis were used to evaluate the effects of the patient’s demographic parameters (age, gender, body weight, race) on imatinib Cmin. The effects of co-medications were not studied because potent cytochrome P450 3A4/3A5 inhibitors and inducers that could affect imatinib PK exposure were prohibited according to the protocol.
Analyses of exposure and response and exposure and safety
For exposure/response and exposure/safety analyses, the data from the two dose groups were combined to increase the sample size for statistical analyses. The association between imatinib Cmin and clinical response and safety parameters was assessed by investigating the differences in the response and Adverse Event (AE) rates across the quartiles of the Cmin levels measured on day 29. The PK Cmin quartiles at day 29 were defined as follows:(6) the lower quartile (Q1) was the 25% of patients with the lowest imatinib Cmin values; quartiles 2 and 3 (Q2 and Q3) comprised patients whose Cmin levels were within the interquartile range; the upper quartile (Q4) was the 25% of patients with the highest imatinib Cmin values. These three PK categories (Q1, Q2–Q3, and Q4) were used for stratification as appropriate.
The response data included MMR rates at 3, 6, 9, and 12 months, and CCyR at 6 and 12 months based on patients with available PK and molecular/cytogenetic sampling. Time to MMR was analyzed by Cmin quartiles at day 29 using the Kaplan-Meier method. Safety data included the most frequently reported hematologic and non-hematologic AEs regardless of assigned relationship. Adverse events were analyzed by Cmin quartiles at day 29 according to the start date of the AE episode (ie, within three or 12 months of initiation of treatment). All analyses reported herein are considered exploratory, and P values have been provided for descriptive purposes.
Ethical considerations
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved by the ethics committee or institutional review boards of all participating centers. All 476 patients randomly assigned to TOPS gave written informed consent to participate in the clinical trial and the PK sampling.
Results
Pharmacokinetic patient populations and dose adjustments
Pharmacokinetic samples were taken at least once in 423 of 476 (89%) patients, including baseline and at months 1, 6, 9, or 12. The baseline PK samples taken before treatment were to confirm the absence of imatinib in blood and thus were not used in the PK data analysis. Overall, 353 patients had at least one Cmin value available, with 240, 251, 226, and 236 patients with samples at months 1, 6, 9, and 12, respectively; 106 patients had samples available at all four time points. The overall median age was 46 years (range 18–75 years), median body weight was 72.4 kg (range 37.5–131.4 kg), median body surface area was 1.86 m2 (range 1.2–2.5 m2), and 56.7% of patients were male. The distribution of demographic characteristics was similar between arms.
Of the patients who remained on treatment at 12 months, 5% of patients in the standard 400 mg dose arm had increased their dose, while 10% had required a dose reduction. In the high-dose arm, 23% of patients were taking less than their assigned dose on day 29, a proportion that reached 37% at month 6 and 38% at month 12.
Imatinib and CGP74588 trough plasma concentrations over dose and over time
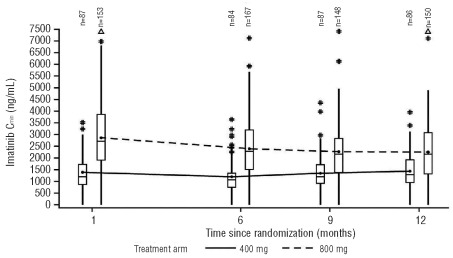
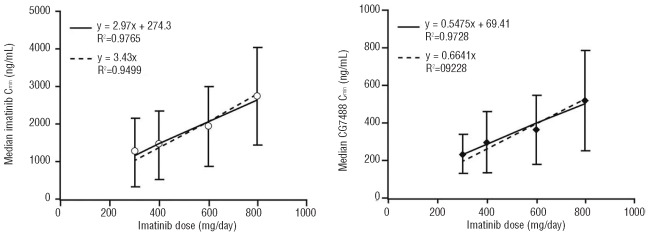
Median imatinib Cmin levels were relatively stable over time in the 400 mg/day arm (Figure 1). There was a slight decrease in imatinib Cmin between months 1 and 6 in the 800 mg/day arm which was likely due to dose adjustments in this period (Online Supplementary Table S1). The Cmin levels for imatinib and CGP74588 appeared to be proportional to the actual dose administered. In a pooled analysis of patients from both arms and all available time points (months 1 [Day 29], 6, 9, and 12), the overall median imatinib Cmin levels were 1040 ng/mL, 1200 ng/mL, 1935 ng/mL, and 2690 ng/mL for the actual 300 mg/day, 400 mg/day, 600 mg/day, and 800 mg/day doses, respectively (Figure 2). The metabolite to parent drug Cmin ratio was approximately 20–25%. The detailed Cmin values for imatinib and CGP74588 and their ratios for each month are summarized in the Online Supplementary Table S2. The intra- and interpatient PK variability for imatinib Cmin was assessed on data from patients with all four Cmin values at months 1, 6, 9, and 12 and without dose changes within the 12-month period. The intra- and interpatient variability was 27.8 and 37.7%, respectively, for the 400 mg/day group (n=40), and 35.7 and 53.8%, respectively, for the 800 mg dose group (n=66).
Figure 1.
Imatinib Cmin over time for the 400 mg/day and 800 mg/day protocol dose groups. Box plots show the median, 25th percentile, 75th percentile, and joined mean values. The whiskers extend up to 1.5 times the interquartile range from the boxes. #: values which exceed 1.5 times the interquartile range; Δ: values > 7500 ng/mL.
Figure 2.
Cmin (mean ± SD)-dose relationship for imatinib (upper panel) and its metabolite CGP74588 (lower panel) over the actual imatinib dose administered in the first 12-month period (pooled analysis of patients from both arms). Dashed line represents the linear regression line forced to the origin to test the dose-to-Cmin proportionality.
Imatinib exposure by patient demographic characteristics
Analysis of the correlation between imatinib Cmin levels and patient demographics showed that the median imatinib Cmin in females in the 400 mg/day dose group was slightly higher (approximately 30%) than in males; 1375 ng/mL (n=38) versus 1060 ng/mL (n=49), respectively. A similar finding was observed for the 800 mg/day group; 2905 ng/mL (n=70) versus 2600 ng/mL (n=83), but the difference was smaller (12%).
The median body weight and median age in the present study were 73 kg and 46 years, respectively. The median body weight was 75.5 kg for males and 65.0 kg for females, a difference of approximately 15%. Patients with higher body weight or body surface area had a lower imatinib Cmin and elderly patients had a higher imatinib Cmin (data not shown); these results were similar to earlier findings.6
Of the 240 patients with day 29 Cmin data, 162 were white, 53 were Asian, and 25 were of other/unknown ethnic group. At the 400 mg/day dose, 54 whites, 19 Asians, and 14 other/unknown had median Cmin values of 1225, 1120, and 1395 ng/mL, respectively. At the 800 mg/day dose, 108 whites, 34 Asians, and 11 other/unknown had median Cmin values of 2695, 2770, and 2900 ng/mL, respectively. The metabolite CGP74588 to imatinib Cmin ratios were similar among different ethnic groups and between different dose levels (Online Supplementary Table S3). The median baseline body weight for Asians was 61 kg versus 76 kg for whites.
Patients with Cmin data represented all Sokal risk groups: 101 had a low Sokal score, 78 had an intermediate Sokal score, and 61 had a high Sokal score. Considering the variability of the data, no major differences in patients’ Cmin values or the metabolite to imatinib Cmin ratios were observed (Online Supplementary Table S4).
Imatinib Cmin levels and their association with clinical responses (MMR and CCyR) at 12 months
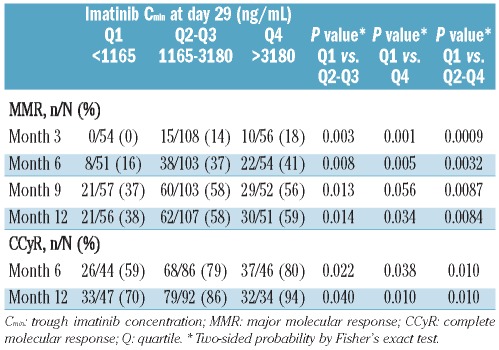
To increase the sample size for statistical analyses, the PK and clinical data were pooled from the two dose groups (Online Supplementary Figure S1); the underlying assumption for the exposure/response correlation analysis was that PK plasma exposure is a better predictor for clinical response than dose. The zero concentrations constituted less than 5% of the data and are not likely to have impacted the correlation analysis. In the pooled population (400 mg/day and 800 mg/day data combined), the lowest quartile (Q1) of the Day 29 Cmin values included patients with imatinib Cmin less than 1165 ng/mL (n=60), the middle 50% of patients (Q2–Q3) had imatinib Cmin between 1165 and 3180 ng/mL (n=120), and the highest quartile (Q4) was defined by imatinib Cmin levels over 3180 ng/mL (n=60). Out of the 60 patients in Q1, two-thirds were from the 400 mg/day arm (41 of 60) and one-third from the 800 mg/day arm (19 of 60). Out of the 60 patients in Q4, 57 (95%) were from the 800 mg/day arm.
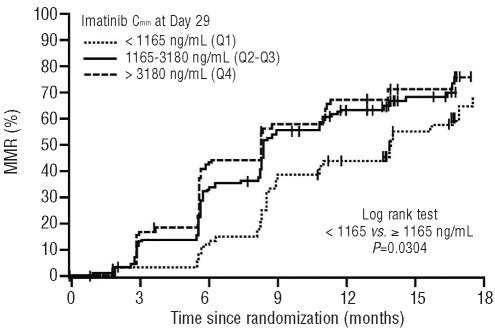
Among evaluable patients with Cmin and RQ-PCR determinations, MMR rates increased with increasing imatinib Cmin levels. The MMR rates at 3, 6, 9, and 12 months for patients with imatinib Cmin less than 1165 ng/mL (Q1) at day 29 were significantly lower than those with imatinib Cmin of 1165 ng/mL or over (Q2–4) (Table 1). The MMR and CCyR rates were similar between Q2 and Q3 (data not shown). The differences between Q1 and all other quartiles combined (Q2–Q4) were significant at months three, six, nine and 12, with P values < 0.01. Patients in the upper quartiles (Q2–Q4) with higher Cmin at Day 29 achieved MMR faster than patients in Q1 (P=0.0304) (Figure 3). The time to MMR was similar between the two upper groups, Q2–Q3 and Q4. The CCyR rates at six and 12 months were also significantly lower in Q1 than in the Q2–Q4 groups combined (Table 1). A receiver operating characteristic (ROC) analysis of MMR on imatinib Cmin was performed, but the analysis did not show a sufficient thresh-old using only imatinib Cmin as a predictor (data not shown).
Table 1.
MMR rates at months 3, 6, 9, and 12, and CCyR rates at 6 and 12 months by imatinib Cmin at day 29.
Figure 3.
Time to MMR by imatinib Cmin at Day 29 (pooled analysis of patients from both arms; Kaplan-Meier analyses). Patients without MMR were censored at last assessment on study treatment. MMR: major molecular response.
Sokal risk groups were evenly distributed between treatment arms and within Cmin quartiles (Online Supplementary Table S4). The MMR rate was lower for patients with high Sokal risk scores (Online Supplementary Figure S2). For the high Sokal risk group, the MMR rate was worse for patients with Cmin levels less than 1165 ng/mL, especially within the first 12 months (Online Supplementary Figure S2). For the low and intermediate Sokal risk groups, patients with Cmin less than 1165 ng/mL had slower time to MMR and lower MMR rates overall, but this trend was not as apparent as for the high Sokal risk group.
Association between imatinib Cmin levels and safety outcomes
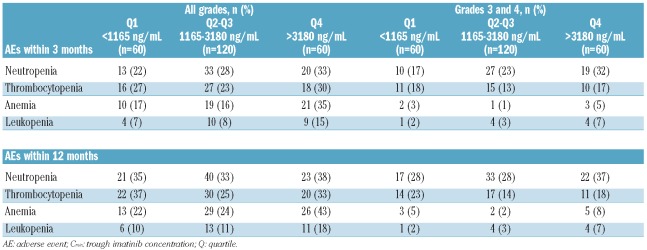
The level of association between imatinib Cmin on Day 29 and the frequency of AEs appeared to be dependent on the type of AE. Higher imatinib Cmin on Day 29 (Q4, Cmin >3180 ng/mL) was associated with the frequency of all-grade neutropenia, anemia, and leukopenia observed within the first three months of therapy and, to a lesser extent, all-grade thrombocytopenia (Table 2). A similar trend of association was observed between imatinib Cmin on Day 29 and anemia and leukopenia within 12 months of treatment. Differences in frequency of neutropenia and thrombocytopenia were less pronounced between Cmin groups, probably due to dose adjustment over time. Grade 3/4 neutropenia within three months and within 12 months was also more common in the Q4 group compared with the lower quartiles. A trend of association was observed between imatinib Cmin and grade 3/4 anemia and leukopenia, but no definitive conclusion could be made due to the low frequencies of these AEs.
Table 2.
Frequency of hematologic AEs (all grades and grades 3 or 4) during the first 3- and 12-month treatment periods by imatinib Cmin on day 29.
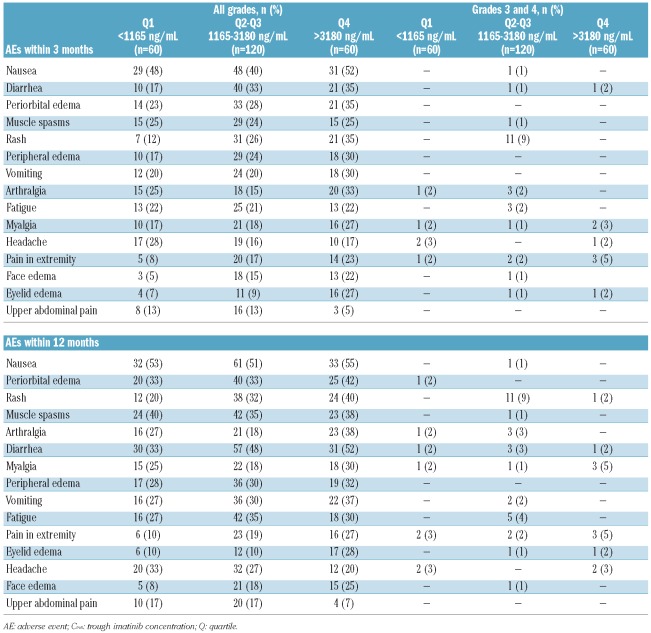
Grade 3/4 non-hematologic AEs were infrequent, and no major association with imatinib Cmin on Day 29 was observed (Table 3). Imatinib Cmin on Day 29 was associated with frequency of all-grade rash, all-cause edema, nausea, diarrhea, vomiting, arthralgia, myalgia, and extremity pain within the first three months of treatment. Muscle spasms, fatigue, and headache were similar among the quartiles, whereas abdominal pain was less frequent among patients in Q4. A similar pattern was observed for all grades of AEs experienced within 12 months.
Table 3.
Frequency of non-hematologic AEs (all grades and grades 3 or 4) during the first 3- and 12-month treatment periods by imatinib Cmin on day 29.
Discussion
Imatinib Cmin was stable over 12 months in this PK analysis of the TOPS study. The slight decrease in Cmin from Day 29 to six months in the 800 mg/day arm was likely due to dose reductions in this high-dose group. There were zero concentration values on Day 29 for both dose groups, especially the 800 mg dose group (n=1 for 400 mg/day and n=10 for 800 mg/day), suggesting toxicity-related dose interruption prior to Day 29 PK sampling. The zero concentrations were included in the PK and correlation analysis to better represent the clinical setting. Removing these values (<5%) did not affect the correlation analysis results (data not shown). When dose adjustment was taken into consideration, imatinib Cmin was proportional to the actual dose administered between 300 mg and 800 mg/day at months 1, 6, 9, and 12. The metabolite to imatinib Cmin ratio also showed similar values within the 12-month period, suggesting that the metabolism and clearance of imatinib were stable over time.
The average and median Cmin values observed with the 400 mg/day imatinib dose in the present study (1425 ng/mL and 1200 ng/mL, respectively) were somewhat higher than those observed in the IRIS study at the same dose level (979 ng/mL and 879 ng/mL, respectively6). All samples in both studies were analyzed in the same laboratory using liquid chromatography and tandem mass spectrometry with deuterated imatinib as the internal standard.21 The exact reason for the difference is unclear but may be related to several factors, such as demographic differences between the patient populations. For example, there were more Asian patients in the TOPS (22% in the PK population) than in IRIS, where nearly 100% of patients were white. The median body weight in the present study was 73 kg versus 82 kg in IRIS. This is likely related to the difference in racial makeup between the two studies. As has been shown previously,6,22 body weight and age were significant covariates for imatinib clearance and plasma exposure in the present study, although their overall impact on imatinib Cmin was small due to the large interpatient PK variability.6 Minor effects due to compliance and food intake cannot be excluded in explaining the differences in Cmin between the two phase III studies.
In the present study, imatinib Cmin levels were correlated with MMR and CCyR rates. Imatinib Cmin levels ≥1165 ng/mL at Day 29 were associated with faster time to MMR (P=0.0304) and higher rates of MMR at months 3, 6, 9, and 12, as well as higher rates of CCyR at months 6 and 12. This suggests that Day 29 imatinib level is a good prognostic parameter for achieving both MMR and CCyR at 12 months. The MMR and CCyR rates were similar between Q2 and Q3, and between the Q2–Q3 (1165–3180 ng/mL) and Q4 (> 3180 ng/mL) groups, suggesting that response rates approach a plateau at 12 months with Cmin levels above 1165 ng/mL in patients with CML-CP. This may help to explain the clinical results of the TOPS study in which CCyR and MMR were achieved faster in the 800 mg/day arm but CCyR and MMR rates at 12 months were comparable between arms.17
As stated earlier, ROC analysis did not show a sufficient threshold using only imatinib Cmin as a predictor, probably due to the heterogeneity of the population with respect to Sokal risk and other factors. As has been shown previously,3,4,6 patients with higher Sokal risk had decreased rates of CCyR and MMR. This is consistent with previous data from patients treated with imatinib on IRIS, where Sokal risk group and imatinib trough level were independent predictors for achievement of CCyR.6 Thus, achieving and maintaining an adequate imatinib trough level (above 1000 ng/mL) might be particularly important for patients with a high Sokal risk score. Further studies are warranted to better characterize the relationship between PK and response considering other factors, such as dose changes or interruptions, race, and transporter-related factors.
Unlike CCyR and MMR response parameters, which are known to be associated with imatinib inhibition of BCR-ABL kinase activity, the underlying cause of AEs may be multifactorial. As a result, the correlation between imatinib concentration and AEs may not be as straightforward as it is with efficacy parameters. This appeared to be the case in TOPS, where a slightly higher incidence of the most frequently reported AEs, such as rash, diarrhea, arthralgia/myalgia and all-cause edema was observed in patients in the highest imatinib Cmin quartile (Q4, Cmin >3180 ng/mL), and these Q4 patients had higher rates of treatment-related neutropenia, anemia, and leukopenia. On the other hand, muscle spasms, fatigue, and headache were similar among the quartiles, and abdominal pain was less frequent among patients in Q4. There were no major differences in the frequency of grade 3/4 non-hematologic AEs among patients in different quartiles. However, PK correlation analysis with grade 3/4 AEs is challenging since grade 3/4 AEs typically result in immediate dose interruption. Thus, the Cmin level associated with grades 3/4 AEs may be underestimated. Grade 3/4 AEs should be avoided since it has been reported that dose interruptions due to grade 3/4 AEs could compromise clinical response.23,24
Numerous factors can contribute to the variability of imatinib Cmin observed in the present study and in clinical practice. Among these is poor adherence to medication, which is an issue for many oral and chronic therapies,25,26 and has been shown to be a concern for imatinib patients.27–29 Since imatinib is primarily metabolized by the cytochrome P450 enzyme CYP3A4 (30), concomitant medications which induce or inhibit CYP3A4 may affect imatinib levels, as can interpatient variability in metabolizing enzyme activities.7,22,31 Drug transporter activities may also impact response.32–39 For example, low activity of the OCT-1 drug influx pump has been associated with suboptimal cytogenetic and molecular response to imatinib,35,40 although some controversial findings related to the involvement of OCT-1 have been reported.36,41 Pharmacokinetic sampling time error could be another source of variability, although all PK samples were collected within three hours more or less than the acceptable sampling window.42
The results of the TOPS PK substudy demonstrate that imatinib Cmin levels are proportional to dose and stable over the 12-month period studied, and support previous findings that imatinib Cmin is associated with response. Imatinib Cmin levels above 1165 ng/mL were associated with improved MMR and CCyR rates at six and 12 months, and Cmin levels above 3180 ng/mL were associated with a higher frequency of some grade 3/4 AEs (e.g. neutropenia). This empirical estimate of effective Cmin (1165 ng/mL) is consistent with the 1000 ng/mL threshold, as indicated in early studies.6,7 Thus, maintaining imatinib Cmin above approximately 1000 ng/mL might be beneficial for achieving an optimal response, especially for patients with high Sokal risk score. However, the upper limit of the therapeutic window could not be easily determined since the extent of association, if any, differed among different AEs. Further studies are needed to better define the therapeutic window for an individual patient considering the individual patient’s BCR/ABL mutation status, Sokal risk scores, and overall AE profiles, as well as factors which could potentially be transporter-related as discussed above. Monitoring the plasma level of imatinib may help to optimize therapy or guide a switch to a more effective agent, such as nilotinib or dasatinib, both of which have been shown to be superior to imatinib in randomized trials of newly diagnosed patients with CML-CP.43,44
Acknowledgments
The authors would like to thank Anthony Gichangi, Yen-Lin Chia, and Eren Demirhan for the valuable discussion and statistical data analysis, and Ovidiu Chiparus for programming support.
Footnotes
Funding: financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Erinn Goldman, PhD for medical editorial assistance with this manuscript.
Data from this manuscript have been previously presented at the American Society of Hematology’s 50th Annual Meeting and Exposition December 6–9, 2008, in San Francisco, CA, USA (oral presentation).
The online version of this article has a Supplementary Appendix.
Study Management Committee
M. D. Anderson Cancer Center, Houston, TX, USA: J. Cortes; Oregon Health Sciences University, Portland, OR, USA: B. Druker; Clinical Investigation Centre 802 INSERM, Poitiers, France: F. Guilhot; Royal Adelaide Hospital, Adelaide, Australia: T. Hughes; University of Bologna, Bologna, Italy: M. Baccarani, PCR Committee: Hanson Institute Centre for Cancer, Adelaide, Australia: S. Branford; Royal Adelaide Hospital, Adelaide, Australia: T. Hughes; Fred Hutchinson Cancer Research Center, Seattle, USA: J. Radich; The Catholic University of Korea, Seoul, Korea: D-W. Kim; University of Naples, Naples, Italy: F. Pane.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.National Comprehensive Cancer Network. Chronic Myelogenous Leukemia. Version 2. 2011. NCCN: Clinical practice guidelines in oncology. [DOI] [PubMed] [Google Scholar]
- 2.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–51. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 4.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 5.Deininger M, O’Brien SG, Guilhot F, Goldman JM, Hochhaus A, Hughes TP, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood. 2009;114(22):462. [abstract 1126] [Google Scholar]
- 6.Larson RA, Druker BJ, Guilhot FA, O’Brien SG, Riviere GJ, Krahnke T, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111(8):4022–8. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- 7.Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2007;109(8):3496–9. doi: 10.1182/blood-2006-07-036012. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi N, Miura M, Scott SA, Kagaya H, Kameoka Y, Tagawa H, et al. Influence of CYP3A5 and drug transporter polymorphisms on imatinib trough concentration and clinical response among patients with chronic phase chronic myeloid leukemia. J Hum Genet. 2010;55(11):731–7. doi: 10.1038/jhg.2010.98. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi N, Wakita H, Miura M, Scott SA, Nishii K, Masuko M, et al. Correlation between imatinib pharmacokinetics and clinical response in Japanese patients with chronic-phase chronic myeloid leukemia. Clin Pharmacol Ther. 2010;88(6):809–13. doi: 10.1038/clpt.2010.186. [DOI] [PubMed] [Google Scholar]
- 10.Cortes J, Giles F, O’Brien S, Thomas D, Garcia-Manero G, Rios MB, et al. Result of high-dose imatinib mesylate in patients with Philadelphia chromosome-positive chronic myeloid leukemia after failure of interferon-alpha. Blood. 2003;102(1):83–6. doi: 10.1182/blood-2003-01-0025. [DOI] [PubMed] [Google Scholar]
- 11.Kantarjian H, Talpaz M, O’Brien S, Garcia-Manero G, Verstovsek S, Giles F, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103(8):2873–8. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 12.Hughes TP, Branford S, White DL, Reynolds J, Koelmeyer R, Seymour JF, et al. Impact of early dose intensity on cytogenetic and molecular responses in chronic- phase CML patients receiving 600 mg/day of imatinib as initial therapy. Blood. 2008;112(10):3965–73. doi: 10.1182/blood-2008-06-161737. [DOI] [PubMed] [Google Scholar]
- 13.Cortes JE, Kantarjian HM, Goldberg SL, Powell BL, Giles FJ, Wetzler M, et al. High-dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: high rates of rapid cytogenetic and molecular responses. J Clin Oncol. 2009;27(28):4754–9. doi: 10.1200/JCO.2008.20.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rea D, Etienne G, Corm S, Cony-Makhoul P, Gardembas M, Legros L, et al. Imatinib dose escalation for chronic phase-chronic myelogenous leukaemia patients in primary suboptimal response to imatinib 400 mg daily standard therapy. Leukemia. 2009;23(6):1193–6. doi: 10.1038/leu.2009.32. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian HM, Larson RA, Guilhot F, O’Brien SG, Mone M, Rudoltz M, et al. Efficacy of imatinib dose escalation in patients with chronic myeloid leukemia in chronic phase. Cancer. 2009;115(3):551–60. doi: 10.1002/cncr.24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jabbour E, Kantarjian HM, Jones D, Shan J, O’Brien S, Reddy N, et al. Imatinib mesylate dose escalation is associated with durable responses in patients with chronic myeloid leukemia after cytogenetic failure on standard-dose imatinib therapy. Blood. 2009;113(10):2154–60. doi: 10.1182/blood-2008-04-154344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortes JE, Baccarani M, Guilhot F, Druker BJ, Branford S, Kim DW, et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J Clin Oncol. 2010;28(3):424–30. doi: 10.1200/JCO.2009.25.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108(1):28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Branford S, Cross NC, Hochhaus A, Radich J, Saglio G, Kaeda J, et al. Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia. 2006;20(11):1925–30. doi: 10.1038/sj.leu.2404388. [DOI] [PubMed] [Google Scholar]
- 20.Branford S, Fletcher L, Cross NC, Muller MC, Hochhaus A, Kim DW, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112(8):3330–8. doi: 10.1182/blood-2008-04-150680. [DOI] [PubMed] [Google Scholar]
- 21.Bakhtiar R, Lohne J, Ramos L, Khemani L, Hayes M, Tse F. High-throughput quantification of the anti-leukemia drug STI571 (Gleevec) and its main metabolite (CGP 74588) in human plasma using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;768(2):325–40. doi: 10.1016/s1570-0232(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 22.Schmidli H, Peng B, Riviere GJ, Capdeville R, Hensley M, Gathmann I, et al. Population pharmacokinetics of imatinib mesylate in patients with chronic-phase chronic myeloid leukaemia: results of a phase III study. Br J Clin Pharmacol. 2005;60(1):35–44. doi: 10.1111/j.1365-2125.2005.02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson RA, Chia YL, Granvil C, Guilhot F, Druker BJ, O’Brien SG, et al. Steady-state imatinib trough levels as well as dose interruptions are associated with clinical response (CCyR and MMR) and adverse events (AEs) in patients with chronic myeloid leukemia (CML) receiving IM as frontline therapy. Blood. 2009;114(22):872. [abstract 2213] [Google Scholar]
- 24.Baccarani M, Druker BJ, Cortes-Franco J, Hughes TP, Kim DW, Pane F, et al. 24 months update of the TOPS study: a phase III, randomized, open-label study of 400mg/d (SD-IM) versus 800mg/d (HD-IM) of imatinib mesylate (IM) in patients (Pts) with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase (CML-CP) Blood. 2009;114(22):142. [abstract 337] [Google Scholar]
- 25.Partridge AH, Avorn J, Wang PS, Winer EP. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94(9):652–61. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 26.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 27.Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–8. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noens L, van Lierde MA, De Bock R, Verhoef G, Zachee P, Berneman Z, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113(22):5401–11. doi: 10.1182/blood-2008-12-196543. [DOI] [PubMed] [Google Scholar]
- 29.Darkow T, Henk HJ, Thomas SK, Feng W, Baladi JF, Goldberg GA, et al. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: a retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics. 2007;25(6):481–96. doi: 10.2165/00019053-200725060-00004. [DOI] [PubMed] [Google Scholar]
- 30.Peng B, Lloyd P, Schran H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet. 2005;44(9):879–94. doi: 10.2165/00003088-200544090-00001. [DOI] [PubMed] [Google Scholar]
- 31.Wojnowski L. Genetics of the variable expression of CYP3A in humans. Ther Drug Monit. 2004;26(2):192–9. doi: 10.1097/00007691-200404000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Thomas J, Wang L, Clark RE, Pirmohamed M. Active transport of imatinib into and out of cells: implications for drug resistance. Blood. 2004;104(12):3739–45. doi: 10.1182/blood-2003-12-4276. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Giannoudis A, Lane S, Williamson P, Pirmohamed M, Clark RE. Expression of the uptake drug transporter hOCT1 is an important clinical determinant of the response to imatinib in chronic myeloid leukemia. Clin Pharmacol Ther. 2008;83(2):258–64. doi: 10.1038/sj.clpt.6100268. [DOI] [PubMed] [Google Scholar]
- 34.White DL, Saunders VA, Dang P, Engler J, Zannettino AC, Cambareri AC, et al. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108(2):697–704. doi: 10.1182/blood-2005-11-4687. [DOI] [PubMed] [Google Scholar]
- 35.White DL, Dang P, Engler J, Frede A, Zrim S, Osborn M, et al. Functional activity of the OCT-1 protein is predictive of long-term outcome in patients with chronic-phase chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2010;28(16):2761–7. doi: 10.1200/JCO.2009.26.5819. [DOI] [PubMed] [Google Scholar]
- 36.Hu S, Franke RM, Filipski KK, Hu C, Orwick SJ, de Bruijn EA, et al. Interaction of imatinib with human organic ion carriers. Clin Cancer Res. 2008;14(10):3141–8. doi: 10.1158/1078-0432.CCR-07-4913. [DOI] [PubMed] [Google Scholar]
- 37.Crossman LC, Druker BJ, Deininger MW, Pirmohamed M, Wang L, Clark RE. hOCT 1 and resistance to imatinib. Blood. 2005;106(3):1133–4. doi: 10.1182/blood-2005-02-0694. [DOI] [PubMed] [Google Scholar]
- 38.Gurney H, Wong M, Balleine RL, Rivory LP, McLachlan AJ, Hoskins JM, et al. Imatinib disposition and ABCB1 (MDR1, P-glycoprotein) genotype. Clin Pharmacol Ther. 2007;82(1):33–40. doi: 10.1038/sj.clpt.6100201. [DOI] [PubMed] [Google Scholar]
- 39.Illmer T, Schaich M, Platzbecker U, Freiberg-Richter J, Oelschlagel U, von Bonin M, et al. P-glycoprotein-mediated drug efflux is a resistance mechanism of chronic myelogenous leukemia cells to treatment with imatinib mesylate. Leukemia. 2004;18(3):401–8. doi: 10.1038/sj.leu.2403257. [DOI] [PubMed] [Google Scholar]
- 40.White DL, Saunders VA, Dang P, Engler J, Venables A, Zrim S, et al. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood. 2007;110(12):4064–72. doi: 10.1182/blood-2007-06-093617. [DOI] [PubMed] [Google Scholar]
- 41.Zach O, Krieger O, Foedermayr M, Zellhofer B, Lutz D. OCT1 (SLC22A1) R61C polymorphism and response to imatinib treatment in chronic myeloid leukemia patients. Leuk Lymphoma. 2008;49(11):2222–3. doi: 10.1080/10428190802322893. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Chia YL, Nedelman J, Schran H, Mahon FX, Molimard M. A therapeutic drug monitoring algorithm for refining the imatinib trough level obtained at different sampling times. Ther Drug Monit. 2009;31(5):579–84. doi: 10.1097/FTD.0b013e3181b2c8cf. [DOI] [PubMed] [Google Scholar]
- 43.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 44.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–70. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]