Abstract
Background
A phase II trial of dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin and rituximab (DA-EPOCH-R) from the National Cancer Institute showed promising activity in untreated diffuse large B-cell lymphoma. The Cancer and Leukemia Group B conducted a study to determine if these results could be reproduced in a multi-institutional setting.
Design and Methods
The study included 69 patients with untreated diffuse large B-cell lymphoma at least 18 years of age and at least stage II. Radiaton therapy was not permitted on study. Median age was 58 years (range 23–83) and 40% had high-intermediate or high International Prognostic Index risk. Immunohistochemical biomarkers for cell of origin and proliferation were performed.
Results
With a median follow up of 62 months, time to progression and overall survival were 81% and 84%, respectively, and time to progression was 87%, 92% and 54% for low/low-intermediate, high-intermediate and high International Prognostic Index risk groups, respectively, at 5-years and beyond. The time to progression and event-free survival of germinal center B-cell lymphoma were 100% and 94%, respectively, and non-germinal center B-cell GCB diffuse large B-cell lymphoma were 67% and 58%, respectively, at 62 months (germinal center vs. non-germinal center B cell P=0.008). DA-EPOCH-R was tolerated without significant grade 4 non-hematologic toxicities.
Conclusions
These results provide the first confirmation by a multi-institutional group that DA-EPOCH-R provides high durable remissions in diffuse large B-cell lymphoma and is effective in both germinal center and non-germinal center B-cell subtypes. The trial was registered at ClinicalTrials.Gov (NCT00032019).
Keywords: diffuse large B-cell lymphoma, DA-EPOCH-rituximab, untreated, outome, molecular
Introduction
Over the past 30 years, efforts to improve chemotherapy strategies for diffuse large B-cell lymphoma (DLBCL) have met with limited success.1–3 While modifications of CHOP chemotherapy led to modest improvements in outcome, these were generally overcome by the addition of rituximab.4,5 Alternative regimens based on aggressive treatment platforms such as ACVBP have improved outcomes in select patient groups, even in the rituximab era, but their applicability is restricted to younger patients due to high acute and long-term toxicities.6–9 The bases for these strategies have generally come from the hypothesis that 'non-cross resistant' drugs and dose intensity will overcome drug resistance, but this has not generally been borne out.10, 11 It is now recognized that treatment failure depends on a complex interplay of factors including tumor biology, tumor volume, pharmacokinetics, and pharmacogenomics.10
Investigators at the National Cancer Institute (NCI) pursued a therapeutic strategy that drew on concepts of drug resistance and pharmacokinetics. Based on studies that showed high tumor proliferation is an adverse prognostic factor in DLBCL, they modeled the effect of drug schedule on tumor cell kill and showed that continuous low-dose drug exposure enhances cell kill of rapidly proliferating tumor cells in vitro.12–14 Furthermore, they hypothesized that variations in drug clearance among patients would significantly impact the drug concentration-response curve in the setting of low steady state concentrations (Css) that are achieved during prolonged continuous infusion schedules. These concepts formed the basis for the dose adjusted (DA)-EPOCH regimen in which doxorubicin, vincristine and etoposide are infused over 96 h, cyclophosphamide and prednisone are administered on a bolus schedule, and doxorubicin, etoposide and cyclophosphamide are pharmacodynamically dose-adjusted based on the neutrophil nadir.15–18 The NCI initially performed a phase II study of DA-EPOCH followed by a study of DA-EPOCH with rituximab in untreated DLBCL, both of which performed well compared to reported outcomes with CHOP and R-CHOP, respectively, in similar patient groups.2–5,17,19 To determine whether the results of the NCI DA-EPOCH-R study were robust and could be translated into the community setting, the Cancer and Leukemia Group B study group (CALGB) performed an independent multi-institutional study of DA-EPOCH-R with analysis of molecular subtype.
Design and Methods
Study design
This multi-center phase II study of DA-EPOCH-R in untreated de novo CD20+ DLBCL enrolled patients at 18 institutions between 15 February 2002 and 28 May 2004. To assure an independent assessment of DA-EPOCH-R, the NCI did not enroll patients on this multicenter study. The minimum follow up required for each patient was three years or until death, whichever occurred first. Data collection was stopped on 15 April 2009 once this time point had been reached. Clinical objectives included response rate, time to progression free and overall survival and toxicity, and experimental end points included tumor immunohistochemical (IHC) biomarker analysis. Seventy-eight patients were enrolled, of which 9 were ineligible; 2 did not start protocol treatment, one patient was taken off study on day one due to rituximab intolerance, one patient refused treatment after one cycle, and 5 patients had ineligible histologies. Central pathology review was conducted by EH in 62 patients.
Eligibility criteria included stages II–IV, human immunodeficiency virus (HIV) negative, negative pregnancy test, adequate major organ function, no central nervous system (CNS) lymphoma, and no evidence of low-grade lymphoma.15,17 Initial evaluation included standard blood tests, whole body computed tomography (CT), and bone marrow biopsy. Standard staging and response criteria were used.20,21 Disease sites were restaged after cycles 4, 6 and 8 (if administered). The study was approved by the Institutional Review Boards of all participating institutions and complied with the Declaration of Helsinki. All patients gave written informed consent. All authors had access to the primary data and approved the manuscript.
Chemotherapy and dose adjustments
DA-EPOCH-R was administered as previously described.15, 17 Patients received 2 cycles beyond CR or stable changes for a minimum of 6 and a maximum of 8 cycles. Pharmacodynamic dose adjustment was based on twice weekly complete blood counts to achieve limited absolute neutropenia count (ANC) below 500/μL with the administration of filgrastim 300 mg from Day 5 until the ANC reached over 5000/μL past the nadir counts.15,17 Strict adherence to the dose adjustment paradigm is mandatory to achieve the results reported herein. Patients with more than one extranodal site and elevated LDH and/or bone marrow involvement by DLBCL received CNS prophylaxis consisting of intrathecal methotrexate 12 mg given on Day 1 (or Day 2) of cycles 3, 4, 5, and 6. Radiation therapy was not permitted on study. Bactrim® DS was administered twice daily for three days per week.
Biomarkers
Immunostaining on whole tissue sections with appropriate primary antibodies was performed at the Pathology Coordinating Office of the CALGB (CD10, clone 56C6; BCL6, PG-B6p; MUM1, clone MUM1p; BCL2, clone 124; Ki67, clone MIB1) using automated immunostainers (Dako, Carpenteria, CA, USA). High pH (9.0) antigen retrieval (Dako) was used for BCL6 and MUM1 while low pH (6.1) was used for CD10, BCL2, and Ki67. LMO2 (clone 1A9-1) was performed at the Cleveland Clinic (Benchmark XT, Ventana Medical Systems, Tucson, AZ, USA). Slides were scored independently in 10% increments by 2 pathologists, with a third review in case of over 20% disagreement for all immunostains (mean score used as final value) except Ki67, for which image analysis (IA, Aperio, Scanscope) and a visual estimate (0=<10%, 1+=10–24%, 2+=25–49%, 3+=50–74%, 4+=75–100%) was used. A 30% cut off for positive staining was used for CD10, BCL6, MUM1 and BCL2 and 60% for Ki67. Classification of tumor biopsies into GCB or non-GCB (i.e. ABC surrogate) subtypes was determined using CD10, BCL6, and MUM1 IHC markers by the validated method of Hans et al.22
Statistical analysis
Overall (OS), time to progression (TTP) and event-free (EFS) survivals were calculated using the Kaplan-Meier method and the significance between Kaplan-Meier curves was calculated using log rank procedure.23,24 Survival end points were calculated from on-study date until death, relapse, progression or last follow up as appropriate. For TTP, deaths among patients without progression or relapse were censored. International Prognostic Index score (IPI) could not be determined in 2 patients due to missing data. Follow up was calculated from study entry until death or close of study analysis for each patient. The analysis of biomarkers was not adjusted for multiple comparisons as these were pre-specified.15 The SAS v9.2 (Cary, NC, USA) statistical package was used for analyses. Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson. CALGB statisticians performed all statistical analyses.
Results
Clinical characteristics and outcome
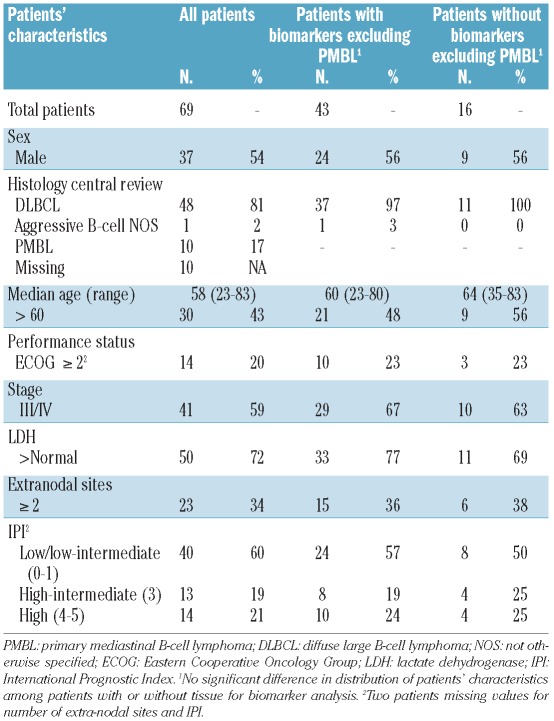
Sixty-nine eligible patients with untreated DLBCL were enrolled (Table 1). Median age was 58 years (range 23–83); 59% were in advanced stage, 72% had an elevated serum lactate dehydrogenase (LDH) level, and 40% had high-intermediate/high risk IPI scores.25 Overall, 58 (84%) patients achieved a complete response (CR), which includes those with CR unconfirmed, and 10 (15%) achieved a partial response (PR). Ten (17%) of those achieving CR and 3 (30%) of those achieving PR have relapsed, half of these relapsed patients had high IPI scores. All episodes of disease progression except one occurred within the first 1.6 years.
Table 1.
Patients’ characteristics.
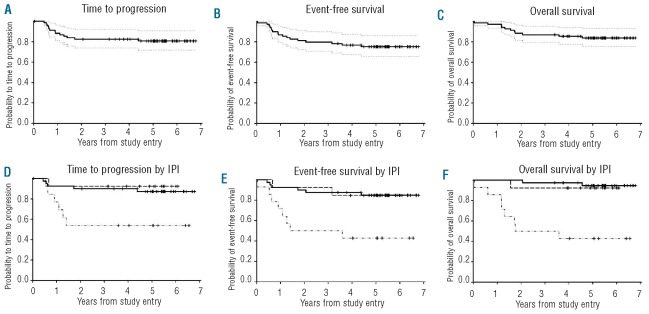
The median (range) follow up of living patients is 5.2 (3.4–6.8) years. At 5-years and beyond, the TTP, EFS and OS are 81%, 75% and 84%, respectively (Figure 1A–C). Three patients died without progression, one on treatment and 2 during follow up from respiratory and cardiac failure. Only high IPI patients were at major risk of progression. At 5 years, the TTP was 54% in high IPI risk patients, whereas those with low/low-intermediate and high-intermediate IPI had low rates of progression with TTP of 87% and 92%, respectively (Figure 1D). Estimates of EFS and OS revealed similar findings (Figure 1E and F).
Figure 1.
Kaplan-Meier plots of survival outcomes of all patients. Outcomes are reported at 5 years (95% Confidence Interval (CI)) and CI range is shown on the curves. (A) PFS 81% (69, 88). (B) EFS 75% (63, 84). (C) OS 84% (73, 91). (D) PFS for IPI risk groups: low/low-intermediate (------------------------) 87% (72, 94), high-intermediate (- - - - - - - - ) 92% (57, 99) and high risk (--- . . --- . . --- ) 54% (25, 76) (P=0.0085). (E) EFS for IPI risk groups: low/low-intermediate 85% (69, 93), high-intermediate 85% (51, 96) and high risk 43% (18, 66) (P<0.0013). (F) OS for IPI risk groups: low/low-intermediate 95% (80, 99), high-intermediate 92% (57, 99) and high risk 43% (18, 66) (P<0.0001).
Clinical and biological prognostic factors
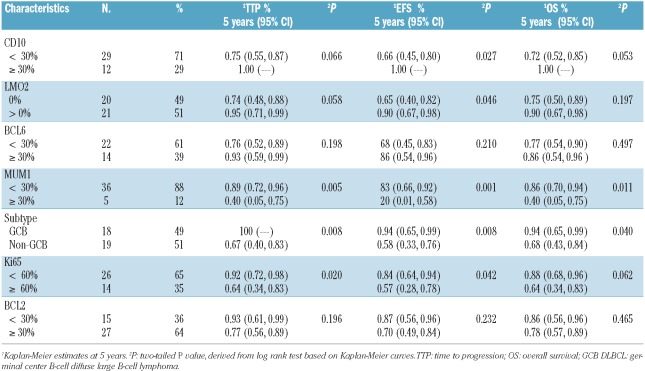
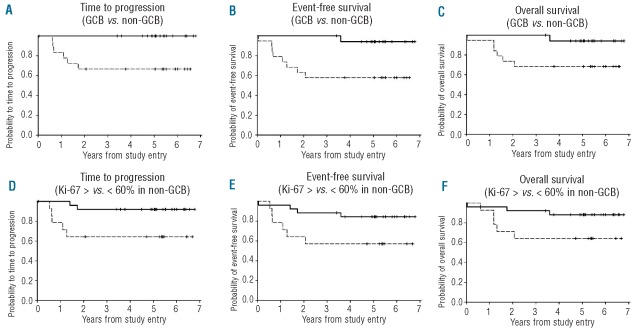
The IPI was significantly associated with TTP (P=0.0085), EFS (P<0.0013) and OS (P<0.0001), which was all driven by the poorer outcome of high-risk patients. Biomarkers were also analyzed in tumor tissue from 52 (76%) patients. To accurately determine the prognostic influence of the cell of origin as defined by the GCB versus non-GCB (ABC surrogate) subgroups, the 10 patients with PMBL were excluded; 9 from the IHC group and one from the group without IHC (Table 1).26 Patients' characteristics were similar in the groups with and without tissue for IHC (Table 1). Tissue was analyzed with biomarkers of cellular differentiation (CD10, LMO2, BCL6, and MUM1), proliferation (Ki67) and apoptosis inhibition (BCL2), and categorized as GCB or non-GCB DLBCL by the Hans method (Table 2).10,14,22 All survival end points were significantly worse in non-GCB compared to GCB DLBCL, though both groups performed well (Table 2; Figure 2A and B). Remarkably, no patients with GCB DLBCL progressed. To control for error in cell categorization by the Hans method, we also assessed outcome using individual markers of cellular differentiation. From 95 to 100% of patients with tumors that expressed LMO2 or CD10, specific markers of germinal center differentiation, are free of progression (Table 2). The less differentiation-specific markers for GCB and non-GCB DLBCL, BCL6 and MUM1, respectively, showed similar results (Table 2). Interestingly, while we observed a significant association between high tumor proliferation (Ki67 ≥ 60%) and survival outcomes, this was only seen in patients with non-GCB DLBCL (Table 2; Figure 2D–F). BCL2 expression was not associated with any survival outcome measure in the combined GCB and non-GCB DLBCL groups (Table 2) or in the non-GCB DLBCL group (data not shown). There were too few events to construct a meaningful multivariate analysis that included IPI score and biomarkers.
Table 2.
Biomarkers in GCB and non-GCB DLBCL and outcome.
Figure 2.
Kaplan-Meier plots of survival outcomes patients with biomarkers. (A) PFS of GCB (-------------------------------) and non-GCB ( - - - - - - - - ) DLBCL (P=0.008). (B) EFS of GCB and non-GCB DLBCL (P=0.008). (C) OS of GCB and non-GCB DLBCL (P=0.04). (D) PFS of Ki67 < 60% (---------------------------) and Ki67 ≥ 60% ( - - - - - - - - - ) in non-GCB DLBCL (P=0.03). (E) EFS of Ki67 < 60% and Ki67 ≥ 60% in non-GCB DLBCL (P=0.04). (F) OS of Ki67 < 60% and Ki67 ≥ 60% in non-GCB DLBCL (P=0.05).
Treatment and toxicity
Toxicity was assessed in all 69 patients on study. Sixty-three (91%) patients completed all treatment cycles. Fifty (72%) patients underwent at least one dose escalation to achieve a nadir ANC below 500/μL, and 65 (94%) patients achieved the desired pharmacodynamic end point. Overall, 25 (36%) patients had fever with neutropenia, 5 (7%) of which were grade 4. Platelet and red cell transfusions were administered to 9 (13%) and 21 (30%) patients, respectively. Gastrointestinal toxicity, such as mucositis or nausea/vomiting, was infrequent and occurred in no more than 5 patients. Similarly, there were infrequent cardiac events, and these only included grade 3 arrhythmia in 4 patients. There was a relatively modest incidence of neuropathy given that vincristine was not capped. Overall, grade 3 motor or sensory neuropathies occurred in 10 (14%) and 7 (10%) patients, respectively, and one patient developed grade 4 motor neuropathy. Most patients experienced significant improvement following treatment. Grade 3 fatigue was observed in 11 (16%) patients. One patient died from a brain hemorrhage during the first cycle.
Discussion
An important goal of this study was to determine whether the outcome of the NCI trial of DA-EPOCH-R could be achieved in a multi-institutional setting.17 Indeed, the positive outcomes of other single institution studies using novel chemotherapy in aggressive lymphomas, including DLBCL, Burkitt's lymphoma and mantle cell lymphoma, could not be confirmed by multi-institutional studies.1,27,28 In the present study, the 5-year TTP and OS were 81% and 84%, respectively, with no late events. Notably, the outcome of DA-EPOCH-R was similar in the low/low-intermediate and high-intermediate IPI groups with 87% and 92% of patients, respectively, progression free at 5-years. Patents with high IPI score had the least favorable outcome with a 5-year TTP of 54%. In the NCI trial of 72 patients, the 5-year TTP and OS were 79% and 80%, respectively, with a median follow up of 54 months. The outcome by IPI was also similar in the NCI trial. Among patients with low/low-intermediate and high IPI, the 5-year TTP was 90% and 47%, respectively. Patients with high-intermediate IPI faired less well in the NCI trial with a 5-year TTP of 67%, but the results are within the confidence intervals of the present study. Notably, in another study of DA-EPOCH-R in high-intermediate and high IPI risk DLBCL, the 2-year EFS and OS were of 68% and 75%, respectively.29 The toxicity profile of DA-EPOCH-R was similar in the CALGB and NCI trials.17 Overall, 91% and 100% of patients in the CALGB and NCI trials, respectively, achieved ANC nadirs below 500 cells/μL, which is the pharmacodynamic end point. The incidence of fever and neutropenia was also similar and occurred in 36% and 47% of patients, respectively. The incidence of gastrointestinal side effects, neuropathy and fatigue were also similar between the two trials.
This CALGB trial provides further evidence that DA-EPOCH-R provides favorable outcomes in DLBCL. Although there are no comparable therapeutic trials with R-CHOP, two retrospective studies and four randomized studies provide outcome results with R-CHOP-based treatment in untreated DLBCL.4,5,30–33 Among these is a retrospective study from the British Columbia Cancer Agency of R-CHOP in 152 patients with a median age of 63 years and high-intermediate/high IPI in 49%.33 In this study, the 3-year progression free survival (defined as TTP) was 65%. In a follow-up paper, this group reported the outcome of R-CHOP at a median follow up of 33 months using the standard and a revised IPI score.34 Using the standard IPI, they reported a 4-year PFS (TTP) of 57% and 51% for high-intermediate and high-risk patients, respectively. Among the high-risk patients, DA-EPOCH-R had a similar TTP of 54%, although with a significantly longer follow up. In contrast, high-intermediate risk patients had an excellent TTP of 92% with DA-EPOCH-R. It should be noted that this study is limited by its short 2-year median follow up and retrospective study design. The randomized MabThera International Trial (MInT) of R-CHOP versus CHOP-like treatments, which included radiotherapy in approximately half the patients, provides outcome results for patients aged 60 years and under with low (0–1) age-adjusted IPI.5 With a median follow up of 34 months, the 4-year EFS was 75% with R-CHOP. Patients on the DA-EPOCH-R study, however, had a significantly poorer prognosis with 40% high-intermediate/high IPI and 43% over the age of 60 years.
Analysis of biomarkers confirm previous findings that DA-EPOCH-R performs better in GCB compared to non-GCB DLBCL.17,35 The robustness of these results is supported by the association of individual markers of cell of origin and outcome in the present study. Our findings are also consistent with the study by Lenz et al. that showed a significant association between outcome and GCB and ABC (i.e. non-GCB by IHC) DLBCL determined by GEP.36 Interestingly, unlike previous results with DA-EPOCH-R, we observed a significant association between high tumor proliferation and poorer outcome in the present study.16,17,35 However, the adverse effect of high tumor proliferation was only present in the non-GCB DLBCL group, which may explain the discordant results with our earlier trials. We did not find any association between BCL2 expression and outcome, which is similar to our previous results.17 It is now recognized that several mechanisms lead to BCL2 overexpression and confer different prognoses, making it an unreliable biomarker of outcome.15,37,38
The favorable outcome of GCB DLBCL as identified by the Hans algorithm in the present study is consistent with prior findings with DA-EPOCH-R.22 In the NCI phase II study of DA-EPOCH-R, GCB DLBCL had a 79% PFS at five years.17 Additionally, a NCI study of DA-EPOCH-R in HIV-associated DLBCL reported a 5-year PFS of 95% in patients with GCB DLBCL.35 Other studies employing the Hans algorithm suggest that GCB DLBCL does not have as favorable an outcome with R-CHOP.39,40 In a recent study from the University of Barcelona, 149 patients with newly diagnosed DLBCL categorized by the Hans algorithm showed no difference in outcome between GCB and non-GCB DLBCL with a 5-year PFS of 54% and 52%, respectively.39 Analysis of the RICOVER-60 trial from the German High-Grade Lymphoma Study Group found a 5-year survival of approximately 50% and 58%, respectively, for GCB and non-GCB DLBCL.40 Furthermore, a trial including 131 patients from the University of Nebraska reported a 3-year EFS of 67% and 52% in GCB and non-GCB DLBCL, respectively.41
It is worthy of note that most clinical studies that have used IHC algorithms to categorize DLBCL as GCB or non-GCB DLBCL have not found any difference in outcome with R-CHOP based treatments.39–42 However, two clinical studies that used gene expression profiling (GEP), the gold standard for molecular classification, have shown differences in outcome of GCB and ABC DLBCL with R-CHOP.36,39 The reason for this discordance may be due to technical variability that might lower accuracy of IHC classification for GCB and ABC (i.e. non-GCB) DLBCL.43 Nonetheless, studies using IHC algorithms with DA-EPOCH-R have shown significantly better disease outcome in GCB compared to non-GCB DLBCL similar to the results with GEP.17, 35
We hypothesize that the efficacy of DA-EPOCH-R in GCB-DLBCL may be related to its effect on BCL6.44,45 BCL6 is a key germinal center B-cell transcription factor that suppresses genes involved in lymphocyte activation, differentiation, cell cycle arrest (p21 and p27Kip1) and DNA damage response genes (p53 and ATR).44 In GCB DLBCL, chromosomal translocations and/or somatic mutations affecting BCL6 enhance its inhibitory effect on the apoptotic stress response and promote proliferation, which are associated with treatment failure.14,44,46–48 Interestingly, inhibition of topoisomerase II leads to downregulation of BCL6 expression by ubiquitin-mediated protein degradation and possibly through transcriptional inhibition.49 This may partially account for the in vitro finding that sustained exposure of tumor cells to the topoisomerase inhibitors, etoposide and doxorubicin, promotes the p53-p21 pathway and activates the check-point kinase (Chk2), effects that are inhibited in cells engineered to over-express BCL6.50,51 The association between topoisomerase II inhibition and BCL6 expression raises the hypothesis that regimens directed against topoisomerase II may be more effective in GCB DLBCL. In this regard, DA-EPOCH-R was designed to inhibit topoisomerase II through several strategies: incorporating two topoisomerase II inhibitors, etoposide and doxorubicin; optimizing topoisomerase II inhibition through a prolonged 96-h infusion; and maximizing steady state concentrations through pharmacodynamic dose adjustment.16
The present study provides the first multi-institutional evidence that DA-EPOCH-R has a favorable outcome in newly diagnosed DLBCL, and confirms the biomarker results from the NCI study. DA-EPOCH-R compares favorably with historical data with R-CHOP, particularly for the treatment of GCB DLBCL. An ongoing phase III trial comparing the outcome of DA-EPOCH-R and R-CHOP in DLBCL within the GCB and ABC DLBCL molecular subtypes will provide a definitive comparison of these regimens (CALGB 50303).
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993;328(14):1002–6. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 2.Pfreundschuh M, Trumper L, Kloess M, Schmits R, Feller AC, Rudolph C, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: results of the NHL-B1 trial of the DSHNHL. Blood. 2004;104(3):626–33. doi: 10.1182/blood-2003-06-2094. [DOI] [PubMed] [Google Scholar]
- 3.Pfreundschuh M, Trumper L, Kloess M, Schmits R, Feller AC, Rube C, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004;104(3):634–41. doi: 10.1182/blood-2003-06-2095. [DOI] [PubMed] [Google Scholar]
- 4.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 5.Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379–91. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 6.Tilly H, Lepage E, Coiffier B, Blanc M, Herbrecht R, Bosly A, et al. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood. 2003;102(13):4284–9. doi: 10.1182/blood-2003-02-0542. [DOI] [PubMed] [Google Scholar]
- 7.Tilly H, Mounier N, Lederlin P, Briere J, Dupriez B, Sebban C, et al. Randomized comparison of ACVBP and m-BACOD in the treatment of patients with low-risk aggressive lymphoma: the LNH87-1 study. Groupe d'Etudes des Lymphomes de l'Adulte. J Clin Oncol. 2000;18(6):1309–15. doi: 10.1200/JCO.2000.18.6.1309. [DOI] [PubMed] [Google Scholar]
- 8.Andre M, Mounier N, Leleu X, Sonet A, Brice P, Henry-Amar M, et al. Second cancers and late toxicities after treatment of aggressive non-Hodgkin lymphoma with the ACVBP regimen: a GELA cohort study on 2837 patients. Blood. 2004;103(4):1222–8. doi: 10.1182/blood-2003-04-1124. [DOI] [PubMed] [Google Scholar]
- 9.Coiffier CH Bertrand, Fermé Christophe, Molina Thierry Jo, Casasnovas Olivier, Gisselbrecht Christian, Bosly Andre, Laurent Guy, Morschhauser Franck, Ghesquieres Herve, Jardin Fabrice, Bologna Serge, Fruchart Christophe, Canioni Danielle, Corront Bernadette, Gabarre Jean, Bonnet Christophe, Janvier Maud, Tilly Herve. A Prospective Randomized Study Comparing Dose Intensive Immunochemotherapy with R-ACVBP vs Standard R-CHOP In Younger Patients with Diffuse Large B-Cell Lymphoma (DLBCL). Groupe d’Etude Des Lymphomes De l’Adulte (GELA) Study LNH03-2B. Proc Am Assoc Heme. 2010;116(21) Abstract 109. [Google Scholar]
- 10.Wilson WH. Drug resistance in diffuse large B-cell lymphoma. Semin Hematol. 2006;43(4):230–9. doi: 10.1053/j.seminhematol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez M, Chabner BA, Pearson D, Steinberg SM, Jaffe ES, Cheson BD, et al. Role of a doxorubicin-containing regimen in relapsed and resistant lymphomas: an 8-year follow-up study of EPOCH. J Clin Oncol. 2000;18(21):3633–42. doi: 10.1200/JCO.2000.18.21.3633. [DOI] [PubMed] [Google Scholar]
- 12.Moskowitz CH, Schoder H, Teruya-Feldstein J, Sima C, Iasonos A, Portlock CS, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in Advanced-stage diffuse large B-Cell lymphoma. J Clin Oncol. 2010;28(11):1896–903. doi: 10.1200/JCO.2009.26.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller TP, Grogan TM, Dahlberg S, Spier CM, Braziel RM, Banks PM, et al. Prognostic significance of the Ki-67-associated proliferative antigen in aggressive non-Hodgkin's lymphomas: a prospective Southwest Oncology Group trial. Blood. 1994;83(6):1460–6. [PubMed] [Google Scholar]
- 14.Wilson WH, Teruya-Feldstein J, Fest T, Harris C, Steinberg SM, Jaffe ES, et al. Relationship of p53, bcl-2, and tumor proliferation to clinical drug resistance in non-Hodgkin's lymphomas. Blood. 1997;89(2):601–9. [PubMed] [Google Scholar]
- 15.Wilson WH, Grossbard ML, Pittaluga S, Cole D, Pearson D, Drbohlav N, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood. 2002;99(8):2685–93. doi: 10.1182/blood.v99.8.2685. [DOI] [PubMed] [Google Scholar]
- 16.Wilson WH, Grossbard ML, Pittaluga S, Cole D, Pearson D, Drbohlav N, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood. 2002;99(8):2685–93. doi: 10.1182/blood.v99.8.2685. [DOI] [PubMed] [Google Scholar]
- 17.Wilson WH, Dunleavy K, Pittaluga S, Hegde U, Grant N, Steinberg SM, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26(16):2717–24. doi: 10.1200/JCO.2007.13.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson WH, Bryant G, Bates S, Fojo A, Wittes RE, Steinberg SM, et al. EPOCH chemotherapy: toxicity and efficacy in relapsed and refractory non-Hodgkin's lymphoma. J Clin Oncol. 1993;11(8):1573–82. doi: 10.1200/JCO.1993.11.8.1573. [DOI] [PubMed] [Google Scholar]
- 19.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Ferme C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23(18):4117–26. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 21.Crowther D, Lister TA. The Cotswolds report on the investigation and staging of Hodgkin's disease. Br J Cancer. 1990;62(4):551–2. doi: 10.1038/bjc.1990.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–81. [Google Scholar]
- 24.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;50:163–70. [PubMed] [Google Scholar]
- 25.A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329(14):987–94. doi: 10.1056/NEJM199309303291402. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 26.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–11. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 27.Mead GM, Sydes MR, Walewski J, Grigg A, Hatton CS, Pescosta N, et al. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt's lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002;13(8):1264–74. doi: 10.1093/annonc/mdf253. [DOI] [PubMed] [Google Scholar]
- 28.Epner EM, Unger J, Miller T, Rimsza L, Spier C, LeBlanc M, Fisher R. A multi center trial of hyperCVAD+rituxan in patients with newly diagnosed mantle cell lymphoma. Blood. 2007;110(11):387. [Google Scholar]
- 29.Garcia-Suarez J, Banas H, Arribas I, De Miguel D, Pascual T, Burgaleta C. Dose-adjusted EPOCH plus rituximab is an effective regimen in patients with poor-prognostic untreated diffuse large B-cell lymphoma: results from a prospective observational study. Br J Haematol. 2007;136(2):276–85. doi: 10.1111/j.1365-2141.2006.06438.x. [DOI] [PubMed] [Google Scholar]
- 30.Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP Versus CHOP Alone or With Maintenance Rituximab in Older Patients With Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2006;24(19):3121–7. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 31.Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9(2):105–16. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 32.Gang AO, Strom C, Pedersen M, d'Amore F, Pedersen LM, Bukh A, et al. R-CHOEP-14 improves overall survival in young high-risk patients with diffuse large B-cell lymphoma compared with R-CHOP-14. A population-based investigation from the Danish Lymphoma Group. Ann Oncol. 2012;23(1):147–53. doi: 10.1093/annonc/mdr058. [DOI] [PubMed] [Google Scholar]
- 33.Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027–33. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 34.Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857–61. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 35.Dunleavy K, Little RF, Pittaluga S, Grant N, Wayne AS, Carrasquillo JA, et al. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 2010;115(15):3017–24. doi: 10.1182/blood-2009-11-253039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359(22):2313–23. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–47. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 38.Iqbal J, Sanger WG, Horsman DE, Rosenwald A, Pickering DL, Dave B, et al. BCL2 Translocation Defines a Unique Tumor Subset within the Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. Am J Pathol. 2004;165(1):159–66. doi: 10.1016/s0002-9440(10)63284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutierrez-Garcia G, Cardesa-Salzmann T, Climent F, Gonzalez-Barca E, Mercadal S, Mate JL, et al. Gene expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood. 2011;117(18):4836–43. doi: 10.1182/blood-2010-12-322362. [DOI] [PubMed] [Google Scholar]
- 40.Ott G, Ziepert M, Klapper W, Horn H, Szczepanowski M, Bernd HW, et al. Immunoblastic morphology but not the immunohistochemical GCB/nonGCB classifier predicts outcome in diffuse large B-cell lymphoma in the RICOVER-60 trial of the DSHNHL. Blood. 2010;116(23):4916–25. doi: 10.1182/blood-2010-03-276766. [DOI] [PubMed] [Google Scholar]
- 41.Fu K, Weisenburger DD, Choi WW, Perry KD, Smith LM, Shi X, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol. 2008;26(28):4587–94. doi: 10.1200/JCO.2007.15.9277. [DOI] [PubMed] [Google Scholar]
- 42.Rayman N, Lam KH, van der Holt B, Koss C, Veldhuizen D, Budel LM, et al. Prognostic Relevance of Immunohistochemical Subclassification of Diffuse Large B-Cell Lymphoma in Two Prospective Phase III Clinical Trials. Clin Lymphoma Myeloma Leuk. 2011;11(1):23–32. doi: 10.3816/CLML.2011.n.003. [DOI] [PubMed] [Google Scholar]
- 43.de Jong D, Rosenwald A, Chhanabhai M, Gaulard P, Klapper W, Lee A, et al. Immunohistochemical Prognostic Markers in Diffuse Large B-Cell Lymphoma: Validation of Tissue Microarray As a Prerequisite for Broad Clinical Applications--A Study From the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol. 2007;25(7):805–12. doi: 10.1200/JCO.2006.09.4490. [DOI] [PubMed] [Google Scholar]
- 44.Phan RT, Dalla-Favera R. The BCL6 protooncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004;432(7017):635–9. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- 45.Phan RT, Saito M, Basso K, Niu H, Dalla-Favera R. BCL6 interacts with the transcription factor Miz-1 to suppress the cyclin-dependent kinase inhibitor p21 and cell cycle arrest in germinal center B cells. Nat Immunol. 2005;6(10):1054–60. doi: 10.1038/ni1245. [DOI] [PubMed] [Google Scholar]
- 46.Paik JH, Jeon YK, Park SS, Kim YA, Kim JE, Huh J, et al. Expression and prognostic implications of cell cycle regulatory molecules, p16, p21, p27, p14 and p53 in germinal centre and non-germinal centre B-like diffuse large B-cell lymphomas. Histopathology. 2005;47(3):281–91. doi: 10.1111/j.1365-2559.2005.02222.x. [DOI] [PubMed] [Google Scholar]
- 47.Ranuncolo SM, Polo JM, Dierov J, Singer M, Kuo T, Greally J, et al. Bcl-6 mediates the germinal center B cell phenotype and lymphomagenesis through transcriptional repression of the DNA-damage sensor ATR. Nat Immunol. 2007;8(7):705–14. doi: 10.1038/ni1478. [DOI] [PubMed] [Google Scholar]
- 48.Pasqualucci L, Migliazza A, Basso K, Houldsworth J, Chaganti RS, Dalla-Favera R. Mutations of the BCL6 protooncogene disrupt its negative autoregulation in diffuse large B-cell lymphoma. Blood. 2003;101(8):2914–23. doi: 10.1182/blood-2002-11-3387. [DOI] [PubMed] [Google Scholar]
- 49.Kurosu T, Fukuda T, Miki T, Miura O. BCL6 overexpression prevents increase in reactive oxygen species and inhibits apoptosis induced by chemotherapeutic reagents in B-cell lymphoma cells. Oncogene. 2003;22(29):4459–68. doi: 10.1038/sj.onc.1206755. [DOI] [PubMed] [Google Scholar]
- 50.Theard D, Coisy M, Ducommun B, Concannon P, Darbon J-M. Etoposide and Adriamycin but Not Genistein Can Activate the Checkpoint Kinase Chk2 Independently of ATM/ATR. Biochemical and Biophysical Research Communications. 2001;289(5):1199–204. doi: 10.1006/bbrc.2001.6095. [DOI] [PubMed] [Google Scholar]
- 51.Siu WY, Lau A, Arooz T, Chow JPH, Ho HTB, Poon RYC. Topoisomerase poisons differentially activate DNA damage check-points through ataxia-telangiectasia mutated-dependent and -independent mechanisms. Mol Cancer Ther. 2004;3(5):621–32. [PubMed] [Google Scholar]